Abstract
Passion fruit-like nano-architectures (NAs) are all-in-one platforms of increasing interest for the translation of metal nanoparticles into clinics. NAs are nature-inspired disassembling inorganic theranostics, which jointly combine most of the appealing behaviors of noble metal nanoparticles with their potential organism excretion. Despite their unique and promising properties, NAs in vivo interactions and potential adverse effects have not yet been investigated. In this study, we employ zebrafish (Danio Rerio) to assess the development toxicity of NAs as well as their uptake and bioaccumulation at different stages of growth. The evaluation of multiple endpoints related to the toxicity clearly indicates that NAs do not induce mortality, developmental defects, or alterations on the hatching rate and behavior of zebrafish. Moreover, the analysis of nanostructures uptake and biodistribution demonstrates that NAs are successfully internalized and present a specific localization. Overall, our results demonstrate that NAs are able to pass through the embryos chorion and accumulate in specific tissues, exhibiting an impressive biocompatibility.
Introduction
Among nanomaterials, noble metal nanoparticles (NPs) are of particular interest to shift the paradigms in cancer treatment. Their intrinsic multi-functionalities are related to their peculiar interaction with electromagnetic waves Localized Surface Plasmon Resonance and the high electron density (Arvizo et al. Citation2012). For instance, gold NPs can act as both radiation dose enhancers and contrast agents for X-ray computed tomography (CT), providing a localized and enhanced action against neoplasms, or due to photon–phonon coupling, can be employed in photo-thermal therapy (PTT) (Hainfeld et al. Citation2008; Lusic and Grinstaff Citation2013; Yang et al. Citation2015).
Nowadays, more than 40 nanosystems for applications in cancer treatment and diagnostic are on the market (Bobo et al. Citation2016). Among them, the large majority are organic nanomaterials composed by lipid nanovesicles or polymer NPs (Caster et al. Citation2017). Remarkably, there is still no approved noble metal nanomaterial for cancer applications (Cassano, Pocoví-Martínez, and Voliani Citation2018). The lack of clinical translation is mainly related to metal NPs persistence in organisms after the designed action, that confines all their intriguing features to the bench-side (Yang et al. Citation2015). Indeed, noble metal NPs are not biodegradable, and their conventional size as theranostic agents is over 20 nm, well above the size threshold of glomerular walls in kidneys (Du et al. Citation2017). The excretion of intact NPs from other pathways, such as hepatic (bile-to-feces) route, is an inefficient process that lead to long-term toxicity and interference with common medical diagnoses (Yang et al. Citation2015).
The reduction of the hydrodynamic diameter (HD) of NPs to ultrasmall range (<6 nm, ultrasmall nanoparticles [USNPs]) enhances their renal clearance efficiency (Zarschler et al. Citation2016). Unluckily, USNPs clearance from the bloodstream is usually excessively fast and their size is too small to observe most of the required features for cancer theranostics, hindering a number of applications (Cassano, Pocoví-Martínez, and Voliani Citation2018).
A groundbreaking advance to overcome the issue of unwanted metal accumulation is the ultrasmall-in-nano approach for the design of novel nanomaterials (Cassano, Pocoví-Martínez, and Voliani Citation2018). By the ultrasmall-in-nano approach, the unique properties of noble metal NPs and their excretion by the renal pathway are jointly combined by the development of degradable nanoplatforms comprising USNPs (Al Zaki et al. Citation2014; Deng et al. Citation2015; Rengan et al. Citation2015). A promising example of ultrasmall-in-nano platform are the nature-inspired passion fruit-like nano-architectures (NAs). NAs are composed of 100 nm hollow silica nanospheres embedding plasmonic USNPs in a polymeric functional matrix, resembling this exotic fruit (Cassano et al. Citation2015, Citation2017). NAs have demonstrated interesting behaviors as drug delivery systems and dual ultrasound-photoacoustic contrast agents (Cassano et al. Citation2016; Avigo et al. Citation2017; Armanetti et al. Citation2018). NAs biodegrade in both physiological fluids and cellular environment in less than 48 h to low-toxic and potentially renal clearable building blocks: biodegradable polymers, endogenous-GSH coated USNPs and silicic acid (Cassano et al. Citation2016; Schmid, Kreyling, and Simon Citation2017; Croissant, Fatieiev, and Khashab Citation2017). Overall, NAs result as an appealing candidate for the translation of metal nano-theranostics to clinics (Cassano et al. Citation2016; Avigo et al. Citation2017).
