Abstract
The use of manufactured nanomaterials is rapidly increasing, while our understanding of the consequences of releasing these materials into the environment is still limited and many questions remain, for example, how do nanoparticles affect living organisms in the wild? How do organisms adapt and protect themselves from exposure to foreign materials? How does the environment affect the performance of nanoparticles, including their surface properties? In an effort to address these crucial questions, our main aim has been to probe the effects of aquatic organisms on nanoparticle aggregation. We have, therefore, carried out a systematic study with the purpose to disentangle the effects of the freshwater zooplankter, Daphnia magna, on the surface properties, stability, and aggregation properties of gold (Au) nanoparticles under different aqueous conditions as well as identified the proteins bound to the nanoparticle surface. We show that Au nanoparticles aggregate in pure tap water, but to a lesser extent in water that either contains Daphnia or has been pre-conditioned with Daphnia. Moreover, we show that proteins generated by Daphnia bind to the Au nanoparticles and create a modified surface that renders them less prone to aggregation. We conclude that the surrounding milieu, as well as the surface properties of the original Au particles, are important factors in determining how the nanoparticles are affected by biological metabolism. In a broader context, our results show how nanoparticles released into a natural ecosystem become chemically and physically altered through the dynamic interactions between particles and organisms, either through biological metabolism or through the interactions with biomolecules excreted by organisms into the environment.
Introduction
Nanoparticles are, due to their growing production and use, increasingly finding their way into our environment and it is, therefore, important to improve our understanding of their effects on organisms, both in terms of metabolism and behavior, and the manner in which these organisms cope with these foreign materials. Furthermore, it is crucial not only to study the effects of nanoparticles on living organisms but also to evaluate to what extent the nanoparticles themselves undergo structural or chemical changes as they are being processed and metabolized through uptake and ingestion by organisms.
Different types of nanoparticles have been shown to undergo surface modifications in a biological milieu, such as being coated by a protein-lipid layer in human plasma (Hellstrand et al. Citation2009) or through protein-driven aggregation in cow serum (Cukalevski et al. Citation2015). A corona of proteins and other biomolecules has been shown to form on nanoparticles (Cedervall et al. Citation2007; Nel et al. Citation2009), which changes over time (Dell’Orco et al. Citation2010; Tenzer et al. Citation2013) and with changes in the environment (Lundqvist et al. Citation2011), the corona composition may even serve as a marker for its path through an organism (Monopoli et al. Citation2012). Moreover, when nanoparticles enter natural ecosystems, e.g. through emissions or via sewage, biomolecules may change the fate and impact of nanoparticles (Lowry et al. Citation2012), although our knowledge of this process is negligible and therefore constitutes the rationale for our study.
The common freshwater zooplankter, Daphnia, feeds by filtering large volumes of water to collect food particles typically ranging from 240 to 640 nm in size (Geller and Muller Citation1981), although they have also been shown to ingest smaller nano-sized particles (Rosenkranz et al. Citation2009; Cedervall et al. Citation2012). The toxicity of metallic nanoparticles on Daphnia magna has been studied extensively, although the reported toxicity varies. Some general conclusions can be drawn, however, including the effects of surface charge on toxicity. For example, gold (Au) nanoparticles with negatively charged ligands have been shown to be less toxic than those carrying positively charged modifications (Lee and Ranville Citation2012; Bozich et al. Citation2014). Moreover, the size of nanoparticles has been shown to play an important role with respect to toxicity (Blinova et al. Citation2010; Dabrunz et al. Citation2011), which is also the case regarding the exposure time of the organism to the particles (Zhu, Chang, and Chen Citation2010; Dabrunz et al. Citation2011). In contrast, Wrench et al. found no dependency of size, coating, aggregation or type of medium on toxicity when Daphnia were exposed to TiO2 and ZnO nanoparticles (Wiench et al. Citation2009). Similarly, Li et al. exposed Daphnia to silver (Ag) nanoparticles and found no correlation between size of the particle, in the range of 36–66 nm, and toxicity (Li et al. Citation2010). The outcome of these studies on biota, however, showed that exposure to any type of nanoparticle led to long-term harmful effects, such as reduced growth rate, increased mortality, and reduced reproduction (Wiench et al. Citation2009; Zhu, Chang, and Chen Citation2010). It is also of importance to understand the aggregation properties of nanoparticles in an aqueous environment, as well as how they are affected by the digestive system of the organisms. Nanomaterials have been detected inside Daphnia after incubation (Scanlan et al. Citation2013; Mattsson et al. Citation2016) possibly by being passively transported through the feeding current of the animal. Changes in particle aggregation state in the surrounding medium are likely to affect the filtration and uptake of nanoparticles by Daphnia.
Feeding and toxicity experiments are often performed using regular tap water (TW), which varies considerably in chemical composition depending on location and type of plumbing material used, such as copper or plastic. TW contains a range of ions and molecules that may influence the aggregation state of the nanoparticles as well. Furthermore, the aqueous medium within the container used in experiments will change with time, as it is being filtered and digested by organisms. In order to evaluate the effects of nanoparticles under different aqueous conditions it is important to characterize them with respect to size distribution both prior to and throughout the duration of an experiment. There are different methods available for measuring particle size, all with their own benefits and limitations, and the estimated size and size distribution will be influenced by the method used. In order to draw meaningful conclusions, it is therefore important not only to rely on one type of measurement or analysis, but to use a combination of techniques.
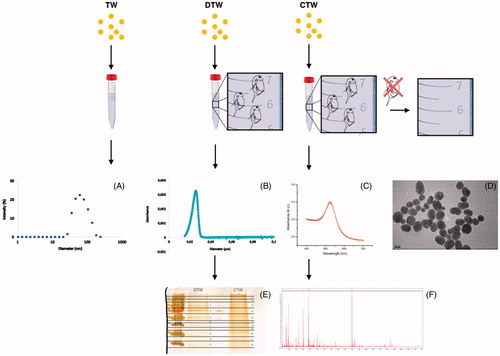
In this study, we use Au nanoparticles as model particles to evaluate how the zooplankter D. magna modifies the surface properties of nanoparticles under a set of different conditions. Changes are evaluated by studying how the aggregation of Au nanoparticles is dependent on the context of the surrounding aqueous medium, as well as on the type of surface modification of the nanoparticles. Moreover, we investigate the fate of Au nanoparticles as they pass through the digestive system of D. magna, i.e. what happens with the nanoparticles in terms of changes in aggregation properties and surface modifications. We employ dynamic light scattering (DLS), differential sedimentation centrifugation (DSC), as well as absorbance spectroscopy to assess particle size before and after the particles have passed through the metabolic machinery of the animal. For comparison, we are also studying the aggregation behavior of bovine serum albumin (BSA)-coated Au nanoparticles, i.e. particles coated with a protein resembling the corona expected to form as the nanoparticles pass through the metabolic system of an organism. We have also identified the proteins bound to the Au particles after incubation with Daphnia or in conditioned water, i.e. water that has been filtered by Daphnia, using mass spectrometry analysis ().
