Abstract
Recent studies reported adverse liver effects and intestinal tumor formation after oral exposure to titanium dioxide (TiO2). Other oral toxicological studies, however, observed no effects on liver and intestine, despite prolonged exposure and/or high doses. In the present assessment, we aimed to better understand whether TiO2 can induce such effects at conditions relevant for humans. Therefore, we focused not only on the clinical and histopathological observations, but also used Adverse Outcome Pathways (AOPs) to consider earlier steps (Key Events). In addition, aiming for a more accurate risk assessment, the available information on organ concentrations of Ti (resulting from exposure to TiO2) from oral animal studies was compared to recently reported concentrations found in human postmortem organs. The overview obtained with the AOP approach indicates that TiO2 can trigger a number of key events in liver and intestine: Reactive Oxygen Species (ROS) generation, induction of oxidative stress and inflammation. TiO2 seems to be able to exert these early effects in animal studies at Ti liver concentrations that are only a factor of 30 and 6 times higher than the median and highest liver concentration found in humans, respectively. This confirms earlier conclusions that adverse effects on the liver in humans as a result of (oral) TiO2 exposure cannot be excluded. Data for comparison with Ti levels in human intestinal tissue, spleen and kidney with effect concentrations were too limited to draw firm conclusions. The Ti levels, though, are similar or higher than those found in liver, suggesting these tissues may be relevant too.
Introduction
The discussions on the food additive E171 (titanium dioxide, TiO2) in the regulatory arena indicate that it is difficult to draw definitive conclusions about its safety due to data gaps and conflicting data. For example, France recently temporarily suspended the allowance of the food additive E171 based on the recommendation of the French institute ANSES to limit the exposure until there is more clarity on the hazard and risks of E171 (ANSES Citation2019, Légifrance Citation2019). On the other hand, the European Food Safety Authority (EFSA) indicates that there are insufficient new indications to reconsider earlier conclusions on the safety of the food additive, and may consider to revisit this recommendation when further information comes available (EFSA Citation2019).
Oral exposure to TiO2 may be due to the intake of the food additive E171 that is widely used in various foods, food supplements and due to swallowing toothpaste (the latter particularly in small children) (Rompelberg et al. Citation2016). The realistic estimated mean intake of TiO2 via food, supplements and toothpaste by children (2–6 year old) in the Netherlands has been estimated to amount 0.67 mg/kg bw/d (Rompelberg et al. Citation2016), and the daily intake by children and adolescents has been estimated to be ∼2 mg/kg bw by EFSA (EFSA ANS Panel Citation2016). In addition, oral exposure to TiO2 can occur due to its presence as a pharmaceutical excipient in medicine (Bachler, von Goetz, and Hungerbuhler Citation2015).
It should be noted that the food additive E171 is known to consist of TiO2 particles with varying size distributions, including a fraction of particles smaller than 100 nm, so-called nanoparticles (NPs). Analysis of several food-grade samples of TiO2 showed that the fraction of particles <100 nm varied between 10 and 49%, based on the number of particles as measured by Electron Microscopy (Weir et al. Citation2012, Peters et al. Citation2014, Yang et al. Citation2014, EFSA FAF Panel Citation2019). Recently, also percentages higher than 50% have been reported (Geiss et al. Citation2020). In addition to differences in size distribution between different forms of TiO2, also differences in crystal structure and surface coatings are possible. In the EU, the crystal structures rutile and anatase are both allowed for use in pigment-grade TiO2 in food (Heringa et al. Citation2016, Rompelberg et al. Citation2016). The TiO2 particles may also be uncoated or alumina and/or silica coated (Warheit, Brown, and Donner Citation2015), though, these coatings seem not to be extensively used in E171 and are likely to be excluded from the future EU specifications of the food additive (EFSA FAF Panel Citation2019).
In biological matrices, the concentration of TiO2 is usually determined by quantifying the level of titanium (Ti). As is argued in Heringa et al. (Citation2018) and Peters et al. (Citation2020), it can be assumed that the Ti found in human tissues almost exclusively originate from oral exposure to TiO2 particles. A potentially alternative source of Ti in organs (at least for internal organs) could come from corrosion of titanium implants, however, in the study by Heringa et al. (Citation2018) two donors with such implants did not have elevated Ti or Ti-particle levels in the internal organs analyzed (i.e. liver and spleen). Another potential alternative source of Ti could come from tattoos (other than black), as their pigments containing Ti, were demonstrated to have reached lymph nodes (Schreiver et al. Citation2017).
The present study intends to place new scientific information on the presence of Ti in human postmortem organs (i.e. liver, spleen, kidney and sections of ileum and jejunum) in the context of potential health effects due to oral exposure to TiO2. To that end, measured levels of Ti in various human postmortem tissues, including intestine (both ileum and jejunum), kidney, liver and spleen, are compared to the Ti levels and effects observed in animal studies.
As there are reports of effects in the liver (Wang et al. Citation2007, Cui et al. Citation2011, Wang et al. Citation2013, Shukla et al. Citation2014, Azim et al. Citation2015, Talamini et al. Citation2019, Cornu, Beduneau, and Martin Citation2020) and intestine (Urrutia-Ortega et al. Citation2016, Bettini et al. Citation2017, Proquin et al. Citation2018, Dorier et al. Citation2019, Talamini et al. Citation2019) in animal studies, we focused on these organs. With regard to the intestine, animal studies predominantly studied the effects on the colon. We generalized the effect concentrations of these studies, in order to be able to compare them with the Ti levels in the small intestinal tissues (ileum and jejunum).
To get a better understanding of whether oral exposure to TiO2 in animal studies is able to induce effects in liver and intestine at concentrations relevant for humans, we not only took into account histopathological and clinical observations in these organs, but also whether earlier events which could lead to these toxicological endpoints have or have not been observed in animal studies. To accomplish this, postulated Adverse Outcomes Pathways (AOPs) describing chains of events leading to the induction of steatosis, edema and fibrosis in the liver and the induction of intestinal tumors are used. These helped to structure the available information from animal studies and to assess whether the associated Key Events (KEs) and the Adverse Outcomes (AOs) are likely to occur due to TiO2 exposure, and at which dose. Please note the qualitative nature of using these AOPs, and that establishing these AOPs was not the aim of the present study. As there are few reports on effects in kidney and spleen as a result of oral TiO2 exposure, the measured Ti levels in these postmortem organs are compared to the Ti levels and effects observed in animal studies in a more general manner, without the use of AOPs.
The quantified Ti content in the human postmortem tissues from 15 individuals have been reported in a separate publication (Peters et al. Citation2020). The findings confirmed the results from our previous study with human postmortem liver and spleen from 15 (other) individuals (Heringa et al. Citation2018). In the present study, these results were combined, thereby expanding the number of subjects in which Ti was measured in liver and spleen. The findings in human intestinal tissues are particularly relevant in view of the discussion on the possible potential of TiO2 to initiate or promote the development of colorectal tumors (Urrutia-Ortega et al. Citation2016, Bettini et al. Citation2017) and intestine-related autoimmune diseases such as Inflammatory Bowel Disease (IBD) including Crohn’s disease (Powell et al. Citation1996, Lomer, Thompson, and Powell Citation2002, Lomer et al. Citation2004, Hummel et al. Citation2014).
Mechanistic insights in effects in liver and intestine after ingestion of TiO2
It is very challenging to draw firm conclusions on toxicity of TiO2. Recent toxicity studies on TiO2 raised concerns for liver effects (liver fibrosis, steatosis and edema) (Wang et al. Citation2007, Cui et al. Citation2011, Wang et al. Citation2013, Shukla et al. Citation2014, Azim et al. Citation2015, Talamini et al. Citation2019) and for a potential enhancement or promotion of intestinal tumor formation (Urrutia-Ortega et al. Citation2016, Bettini et al. Citation2017, Proquin et al. Citation2018, Talamini et al. Citation2019), after ingestion. On the other hand, there are also toxicity studies showing no effect on liver and intestine, despite prolonged exposure and high doses (NCI Citation1979, Warheit, Brown, and Donner Citation2015, Blevins et al. Citation2019). In order to improve the assessment whether such effects occur due to TiO2 exposure, we moved beyond the traditional toxicological hazard assessment based on morphological, histopathological and clinical observations. We included information from in vivo studies using advanced techniques to assess events in the pathway to specific adverse effects in liver and intestine. The concept of AOP1 was used, taking into account all steps on the molecular, cellular and organ levels (called Key Events (KEs)), to assign information on early effects, effects that go beyond these early effects and adverse effects at the right place within the pathway (Vinken et al. Citation2020). An overview was developed of the oral studies with TiO2 supporting and not-supporting KEs leading to liver and intestinal effects, structuring the available information according to the sequence of KEs in an AOP. In this way we have obtained a well-organized and more complete picture of the available information that supports the concern for potential health effects in liver and intestine due to (oral) exposure to TiO2.
Liver
The suggested AOP for liver effects () is a compilation of two existing AOPs (AOP 144 and AOP 34 of the AOP-Wiki2), amended with information from recent research. We used events leading to liver fibrosis as described in AOP 144 (https://aopwiki.org/aops/144) as the basis. This pathway is considered relevant for TiO2 that consists of small particles of which 10–49% are nanosized, as nanosized particles are listed as one of the stressors. We have also amended and extended this AOP by adding the connection to the AOP describing the pathway leading to hepatic steatosis (AOP 34) (color-coded in ). The generation of Reactive Oxygen Species (ROS) (KE2 in ) was added with a feedback mechanism from leukocyte recruitment. These changes are based on additional information from recent studies (Gerloff et al. Citation2017, Abbasi-Oshaghi, Mirzaei, and Pourjafar Citation2019, Talamini et al. Citation2019), and the AOP for hepatic steatosis (AOP 34) (https://aopwiki.org/aops/34). Also, in line with AOP 34, liver steatosis was added as AO (AO3), and increased liver weight as Associated Event (AE) based on findings from studies with TiO2 (Shukla et al. Citation2014, Azim et al. Citation2015), and expert judgment. Finally, we have added KE6.1 ‘Liver inflammation,’ leading to ‘Liver edema’ (AO2) and ‘Increased liver weight’ (AE), based on expert judgment and confirming studies (Cui et al. Citation2011, Shukla et al. Citation2014). These extensions fitted in the primary structure provided by AOP 144 and were supported by KE-related assays from the hazard studies described below.