So far, complete toxicological evaluations and fate investigations of NAs are required before in vivo efficacy studies. Zebrafish is considered a valuable in vivo model for effective and rapid evaluation and screening of the whole-body toxicity and biodistribution of nanomaterials (D'Amora et al. Citation2016, Citation2017). Zebrafish is a small size animal with high fecundity rate and external fertilization (Fako and Furgeson Citation2009; Ko et al. Citation2011). It shares a high homology with humans, as genome and physiology of different organs, including the digestive and cardiovascular systems (MacRae and Peterson Citation2015). Furthermore, zebrafish embryos and larvae are transparent and their development is rapid, enabling the investigation over potential effects of nanomaterials or drugs exposure by means of optical microscopy (Cheng, Flahaut, and Cheng Citation2007). In addition, zebrafish embryos are surrounded by a protective membrane, the chorion, that presents pore channels (∼0.6 µm) that allows the compounds to be internalized by passive diffusion (OECD Citation2013). In this work, we employ zebrafish as a vertebrate model in order to evaluate the potential NAs toxicity during the animal development. Taking into account various parameters, including malformation incidence, hatching and survival rates, cardiac and swimming activities we assess the biocompatibility of NAs. Furthermore, we characterize the NAs uptake and biodistribution in zebrafish embryos and larvae by ICP-AES and spinning disk microscopy, shedding light on their in vivo internalization, degradation, and excretion. The findings reported here represent a significant step forward toward the translation of metal nano-theranostics to clinics.
Methods
Synthesis of dye-functionalized poly(L-lysine)
Poly(L-lysine) hydrobromide 15–30 kDa (PL, 1.5 mg) was dissolved in PBS (780 μL) and AlexaFluor647-NHS ester (200 μg) was added to the solution. The reaction mixture was kept under stirring overnight at RT and used without further purification.
Synthesis of NAs
Gold USNPs with a diameter of 2.8 ± 0.4 nm were prepared according to the following procedure. To 20 mL of milliQ water were added 10 μL of poly(sodium 4-styrene sulfonate) 70 kDa (30% aqueous solution) and 200 μL of HAuCl4 (25 mM aqueous solution). During vigorously stirring, 200 μL of NaBH4 (4 mg/mL aqueous solution) were added quickly, and the mixture was stirred vigorously for other 2 minutes assuming a brilliant orange color. About 200 μL of dye-modified PL (20 mg/mL aqueous solution) were added to the gold USNPs solution under gentle stirring for 30 min. The aggregates were purified from free USNPs by centrifugation (16 000 rcf, 3 min) and the precipitate was dispersed in 2 mL of milliQ water. The aggregates were employed as templates for the silica shell composition by performing a modified Stöber process. In a 100 mL round-bottomed flask were added 70 mL of absolute ethanol followed by 2.4 mL of ammonium hydroxide solution (30% aqueous solution), and 40 μL of tetraethyl orthosilicate (TEOS, 98%). The solution was allowed to stir for 2 min at RT. Then, 2 mL of the aggregates were added to the reaction flask and the solution was allowed to stir for further 3 h at RT. NAs were purified by centrifugation and dispersed in 1 mL milliQ water, obtaining a pink-iridescent solution. A short spin centrifugation was finally employed to discriminate NAs with diameter over 150 nm. By this protocol are usually obtained around 1.5 mg NAs. The protocol was tested to up to 15 mg. NAs can be stored for at least 1 year frozen in milliQ water or at RT in ethanol.
UV–vis spectroscopy
Extinction spectra were collected by means of a double beam spectrophotometer Jasco V-550 UV/VIS equipped with quartz cuvettes of 1.5 mm path length. PBS buffer at pH 7.4 was employed as solvent.
Gold quantification in NAs
NAs were dissolved in 1 mL aqua regia (prepared with ICP grade HCl and HNO3) and digested under microwave irradiation (200 °C/15 min) in Teflon-lined vessels. The resulting solution was diluted to 10 mL with ICP grade water, and Au content was determined by ICP-MS analysis against a standard calibration curve.