Materials and methods
Preparation of Au nanoparticles
Citrate-coated Au nanoparticles were prepared by the protocol described by Lèvy et al. (Citation2004). Briefly, doubly deionized water (250 mL) was heated to reflux in a 500 mL round-bottomed flask, equipped with a stir bar, and citric acid (1% in water, 12.5 mL) and chloroauric acid (100 mM, 1.5 mL) were added. The resulting solution was allowed to stir for 20 min before the heat source was removed and the solution allowed to cool to room temperature. The Au particles were used as such without further purification. The process provided a monodisperse solution of 25 nm gold nanoparticles, as verified by DLS, DSC, and absorbance spectroscopy, as described below under Data Analysis. After the pH was adjusted to 7.0, the particles were used without purification. The calculated concentration of Au nanoparticles were 1.1*1012 nps/mL or 174 mg/L based on a radius of 12.5 nm.
Incubation experiments
Adult D. magna, approximate 3 mm in length, were used in all experiments described. The D. magna originate from Lake Bysjön, Skåne, Sweden (55°50′15.3″ N, 13°17′16.4″ E) and have been kept under controlled laboratory conditions for more than 100 generations. At time zero, Au nanoparticles were added to tubes containing: (1) TW, (2) conditioned water (CTW) or (3) TW incubated with D. magna (DTW) for the duration of the experiment (24 h). Conditioned tap water (CTW) was prepared by the addition of 10 Daphnia to each tube containing TW. The Daphnia were then allowed to filtrate the water for 24 h and were then removed prior to the addition of the Au nanoparticles. At time zero, Au particles were added and removed for analysis at four different time points, 0 h i.e. right after the particles were added, 1, 4 and 24 h, for a total incubation time of 24 h at ambient temperature. The TW originated from lake Bolmen, southern Sweden and the yearly (12 months) mean values were: pH 8.5, hardness (Ca + Mg) 26 mg/L, conductivity 17 mS/m, a total organic carbon content of 2.1 mg/L, and an alkalinity of 56 mg/L.
Incubation of Au nanoparticles in TW, CTW, or DTW
Twelve 15 mL Falcon tubes with 9.0 mL each of TW, CTW, or DTW (four tubes for each condition) and 1.0 mL (10% v/v) of Au nanoparticles, i.e. 1.1*1012 Au nanoparticles in each tube or 17.4 mg/L. The DTW tubes contained five Daphnia.
Control experiment: batch effect
A different batch of Au nanoparticles, prepared by the same protocol as described above, were used in the incubation experiment with TW, CTW, or DTW, to ensure consistency in results.
Control experiment: volume effects
Citrate-coated Au nanoparticles were added to the three different conditions (TW, CTW, or DTW) in Eppendorf tubes containing 100 μL of particles in 0.900 mL of medium (10% v/v) in which DTW contained three Daphnia per tube. For each condition, 12 Eppendorf tubes were used, with 3 tubes for each time point (0, 1, 4, and 24h). The Eppendorf tubes from the same time point were mixed prior to analysis.
Identification of Au nanoparticles in Daphnia gut
The same batch of Au nanoparticles was used as described above. In order to be able to remove Daphnia at different time points, 15 mL Falcon tubes were used with 13.5 mL of medium (TW, CTW, or DTW) and 1.5 mL of particles (10% v/v) with three replicates of each condition. DTW contained 10 Daphnia at time zero. At five different time points (1, 3, 5, 12, and 24 h) one Daphnia was removed together with 1.5 mL of medium in order to maintain the same Daphnia to medium concentration of 1 Daphnia per 1.5 mL during the course of the experiment and analyzed under a microscope.
Incubation of BSA coated AU nanoparticles in TW, CTW, or DTW
BSA-coated Au nanoparticles were added to the three different aqueous conditions (TW, CTW, or DTW). For each condition twelve Eppendorf tubes were used, with three tubes for each time point (1 min, 1, 4 and 24 h) with 1.0 mL of medium (TW, CTW, and DTW) and 10% particles by volume. At each time point, the three replicates were mixed before removed for further analysis. For DTW we used three Daphnia in each tube.
Data analysis
Optical microscope analysis
Images of the exposed Daphnia were recorded with Olympus SZX7 microscope with an Infinity 1 camera.
DSC analysis
The size distribution was determined by DSC on a DC24000 UHR Disc centrifuge, CPS Instruments, Inc., USA, at 18 °C. Samples of 100 µl were loaded onto a 12–24% sucrose gradient with detection at 405 nm. From each sample three replicates were analyzed with a delay of 7 min between measurements. Before each run, the system was calibrated by loading 100 µl standard polystyrene particles with a size of 483 nm.
Absorbance spectroscopy
Absorbance spectra were recorded between 400 and 700 nm employing a UV-1800 spectrophotometer, Shimadzu, Japan, using a cuvette with 1 cm path length at 23 °C.
DLS analysis
Samples of 100 μL were pipetted into a 96 well plate and measured by DLS in a DynaPro Plate Reader-II, Wyatt technology, equipped with an 830 nm laser under the sample. Scattered light was detected at 158° and for the correlation function regularization fitting was used. Each sample was measured at 25 °C, 10 times with a delay of 5 min between measurements.
Transmission electron microscope analysis
TEM was performed on a JEOL JEM-1400 instrument. The microscope was charged to 120 kV prior to use. AuNP samples (15 µL) were placed on the surface of a glass microscope slide and allowed to dry at ambient temperature for 10 min and were then adhered to a Formvar 200 mesh copper grid (Ted Pella, Inc.) by sliding the grid on the surface of the slide. The grid was stored in a desiccator for 12 h before imaging. Images were taken with a magnification ranging from x200k to x1.2M.
Characterization of Au nanoparticles in water
The size of the Au nanoparticles in ultrapure Millipore water was characterized by DLS, DSC, and UV-visible spectroscopy. The hydrodynamic diameter was determined to be 29 nm by DLS using a cumulative fit of the data and 25 nm by DSC using the absorbance size distribution mode, . The UV maximum is observed at 529 nm, which corresponds to an Au nanoparticle size of around 25 nm.