Figure 1. Compilation of two Adverse Outcome Pathways (AOPs) leading to effects on the liver by TiO2. Events resulting in the adverse outcomes liver fibrosis, steatosis and edema are based on the AOP 144 on liver inflammation of the AOP-Wiki (https://aopwiki.org/aops/144). Adaptations based on recent studies, AOP 34 with hepatic steatosis as adverse outcome (https://aopwiki.org/aops/34) and expert judgment, are included. After the molecular initiating event (MIE), a series of key events (KE) take place that lead to an adverse outcome (AO) or and associated event (AE). ECM: extra-cellular matrix; HSC: hepatic stellate cell; ROS: reactive oxygen species.
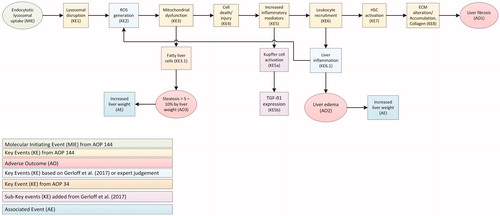
presents a comprehensive overview of all the available oral in vivo studies with information on events leading to liver effects as a result of exposure to TiO2. By visualizing both positive (+) and negative (−) effects or toxicity observations, along with information on the dose and exposure duration. In total, 13 in vivo oral exposure rodent studies using TiO2 were included for investigation of the suggested AOP leading to liver effects. More detailed information on the dosing, the animals, the properties of the TiO2 used and the effects observed (if any) in the studies are provided in the Supplementary Information (Supplementary Information 1). Several studies indicated that TiO2 could initiate events that can eventually lead to liver fibrosis, liver steatosis and/or liver edema. On the other hand, no indications of liver fibrosis, liver steatosis and liver edema were found in a number of other, sometimes older, long-term in vivo studies in which animals were highly dosed.
Table 1. Overview of the oral in vivo studies with TiO2 investigating the suggested adverse outcome pathway (AOP) leading to liver fibrosis, steatosis and edema, expressed against dose level ().
It should be noted that the characterization of the TiO2 composition in the National Cancer Institute (NCI) carcinogenicity study from 1979 is very limited (NCI Citation1979), in contrast to more detailed information provided in recent studies. It is also not always clear if the lack of toxicity reported in some studies is due to the absence of adverse effects, or because these endpoints were not investigated. For example, in the NCI study changes in liver weight were not recorded, nor is a list of examined effects available (NCI Citation1979). Furthermore, the 90-day study that administered a dose of 1000 mg/kg bw/d by Warheit, Brown, and Donner (Citation2015) used a commercially available TiO2 with an alumina surface coating. As long as the coating is not dissolved, the body is exposed to alumina rather than to the TiO2 core. To which extent dissolution of the coating occurs, especially during transit through the acidic conditions of the stomach, is unknown. Although TiO2 with these physicochemical properties can presently be applied in Europe in food according to the recommended specifications of the food additive, coated TiO2 is not expected to be included in the specifications of the food additive E171 in Europe in the future (EFSA FAF Panel Citation2019). Warheit, Brown, and Donner (Citation2015) also studied two other forms of commercially available uncoated pigment TiO2 (both rutile) in a 28-day oral study at the extremely high dose of 24 000 mg/kg bw/d, without finding any effects.
Using the AOP given in , we were able to provide a clear structure for assessment of effects, e.g. investigating assays beyond those reported by standard OECD Test Guidelines (TG). A number of in vivo studies show that KEs potentially leading to liver fibrosis, steatosis and/or edema can occur, or found evidence of an AE, i.e. increased liver weight. The overview shows that a substantial part of the KE-related early effects are triggered (). These effects were already observed in sub-chronic studies at doses of 5–150 mg/kg bw/d, doses much lower than used in the negative studies mentioned above. Only two studies recorded an AO, i.e. liver fibrosis (Gu et al. Citation2015), and liver edema (Wang et al. Citation2013). Increased liver weight, an AE, was found in three studies (Wang et al. Citation2007, Shukla et al. Citation2014, Azim et al. Citation2015). The Molecular Initiating Event (MIE) (Endocytotic lysosomal uptake), KE1 (Lysosomal disruption), KE7 (Hepatic Stellate Cell (HSC) activation) and KE8 (Extra-Cellular Matrix (ECM) alteration/Accumulation, Collagen) were not investigated in any (in vivo) study (). Many in vitro studies give additional evidence related to the presently used AOPs on liver fibrosis, steatosis and edema. In vitro studies covering the MIE (Endocytotic lysosomal uptake), KE1 (Lysosomal disruption), KE2 (ROS generation), KE3 (Mitochondrial dysfunction) and KE4 (Cell injury/death) often report positive effects for TiO2 (tested concentrations between 50 and 300 µg/ml) (Jin et al. Citation2008, Natarajan et al. Citation2015, Abbasi-Oshaghi, Mirzaei, and Pourjafar Citation2019).
Intestine
The mechanism of action regarding the intestine after oral exposure to TiO2 is not completely understood. It is suggested that TiO2 might induce or promote colon tumors via ROS generation and inflammation (Bettini et al. Citation2017, Proquin et al. Citation2018). In case of excess ROS generation, oxidative stress might be induced leading to tissue damage. An AOP for TiO2 was postulated by Braakhuis et al. (Citation2021) which we used to gather available information on each of the KEs (). and present comprehensive overviews of all the available oral in vivo studies with information relevant for investigation of the suggested AOP leading to intestinal tumors as a result of exposure to TiO2 for rats and mice, respectively. In total, 18 in vivo oral exposure studies using different types of TiO2 such as food additive E171 or specific TiO2 NPs, were included.
Figure 2. Adverse Outcome Pathways (AOP) for the intestine leading to tumor formation as a result of oral TiO2 exposure. The AOP has been postulated by Braakhuis et al. (Citation2021) for TiO2 related colon carcinogenicity after oral exposure. After the molecular initiating event (MIE), a series of key events (KE) take place that lead to the adverse outcome (AO). ROS: reactive oxygen species.
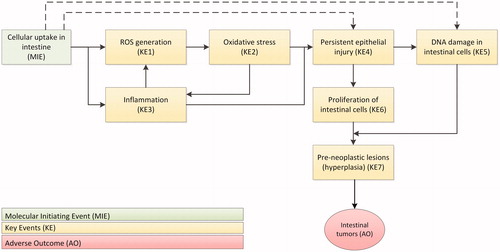
Table 2. Overview of oral in vivo studies investigating the suggested adverse outcome pathway (AOP) leading to TiO2 related colon carcinogenicity after oral exposure in rats.
Table 3. Overview of oral in vivo studies investigating the suggested adverse outcome pathway (AOP) leading to TiO2 related colon carcinogenicity after oral exposure in mice.
For intestinal tumor formation and promotion, it seems there is a dose dependent increase in generation of ROS, oxidative stress and inflammation (Trouiller et al. Citation2009, Wang et al. Citation2013, Grissa et al. Citation2015, Urrutia-Ortega et al. Citation2016, Bettini et al. Citation2017, Martins et al. Citation2017, Hu et al. Citation2018). These KEs fit into a mechanism potentially leading to tumor formations. However, for the later KEs, persistent epithelial injury (KE4) and proliferation of intestinal cells (KE6), insufficient information is available. Only a single study measured epithelial injury (Sycheva et al. Citation2011), and two studies measured proliferation of intestinal cells (Sycheva et al. Citation2011, Proquin et al. Citation2018) (). More information on these KEs is needed to evaluate whether the AOP is operative in rats and mice. For the induction of pre-neoplastic lesions (KE7) such as hyperplasia in the colon, contradicting results have been reported. Two studies using high doses of 175–24 000 mg/kg bw/d showed no induction of hyperplasia (NCI Citation1979, Warheit, Brown, and Donner Citation2015), whereas three more recent studies did observe induction of hyperplasia at relevant (comparable to human daily intake) doses of 5–10 mg/kg bw/d (Urrutia-Ortega et al. Citation2016, Bettini et al. Citation2017, Proquin et al. Citation2018). A recent study in which both lower and higher doses of TiO2 in the diet were applied, revealed no induction of hyperplasia in the colon after 100 days (Blevins et al. Citation2019). The authors suggested that the differences between studies might be explained by the difference in the administration of TiO2. A dispersion of TiO2 in water could for instance lead to different protein corona formation affecting the particle characteristics of TiO2 and thereby potentially negatively influencing its bioavailability, intestinal uptake and subsequent effects.
Discussion on the mechanistic insights in effects in liver and intestine after ingestion of TiO2
As shown in , in some studies, effects on liver and intestine resulting from exposure to TiO2 were found, whereas in others no effects were observed. The following aspects might explain the observed discrepancies.
First, differences might be due to the properties of the TiO2 used: variability in crystal structure, particle size distribution, and/or coating. For example, the size of the TiO2 particles used in the oral studies investigating liver effects varied from 5 nm to 220 nm (NCI Citation1979, Wang et al. Citation2007, Cui et al. Citation2010, Citation2011, Wang et al. Citation2013, Shukla et al. Citation2014, Azim et al. Citation2015, Gu et al. Citation2015, Warheit, Brown, and Donner Citation2015, Hu et al. Citation2018, Abbasi-Oshaghi, Mirzaei, and Pourjafar Citation2019, Talamini et al. Citation2019). The particle characteristics of the TiO2 used are sometimes poorly described, especially in older studies, or even absent in case of the 2-year oral carcinogenicity performed by NCI (NCI Citation1979). Hence, an assessment whether the same or similar material is used in different studies can often not be made. Different types of TiO2 may be absorbed to a different extent, the distribution over and elimination from tissues can vary, whereas also the potency to exert a hazard may differ. Only a limited number of studies in explicitly applied food-grade TiO2 (E171) (Urrutia-Ortega et al. Citation2016, Bettini et al. Citation2017, Proquin et al. Citation2018, Blevins et al. Citation2019, Talamini et al. Citation2019), or at least in part of their experiments (Warheit, Brown, and Donner Citation2015). The TiO2 used in some other studies may also be in line with food grade TiO2 (see the Supplementary Information for details on the TiO2 used per study).