Transmission electron microscopy (TEM)
TEM measurements were carried out on a ZEISS Libra 120 TEM operating at an accelerating voltage of 120 kV, equipped with an in-column omega filter. The colloidal solutions of NAs were deposited on 300-mesh carbon-coated copper grids and air dried overnight before being imaged.
Zebrafish husbandry
Wild-type adult zebrafish were kept in a recirculating aquarium on a 14 h/10 h light/dark photoperiod at 28 °C. Zebrafish were crossed as described in the “Zebrafish Book” (Westerfield Citation2007).
Exposure of zebrafish to NAs
Healthy embryos were collected at the same developmental stage (4 hpf) and were placed in 24 well plates in embryo medium [5 mM NaCl, 0.17 mM KCl, 0.33 mM CaCl2, and 0.33 mM MgSO4 (pH 7.2–7.3)] (5 embryos in 1 mL of medium/well). Eggs were treated with different NAs dilutions in embryo medium (5, 10, 50, and 100 μg/mL) and embryo medium as negative control until 120 hpf at 28 °C. Survival rate, hatching rate, malformations, heart rate and distance of swimming were assessed under a stereomicroscope (Stereomicroscope, SMZ18, Nikon) (Nikon, Tokyo, Japan) attached to a CCD camera at 4–120 hpf. The locomotor activity was recorded using an EthoVision video tracking software (Noldus Information Technology, Wageningen, Netherlands). Larvae of 96 hpf were acclimated to the light conditions for 20 min, and then recorded in embryos medium for 3 min. To achieve robust statistical analysis, all experiments were performed in triplicate each using 80 embryos. All animal experiments were performed in full compliance with the revised directive 2010/63/EU.
Uptake and biodistribution of NAs
Zebrafish embryos and larvae treated with 100 μg/mL of NAs were carefully rinsed with embryo medium, anesthetized with 0.016% (w/v) tricaine (ethyl 3-aminobenzoate methanesulfonate) (Sigma-Aldrich) and placed in a plate with medium. Imaging was performed by means of a Nikon spinning-disk confocal microscope (Nikon, Tokyo, Japan), equipped with a Nikon CFI Plan Fluor 4×/0.2 air objective (Nikon). NAs were excited with a 640 nm laser and detected at 675/725 nm. Images were acquired with an exposure time of 100 ms. Eight embryos/larvae for each developmental stage were imaged.
Quantification of au accumulated in zebrafish
Zebrafish embryos and larvae treated with 5, 10, 50, and 100 μg/mL of NAs were collected at 24, 48, and 72 hpf and rinsed with embryo medium. Embryos at 24 and 48 hpf were dechorionated. Samples were kept at −20 °C until further analysis. Five embryos or larvae were lyophilized for 48 h, kept in NaOH pH = 10 for 6 h and then digested with 500 µL of aqua regia overnight. The day after milli-Q water was added to each sample, until a final volume of 10 mL and the amount of gold was evaluated by ICP-AES at λ = 208 nm (iCAP 6300 ICP Spectrometer, Thermo Scientific, US). Experiments were performed in duplicate.
Statistical analysis
Values were expressed as means ± the standard deviation. Data were analyzed by one-way analysis of variance (ANOVA) in combination with Holm–Sidak post hoc. A value of p ≤ 0.01 was considered statistically significant.