Table 1. Size of Au-nanoparticles measured with three different methods, including dynamic light scattering (DLS), differential sedimentation centrifugation (DSC), and absorbance spectroscopy.
Nanoparticle corona mass spectrometry analysis
Identification of Daphnia proteins bound to Au nanoparticles
After 24 h of exposure (CTW or DTW), the four replicates were combined in a 50 mL tube and centrifuged for 4 h at 104 g. The content of the pellet was analyzed by dissolving it in SDS-PAGE loading buffer, incubated in 95 °C and quickly spin before the proteins were separated by a 12% polyacrylamide gel (with 1% sodium dodecyl sulfate (SDS)) electrophoresis and silver stained (Figure S1). From each group (CTW or DTW), 11 bands were cut out and further analyzed.
Protein digestion
Gel pieces (1 × 1 mm) were washed twice in 500 µl 50 mM ammonium bicarbonate/50% acetonitrile to remove the silver stain, then 500 µl of 100% acetonitrile was added to dehydrate the gel pieces. Digestion was performed by adding 25 µl 12 ng/µl sequencing-grade modified trypsin (Promega, Madison, WI, USA) in 50 mM ammonium bicarbonate/10% acetonitrile and incubation on ice for 2 h. Thereafter, 25 µl of 50 mM ammonium bicarbonate +10% acetonitrile in water was added to cover the gel pieces and the gel samples were digested overnight at 37 °C. The next day the peptide-containing solution above the gel pieces was withdrawn, and the peptides further extracted from the gel pieces by the addition of 2:1 50% acetonitrile:1% formic acid (FA) in water, for 2 h at 37 °C. The extracted peptide solutions were pooled, dried in a fume hood overnight and resuspended the next day in 10 µl of 2% acetonitrile:0.1% FA in water before they were analyzed on the mass spectrometer.
Peptide separation and mass spectrometry
Peptides were subjected to reversed phase nano-LC source (Proxeon Biosystems, Odense, Denmark) coupled to an LTQ-Orbitrap Velos Pro mass spectrometer equipped with a nano Easy spray ion source (Thermo Fisher Scientific, Stockholm, Sweden). The chromatographic separation was performed on a 2 cm C18 Acclaim PepMap precolumn (75 μm i.d.) packed with three resin from Thermo Fisher, and a 15 cm C18 EASY-Spray LC Capillary Separation Column (75 μm i.d.) packed with 3 μm resin from Thermo Fisher. The gradient was created by solvent A (0.1% (v/v) FA in water and solvent B (0.1% (v/v) FA in 100% (v/v) acetonitrile) and the gradient was run as follow; 5–30% during 40 min, 30–50% during 20 min and 50–95% during 5 min and then held constant at 95% for 10 min. A flow rate of 300 nl/min was used throughout the whole gradient.
An MS scan (400–1400 m/z) was recorded using an Orbitrap mass analyzer set at a resolution of 60,000 at 400 m/z, 1 × 106 automatic gain control target and 500 ms maximum ion injection time. The MS was followed by data-dependent collision-induced dissociation MS/MS scans on the four most intense ions in the LTQ at 2500 signal threshold, 30,000 automatic gain control target, 300-ms maximum ion injection time, 3.0 m/z isolation width, 10 ms activation time at 35 normalized collision energy and dynamic exclusion enabled for 30 or 600 s with two repeats, auxiliary gas flow; S-lens 60%; ion transfer tube temperature, 275 °C.
Data analysis and protein identification
Raw files typically containing 7000 scans each were converted to mgf-format by Mascot Distiller (version 2.6) and identification of proteins were carried out with the Mascot Daemon software (version 2.4). The following search settings were used: trypsin as protease, two allowed missed cleavage sites, 10 ppm MS accuracy for peptides and 0.15 Da MS/MS accuracy, variable modifications: Oxidation (M). The files were searched against an in-house created database containing D. Magna proteins (downloaded from Uniprot 161215, 506,807 Entries).
Results and discussion
Characterization of Au nanoparticle size distribution
Since it is well known that salinity, pH, and the presence of various organic additives can change the stability of a nanoparticle dispersion and, hence, its aggregation state, we used three different aqueous conditions to test the effects of the surrounding medium on the size distribution of the nanoparticles. In the first condition, we used regular TW and in the second, referred to as CTW, we used TW that had first been digested by Daphnia for 24h, which were then removed. In the third condition, we used TW with Daphnia present throughout the experiment (DTW). Different types of analyses were then used in order to assess the size of the particles after incubation. DLS, absorbance spectroscopy, and DSC provided the size of the particles, including any corona present. In contrast, TEM only provides the Au particle size, since a biomolecule-based corona cannot be visualized by this technique. In order to identify any proteins bound to the particles, analysis by mass spectrometry was applied to extracts of collected particles that had been incubated in DTW and CTW.
No acute toxic effects of Au nanoparticles on Daphnia were seen under any of the conditions used, which is consistent with previously published data (Lee and Ranville Citation2012). The next step was to evaluate the extent to which Daphnia might have affected the properties of Au nanoparticles.
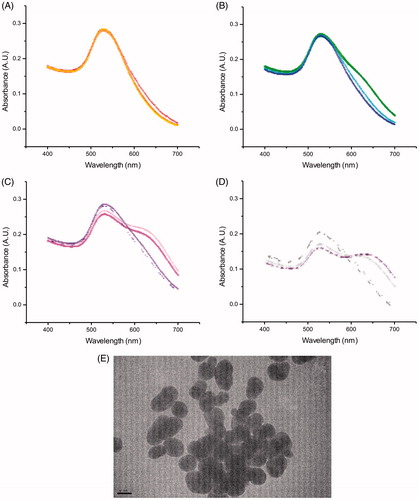
The behavior of the particles under the different aqueous conditions followed a clear trend. Incubation of Au particles in TW led to a rapid increase in nanoparticle aggregate size. A considerable degree of aggregation was observed under all experimental conditions. The DSC data showed a steady increase, up to 300%, in size over time. The DLS data, displayed a 3000% increase in size, from 29 nm to close to a micrometer in diameter after 24 h of incubation. The presence of large aggregates was also indicated by absorbance spectroscopy in the visible range. Monomeric Au nanoparticles displayed a λmax of around 520–530 nm, depending on individual particle size. In the aggregated samples, a second maximum around 632 nm was observed after about 1 h of incubation. After 24 h, this signal was further shifted to 700 nm, with a concomitant increase in intensity (), consistent with a continued aggregation over time. The observed aggregation was likely driven by low ionic strength, which might lead to a dilution of the stabilizing citrate molecules on the Au nanoparticle surface. This result emphasizes the importance of characterizing the aggregation state of the Au nanoparticles in the medium used for experiments or analyses. There was a discrepancy between the DSC data and those obtained by DLS and absorbance spectroscopy, in which the DSC data indicated less aggregation. However, this discrepancy was likely due to the breaking apart of the agglomerates during the separation process due to the centrifugal force of the centrifugation and/or by the sucrose gradient used.