Secondly, the formulation of TiO2 might be an important factor. In some studies TiO2 was suspended in water and animals were exposed via oral gavage or by dripping water into the mouth, while in other studies TiO2 is mixed with the diet. The formulation of the oral administration matrix can affect the particle characteristics of TiO2, for example due to protein corona formation, and thereby potentially influence its bioavailability, intestinal uptake and subsequent effects (Blevins et al. Citation2019). This supposition is supported by the observation that TiO2 suspended in water (with or without a dispersant, like 0.5% (w/v) hydroxypropyl-methylcellulose or 1% Tween 80) and given via oral gavage, intragastric administration or in drinking water, induced ROS generation, oxidative stress, inflammation and/or hyperplasia (NCI Citation1979, Wang et al. Citation2007, Trouiller et al. Citation2009, Cui et al. Citation2010, Citation2011, Sycheva et al. Citation2011, Wang et al. Citation2013, Chen et al. Citation2014, Shukla et al. Citation2014, Azim et al. Citation2015, Grissa et al. Citation2015, Gu et al. Citation2015, Mohamed Citation2015, Shi et al. Citation2015, Warheit, Brown, and Donner Citation2015, Mohamed and Hussien Citation2016, Urrutia-Ortega et al. Citation2016, Bettini et al. Citation2017, Hu et al. Citation2018, Proquin et al. Citation2018, Abbasi-Oshaghi, Mirzaei, and Pourjafar Citation2019, Talamini et al. Citation2019). In contrast, two other studies that exposed animals to TiO2 via the diet reported no induction of adverse effects (NCI Citation1979, Blevins et al. Citation2019).
Other aspects that may lead to discrepancy between studies are the differences in rodent species and strains used, the exposure concentrations and duration, and the specific effects which were evaluated. Furthermore, information on the relationship between dose and gastrointestinal uptake is rarely available. It is known that large agglomerates of TiO2 particles can be formed at high concentrations, complicating uptake and reducing bioavailability. Increased agglomeration in gut conditions has been shown for silica particles (Peters et al. Citation2012), which is in line with observations in a rat study that the lowest concentration resulted in the highest absorption (van der Zande et al. Citation2014). A high degree of agglomeration may therefore also reduce the absorption of TiO2 from the gastrointestinal tract. As a result, internal organ or tissue concentrations are not expected to increase linearly with increasing dose, whereas it is likely that potential effects depend on the internal concentration. This may be an explanation for the absence of effects in high dose studies compared to the finding of effects in low dose studies. Recent studies show, however, agglomeration/aggregation of silica NPs does not necessarily reduce their toxicity (Murugadoss et al. Citation2020). Hence, to better take the internal concentration into account in risk assessment, the available information on organ and tissue concentrations from oral in vivo animal studies is gathered and compared to concentrations found in humans.
Ti measurements in organs and tissues
In human
Ti determined in human organs and tissues is believed to be predominately caused by the presence of TiO2 particles, which, in individuals without an occupational inhalatory exposure to TiO2, almost exclusively originates from oral exposure to TiO2 particles. Still, potentially a limited part could be caused by other Ti compounds (Rompelberg et al. Citation2016, Heringa et al. Citation2018, Locci et al. Citation2019, Peters et al. Citation2020). Quantified Ti content (as well as Ti-particle content) in several human postmortem tissues have been reported separately (Heringa et al. Citation2018, Peters et al. Citation2020). The tissues included liver and spleen (Heringa et al. Citation2018, Peters et al. Citation2020), and kidney and two sections of intestinal tissue (Peters et al. Citation2020). Liver, spleen, kidney and specific parts of the intestine are among the organs to which NPs, including TiO2, are known to distribute to and/or accumulate in (Kreyling et al. Citation2017, EFSA Scientific Committee et al. Citation2018). The postmortem organs and tissues were obtained from 30 individuals whose bodies were donated to the Department of Anatomy of the University Medical Center Utrecht for educational and research purposes, and were collected for two separate studies (Heringa et al. Citation2018, Peters et al. Citation2020). Details on the 15 human subjects per study with respect to gender and age, as well as for the combined studies can be found in . All but one individual, who was of Asian origin, had a Caucasian ethnicity, and all individuals lived in the Netherlands most or all of their lives (Heringa et al. Citation2018, Peters et al. Citation2020). From the 15 human subjects of the first study, two had titanium implants, which, however, did not lead to elevated Ti levels in the organs analyzed (Heringa et al. Citation2018). The presence of implants was not recorded in the second study (Peters et al. Citation2020). The presence of tattoos had not been recorded either, however, it is considered highly unlikely that many of the subjects were (extensively) tattooed, also taken their age of, on average, 86 years into account (). Note that there are no data available on the human post mortem organs and tissues regarding (histo)pathology.
Table 4. Gender and age of the subjects from Heringa et al. (Citation2018) and Peters et al. (Citation2020), and of the combined studies.
The same method for the analysis of Total Ti as has been described in Peters et al. (Citation2018) has been used by Heringa et al. (Citation2018) and Peters et al. (Citation2020). Therefore, these separate, independent data sets can be combined. The total Ti levels in the human postmortem organs and tissues in each study, as well as combined are reported in . As the analysis of Ti-particles has been performed with different recovery values, the separate data sets on Ti-particle levels were not combined. There is no significant difference between the measured Total Ti detected in liver between both groups (p-value: 0.98), according to the two-tailed Welch’s t-test (unequal variances t-test), but the different group sizes (7 and 11) hamper a good comparison. There is no significant difference between the measured Ti detected in spleens between both groups (p-value: 0.83), either.
Table 5. Total Ti levels in postmortem human organs/tissues from Heringa et al. (Citation2018) and Peters et al. (Citation2020), as well as combined total Ti levels (for liver and spleen).
In animal studies
Ti levels in organs and tissues have been measured in a number of different types of animal studies (Jani, McCarthy, and Florence Citation1994, Wang et al. Citation2007, Onishchenko et al. Citation2012, Cho et al. Citation2013, Wang et al. Citation2013, Geraets et al. Citation2014, Janer et al. Citation2014, Kim and Park Citation2014, Tassinari et al. Citation2014, Gu et al. Citation2015, MacNicoll et al. Citation2015, Mohamed Citation2015, Donner et al. Citation2016, Ammendolia et al. Citation2017, Farrell and Magnuson Citation2017, Kreyling et al. Citation2017, Martins et al. Citation2017, Hu et al. Citation2018, Hu et al. Citation2020, Lee et al. Citation2019, Talamini et al. Citation2019). Some are kinetic studies, others are general toxicity studies or studies aimed at specific effects, measuring Ti levels for different reasons (see Supplementary Information 2). Similar to the comparability aspects of in vivo data and methodologies discussed earlier, studies reporting Ti content differ in experimental conditions, such as species, dosing, exposure duration, and type of TiO2 used. They also vary greatly in the sample preparation and analytical techniques applied in order to detect and quantify the level of Ti. Sometimes, small group sizes, variability in the data, high background levels or just (too) small concentration differences hamper the derivation of significant Ti levels in specific organs and tissues. This makes comparing these study results difficult, and these aspects should also be taken into account when relating the results to the Ti levels found in the human post mortem tissues. Still, risk assessment of (nano)particles such as TiO2 should preferably be performed taking organ levels into account.
Absorption of TiO2 from the gastrointestinal tract and subsequent distribution and potential accumulation may differ between humans exposed via food and animals in in vivo studies. Species differences and differences caused by experimental conditions, including type of TiO2 used, dose and exposure duration, likely affect tissue distribution and organ concentration.
In acute studies, the presence of TiO2 in organs is highly dependent on the moment of measurement, as is illustrated in the biodistribution study by Kreyling et al. (Citation2017) in which rats were exposed to a single low dose ( ∼30–80 µg/kg bw) of radiolabeled TiO2, and the level of radioactivity was measured in different organs and tissues at different timepoints after dosing (1, 4, 24 hours and 1 week). Only liver radioactivity could already be measured after 1 hour, and peaked at 4 hours in this organ, while in spleen and kidney radioactivity could first be detected after 4 hours and increased in concentration up to 1 week (Kreyling et al. Citation2017). Geraets et al. (Citation2014) showed for several types of TiO2 that 54–86% of the amount found directly after five daily iv doses remained in the liver and spleen for up to 90 days, suggesting that these particles can remain in tissues for a long period of time. Therefore, we prefer to use chronic or subchronic studies where accumulation has taken place and the organ concentrations are not so much dependent on the exact moment of sampling.
Determination of Ti levels in organs can be a challenge given the limit of detection. In a number of studies the Ti levels in organs were below the limit of detection or did not differ significantly from the control. Especially when doses are relatively few, the organ concentrations may not be high enough for detection or may not have increased enough compared to the control (Geraets et al. Citation2014, Farrell and Magnuson Citation2017, Talamini et al. Citation2019). Note that also a toxicokinetic 90-day study (according OECD TG 408) with higher doses of TiO2 NPs (260–1042 mg/kg bw/d) did not report significant increased Ti levels compared to the control (Cho et al. Citation2013).
The Ti levels in liver, spleen, kidney or intestinal tissues from animal studies in which these were quantified and significantly different from the control, have been summarized in , together with values to relate to human risk assessment.
Table 6. Significant different (from the control value mentioned) Ti levels in liver, spleen, kidney and intestinal tissues as a result of different doses of TiO2 as reported in different animal studies with oral exposure (duration of the study between parentheses; d: day, w: week).
Comparing Ti levels in human organs and tissues with animal studies
We compared the Ti levels in liver, spleen, kidney and intestine in human to the Ti levels detected in animals organs and tissues from selected studies (that quantified Ti in these organs and reported significant changes compared to a vehicle control as a result of exposure to TiO2), below. The comparison is discussed per organ/tissue in the paragraphs below and also includes the associated toxicological effects detected in the respective studies, and a relation to risk assessment.
Liver
In the human liver samples, Ti levels ranging from 0.01 up to 0.16 mg/kg liver with a P50 value of 0.03 mg/kg liver were measured () as a result of life-long exposure. Significant increased Ti levels ranging from 0.94 up to ∼4.0 mg/kg liver were reported in animal studies ().