Results
Production and characterizations of NAs
Supporting Information Figure S1A reports the standard and patented production protocol for NAs (Cassano et al. Citation2015). Briefly, poly(L-lysine) 15–30 kDa (PL) is covalently functionalized with commercial AlexaFluor647-NHS ester on about 5% of amines and employed for the synthesis of NAs (Cassano et al. Citation2016; Avigo et al., Citation2017). Poly(sodium 4-styrene sulfonate) 70 kDa (PSS)-coated gold USNPs showing a diameter of 2.8 ± 0.4 nm (Supporting Information Figure S2) are aggregated by means of ionic interactions with dye-modified PL. We employ polymer templates to grow the silica shells by a modified Stöber method, resulting in NAs of 100.1 ± 5.4 nm in diameter and 20.2 ± 1.6 nm of wall thickness (Supporting Information Figures S2 and S3). Supporting Information Figure S1B reports the optical behaviors of NAs in phosphate buffer (PBS) and shows a typical Rayleigh scattering background due to the silica shells including the absorption band of the dye and the LSPR of gold USNPs aggregates. AlexaFluor647 is inserted in NAs in order to exploit the performance of spinning disk microscopy to investigate the internalization and biodistribution of the nanomaterial in zebrafish. NAs produced by following this standard protocol show a ζ-potential of −26 mV, a HD of 140 nm and contain about 6% w/w gold/NAs and 3.4 nmol/mg dye/NAs (Avigo et al., Citation2017). The cost of NAs is estimated in about 1 euro/mg by considering the actual price of their components. It is also worth to notice that the entire production requires less than 4 h, the protocol is extremely versatile and scalable up to 15 mg NAs/synthesis (Pocovı́-Martı́nez, Cassano, and Voliani 2018).
Biosafety assessment
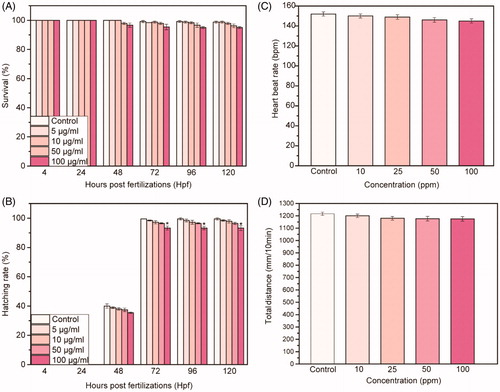
We perform a biosafety assessment () by soaking zebrafish embryos collected at 4 hpf in solution of passion fruit-like nano-architectures at different concentrations (5, 10, 50, and 100 μg/mL). In order to investigate the interactions of NAs with zebrafish, we determine multiple toxicity endpoints throughout the whole exposure period (from 4 up to 120 hpf). The survival graphic of treated embryos/larvae indicates a dose dependent effect of NAs, with no significant difference (p ≤ 0.01) in comparison to the control group (). The time of hatching rate presents a similar trend, with a dose-dependent behavior (). The hatching rate is investigated between 48 and 72 hpf as in the control conditions. Only at 100 µg/mL and between 72 and 120 hpf (), the hatching rate shows a relevant difference (p ≤ 0.01) with respect to the untreated larvae. In addition, we test the cardiac and swimming activity of zebrafish larvae exposed to NAs. As reported in , larvae at 96 hpf do not present cardiac effects even at the higher concentration of NAs. Indeed, the number of heartbeats is similar between treated and untreated larvae. Moreover, the total distance of swimming covered by treated larvae shows no reductions ().
Figure 1. (A) Morphology of zebrafish treated with 100 µg/mL of NAs at the different time points investigated (4, 24, 48, 72, 96, and 120 hpf). Scale bars = 500 µm. (B) Malformations rates for each abnormality versus NAs concentration in different larvae’s parts at 96 hpf (head, tail, heart, yolk sac). Data are expressed as means ± standard deviations from three independent experiments; number of fish in each control and treated groups in one experiment = 80 (*p ≤ 0.01).
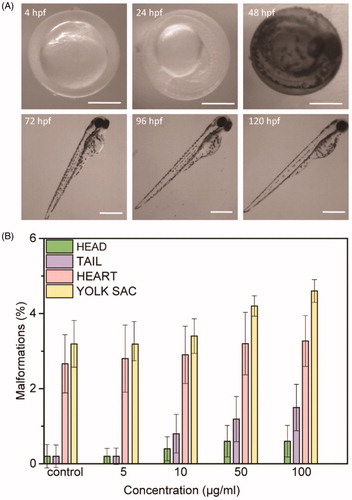
Figure 2. (A) Survival rate and (B) hatching rate of embryos treated with different concentration of NAs up to 120 hpf. (C) Heart beat rate and (D) total distance of swimming of larvae at 96 hpf treated with different concentration of NAs. Data are expressed as means ± standard deviations from three independent experiments; number of fish in each control and treated groups in one experiment =80 (*p ≤ 0.01).