Figure 1. Au nanoparticles were incubated under three conditions: TW (tap water), DTW (tap water containing Daphnia) and CTW (conditioned tap water, in which Daphnia had filtered the water for 24 h before removal). The media were then analysed with dynamic light scattering (A), disc centrifugation (B), absorbance spectroscopy (C) and trans emission electron microscopy (D). The particles and proteins in DTW and CTW were further analysed by SDS gel chromatrography (E) and mass spectrometry (F).
Size and aggregation of Au nanoparticles were also affected in CTW and DTW treatments, but to a lesser extent and more slowly than in TW. In CTW, an increase in size was clearly observed by the DLS analysis. While the DSC and the absorbance spectroscopy data were generally consistent with this finding, the absorbance data, in particular, were less clear as some samples lacked the second absorbance peak at 600–700 nm. In DTW, the size increase was initially modest, as seen in the data collected after 1 h of incubation, but after 4 h it increased significantly. According to DSC, two distinct populations of particles were present, with an average diameter of 32 and 57 nm, respectively. The absorbance spectra, at time 1 min, displayed a single λmax at 529 nm, consistent with a particle diameter of 25 nm. Over time, a second maximum appeared around 640 nm, which confirms that the Au nanoparticles aggregated in DTW. The differences in aggregate size of Au nanoparticles incubated in TW, CTW, and DTW, strongly indicate that Au nanoparticles become modified through the Daphnia metabolism, likely due to the biomolecules secreted by the animals forming a corona around the particles.
provides a summary of the average size of Au particles incubated in regular TW, in tap water with Daphnia (DTW) or in CTW, as measured with DLS, DSC, and absorbance spectroscopy. The overall outcome was likely a combination of a difference in the range and concentration of salts and biomolecules present in the type of TW used, as discussed in more detail below. In some cases, it was difficult to discern the number of actual size distributions present because of the relatively small difference in particle sizes. The DLS data generally showed a larger, less well defined, peak which indicate the presence of aggregates, but since the size of the aggregates differed between replicates we only denote the presence of aggregates with a ‘yes’ instead of providing an exact size. However, the absorbance spectroscopy data suggest that the modification of the Au nanoparticles was different after incubation with Daphnia (DTW) compared to in conditioned water (CTW). The λmax for TW 527–528 nm and for CTW 529–530 nm indicates an Au nanoparticle size of around 25 nm. After 4 h incubation with Daphnia (DTW), the wavelength maximum shifted by 2–3 nm to a longer wavelength, and . This result is consistent with the presence of molecules bound to the surface, affecting the plasmonic field and shifting the absorbance to longer wavelengths. This increase in wavelength was not seen after incubation in conditioned water (CTW), indicating differences in the surface modification when particles had passed through the Daphnia digestive system. In both DTW and CTW, as described above, a second λmax at 637–700 nm was also observed after incubation, consistent with the presence of aggregates.
Figure 2. Absorbance spectra of Au nanoparticles when incubated in tap water (TW) at (A) 0 hour, i.e. the samples were taken right after the particles were added to the test tubes, (B) 1 hour, (C) 4 hour and (D) 24 hours (4 samples each), with (E) TEM image from particles in tap water (TW).
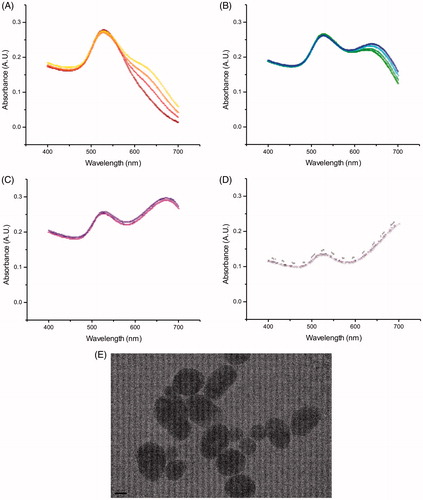
Figure 3. Absorbance spectra of Au nanoparticles when incubated with Daphnia (DTW) at (A) 0 h, i.e. a sample taken right after the particles were added to the test tubes, (C) 1 h, (E) 4 h and (G) 24 h (four samples each), with TEM images at time points (B) 0 h, (D) 1 h, (F) 4 h and (H) 24 h.
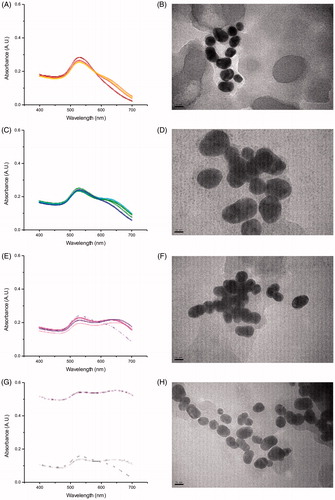
Table 2. Diameter of Au nanoparticles at different incubation times in TW, CTW and DTW, as determined by DLS, DSC and absorbance spectroscopy (N = 4 for each time point).
Metabolism of Au nanoparticles by Daphnia
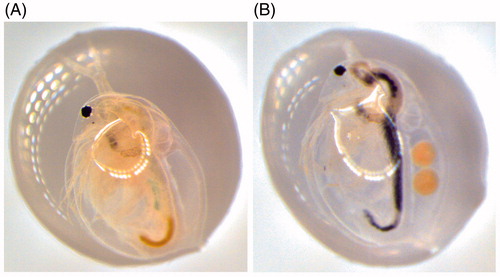
The average size and size distribution of the particles were analyzed after incubation with Daphnia (DTW) and after incubation in Daphnia conditioned water (CTW). The important difference between the two experimental conditions was that, when incubated with Daphnia (DTW), the particles were filtered by Daphnia and entered its digestive system, which might have exposed them to either (1) a higher concentration of biomolecules and/or (2) a different and more diverse set of biomolecules than when the water was only pre-conditioned with Daphnia (CTW). Daphnia can ingest nano- and micro-sized (20–70 μm) particles (Rosenkranz et al. Citation2009; Zhu et al. Citation2009) and it is likely that some particles will pass through the digestive system since Daphnia has a high filtering rate, up to 18.5 mL/h (per mg dry wt Daphnia) (Burns Citation1969) and after 10 min of filtration they can replace up to 3.1% of their body weight (Stobbart, Keating, and Earl Citation1977). Moreover, in case of a low food level, they can ingest sediment particles by scraping the bottom or stirring up sediments (Gillis et al. Citation2005), which reduce any potential problems with aggregation and/or accumulation of particles at the bottom of the test tubes. However, to confirm that Au nanoparticles of this size actually ended up in the digestive system we retrieved Daphnia at different time points of incubation and analyzed them under a microscope. After 4 h, Au nanoparticles were clearly visible inside the digestive system, . Furthermore, in samples where Daphnia had shed their outer carapace, i.e. their exoskeleton, these were shown to carry bound Au nanoparticles. This result shows that in addition to being metabolized by the animals, the Au nanoparticles also interact directly with the carapace, in agreement with other studies (Wray and Klaine Citation2015; Botha, Boodhia, and Wepener Citation2016).