The Ti level of 0.94 mg/kg liver reported in a 3-week study (exposure 3 times a week) with mice exposed to a low concentration of 5 mg/kg food-grade TiO2 was associated with molecular and cellular alterations in the inflammatory response in the liver (i.e. necro-inflammatory foci containing tissue monocytes/macrophages; KE5 in ) (Talamini et al. Citation2019). A similar effect (as determined by genome analysis by RNA sequencing) was seen at the elevated TiO2 levels of ∼2.8 mg/kg liver, in mice sub-chronically exposed to TiO2 P25 NPs at a dose of 50 mg/kg bw/d (Hu et al. Citation2018). However, it should be noted that the TiO2 P25 NPs in this study are smaller than the TiO2 particles present in the white pigment that is used as food additive. Serious liver effects (hepatic injury, as KE4 in , and increased liver weight (AE)) were seen at a level of ∼4.0 mg/kg liver in mice as a result of a single dose of 5000 mg/kg bw of 80 nm rutile-like TiO2 NPs two weeks post-dose (Wang et al. Citation2007). An equal dose of two other types of TiO2 particles (25 nm rutile-like NPs and 155 nm anatase particles), however, did not lead to a significantly increased Ti level in liver in this study (Wang et al. Citation2007). Also Gu et al. (Citation2015) noted liver effects (tissue fiber fractures, increased ROS generation and inflammatory markers, all seen as KEs in the AOP leading to effects on the liver ()) at a level of ∼1.9 mg/kg liver in mice as a result of a 28 week exposure to 64 mg/kg bw/d anatase TiO2 NPs (18 nm), but no increased Ti organ concentration, nor effects on the liver were seen with an equal dose of larger (120 nm) anatase TiO2 particles.
Because absorption from the gastrointestinal tract may differ with species, TiO2 dose and type, and because TiO2 organ and tissue levels may increase in time, risk assessment of such particles should preferably be performed based on TiO2 levels in organs and tissues. In an earlier assessment, based on a No Observed Adverse Exposure Level (NOAEL) of 10 mg/kg bw/d from an animal study that showed liver edema in young rats at doses of 50 mg/kg bw/d and higher (Wang et al. Citation2013), toxicokinetic modeling was used to estimate the liver concentration at the end of that key toxicity study (Heringa et al. Citation2016). A tissue level associated to effects in animals of 1.2 mg/kg liver was estimated, and a toxicologically safe tissue level of 0.008 mg/kg liver for TiO2 was calculated for humans (Heringa et al. Citation2016). As Ti accounts for 60% of the molecular weight of TiO2, this equals an animal effect tissue level of 0.72 mg/kg liver, and a human safe tissue level for Ti of 0.0048 mg/kg liver, respectively, assuming TiO2 is responsible for all Ti present.
In 18 of the total of 30 livers (), Ti levels were measured above the Limit Of Detection (LOD) (0.01 mg/kg), with a P50 value of 0.03 mg/kg being around 6 times higher than the calculated safe tissue level (note that also some of the livers with a Ti level below the LOD may contain a Ti level above the safe tissue level as this is lower than the LOD). In line with our previous studies, this indicates that adverse effects on the liver as a result of TiO2 exposure cannot be excluded. Furthermore, now we have also compared human liver concentrations to measured (rather than modeled) liver concentrations from animal studies in which effects were found. The margin between the median Ti level in human liver, and the Ti level in livers from animals in experiments showing effects on liver is small (e.g. a factor ∼30 compared to the 3-week mice study, in which inflammatory responses in the liver were found (Talamini et al. Citation2019)). For the highest Ti level found in human liver (0.16 mg/kg liver) this factor is less than 6. This further emphasizes that adverse effects on the liver as a result of TiO2 exposure cannot be excluded, as typically a considerable larger factor to account for intra- and interspecies differences and variability would be needed.
When considering the overview of KEs and AOs as structured by an AOP for liver effects (), in conjunction with the information on liver concentrations in these toxicity studies () and from the human, real life situation, it can be concluded that early effects occur in many toxicity studies at liver concentrations slightly higher (a factor 6 or more) than found in human livers. However, there are also studies that do not show effects (including specific KEs) at higher tissue concentrations of ∼2.1 mg/kg liver (Martins et al. Citation2017, Hu et al. Citation2018) (). Hence, the conclusion that effects on the liver as a result of oral TiO2 exposure cannot be excluded, remains standing. Yet, not in all situations such effects seem to occur. These differences may be due to other aspects, such as physicochemical properties of the TiO2, or whether the material is administered well dispersed in solution or in food. In any case, under specific conditions, the margin between Ti levels in livers found in humans and Ti levels modeled or measured in animal studies showing effects is considered to be too limited to exclude the possibility of liver effects occurring as a result of realistic human exposure levels.
Intestine
In the intestinal tract usually at least four zones are distinguished: duodenum, jejunum, ileum and colon. The gut epithelium is composed of enterocytes, and mucus secreting cells (Goblet cells). The ileum and jejunum regions contain Peyer’s patches, a gut associated lymphoid tissue where M-cells are located responsible for the uptake of particles (Frey et al. Citation2019, Kobayashi et al. Citation2019). NPs are usually believed to be taken up by Goblet cells and M-cells (Brun et al. Citation2014), although this process is dependent on the particle size (Powell et al. Citation2010).
The human intestinal samples analyzed (), were taken at the end of the ileum and at the beginning of the jejunum (Peters et al. Citation2020). The median Ti level was higher in the ileum than in the jejunum, though the highest value of 2.04 mg/kg intestinal tissue was measured in jejunum. The human Ti levels range from 0.02 up to 2.04 mg/kg jejunum with a P50 value of 0.14 mg/kg, and from 0.06 up to 1.41 mg/kg ileum with a P50 value of 0.26 mg/kg (). Surprisingly, these values were much higher compared to liver and spleen, organs in which accumulation of TiO2 is expected to take place. This may be because the intestinal cells are in direct contact with the relatively high concentration of TiO2 in the gut lumen, which can explain the higher concentrations found in intestinal tissues, and accumulation can also take place in the M-cells. The variation in Ti-level in the intestinal tissues is higher than in the other tissues, which can be explained by the differences between individuals in the presence of the Peyer’s patches in the tissue samples analyzed.
There are only few studies in which Ti has been analyzed or quantified in intestinal tissues of animal studies (Jani, McCarthy, and Florence Citation1994, Janer et al. Citation2014, MacNicoll et al. Citation2015, Ammendolia et al. Citation2017, Kreyling et al. Citation2017, Hu et al. Citation2018, Talamini et al. Citation2019). Most of them did not focus on effects on the intestinal tissue (Jani, McCarthy, and Florence Citation1994, Janer et al. Citation2014, MacNicoll et al. Citation2015, Hu et al. Citation2018), or uptake in intestinal tissues (Jani, McCarthy, and Florence Citation1994, Kreyling et al. Citation2017). Janer et al. (Citation2014) studied Ti levels in several intestinal tissues, 24 hours after a single dose of 100 mg/kg bw in rats, but did not find significant increased levels. Also another single dose study did not report significant (increased) levels in intestinal tissues 4 days post-dose after 5 mg/kg bw (MacNicoll et al. Citation2015). Kreyling et al. (Citation2017) detected an increased amount of radiolabeled TiO2 in the small intestinal wall 1 hr post-dose, and an increased amount in the colon 4 and 24 hours post-dose. These studies indicate the time point after a single dose is important and suggest that (sub)chronic exposure is needed in order to cause detectable, increased Ti levels in intestinal tissues, probably as well as subsequent effects. After a 5-day exposure to a low dose of 2 mg/kg bw/d TiO2 NPs, Ammendolia et al. (Citation2017) reported an increased Ti level in the small intestine of 0.13 mg/kg intestine, but no local effects on the intestine as a result of this were studied in vivo (though some effects on villi were reported). Talamini et al. (Citation2019) is the only study in which significant increased Ti levels in intestinal tissues (i.e. colon) were measured along with local effects in this tissue. In that study, a significantly increased Ti level of 1.07 mg/kg colon (compared to ∼0.6 mg/kg colon in the vehicle control) as a result 3 weeks (3 times a week) 5 mg/kg bw food-grade TiO2 was measured (Talamini et al. Citation2019). Interestingly, in this study no significant increased level was found in the small intestine (Talamini et al. Citation2019). The internal concentrations were accompanied by an increase in the expression of interleukin (IL)-1β and downregulation of tumor necrosis factor (TNF)-α and intracellular adhesion molecule (ICAM)-1, as determined in the whole intestine, which indicate increased ROS generation and inflammatory status (Talamini et al. Citation2019). These effects can be seen as early effects that could potentially lead to an AO (). One other study in which Ti levels were quantified in intestinal tissues (i.e. small intestine) and significantly higher levels of Ti were found, was performed by Hu et al. (Citation2018). In this sub-chronic study with mice exposed to 10 or 50 mg/kg bw/d TiO2 P25 NPs, Ti levels were relatively high in small intestine, i.e. ∼3.4 or ∼5.3 mg/kg small intestine, respectively (Hu et al. Citation2018). Increased ROS generation and inflammatory markers were detected in liver and serum, but no local effects in the intestinal tissue were studied.
The P50 values of Ti levels human intestinal tissue differ a factor 4–8 from the Ti level found in colon in which effects were observed in mice. In two human donors Ti levels in intestinal tissues were detected which were higher (Peters et al. Citation2020), up to a factor two over the concentration of 1.07 mg/kg colon detected by Talamini et al. (Citation2019). Ideally, there is a considerable margin between these tissue concentrations in order to exclude risks for humans. Considering the overlap, it seems feasible that molecular and cellular alterations in the inflammatory response in the intestine as found by Talamini et al. (Citation2019), could occur in humans too. However, no studies are available with human tissue concentrations in combination with AO effects.
Hence, it seems feasible that early effects (KE1–5, ) occur at concentrations that occur in human intestinal tissues. However, and indicate that in three studies the latest KE (KE7) is triggered (Urrutia-Ortega et al. Citation2016, Bettini et al. Citation2017, Proquin et al. Citation2018), whereas a number of other studies investigated this aspect but found no effects related to this KE (NCI Citation1979, Warheit, Brown, and Donner Citation2015, Blevins et al. Citation2019). As the concentration in the intestinal tissues was not measured, a comparison on tissue concentration cannot be made, but daily doses administered in these studies were only slightly higher than the estimated human intake. Therefore, presently the information available is insufficient to draw firm conclusions.
Spleen
In the human spleen, Ti levels ranging from 0.02 up to 0.4 mg/kg spleen with a P50 value of 0.04 mg/kg spleen were measured () as a result of life-long exposure. Significantly increased Ti levels ranging from 0.046 up to ∼3.7 mg/kg spleen were reported in animal studies (). The Ti level of 0.046 mg/kg spleen was found by Tassinari et al. (Citation2014) after a 5-day exposure of rats to TiO2 NPs at a dose of 2 mg/kg bw/d. The white-to-red pulp area ratio was altered as a result of this exposure, indicating an immunological effect (Tassinari et al. Citation2014). Higher levels of ∼2.7–3.7 mg/kg spleen were reported by Hu et al. (Citation2018), however, they did not study the adverse effects on the spleen. No effects were seen at a level of ∼0.6 mg/kg spleen in mice as a result of a single dose of 5000 mg/kg bw to 3 types of specific TiO2 materials two weeks post-dose, either (Wang et al. Citation2007).