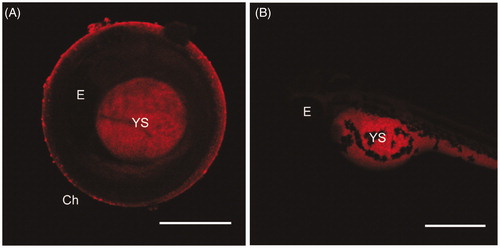
Uptake and biodistribution of NAs
We employ spinning disk microscopy to investigate the uptake of NAs in embryos and larvae. This technique provides the best equilibrium between fast imaging speed and optical sections. demonstrates that: (i) embryos are still surrounded by the chorion at 48 hpf, (ii) NAs enter into embryo through the chorion pores, and (iii) the nanostructures accumulated specifically in the yolk sac of the embryo with a tissue dependent affinity. The localization of NAs in the yolk-sac can be attributed to the interactions of the nanostructures with lipid-rich yolk cells during the zebrafish early life stages. As observed from the evaluation of possible presence of malformations induced from NAs (), the low percentage of yolk sac edema indicates that these nanostructures do not induce any detectable toxicity in this organ. On the other hand, the fluorescence collected on the surface of the chorion indicates that NAs also accumulate on the protective membrane of the treated embryo. Intact NAs can be also absorbed by larvae by swallowing and skin-absorption at the subsequent stage of growth (72 hpf). The presence of high fluorescence signal in the yolk sac and, especially near the intestine of larvae at 72 hpf, indicates that a high amount of NAs or their building blocks (dye-modified PL) are able to enter the digestive system. Moreover, the accumulation of NAs in the intestine proposes that they are removed from the larva through metabolism, by excretion from the model. Thus, the absorption, distribution, metabolism, and excretion (ADME) of NAs are suggested on the basis of their localization in the different organs. NAs penetrate and accumulate in the yolk sac and subsequently they are in part excreted through the gut. Even if the localization of NAs in the gut and intestine could cause toxic effects, leading to intestinal disorders, the absence of malformations and the supposed short time of accumulation in both organs suggest the absence of induced adverse effects. In it is visible that NAs are accumulated in the yolk sac and in the intestine of larvae at 72 hpf.
Quantification of Au accumulated in zebrafish
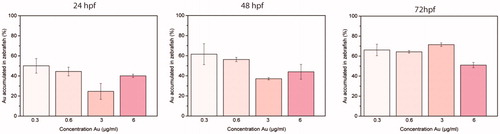
In order to quantitatively evaluate the accumulation of NAs in zebrafish embryos and larvae at different stages of development, we perform ICP-AES analysis. We treat embryos and larvae with 5, 10, 50, and 100 μg/mL of NAs (respectively, 0.3, 0.6, 3, and 6 µg/mL in gold) for 24, 48, and 72 hpf (). Embryos at 24 and 48 hpf are dechorionated before the analysis in order to quantify the NAs absorbed in the models, avoiding to include the nanostructures accumulated on the chorion surface.
Figure 4. Normalized percentages of gold measured by ICP-AES in zebrafish embryos and larvae at 24, 48, and 72 hpf treated with 5, 10, 50, and 100 µg/mL of NAs (respectively, 0.3, 0.6, 3, and 6 µg/mL in gold). Five embryos/larvae were analyzed per condition. Data are expressed as means ± standard deviations from three independent experiments; number of fish in each control and treated groups in one experiment = 80 (*p ≤ 0.01).AQ1: Please note that the Funding section(s) has/have been created from information provided through CATS. Please correct if this is inaccurate.
Discussion
NAs are disassembling nanocomposites able to jointly combine most of the appealing features of noble metal nanoparticles with their potential organism excretion (Cassano et al. Citation2016, Citation2017). In NAs, the silica nanocapsule gives to the system the best size to enhance their passive accumulation in tumor targets and provides a straightforward modifiable/functionalizable surface (Cassano et al. Citation2016). Strictly packed USNPs confer the physical behavior needed for theranostic applications, while the polymer can be covalently functionalized with active molecules without affecting its rolling properties (Cassano et al. Citation2016; Avigo et al. Citation2017). Due to the peculiar structure of NAs, the key-question of regulatory agencies related to the excretion rate of nanomaterials after the designed action will be unraveled. Indeed, NAs biodegrade in less than 48 h to low-toxic and potentially renal clearable building blocks: biodegradable polymers, endogenous-GSH coated USNPs and silicic acid. The innovative design of NAs brings again to the forefront noble metals for theranostics by overcoming toxicity issues related to long-term accumulation in organs. In the next future, our approach could unlock the true potential of noble metal nanotheranostics for cancer treatment and beyond.