Proteins associated with Au nanoparticles
The Au nanoparticles incubated with Daphnia (DTW) or in conditioned water (CTW) showed less increase in size compared to Au nanoparticles in TW (). Furthermore, absorbance spectroscopy data suggest that they were coated with a corona consisting of biomolecules originating from Daphnia. Proteins can, depending on identity and concentration, destabilize or stabilize nanoparticles (Cukalevski et al. Citation2015). A possible explanation for the difference in aggregation between the three conditions (DTW, CTW, and TW) can, in part, be because of differences in the protein binding. To explore this hypothesis, we analyzed the protein content of the corona from the Au nanoparticles incubated in DTW and CTW, after Au nanoparticles were collected by centrifugation. Proteins bound to the Au nanoparticles were desorbed by treatment with SDS and the resulting protein mixture separated by gel electrophoresis, Figure S1. A higher amount of proteins, as judged from the staining of the gel, appeared to be bound to the Au nanoparticles in CTW than in DTW. Furthermore, a larger number of proteins (175 vs. 90) were bound to the Au nanoparticles in CTW, compared to when incubated together with Daphnia (DTW), . The fact that, in the CTW, Au nanoparticles bound a larger number of proteins is consistent with the aggregate size results. In the case of Au nanoparticles characterized from DTW and CTW, the aggregates were smaller compared to in TW, consistent with the hypothesis that proteins on the particle surfaces were stabilizing the dispersion and preventing aggregation of the nanoparticles in CTW and DTW. A total of 265 proteins were identified from 22 bands isolated by gel electrophoresis, Table S1 and Figure S1. However, out of the 265 proteins only 8 were identical between the two tested conditions. There was a difference between the amount and number of proteins bound to Au nanoparticles in CTW and DTW, which is likely a result of Daphnia filtered nanoparticles first coming in contact with proteins in the digestive system. These proteins may differ from the proteins secreted. Thus, Au nanoparticles in CTW will never come in contact with the same proteins as Au nanoparticles in DTW. The binding of Daphnia-derived proteins to Au nanoparticles only partly explains the stabilization of the particles.
Protein (BSA) conjugated Au nanoparticles
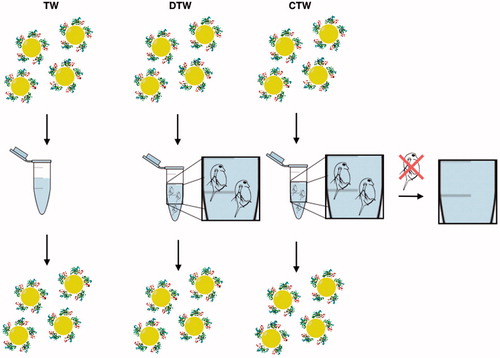
In the standard synthesis of Au nanoparticles, citrate is used to reduce chloric auric acid, HAuCl4, to Au. At the same time as Au is generated and associates to form nanoparticles, citrate adheres to the surface of the growing nanoparticles. The negatively charged citrate coating at neutral pH serves as a shield to prevent aggregation of the nanoparticles. Our results suggest that the citrate coating of the Au nanoparticles was modified by Daphnia during digestion, presumably by secreted biomolecules, particularly proteins. In order to test this hypothesis, we performed a control experiment by using a sample from the same batch of synthetic citrate-coated Au nanoparticles as in the previous experiments but modified with the protein BSA. The Au nanoparticles were incubated with BSA in a 1:1 molar ratio in Millipore water for 12 h. The BSA conjugated Au nanoparticles were then analyzed under the same conditions as described above in TW, CTW, and DTW. These particles were not toxic to Daphnia under any conditions over a time period of 24 h, i.e. no mortality was observed. Furthermore, no aggregation of the BSA conjugated Au nanoparticles was observed under any of the conditions, clearly showing that BSA stabilized the Au nanoparticle dispersion and that, if further modifications occurred after incubation with Daphnia, it did not affect the stability of the Au nanoparticles, , Figures S2–S4. The presence of a second peak observed in the DLS data () indicates the presence of biomolecules originating from the Daphnia in both CTW and DTW. The BSA-coated nanoparticles do not aggregate and were confirmed with TEM images. The TEM images of the BSA-coated nanoparticles in TW (Figure S2) show that they are more clearly separated from one another compared to the non-coated nanoparticles in TW (). The dimension of the space between particles is consistent with a monolayer of BSA molecules surrounding each particle.
Table 3. Diameter of Au nanoparticles at different time points for BSA-coated particles (N = 3 for each time point).
Differences in surface chemistry are important as the citrate stabilized Au nanoparticles, which were initially used, were affected by incubation in water (TW), conditioned water (CTW) and when exposed to Daphnia (DTW). Interestingly, BSA conjugated Au nanoparticles were not affected under any of these conditions. The effect of BSA on the nanoparticles was presumably very similar to that of citrate as BSA is a negatively charged protein at physiological conditions and thereby creates a negatively charged coating around the nanoparticle (). It is also considerably larger in size than citrate, which might further aid in the coating of the Au nanoparticles and thereby shield them from one another. When citrate is present, the BSA binds to the gold surface by an electrostatic mechanism (Brewer et al. Citation2005), which makes the particles less stable than when BSA only binds to the gold particle directly. However, our results suggest that the BSA surface is more stable when present on nanoparticles coated with citrate rather than clean Au particles themselves. Hence, if an organism in a natural ecosystem ingests or interacts with nanoparticles, it is likely that most of the particles have a surface modification made up by more than one type of biomolecules. We used BSA as a model protein to investigate if a coating of Au by proteins or biomolecules can affect how Daphnia modify the particles.