Regarding risk assessment, based on the provisional NOAEL for spleen from Tassinari et al. (Citation2014), and toxicokinetic modeling accounting for life-long accumulation, a tissue level associated with effects in animals of 21 mg/kg spleen was estimated, and a toxicological safe tissue level of 0.14 mg/kg spleen for TiO2 for humans was calculated (Heringa et al. Citation2016). As Ti accounts for 60% of the weight of TiO2, this equals an animal effect tissue level of 12.6 mg/kg spleen, and a human safe tissue level for Ti of 0.084 mg/kg, respectively, assuming TiO2 is responsible for all Ti present.
The P50 value measured in spleen was a factor ∼2 below the safe human tissue level. In seven spleens, a Ti level above this safe human tissue level was measured. Although the concentration at which alteration in white-to-red pulp area ratio was noticed in an animal study is in the range of concentrations found in humans (Tassinari et al. Citation2014), the relevance of this effect for humans is not clear. Gu et al. (Citation2015) reported decreased folliculi lymphaticus in the spleen as a result of a 28-week exposure of mice to 64 mg/kg bw/d TiO2 NPs (18 nm), however, they did not look at the organ concentration of the spleen. Other animal studies which looked at histopathological changes in the spleen as a result of TiO2 exposure did not find effects on the spleen (NCI Citation1979, Wang et al. Citation2007, Bu et al. Citation2010, Wang et al. Citation2013, Auttachoat et al. Citation2014). Hence, one study indicates that effects occur at spleen levels that equals the P50 value of Ti found in human spleens, whereas many other studies did not find effects. One other study that determined spleen concentrations in combination with studying effects did not find an effect at spleen concentrations above the highest dose observed in human (∼0.6 mg/kg spleen), after a single dose of 5000 mg/kg bw (Wang et al. Citation2007). Therefore, conclusions cannot be drawn based on the present data.
Kidney
In the human kidney, Ti levels ranging from 0.01 up to 0.37 mg/kg kidney with a P50 value of 0.06 mg/kg kidney were measured () as a result of life-long exposure. These levels are higher than have been detected in liver, and similar to the levels in spleen (). Higher kidney than liver concentrations are also found in most animal studies (Cho et al. Citation2013, Kim and Park Citation2014, Martins et al. Citation2017, Hu et al. Citation2018).
Significant increased Ti levels ranging from ∼0.4 up to ∼5.2 mg/kg kidney were reported (). The level of ∼0.4 mg/kg kidney was a result of an experiment with mice with single dose of 5000 mg/kg bw to 25 nm or 80 nm rutile-like TiO2 NPs, after two weeks (Wang et al. Citation2007). As a result of this exposure, serious changes in blood markers (indicating kidney disfunction) and histopathological changes (filled renal tubule by proteinic liquids, serious swelling of the glomerulus) were noted (Wang et al. Citation2007). In the same study, no significant increased Ti level in kidney was found with 155 nm anatase TiO2 particles, however, still serious swelling of the glomerulus was found (Wang et al. Citation2007). Higher levels of ∼2.8 and ∼3.9–5.2 mg/kg kidney were reported in sub-chronic studies (), however, these did not study effects on kidney (Martins et al. Citation2017, Hu et al. Citation2018). Some studies reported histopathological changes in the kidney as a result of (relatively high) exposure to TiO2, however, did not find a significant changed Ti level in the kidney (nor in other organs) (Wang et al. Citation2013), or did not study Ti levels in organs (Al-Rasheed et al. Citation2013, Gu et al. Citation2015). Hence, the present data set is too limited and scattered to allow drawing conclusions.
Additional human data
Only very few studies investigated Ti-particle levels in human tissues, for instance Powell et al. (Citation1996) that detected TiO2 particles in gut associated lymphoid tissue. Two other, recent studies of relevance are discussed here.
First, Ti levels in human pancreas were recently reported by Heller, Coffman, and Friedman (Citation2019). The reported levels of Ti (0.75–3.78 mg/kg) in pancreas are remarkably high, 25–45 times higher than the levels determined in human liver and spleen. The authors link this finding to diabetes 2 associated with high body mass index (Heller, Coffman, and Friedman Citation2019). On the other hand, in sub-chronic studies with mice exposed to TiO2 NPs, the concentration Ti in pancreas was low compared to other organs (Hu et al. Citation2018), or similar to the level determined in liver (Gu et al. Citation2015, Hu et al. Citation2020). Hence, given the very limited number of studies that either indicate high concentrations and potential effects or low concentrations and no effects, it is recommended for future studies to include determination of concentrations and effects in the pancreas.
Furthermore, the presence of Ti particles have been qualitatively detected in human liver and kidney by Locci et al. (Citation2019). They determined size, shape and elemental composition of particles in postmortem liver and kidney from 35 subjects from Sardinia, Italy (Locci et al. Citation2019). Quantitative determinations of particles, e.g. number or mass per kg organ, were not performed. Instead, the number of particles in a defined area of a field-emission scanning electron microscopy sample, as prepared from a slice of 5 g tissue sample was determined. Elemental composition of the particles was determined by energy-dispersive X-ray spectroscopy. No major differences in the determined number of particles, size and morphology between liver and kidney were observed. No relationship between gender, age and detected particles was observed. They found particles, as aggregates or agglomerates, ranging between 50 nm and 100 µm and often consisting of multiples associations of elements. Ti comprised about 2% of the elemental composition over all particles determined, whereas Si and Fe comprised 61 and 23% (note that C and O were not included in the elemental composition) (Locci et al. Citation2019).
Discussion
On a daily basis, humans are exposed orally to TiO2 via food, food supplements, tooth paste (i.e. for young children) and medicines (Bachler, von Goetz, and Hungerbuhler Citation2015, Rompelberg et al. Citation2016). Analysis of postmortem tissues indicates that these particles are taken up by the intestine and subsequently transported to secondary organs such as liver and spleen, where they can accumulate (Heringa et al. Citation2018, Peters et al. Citation2020). Current legislation of the food additive E171 is based on the lack of effects in the chronic study by NCI (Citation1979), investigating only traditional toxicological endpoints (NCI Citation1979). As there is information from recent studies showing toxicological responses, we decided to include these studies as an evidence of possible adverse effects. However, there are also recent studies in which no adverse effects were demonstrated.
For both liver and the intestine, postulated AOPs were used describing steps that can lead to liver steatosis, edema and fibrosis, and to intestinal tumor formation as a result of oral exposure to TiO2. Using the AOP approach allowed us to take into consideration not only morphological, histopathological and clinical observations, but also the available data from in vivo animal studies investigating some of the KEs in the AOPs. We did not seek to prove the AOPs, but we used postulated AOPs to obtain an organized picture of the available information. The AOPs helped to get a more structured and better representation of early effects that may have limited health implications and can be reversible, versus effects that go beyond these early effects and adverse effects such as organ toxicity. The AOP construct facilitated in assigning qualitatively the investigated endpoints and available assays to the right place in the chain of events leading to the ‘point of no return’: the AO. The structure also helps to show the data gaps and formulate more focused questions to aid the relevance of future studies for risk assessment. There are indeed many data gaps (as illustrated by the blank ‘NDs’ in ). The data gaps and research recommendations related to a potential carcinogenic effect of TiO2 are described and discussed in detail in Braakhuis et al. (Citation2021). Altogether, the methodology helped us to organize the available information and to get a more complete representation of toxicological pathways leading to the organ toxicity, better list the uncertainties and formulate more focused questions (Vinken et al. Citation2020). Note that the postulated AOPs are solely based on rodent data, and this should be taken into account when translating their outcome to the human situation (Braakhuis et al. Citation2021).
The general comparison of studies is hampered by differences in various aspects. It remains unknown to what extent the observed discrepancies are caused by factors such as the properties of TiO2 (including the fraction of TiO2 NPs), dose, exposure duration and animal species. We noted that differences in administration methodology (suspension in water versus dietary exposure) may greatly affect the toxicity. A suspension of TiO2 in water could for instance lead to different protein corona formation affecting the particle characteristics of TiO2, thereby potentially negatively influencing its bioavailability, intestinal uptake and subsequent effects. On the other hand, a suspension in water could also enable aggregation/agglomeration potentially negatively influencing bioavailability. To take into account differences in absorption from the gastrointestinal tract, as well as accumulation in tissues (e.g. by different species and between different types of TiO2), the information available on Ti tissue concentrations was considered.
For the effects on liver (), the analysis shows KEs in line with the AOP of liver fibrosis, steatosis and/or edema (KE2–KE6), are triggered in most studies that assessed those (). Two studies also observed AOs: liver fibrosis (AO1 in ) (Gu et al. Citation2015), and liver edema (AO2 in ) (Wang et al. Citation2013). Nevertheless, there are also a number of other studies that do not find any AOs (NCI Citation1979, Warheit, Brown, and Donner Citation2015, Blevins et al. Citation2019). The KEs are, unfortunately, not investigated in most of these studies (). When comparing Ti levels in liver, only a factor of 30 and 6 is observed between liver concentrations found in mice associated with inflammatory responses (KE6, ) in the liver, and the P50 value and highest liver concentration found in humans, respectively. Unfortunately, TiO2 uptake and/or tissue concentrations have not been investigated in studies that used dietary exposure to TiO2. This makes it difficult to extrapolate and conclude whether there is a health risk due to exposure to TiO2 in food. Looking at the oral intake, it cannot be ignored that TiO2 seems to be able to trigger KEs in the liver AOP at doses that are within one or two orders of magnitude of the average daily oral intake of E171 estimated for the Dutch, or European population. Whether this subsequently results in irreversible adverse liver effects in humans remains unknown, given the discrepancies in observations of later KEs and as the number of studies that investigated the AO (i.e. liver fibrosis, liver edema, liver steatosis or intestinal tumors) is limited. Generally, KEs closer to the AO are more reliable in predicting toxic effects (Hu et al. Citation2018, Martins et al. Citation2017). The earlier KEs (i.e. ROS generation, inflammation) could be reversible depending on the dose and the exposure duration. It might be that persistent and/or higher ROS generation and inflammation are needed to induce subsequent KEs such as tissue injury and histopathological lesions.