Optical imaging at different time points of exposure (4, 24, 48, 72, 96, and 120 hpf) confirms the normal growth of treated embryos and larvae (). NAs exposure induces negligible developmental defects in different parts of the zebrafish, including head, tail, heart, yolk sac, and fin fold ( and Supporting Information Figure S4). The small percentage of animals’ abnormalities suggests a nontoxic effect induced by NAs. Remarkably, the percentage of malformations reaches a value of 4.6% only for defects of the yolk sac. Data reported in reveal no significant toxic effects of NAs, according to the OECD guidelines (OECD Citation2013). If the survival and hatching percentages are, respectively, ≥90% and ≥80%, a nanomaterial should be assumed as nontoxic (OECD Citation2013). Taken together, results reported in indicate that NAs have a concentration dependent behavior on the survival and hatching rates but exert no detectable toxicity in zebrafish during the development. In addition, tests on the cardiac and swimming activity of zebrafish larvae exposed to NAs () confirm that NAs and the building blocks resulting from their degradation have no adverse effects on the larval behavior.
The red fluorescence collected from the samples () is related to the commercial dye covalently inserted in NAs, confirming their successful internalization in embryos. It is worth to notice here that the fluorescence can be related to either intact or degraded NAs. Indeed, while NAs degrade to the building blocks in less than 48 h in the cellular environment, they are stable for up to 5 days in buffer at neutral pH, such as the embryo medium (Cassano et al. Citation2015, Citation2016). These results, together with the absence of NAs in the circulatory system or other regions of the zebrafish, suggest that after 72 hpf the nanostructures (both intact or degraded) might be removed from the larva. Overall, our findings demonstrate the nontoxicity and good biocompatibility of NAs in zebrafish during their development and are in good agreement with previous toxicological and biodegradation experiments performed on 2D cell cultures (Cassano et al. Citation2016).
The amount of gold () is already detectable by ICP-AES at the lower concentration of NAs (5 and 10 μg/mL) and, as expected, increases in a time-dependent manner. At 24 and 48 hpf, NAs enter in the embryos by passive diffusion through the pores of the chorion, while at 72 hpf the uptake occurs through skin-absorption and swallowing. At 50 and 100 μg/mL, the quantity of gold internalized by zebrafish embryos and larvae seems lower in comparison to 5 and 10 μg/mL. This discrepancy might be attributed to aggregation of the nanostructures at high concentrations that partially prevent or force the crossing of chorion pores from NAs. On the other hand, at 72 hpf, due to the absence of the chorion, the uptake of NAs is not influenced by possible aggregation. Furthermore, it should be noted that the amount of gold can result from an equilibrium between the gold comprised in NAs accumulated in samples and gold released from biodegraded NAs.
Conclusion
In summary, we demonstrate the significant biocompatibility of passion fruit-like Nano-Architectures in a vertebrate organism. The analysis of different endpoints demonstrates that NAs do not induce alterations in the zebrafish development, indicating their nontoxic effects, according to the normative guidelines. Moreover, we demonstrate that these nanostructures are successfully taken up by zebrafish during the development and accumulate in specific tissues. These results confirm the biosafety of NAs and represent an encouraging advance for both efficacy investigations on murine models, and possible translation of metal nano-theranostics to clinics. Indeed, our findings can be easily employed to predict the effects of NAs on other vertebrates due to the good genetic and physiological similarity between zebrafish and humans.
Supporting Information
Download MS Word (2 MB)Acknowledgments
Istituto Italiano di Tecnologia is greatly acknowledged for funding. S.G. and M.d.A. acknowledge the COST Action CA 15107 “Multi-Functional Nano-Carbon Composite Materials Network (Multi-Comp).” The authors wish to thank Dr. Filippo Drago (IIT) for ICP measurements, Nikon Imaging Center@IIT, IIT Nanochemistry and Nanophysics departments for access to facilities.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Al Zaki, A., D. Joh, Z. Cheng, A. L. B. De Barros, G. Kao, J. Dorsey, and A. Tsourkas. 2014. “Gold-Loaded Polymeric Micelles for Computed Tomography-Guided Radiation Therapy Treatment and Radiosensitization.” ACS Nano 8 (1): 104–112. doi:10.1021/nn405701q.