Figure 6. Au nanoparticles were incubated under three conditions TW (tap water), DTW (tap water containing Daphnia) and CTW (conditioned tap water in which Daphnia had filtered the water during 24 h prior to being removed). The water was then analyzed with DLS, DSC, absorbance spectroscopy and TEM. In TW the particles aggregate. In DTW and CTW proteins and other biomolecules present bind to the particles affecting the size of the aggregates. As shown in the Venn diagram, the overlap in the number of identified proteins (n = 8) is very low compared to the overall number of proteins present in DTW (red, n = 90) vs. CTW (blue, n = 175).
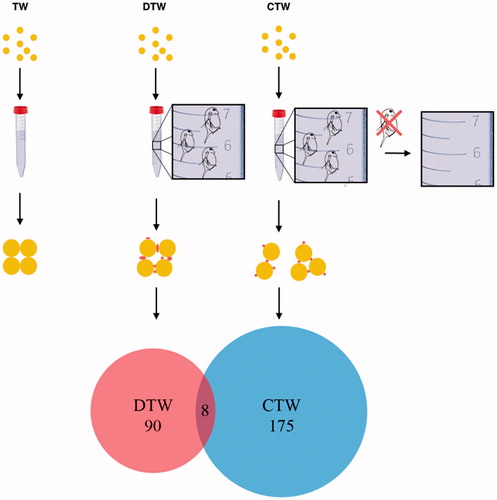
Figure 7. BSA-coated Au nanoparticles were incubated under three conditions TW (tap water), DTW (tap water containing Daphnia) and CTW (conditioned tap water, pre-incubated with Daphnia for 24 h before removal). The media were then analyzed with DLS, DSC, UV–VIS and TEM showing that the particles do not aggregate but remain in their initial state.
Volume and match effects
The volume of the test tubes used in the incubation experiments can affect the size distribution of the nanoparticles (Baumann et al. Citation2014) and, in addition, there might be a batch dependency. Therefore, we performed an experimental setup with two different batches in two different volumes each (1.5 and 15 mL tubes). For the second batch, we registered similar size distributions for DTW and CTW with all used techniques, Table S2, Figures S5–S7. However, for the TW treatment, there was no second maximum registered, indicating larger aggregates with absorbance spectroscopy. When changing the volume by a factor of 10, while maintaining the same concentration of particles, discrepancies were observed among the DLS, DSC, and absorbance spectroscopy data. However, overall, the Au nanoparticles aggregated to a lesser degree in the small 1.5 mL tubes compared to in the larger 15 mL tubes, regardless of treatment, Table S3, Figures S8–S10. A difference between the large and the small tubes is that the ratio between the volume of Au nanoparticles and the surface area of the tubes is smaller. A difference in surface area potentially changes the fraction of Au nanoparticles that interacts with the wall, as well as the manner by which the secreted biomolecules interact with the surface. Importantly, a change in volume also affects the water flow in the tubes when filtered by Daphnia.
Mechanisms and importance
The size and aggregation properties of individual Au nanoparticles are strongly affected by the particle coating. However, the citrate shield, initially coating the synthesized Au particles used in this study, may be affected by the surrounding medium. Here, we show that when the particles are incubated in pure TW a considerable amount of aggregation is observed among the Au nanoparticles, consistent with a shedding of the repelling citrate due to dilution or displacement by other small molecules or ions. The particles were also affected by water containing Daphnia (DTW), but here the effect was different in that less aggregation was observed. Similarly, the Au particles were affected by TW pre-conditioned with Daphnia (CTW). In both cases, less aggregation was likely a result of surface modifications of the nanoparticles by biomolecules produced by Daphnia. Although both conditions caused aggregation, it was more pronounced in water that had only been filtered through Daphnia rather than in water incubated with water where Daphnia had been swimming. The aggregates were also larger in size due to coating by molecules presumably consisting mainly of proteins. These were identified and we show that the number of identified Daphnia proteins on Au particles in CTW was higher than the number of proteins on the particles that had passed through the Daphnia digestive system (DTW). This finding suggests that the binding of Daphnia proteins to the Au nanoparticles explains the stabilization of the particles, thereby preventing aggregation. In conditioned water, the particles bound a larger number of proteins compared to when digested by Daphnia, and the size of the aggregates was smaller, which confirms that the proteins on the Au particle surface were stabilizing the particles and prevented them from forming aggregates. Proteins can aggregate nanoparticles due to depletion forces, i.e. the entropy for the system as a whole increases if the big objects (nanoparticles) are brought together giving the small objects (proteins) more possible configurations. In addition, there may be specific effects due to protein-nanoparticles interactions by which proteins can both aggregate and stabilize a nanoparticle dispersion depending on if there is a protein bridge between particles or not. However, even bridging proteins stabilize them at high protein concentration and stabilize the dispersion (Cukalevski et al. Citation2015), which supports the explanation for our conditions outlined in and . These results are consistent with the idea that a modification of the Au nanoparticles by Daphnia-derived biomolecules prevents aggregation, compared to when the particles are incubated in pure TW.
Many different nanoparticles bind to proteins and other biomolecules which may depend on the type, size, and surface modification of the nanoparticle. In contrast to our work in which protein coating prevented particle aggregation, Nasser and Lynch showed that the proteins released by Daphnia affect the original nanoparticle surface (Citation2016). With respect to polystyrene nanoparticles, they found that the protein coating resulted in promoted aggregation. In our case, a protein corona was generated by circulation through Daphnia as well as by biomolecules produced by Daphnia in pre-conditioned water (CTW). Which proteins bind to the nanoparticles depend on the properties of the particles, such as charge and size. In addition, our study suggests that Au nanoparticles become modified by a range of proteins when incubated in TW containing biomolecules produced by Daphnia. Whether this is a consequence of a deliberate mode of detoxification used by Daphnia to handle foreign materials or simply an association of proteins due to complementary physical properties of the protein surface with the nanoparticle surface, or both, is an interesting future direction of study. Our findings are in agreement with previous studies showing that different media commonly used in Daphnia experiments affect the aggregation state of Au and other metal nanoparticles (Baalousha Citation2009; Bian et al. Citation2011; Cupi, Hartmann, and Baun Citation2015), as well as polymeric particles (Nasser and Lynch Citation2016). However, in addition, we show that particles modified with a protein layer before they are introduced to Daphnia, affect the particle aggregation state in a different way than when they are modified by the Daphnia digestive system.