Similarly, for intestinal effects, the analysis shows that for the earlier KEs such as generation of ROS, oxidative stress, and inflammation (KE1–KE3, ), there is evidence that oral exposure to TiO2 (including NPs) can trigger these KEs ( and ). The body can resolve the induced earlier KEs without progressing to later KEs and an AO. However, it remains unclear whether those later KEs and AO could be induced upon prolonged exposure to TiO2. The results from animal studies are contradicting as some do show the induction of hyperplasia of the colon or associated events (Bettini et al. Citation2017, Proquin et al. Citation2018, Urrutia-Ortega et al. Citation2016), while other studies do not (Blevins et al. Citation2019, NCI Citation1979, Warheit, Brown, and Donner Citation2015). The reason for not observing hyperplasia or tumors at high doses remains unclear. This is complicated by the fact that the internal dose in organs has not been measured in the majority of the studies, making it impossible to relate the internal dose to the observed effects. Of the few studies in which Ti levels in intestinal tissue are determined, one study found an increased Ti level in colon after 3 weeks of exposure (3 times a week) to food-grade TiO2 that was accompanied by increased ROS generation and inflammatory status (KE1 and KE3, ) (Talamini et al. Citation2019), whereas others did not find increased tissue concentrations and local effects. For the studies that used high concentrations (>1000 mg/kg bw/d) it is unknown whether large agglomerates were formed that might have hampered the uptake and bioavailability of the TiO2 particles. For the later KEs such as epithelial cell proliferation, pre-neoplastic lesions (KE6 and KE7, ) and for the AO, available studies are inconsistent and lacking information on tissue concentrations ( and ). In the paper by Braakhuis and colleagues, the AOP leading to intestinal tumors has been analyzed semi-quantitatively taking the doses at which effects were observed in animals into account. The semi-quantitative approach shows that there is evidence that the AOP might be operative for TiO2; to reduce uncertainties, specific future studies are recommended (Braakhuis et al. Citation2021).
For kidney and spleen, data are too scattered, and limited, to draw conclusions on potential health risks. However, the Ti level in these tissues found in humans is similar or higher than in liver. Also a recent study suggest that Ti levels in human pancreas are high (Heller, Coffman, and Friedman Citation2019).
Another AO that is receiving increasing attention, but that is not included in the AOP regarding the intestine in , is the possibility that TiO2 may exacerbate inflammation in patients with IBD such as Crohn’s disease (Hummel et al. Citation2014, Lomer et al. Citation2004, Powell et al. Citation1996, Ruiz et al. Citation2017). In a mouse model, TiO2 NPs were shown to exacerbate acute colitis (Ruiz et al. Citation2017). A pilot study in IBD patients showed that a diet low in exogenous particles such as TiO2 and aluminosilicates appears to alleviate the symptoms of Crohn’s disease (Lomer, Thompson, and Powell Citation2002), though another study could not confirm this (Lomer et al. Citation2005).
Conclusions and recommendations
The available data is, however, sufficient to conclude that TiO2 (including NPs) has the potential to generate ROS, induce oxidative stress and inflammation in liver and intestinal tissue, but it is unknown whether these events subsequently result in irreversible adverse effects in humans. To allow for conclusions whether TiO2 leads to AOs, and, if so, under which conditions this is possible, further studies would be needed.
With regard to kidney and spleen, data are too limited to draw conclusions on potential health risks. The level presence of TiO2 in these organs, and in addition in pancreas as has been reported, indicate that it is relevant to gain further knowledge on potential effects of TiO2 on these tissues. In addition, more information is needed on the potential effects of TiO2 exposure in patients with IBD.
In line with a recent publication on the intestinal AOP potentially induced by TiO2 (Braakhuis et al. Citation2021), we recommend the performance of chronic in vivo studies using well-characterized food-grade TiO2, including measurement of Ti levels in key tissues (liver, spleen, kidney, intestinal tissues and pancreas), assessment of KEs ( and ) and AOs. Furthermore, it is recommended to investigate whether administration via diet and well-dispersed in water affects the uptake, tissue concentrations and toxicity. The same holds for systematic research on the relationships between the properties of the TiO2 and toxicokinetic behavior (uptake, tissue distribution, accumulation) as well as toxicity. Furthermore, the present document can be seen as a start toward a weight of evidence approach assessing the risk of oral exposure to TiO2. We recommend, as a next step, to include all aspects of reliability, relevance, consistency and transparency of a complete weight of evidence approach (EFSA Scientific Committee Citation2017). Finally, it is recommended that future studies are performed with realistic to high doses (e.g. 1–1000 mg/kg bw/d). At extremely high doses (even up to 24 000 mg/kg bw/d, ) the dose itself may paralyze the gastrointestinal tract, leading to situations that have no relevance for real life.
Supplemental Material
Download Zip (64.6 KB)Acknowledgements
The authors thank Jacqueline Castenmiller of the Netherlands Food and Consumer Product Safety Authority (NVWA-BuRO) for support and fruitful discussions. Susan Dekkers, Joke Herremans and Emiel Rorije (all from RIVM) are acknowledged for their useful comments on the paper.
Disclosure statement
The authors declare that they have no competing interests.
Notes
Additional information
Funding
Notes
1 An AOP describes a sequential chain of causally linked events at different levels of biological organisation that lead to an adverse health or ecotoxicological effect. AOPs are the central element of a toxicological knowledge framework being built to support chemical risk assessment based on mechanistic reasoning (https://www.oecd.org/chemicalsafety/testing/adverse-outcome-pathways-molecular-screening-and-toxicogenomics.htm).
2 The AOP-Wiki is a collaborative, international effort and serves as a component of the OECD-sponsored AOP Knowledgebase (AOP-KB) effort. This wiki is hosted by the Society for the Advancement of Adverse Outcome Pathways (SAAOP). The AOP-KB represents the central repository for all AOPs developed as part of the OECD AOP Development Effort by the Extended Advisory Group on Molecular Screening and Toxicogenomics. All AOPs from the AOP Knowledgebase are available at https://aopwiki.org/.
References
- Abbasi-Oshaghi, E., F. Mirzaei, and M. Pourjafar. 2019. “NLRP3 Inflammasome, Oxidative Stress, and Apoptosis Induced in the Intestine and Liver of Rats Treated with Titanium Dioxide Nanoparticles: In Vivo and In Vitro Study.” International Journal of Nanomedicine 14: 1919–1936. doi:10.2147/IJN.S192382.
- Al-Rasheed, N. M., L. M. Faddah, A. M. Mohamed, N. A. Abdel Baky, N. M. Al-Rasheed, and R. A. Mohammad. 2013. “Potential Impact of Quercetin and Idebenone against Immuno- Inflammatory and Oxidative Renal Damage Induced in Rats by Titanium Dioxide Nanoparticles Toxicity.” Journal of Oleo Science 62 (11): 961–971. doi:10.5650/jos.62.961.
- Ammendolia, M. G., F. Iosi, F. Maranghi, R. Tassinari, F. Cubadda, F. Aureli, A. Raggi, F. Superti, A. Mantovani, and B. De Berardis. 2017. “Short-Term Oral Exposure to Low Doses of Nano-Sized TiO2 and Potential Modulatory Effects on Intestinal Cells.” Food and Chemical Toxicology: An International Journal Published for the British Industrial Biological Research Association 102: 63–75. doi:10.1016/j.fct.2017.01.031.
- ANSES. 2019. AVIS de l’agence nationale de sécurité sanitaire de l’alimentation, de l’environnement et du travail – relatif aux risques liés à l’ingestion de l’additif alimentaire E171. Available from: https://www.anses.fr/fr/system/files/ERCA2019SA0036.pdf [Accessed 26 August 2019].
- Auttachoat, W., C. E. McLoughlin, K. L. White, Jr., and M. J. Smith. 2014. “Route-Dependent Systemic and Local Immune Effects following Exposure to Solutions Prepared from Titanium Dioxide Nanoparticles.” Journal of Immunotoxicology 11 (3): 273–282. doi:10.3109/1547691X.2013.844750.
- Azim, S. A., H. A. Darwish, M. Z. Rizk, S. A. Ali, and M. O. Kadry. 2015. “Amelioration of Titanium Dioxide Nanoparticles-Induced Liver Injury in Mice: possible Role of Some Antioxidants.” Experimental and Toxicologic Pathology : official Journal of the Gesellschaft Fur Toxikologische Pathologie 67 (4): 305–314. doi:10.1016/j.etp.2015.02.001.
- Bachler, G., N. von Goetz, and K. Hungerbuhler. 2015. “Using Physiologically Based Pharmacokinetic (PBPK) Modeling for Dietary Risk Assessment of Titanium Dioxide (TiO2) Nanoparticles.” Nanotoxicology 9 (3): 373–380. doi:10.3109/17435390.2014.940404.
- Bettini, S., E. Boutet-Robinet, C. Cartier, C. Comera, E. Gaultier, J. Dupuy, N. Naud, et al. 2017. “Food-Grade TiO2 Impairs Intestinal and Systemic Immune Homeostasis, Initiates Preneoplastic Lesions and Promotes Aberrant Crypt Development in the Rat Colon.” Scientific Reports 7: 40373. doi:10.1038/srep40373.
- Blevins, L. K., R. B. Crawford, A. Bach, M. D. Rizzo, J. Zhou, J. E. Henriquez, D. M. I. Olive Khan, et al. 2019. “Evaluation of Immunologic and Intestinal Effects in Rats Administered an E 171-Containing Diet, a Food Grade Titanium Dioxide (TiO2).” Food and Chemical Toxicology : An International Journal Published for the British Industrial Biological Research Association 133: 110793. doi:10.1016/j.fct.2019.110793.
- Braakhuis, H. M., I. Gosens, M. Heringa, A. G. Oomen, R. J. Vandebriel, M. Groenewold, and F. R. Cassee. 2021. “Mechanism of Action of TiO2: Recommendations to Reduce Uncertainties Related to Carcinogenic Potential.” Annual Review of Pharmacology and Toxicology 61 (1). doi:10.1146/annurev-pharmtox-101419-100049.
- Brun, E., F. Barreau, G. Veronesi, B. Fayard, S. Sorieul, C. Chaneac, C. Carapito, et al. 2014. “Titanium Dioxide Nanoparticle Impact and Translocation Through Ex Vivo, In Vivo and In Vitro Gut Epithelia.” Particle and Fibre Toxicology 11: 13. doi:10.1186/1743-8977-11-13.