- Armanetti, P., S. Pocoví-Martínez, and A. Flori. 2018. “Dual Photoacoustic/Ultrasound Multi-Parametric Imaging from Passion Fruit-like Nano-Architectures.” Nanomedicine Nanotechnology, Biology, and Medicine 12: 1663–1701.
- Arvizo, R. R., S. Bhattacharyya, R. A. Kudgus, K. Giri, R. Bhattacharya, and P. Mukherjee. 2012. “Intrinsic Therapeutic Applications of Noble Metal Nanoparticles: Past, Present and Future.” Chemical Society Reviews 41 (7): 2943–2970. doi:10.1039/c2cs15355f.
- Avigo, C., D. Cassano, C. Kusmic, V. Voliani, and L. Menichetti. 2017. “Enhanced Photoacoustic Signal of Passion Fruit-like Nanoarchitectures in a Biological Environment.” The Journal of Physical Chemistry C 121 (12): 6955–6961. doi:10.1021/acs.jpcc.6b11799.
- Bobo, D., K. J. Robinson, J. Islam, K. J. Thurecht, and S. R. Corrie. 2016. “Nanoparticle-Based Medicines: A Review of FDA-Approved Materials and Clinical Trials to Date.” Pharmaceutical Research 33 (10): 2373–2387. doi:10.1007/s11095-016-1958-5.
- Cassano, D., D. Rota Martir, G. Signore, V. Piazza, and V. Voliani. 2015. “Biodegradable Hollow Silica Nanospheres Containing Gold Nanoparticle Arrays.” Chemical Communications 51 (49): 9939–9941. doi:10.1039/C5CC02771C.
- Cassano, D., M. Santi, V. Cappello, S. Luin, G. Signore, and V. Voliani. 2016. “Biodegradable Passion Fruit-like Nano-Architectures as Carriers for Cisplatin Prodrug.” Particle & Particle Systems Characterization 33 (11): 818–824. doi:10.1002/ppsc.201600175.
- Cassano, D., J. David, S. Luin, and V. Voliani. 2017. “Passion Fruit-like Nano-Architectures: A General Synthesis Route.” Scientific Reports 7: 43795. doi:10.1038/srep43795.
- Cassano, D., S. Pocoví-Martínez, and V. Voliani. 2018. “Ultrasmall-in-Nano Approach: Enabling the Translation of Metal Nanomaterials to Clinics.” Bioconjugate Chemistry 29 (1): 4–16. doi:10.1021/acs.bioconjchem.7b00664.
- Caster, J. M., A. N. Patel, T. Zhang, and A. Wang. 2017. “Investigational Nanomedicines in 2016: A Review of Nanotherapeutics Currently Undergoing Clinical Trials.” Wiley Interdiscip. Rev. Nanomedicine Nanobiotechnology 42: 742–755. doi:10.1002/wnan.1416.
- Cheng, J., E. Flahaut, and S. H. Cheng. 2007. “Effect of Carbon Nanotubes on Developing Zebrafish (Danio rerio) Embryos.” Environmental Toxicology and Chemistry 26 (4): 708–716.
- Croissant, J. G., Y. Fatieiev, and N. M. Khashab. 2017. “Degradability and Clearance of Silicon, Organosilica, Silsesquioxane, Silica Mixed Oxide, and Mesoporous Silica Nanoparticles.” Advanced Materials 29 (9): 1604634. doi:10.1002/adma.201604634.
- D'Amora, M., M. Rodio, J. Bartelmess, G. Sancataldo, R. Brescia, F. Cella Zanacchi, A. Diaspro, and S. Giordani. 2016. “Biocompatibility and Biodistribution of Functionalized Carbon Nano-Onions (f-CNOs) in a Vertebrate Model.” Scientific Reports 6: 33923.