Conclusion
In conclusion, our results show that the choice of exposure route, including incubation time, type of surrounding medium and incubation volume, affect the aggregation of Au nanoparticles. Moreover, nanoparticles that spread in nature may not only affect organisms but the metabolism of organisms may also affect the chemical properties and function of nanoparticles. Our study highlights the importance of, and provides the recommendation for, characterizing the particles throughout an experiment and to test the effect of nanoparticles using several different methods. Moreover, we emphasize that the complex and intricate interplay between man-made materials and the biological environment leads to alterations in the originally intended properties of the particles which are poorly understood and require further investigations. The overall conclusion is that the activity of animal feeding affects the size and aggregation state of nanoparticles, which is important for the understanding on how the release and spread of nanoparticles into natural ecosystems may alter the properties of nanoparticles. Hence, the biological coating of nanoparticles affects their performance in ways that are difficult to predict and when organisms modify particles, a corona is generated around them that may be protective or, alternatively, could lead to an increased toxicity of the particles. The present work and studies by others suggest that the resulting protein-based surface acts as a shield that detoxifies the particles. While this might be advantageous to the organism, the coating may also potentially disturb the balance of biomolecules within the organism such that it produces a toxic effect over time. Further studies are urgently needed to follow up on the consequences of these findings in natural ecosystems if we are to manage and direct the production of nanomaterials to products with minimal effects on natural ecosystems.
Supplementary Tables 1-3, Figures 1-10
Download MS Word (8.5 MB)Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Baalousha, M. 2009. “Aggregation and Disaggregation of Iron Oxide Nanoparticles: Influence of Particle Concentration, pH and Natural Organic Matter.” The Science of the Total Environment 407 (6): 2093–2101. doi:10.1016/j.scitotenv.2008.11.022.
- Baumann, J., Y. Sakka, C. Bertrand, J. Koser, and J. Filser. 2014. “Adaptation of the Daphnia sp. acute Toxicity Test: miniaturization and Prolongation for the Testing of Nanomaterials.” Environmental Science and Pollution Research 21 (3): 2201–2213. doi:10.1007/s11356-013-2094-y.
- Bian, S. W., I. A. Mudunkotuwa, T. Rupasinghe, and V. H. Grassian. 2011. “Aggregation and Dissolution of 4 Nm ZnO Nanoparticles in Aqueous Environments: influence of pH, Ionic Strength, Size, and Adsorption of Humic Acid.” Langmuir 27 (10): 6059–6068. doi:10.1021/la200570n.
- Blinova, I., A. Ivask, M. Heinlaan, M. Mortimer, and A. Kahru. 2010. “Ecotoxicity of Nanoparticles of CuO and ZnO in Natural Water.” Environmental Pollution (Barking, Essex: 1987) 158 (1): 41–47. doi:10.1016/j.envpol.2009.08.017.
- Botha, T. L., K. Boodhia, and V. Wepener. 2016. “Adsorption, Uptake and Distribution of Gold Nanoparticles in Daphnia Magna following Long Term Exposure.” Aquatic Toxicology 170: 104–111. doi:10.1016/j.aquatox.2015.11.022.
- Bozich, J. S., S. E. Lohse, M. D. Torelli, C. J. Murphy, R. J. Hamers, and R. D. Klaper. 2014. “Surface Chemistry, Charge and Ligand Type Impact the Toxicity of Gold Nanoparticles to Daphnia Magna.” Environmental Science-Nano 1 (3): 260–270. doi:10.1039/C4EN00006D.
- Brewer, S. H., W. R. Glomm, M. C. Johnson, M. K. Knag, and S. Franzen. 2005. “Probing BSA Binding to Citrate-Coated Gold Nanoparticles and Surfaces.” Langmuir: The ACS Journal of Surfaces and Colloids 21 (20): 9303–9307. doi:10.1021/la050588t.
- Burns, C. W. 1969. “Relation between Filtering Rate, Temperature, and Body Size in 4 Species of Daphnia.” Limnology and Oceanography 14 (5):693. doi:10.4319/lo.1969.14.5.0693.
- Cedervall, T., L. A. Hansson, M. Lard, B. Frohm, and S. Linse. 2012. “Food Chain Transport of Nanoparticles Affects Behaviour and Fat Metabolism in Fish.” Plos One 7 (2): e32254. doi:10.1371/journal.pone.0032254.
- Cedervall, T., I. Lynch, S. Lindman, T. Berggard, E. Thulin, H. Nilsson, K. A. Dawson, and S. Linse. 2007. “Understanding the Nanoparticle-Protein Corona Using Methods to Quantify Exchange Rates and Affinities of Proteins for Nanoparticles.” Proc Natl Acad Sci U S A 104 (7): 2050–2055. doi:10.1073/pnas.0608582104.
- Cukalevski, R., S. A. Ferreira, C. J. Dunning, T. Berggard, and T. Cedervall. 2015. “IgG and Fibrinogen Driven Nanoparticle Aggregation.” Nano Research 8 (8): 2733–2743. doi:10.1007/s12274-015-0780-4.
- Cupi, D., N. B. Hartmann, and A. Baun. 2015. “The Influence of Natural Organic Matter and Aging on Suspension Stability in Guideline Toxicity Testing of Silver, Zinc Oxide, and Titanium Dioxide Nanoparticles with Daphnia Magna.” Environmental Toxicology and Chemistry 34 (3): 497–506. doi:10.1002/etc.2855.
- Dabrunz, A., L. Duester, C. Prasse, F. Seitz, R. Rosenfeldt, C. Schilde, G. E. Schaumann, and R. Schulz. 2011. “Biological Surface Coating and Molting Inhibition as Mechanisms of TiO2 Nanoparticle Toxicity in Daphnia Magna.” Plos One 6 (5): e20112. doi:10.1371/journal.pone.0020112.
- Dell'Orco, D., M. Lundqvist, C. Oslakovic, T. Cedervall, and S. Linse. 2010. “Modeling the Time Evolution of the Nanoparticle-Protein Corona in a Body Fluid.” Plos One 5 (6): e10949. doi:10.1371/journal.pone.0010949.
- Geller, W., and H. Muller. 1981. “The Filtration Apparatus of Cladocera: Filter mesh-sizes and Their Implications on Food Selectivity.” Oecologia 49 (3): 316–321. doi:10.1007/BF00347591.