- Bu, Q., G. Yan, P. Deng, F. Peng, H. Lin, Y. Xu, Z. Cao, et al. 2010. “ NMR-Based Metabonomic Study of the Sub-Acute Toxicity of Titanium Dioxide Nanoparticles in Rats after Oral Administration.” Nanotechnology 21 (12): 125105. doi:10.1088/0957-4484/21/12/125105.
- Chen, Z., D. Zhou, S. Han, S. Zhou, and G. Jia. 2019. “Hepatotoxicity and the Role of the Gut-Liver Axis in Rats after Oral Administration of Titanium Dioxide Nanoparticles.” Particle and Fibre Toxicology 16 (1): 48. doi:10.1186/s12989-019-0332-2.
- Chen, Z., Y. Wang, T. Ba, Y. Li, J. Pu, T. Chen, Y. Song, et al. 2014. “Genotoxic Evaluation of Titanium Dioxide Nanoparticles In Vivo and In vitro.” Toxicology Letters 226 (3): 314–319. doi:10.1016/j.toxlet.2014.02.020.
- Cho, W. S., B. C. Kang, J. K. Lee, J. Jeong, J. H. Che, and S. H. Seok. 2013. “Comparative Absorption, Distribution, and Excretion of Titanium Dioxide and Zinc Oxide Nanoparticles after Repeated Oral administration.” Particle and Fibre Toxicology 10: 9. doi:10.1186/1743-8977-10-9.
- Cornu, R., A. Beduneau, and H. Martin. 2020. “Influence of Nanoparticles on Liver Tissue and Hepatic Functions: A Review.” Toxicology 430: 152344. doi:10.1016/j.tox.2019.152344.
- Cui, Y., H. Liu, M. Zhou, Y. Duan, N. Li, X. Gong, R. Hu, M. Hong, and F. Hong. 2011. “Signaling Pathway of Inflammatory Responses in the Mouse Liver Caused by TiO2 Nanoparticles.” Journal of Biomedical Materials Research. Part A 96 (1): 221–229. doi:10.1002/jbm.a.32976.
- Cui, Y., X. Gong, Y. Duan, N. Li, R. Hu, H. Liu, M. Hong, et al. 2010. “Hepatocyte Apoptosis and Its Molecular Mechanisms in Mice Caused by Titanium Dioxide nanoparticles.” Journal of Hazardous Materials 183 (1-3): 874–880. doi:10.1016/j.jhazmat.2010.07.109.
- Donner, E. M., A. Myhre, S. C. Brown, R. Boatman, and D. B. Warheit. 2016. “In Vivo Micronucleus Studies with 6 Titanium Dioxide Materials (3 Pigment-Grade & 3 Nanoscale) in Orally-Exposed Rats.” Regulatory Toxicology and Pharmacology: RTP 74: 64–74. doi:10.1016/j.yrtph.2015.11.003.
- Dorier, M., D. Béal, C. Tisseyre, C. Marie-Desvergne, M. Dubosson, F. Barreau, E. Houdeau, N. Herlin-Boime, T. Rabilloud, and M. Carriere. 2019. “The Food Additive E171 and Titanium Dioxide Nanoparticles Indirectly Alter the Homeostasis of Human Intestinal Epithelial Cells: In Vitro.” Environmental Science: Nano 6 (5): 1549–1561. doi:10.1039/C8EN01188E.
- EFSA ANS Panel. 2016. “Re-Evaluation of Titanium Dioxide (E 171) as a Food Additive.” EFSA Journal 14 (9): 4545.
- EFSA FAF Panel. 2019. “Scientific Opinion on the Proposed Amendment of the EU Specifications for Titanium Dioxide (E 171) with Respect to the Inclusion of Additional Parameters Related to Its Particle Size Distribution.” EFSA Journal 17 (7): 5760.
- EFSA Scientific Committee, Hardy, A., D. Benford, T. Halldorsson, M. J. Jeger, H. K. Knutsen, S. More, H. Naegeli, et al. 2018. “Guidance on Risk Assessment of the Application of Nanoscience and Nanotechnologies in the Food and Feed Chain: Part 1, Human and Animal Health.” EFSA Journal 16 (7): 5327. doi:10.2903/j.efsa.2018.5327.
- EFSA Scientific Committee, Hardy, A., D. Benford, T. Halldorsson, M. J. Jeger, H. K. Knutsen, S. More, H. Naegeli, et al. 2017. “Scientific Opinion on the Guidance on the Use of the Wei Ght of Evidence Approach in Scientific Assessments.” EFSA Journal 15 (8): 4971. doi:10.2903/j.efsa.2017.4971.
- EFSA. 2019. “EFSA Statement on the Review of the Risks Related to the Exposure to the Food Additive Titanium Dioxide (E 171) Performed by the French Agency for Food, Environmental and Occupational Health and Safety (ANSES).” EFSA Journal 17 (6): 5714.
- Farrell, T. P., and B. Magnuson. 2017. “Absorption, Distribution and Excretion of Four Forms of Titanium Dioxide Pigment in the Rat.” Journal of Food Science 82 (8): 1985–1993. doi:10.1111/1750-3841.13791.
- Frey, A., K. Ramaker, N. Röckendorf, B. Wollenberg, I. Lautenschläger, G. Gébel, A. Giemsa, M. Heine, D. Bargheer, and P. Nielsen. 2019. “Fate and Translocation of (Nano)Particulate Matter in the Gastrointestinal Tract.” In Biological Responses to Nanoscale Particles. Nanoscience and Technology, edited by P. Gehr and R. Zellner, 281–327. Cham: Springer.
- Geiss, O., J. Ponti, C. Senaldi, I. Bianchi, D. Mehn, J. Barrero, D. Gilliland, R. Matissek, and E. Anklam. 2020. “Characterisation of Food Grade Titania with Respect to Nanoparticle Content in Pristine Additives and in Their Related Food Products.” Food Additives & Contaminants. Part A, Chemistry, Analysis, Control, Exposure & Risk Assessment 37 (2): 239–253. doi:10.1080/19440049.2019.1695067.
- Geraets, L., A. G. Oomen, P. Krystek, N. R. Jacobsen, H. Wallin, M. Laurentie, H. W. Verharen, E. F. Brandon, and W. H. de Jong. 2014. “Tissue Distribution and Elimination after Oral and Intravenous Administration of Different Titanium Dioxide Nanoparticles in Rats.” Particle and Fibre Toxicology 11: 30doi:10.1186/1743-8977-11-30.
- Gerloff, K., B. Landesmann, A. Worth, S. Munn, T. Palosaari, and M. Whelan. 2017. “The Adverse Outcome Pathway Approach in Nanotoxicology.” Computational Toxicology 1: 3–11. doi:10.1016/j.comtox.2016.07.001.
- Grissa, I., J. Elghoul, L. Ezzi, S. Chakroun, E. Kerkeni, M. Hassine, L. El Mir, M. Mehdi, H. Ben Cheikh, and Z. Haouas. 2015. “Anemia and Genotoxicity Induced by sub-chronic intragastric treatment of rats with titanium dioxide nanoparticles.” Mutation Research – Genetic Toxicology and Environmental Mutagenesis 794: 25–31. doi:10.1016/j.mrgentox.2015.09.005.
- Gu, N., H. Hu, Q. Guo, S. Jin, C. Wang, Y. Oh, Y. Feng, and Q. Wu. 2015. “Effects of Oral Administration of Titanium Dioxide Fine-Sized Particles on Plasma Glucose in Mice.” Food and Chemical Toxicology : An International Journal Published for the British Industrial Biological Research Association 86: 124–131. doi:10.1016/j.fct.2015.10.003.
- Heller, A., S. S. Coffman, and K. A. Friedman. 2019. “Obesity-Dependent Accumulation of Titanium in the Pancreas of Type 2 Diabetic Donors.” Chemical Research in Toxicology 32 (7): 1351–1356. doi:10.1021/acs.chemrestox.8b00304.
- Heringa, M. B., L. Geraets, J. C. van Eijkeren, R. J. Vandebriel, W. H. de Jong, and A. G. Oomen. 2016. “Risk Assessment of Titanium Dioxide Nanoparticles via Oral Exposure, Including Toxicokinetic Considerations.” Nanotoxicology 10 (10): 1515–1525. doi:10.1080/17435390.2016.1238113.
- Heringa, M. B., R. J. B. Peters, R. L. A. W. Bleys, M. K. van der Lee, P. C. Tromp, P. C. E. van Kesteren, J. C. H. van Eijkeren, A. K. Undas, A. G. Oomen, and H. Bouwmeester. 2018. “Detection of Titanium Particles in Human Liver and Spleen and Possible Health Implications.” Particle and Fibre Toxicology 15 (1): 15. doi:10.1186/s12989-018-0251-7.
- Hu, H., B. Zhang, L. Li, Q. Guo, D. Yang, X. Wei, X. Fan, et al. 2020. “The Toxic Effects of Titanium Dioxide Nanoparticles on Plasma Glucose Metabolism Are More Severe in Developing Mice than in Adult Mice.” Environmental Toxicology 35 (4): 443–456. doi:10.1002/tox.22880.
- Hu, H., L. Li, Q. Guo, H. Zong, Y. Yan, Y. Yin, Y. Wang, et al. 2018. “RNA Sequencing Analysis Shows That Titanium Dioxide Nanoparticles Induce Endoplasmic Reticulum Stress, Which Has a Central Role in Mediating Plasma Glucose in Mice.” Nanotoxicology 12 (4): 341–356. doi:10.1080/17435390.2018.1446560.
- Hummel, T. Z., A. Kindermann, P. C. Stokkers, M. A. Benninga, and F. J. ten Kate. 2014. “Exogenous Pigment in Peyer Patches of Children Suspected of Having IBD.” Journal of Pediatric Gastroenterology and Nutrition 58 (4): 477–480. doi:10.1097/MPG.0000000000000221.
- Janer, G., E. Mas del Molino, E. Fernandez-Rosas, A. Fernandez, and S. Vazquez-Campos. 2014. “Cell Uptake and Oral Absorption of Titanium Dioxide Nanoparticles.” Toxicology Letters 228 (2): 103–110. doi:10.1016/j.toxlet.2014.04.014.
- Jani, P. U., D. E. McCarthy, and A. T. Florence. 1994. “Titanium Dioxide (Rutile) Particle Uptake from the Rat GI Tract and Translocation to Systemic Organs after Oral Administration.” International Journal of Pharmaceutics 105 (2): 157–168. doi:10.1016/0378-5173(94)90461-8.