- D'Amora, M., A. Camisasca, S. Lettieri, and S. Giordani. 2017. “Toxicity Assessment of Carbon Nanomaterials in Zebrafish during Development.” Nanomaterials 7 (12): 414. doi:10.3390/nano7120414.
- Deng, H., F. Dai, G. Ma, and X. Zhang. 2015. “Theranostic Gold Nanomicelles Made from Biocompatible Comb-like Polymers for Thermochemotherapy and Multifunctional Imaging with Rapid Clearance.” Advanced Materials (Deerfield Beach, Fla.) 27 (24): 3645–3653. doi:10.1002/adma.201501420.
- Du, B., X. Jiang, A. Das, Q. Zhou, M. Yu, R. Jin, and J. Zheng. 2017. “Glomerular Barrier Behaves as an Atomically Precise Bandpass Filter in a Sub-Nanometre Regime.” Nature Nanotechnology 12 (11): 1096–1102. doi:10.1038/nnano.2017.170.
- Fako, V. E., and D. Y. Furgeson. 2009. “Zebrafish as a Correlative and Predictive Model for Assessing Biomaterial Nanotoxicity.” Advanced Drug Delivery Reviews 61 (6): 478–486. doi:10.1016/j.addr.2009.03.008.
- Hainfeld, J. F., F. A. Dilmanian, D. N. Slatkin, and H. M. Smilowitz. 2008. “Radiotherapy Enhancement with Gold Nanoparticles.” The Journal of Pharmacy and Pharmacology 60 (8): 977–985. doi:1211/jpp.60.8.0005.
- Ko, S.-K., X. Chen, J. Yoon, and I. Shin. 2011. “Zebrafish as a Good Vertebrate Model for Molecular Imaging Using Fluorescent probes.” Chemical Society Reviews 40 (5): 2120–2130. doi:10.1039/c0cs00118j.
- Lusic, H., and M. W. Grinstaff. 2013. “X-ray-computed tomography contrast agents.” Chemical Reviews 113 (3): 1641–1666. doi:10.1021/cr200358s.
- MacRae, C. A., and R. T. Peterson. 2015. “Zebrafish as Tools for Drug Discovery.” Nature Reviews Drug Discovery 14 (10): 721–731. doi:0.1038/nrd4627.
- OECD. 2013. Test No. 236: Fish Embryo Acute Toxicity (FET) Test. Economic Co-operation and Development (OECD) Test Guideline (236). Paris: OECD Publishing.
- Pocovı́-Martı́nez, S.,. D. Cassano, and V. Voliani. 2018. “Naked Nanoparticles in Silica Nanocapsules: A Versatile Family of Nanorattle Catalysts.” ACS Applied Nano Materials 1 (4): 1836–1840. doi:10.1021/acsanm.8b00247.
- Rengan, A. K., A. B. Bukhari, A. Pradhan, R. Malhotra, R. Banerjee, R. Srivastava, and A. De. 2015. “In Vivo Analysis of Biodegradable Liposome Gold Nanoparticles as Efficient Agents for Photothermal Therapy of Cancer.” Nano Letters 15 (2): 842–848. doi:10.1021/nl5045378.
- Schmid, G., W. G. Kreyling, and U. Simon. 2017. “Toxic Effects and Biodistribution of Ultrasmall Gold nanoparticles.” Archives of Toxicology 91 (9): 3011–3037. doi:10.1007/s00204-017-2016-8.
- Westerfield, M. 2007. The Zebrafish Book. A Guide for the Laboratory Use of Zebrafish (Danio rerio). 5th ed. Eugene: University of Oregon Press.
- Yang, X., M. Yang, B. Pang, M. Vara, and Y. Xia. 2015. “Gold Nanomaterials at Work in Biomedicine.” Chemical Reviews 115 (19): 10410–10488. doi:10.1021/acs.chemrev.5b00193.
- Zarschler, K., L. Rocks, N. Licciardello, L. Boselli, E. Polo, K. P. Garcia, L. De Cola, H. Stephan, and K. A. Dawson. 2016. “Ultrasmall Inorganic Nanoparticles: State-of-the-Art and Perspectives for Biomedical Applications.” Nanomedicine Nanotechnology, Biology, and Medicine 12 (6): 1663–1701. doi:10.1016/j.nano.2016.02.019.