- Gillis, P. L., P. Chow-Fraser, J. F. Ranville, P. E. Ross, and C. M. Wood. 2005. “Daphnia Need to Be Gut-Cleared Too: The Effect of Exposure to and Ingestion of Metal-Contaminated Sediment on the Gut-Clearance Patterns of D. magna.” Aquatic Toxicology (Amsterdam, Netherlands) 71 (2): 143–154. doi:10.1016/j.aquatox.2004.10.016.
- Hellstrand, E., I. Lynch, A. Andersson, T. Drakenberg, B. Dahlback, K. A. Dawson, S. Linse, and T. Cedervall. 2009. “Complete High-Density Lipoproteins in Nanoparticle Corona.” The Febs Journal 276 (12): 3372–3381. doi:10.1111/j.1742-4658.2009.07062.x.
- Lee, B. T., and J. F. Ranville. 2012. “The Effect of Hardness on the Stability of Citrate-Stabilized Gold Nanoparticles and Their Uptake by Daphnia Magna.” Journal of Hazardous Materials 213–214: 434–439. doi:10.1016/j.jhazmat.2012.02.025.
- Levy, R., N. T. Thanh, R. C. Doty, I. Hussain, R. J. Nichols, D. J. Schiffrin, M. Brust, and D. G. Fernig. 2004. “Rational and Combinatorial Design of Peptide Capping Ligands for Gold Nanoparticles.” Journal of the American Chemical Society 126 (32): 10076–10084. doi:10.1021/ja0487269.
- Li, T., B. Albee, M. Alemayehu, R. Diaz, L. Ingham, S. Kamal, M. Rodriguez, and S. W. Bishnoi. 2010. “Comparative Toxicity Study of Ag, Au, and Ag-Au Bimetallic Nanoparticles on Daphnia Magna.” Analytical and Bioanalytical Chemistry 398 (2): 689–700. doi:10.1007/s00216-010-3915-1.
- Lowry, G. V., K. B. Gregory, S. C. Apte, and J. R. Lead. 2012. “Transformations of Nanomaterials in the Environment.” Environmental Science & Technology 46 (13): 6893–6899. doi:10.1021/es300839e.
- Lundqvist, M., J. Stigler, T. Cedervall, T. Berggard, M. B. Flanagan, I. Lynch, G. Elia, and K. Dawson. 2011. “The Evolution of the Protein Corona around Nanoparticles: A Test Study.” ACS Nano 5 (9): 7503–7509. doi:10.1021/nn202458g.
- Mattsson, K., Adolfsson, K. M. T., Ekvall M. T., Borgstrom S., Linse L. A., Hansson T., Cedervall C., and N. Prinz. 2016. “Translocation of 40 Nm Diameter Nanowires through the Intestinal Epithelium of Daphnia Magna.” Nanotoxicology 10 (08): 1160–1167. doi:10.1080/17435390.2016.1189615.
- Monopoli, M. P., C. Aberg, A. Salvati, and K. A. Dawson. 2012. “Biomolecular Coronas Provide the Biological Identity of Nanosized Materials.” Nature Nanotechnology 7 (12): 779–786. doi:10.1038/nnano.2012.207.
- Nasser, F., and I. Lynch. 2016. “Secreted Protein Eco-Corona Mediates Uptake and Impacts of Polystyrene Nanoparticles on Daphnia Magna.” J Proteomics 137: 45–51. doi:10.1016/j.jprot.2015.09.005.
- Nel, A. E., L. Madler, D. Velegol, T. Xia, E. M. Hoek, P. Somasundaran, F. Klaessig, V. Castranova, and M. Thompson. 2009. “Understanding Biophysicochemical Interactions at the Nano-Bio Interface.” Nature Materials 8 (7): 543–557. doi:10.1038/nmat2442.
- Rosenkranz, P., Q. Chaudhry, V. Stone, and T. F. Fernandes. 2009. “A Comparison of Nanoparticle and Fine Particle Uptake by Daphnia Magna.” Environmental Toxicology and Chemistry 28 (10): 2142–2149. doi:10.1897/08-559.1.
- Scanlan, L. D., Reed, R. B. A. V., Loguinov P., Antczak A., Tagmount S., Aloni D. T., Nowinski P. et al. 2013. “Silver Nanowire Exposure Results in Internalization and Toxicity to Daphnia Magna.” ACS Nano 7 (12):10681–10694. doi:10.1021/nn4034103
- Schaumann, G. E., Philippe, A. M., Bundschuh G., Metreveli S., Klitzke D., Rakcheev A. Grun, et al. 2015. “Understanding the Fate and Biological Effects of Ag- and TiO(2)-Nanoparticles in the Environment: The Quest for Advanced Analytics and Interdisciplinary Concepts.” Science and Total Environment 535: 3–19. doi:10.1016/j.scitotenv.2014.10.035.
- Stobbart, R. H., J. Keating, and R. Earl. 1977. “Study of Sodium Uptake by Water Flea Daphnia-Magna.” Comparative Biochemistry and Physiology a-Physiology 58 (3): 299–309. doi:10.1016/0300-9629(77)90387-5.
- Tenzer, S., Docter, D. J., Kuharev A., Musyanovych V., Fetz R., Hecht F. Schlenk, et al. 2013. “Rapid Formation of Plasma Protein Corona Critically Affects Nanoparticle Pathophysiology.” Nature Nanotechnology 8 (10): 772. U1000. doi:10.1038/nnano.2013.181.
- Wiench, K., W. Wohlleben, V. Hisgen, K. Radke, E. Salinas, S. Zok, and R. Landsiedel. 2009. “Acute and Chronic Effects of Nano- and Non-Nano-Scale TiO2 and ZnO Particles on Mobility and Reproduction of the Freshwater Invertebrate Daphnia Magna.” Chemosphere 76 (10): 1356–1365. doi:10.1016/j.chemosphere.2009.06.025.
- Wray, A. T., and S. J. Klaine. 2015. “Modeling the Influence of Physicochemical Properties on Gold Nanoparticle Uptake and Elimination by Daphnia Magna.” Environmental Toxicology and Chemistry 34 (4): 860–872. doi:10.1002/etc.2881.
- Zhu, X. S., Y. Chang, and Y. S. Chen. 2010. “Toxicity and Bioaccumulation of TiO2 Nanoparticle Aggregates in Daphnia Magna.” Chemosphere 78 (3): 209–215. doi:10.1016/j.chemosphere.2009.11.013.
- Zhu, X. S., L. Zhu, Y. S. Chen, and S. Y. Tian. 2009. “Acute Toxicities of Six Manufactured Nanomaterial Suspensions to Daphnia Magna.” Journal of Nanoparticle Research 11 (1): 67–75. doi:10.1007/s11051-008-9426-8.