- Jin, C. Y., B. S. Zhu, X. F. Wang, and Q. H. Lu. 2008. “Cytotoxicity of Titanium Dioxide Nanoparticles in Mouse Fibroblast Cells.” Chemical Research in Toxicology 21 (9): 1871–1877. doi:10.1021/tx800179f.
- Kim, H., and K. Park. 2014. “Excretion, Tissue Distribution and Toxicities of Titanium Oxide Nanoparticles in Rats after Oral Administration over Five Consecutive Days.” Korean Journal of Environmental Health Sciences 40 (4): 294–303. doi:10.5668/JEHS.2014.40.4.294.
- Kobayashi, N., D. Takahashi, S. Takano, S. Kimura, and K. Hase. 2019. “The Roles of Peyer’s Patches and Microfold Cells in the Gut Immune System: Relevance to Autoimmune Diseases.” Frontiers in Immunology 10: 2345doi:10.3389/fimmu.2019.02345.
- Kreyling, W. G., U. Holzwarth, C. Schleh, J. Kozempel, A. Wenk, N. Haberl, S. Hirn, et al. 2017. “Quantitative Biokinetics of Titanium Dioxide Nanoparticles after Oral Application in Rats: Part 2.” Nanotoxicology 11 (4): 443–453. doi:10.1080/17435390.2017.1306893.
- Lee, J., J. S. Jeong, S. Y. Kim, M. K. Park, S. D. Choi, U. J. Kim, K. Park, E. J. Jeong, S. Y. Nam, and W. J. Yu. 2019. “Titanium Dioxide Nanoparticles Oral Exposure to Pregnant Rats and Its Distribution.” Particle and Fibre Toxicology 16 (1): 31. doi:10.1186/s12989-019-0313-5.
- Légifrance. 2019. Arrêté du 17 avril 2019 portant suspension de la mise sur le marché des denrées contenant l’additif E 171 (dioxyde de titane - TiO2). Accessed 26 August 2019. https://www.legifrance.gouv.fr/affichTexte.do;jsessionid=4C4616BE5A75DEC2E7DA786B41904851.tplgfr42s_1?cidTexte=JORFTEXT000038410047&dateTexte=29990101
- Locci, E., I. Pilia, R. Piras, S. Pili, G. Marcias, P. Cocco, F. De Giorgio, et al. 2019. “Particle Background Levels in Human Tissues—PABALIHT Project. Part I: A Nanometallomic Study of Metal-Based Micro- and Nanoparticles in Liver and Kidney in an Italian Population Group.” Journal of Nanoparticle Research 21 (3): 45. doi:10.1007/s11051-019-4480-y.
- Lomer, M. C., C. Hutchinson, S. Volkert, S. M. Greenfield, A. Catterall, R. P. Thompson, and J. J. Powell. 2004. “Dietary Sources of Inorganic Microparticles and Their Intake in Healthy Subjects and Patients with Crohn’s disease.” British Journal of Nutrition 92 (6): 947–955. doi:10.1079/bjn20041276.
- Lomer, M. C., R. P. Thompson, and J. J. Powell. 2002. “Fine and Ultrafine Particles of the Diet: Influence on the Mucosal Immune Response and Association with Crohn's Disease.” The Proceedings of the Nutrition Society 61 (1): 123–130. doi:10.1079/pns2001134.
- Lomer, M. C., S. L. Grainger, R. Ede, A. P. Catterall, S. M. Greenfield, R. E. Cowan, F. R. Vicary, et al. 2005. “Lack of Efficacy of a Reduced Microparticle Diet in a Multi-Centred Trial of Patients with Active Crohn’s Disease.” European Journal of Gastroenterology & Hepatology 17 (3): 377–384.
- MacNicoll, A., M. Kelly, H. Aksoy, E. Kramer, H. Bouwmeester, and Q. Chaudhry. 2015. “A Study of the Uptake and Biodistribution of Nano-Titanium Dioxide Using In Vitro and In Vivo Models of Oral Intake.” Journal of Nanoparticle Research 17 (2): 66. doi:10.1007/s11051-015-2862-3.
- Martins, A. D. C., Jr., L. F. Azevedo, C. C. de Souza Rocha, M. F. H. Carneiro, V. P. Venancio, M. R. de Almeida, L. M. G. Antunes, et al. 2017. “Evaluation of Distribution, Redox Parameters, and Genotoxicity in Wistar Rats Co-Exposed to Silver and Titanium Dioxide Nanoparticles.” Journal of Toxicology and Environmental Health. Part A 80 (19–21): 1156–1165. doi:10.1080/15287394.2017.1357376.
- Mohamed, H. R. 2015. “Estimation of TiO2 Nanoparticle-Induced Genotoxicity Persistence and Possible Chronic Gastritis-Induction in Mice.” Food and Chemical Toxicology : An International Journal Published for the British Industrial Biological Research Association 83: 76–83. doi:10.1016/j.fct.2015.05.018.
- Mohamed, H. R., and N. A. Hussien. 2016. “Genotoxicity Studies of Titanium Dioxide Nanoparticles (TiO2NPs) in the Brain of Mice.” Scientifica 2016: 6710840. doi:10.1155/2016/6710840.
- Murugadoss, S., S. van den Brule, F. Brassinne, N. Sebaihi, J. Mejia, S. Lucas, J. Petry, et al. 2020. “Is Aggregated Synthetic Amorphous Silica Toxicologically Relevant?” Particle and Fibre Toxicology 17 (1): 1. doi:10.1186/s12989-019-0331-3.
- Natarajan, V., C. L. Wilson, S. L. Hayward, and S. Kidambi. 2015. “Titanium Dioxide Nanoparticles Trigger Loss of Function and Perturbation of Mitochondrial Dynamics in Primary Hepatocytes.” PLoS One 10 (8): e0134541. doi:10.1371/journal.pone.0134541.
- NCI. 1979. “Bioassay of Titanium Dioxide for Possible Carcinogenicity.” National Cancer Institute Carcinogenesis Technical Report Series 97: 1–123.
- Onishchenko, G. E., M. V. Erokhina, S. S. Abramchuk, K. V. Shaitan, R. V. Raspopov, V. V. Smirnova, L. S. Vasilevskaya, I. V. Gmoshinski, M. P. Kirpichnikov, and V. A. Tutelyan. 2012. “Effects of Titanium Dioxide Nanoparticles on Small Intestinal Mucosa in rats.” Bulletin of Experimental Biology and Medicine 154 (2): 265–270. doi:10.1007/s10517-012-1928-9.
- Peters, R. J. B., A. G. Oomen, G. van Bemmel, L. van Vliet, A. K. Undas, S. Munniks, R. L. A. W. Bleys, P. C. Tromp, W. Brand, and M. van der Lee. 2020. “Silicon Dioxide and Titanium Dioxide Particles Found in Human Tissues.” Nanotoxicology 14 (3): 420–432. doi:10.1080/17435390.2020.1718232.
- Peters, R. J. B., A. K. Undas, J. Memelink, G. van Bemmel, S. Munniks, H. Bouwmeester, P. Nobels, W. Schuurmans, and M. K. van der Lee. 2018. “Development and Validation of a Method for the Detection of Titanium Dioxide Particles in Human Tissue with Single Particle ICP-MS.” Current Trends in Analytical and Bioanalytical Chemistry 2 (1): 74–84. doi:10.36959/525/442.
- Peters, R. J., G. van Bemmel, Z. Herrera-Rivera, H. P. Helsper, H. J. Marvin, S. Weigel, P. C. Tromp, A. G. Oomen, A. G. Rietveld, and H. Bouwmeester. 2014. “Characterization of Titanium Dioxide Nanoparticles in Food Products: Analytical Methods to Define Nanoparticles.” Journal of Agricultural and Food Chemistry 62 (27): 6285–6293. doi:10.1021/jf5011885.
- Peters, R., E. Kramer, A. G. Oomen, Z. E. Rivera, G. Oegema, P. C. Tromp, R. Fokkink, et al. 2012. “Presence of Nano-Sized Silica During In Vitro Digestion of Foods Containing Silica as a Food Additive.” ACS Nano 6 (3): 2441–2451. doi:10.1021/nn204728k.
- Powell, J. J., C. C. Ainley, R. S. Harvey, I. M. Mason, M. D. Kendall, E. A. Sankey, A. P. Dhillon, and R. P. Thompson. 1996. “Characterisation of Inorganic Microparticles in Pigment Cells of Human Gut Associated Lymphoid Tissue.” Gut 38 (3): 390–395. doi:10.1136/gut.38.3.390.
- Powell, J. J., N. Faria, E. Thomas-McKay, and L. C. Pele. 2010. “Origin and Fate of Dietary Nanoparticles and Microparticles in the Gastrointestinal Tract.” Journal of Autoimmunity 34 (3): J226–J233. doi:10.1016/j.jaut.2009.11.006.
- Proquin, H., M. J. Jetten, M. C. M. Jonkhout, L. G. Garduno-Balderas, J. J. Briede, T. M. de Kok, Y. I. Chirino, and H. van Loveren. 2018. “Gene Expression Profiling in Colon of Mice Exposed to Food Additive Titanium Dioxide (E171).” Food and Chemical Toxicology: An International Journal Published for the British Industrial Biological Research Association 111: 153–165. doi:10.1016/j.fct.2017.11.011.
- Rompelberg, C., M. B. Heringa, G. van Donkersgoed, J. Drijvers, A. Roos, S. Westenbrink, R. Peters, G. van Bemmel, W. Brand, and A. G. Oomen. 2016. “Oral Intake of Added Titanium Dioxide and Its Nanofraction from Food Products, Food Supplements and Toothpaste by the Dutch Population.” Nanotoxicology 10 (10): 1404–1414. doi:10.1080/17435390.2016.1222457.
- Ruiz, P. A., B. Moron, H. M. Becker, S. Lang, K. Atrott, M. R. Spalinger, M. Scharl, et al. 2017. “Titanium Dioxide Nanoparticles Exacerbate DSS-Induced Colitis: Role of the NLRP3 Inflammasome.” Gut 66 (7): 1216–1224. doi:10.1136/gutjnl-2015-310297.
- Schreiver, I., B. Hesse, C. Seim, H. Castillo-Michel, J. Villanova, P. Laux, N. Dreiack, et al. 2017. “Synchrotron-Based ν-XRF Mapping and μ-FTIR Microscopy Enable to Look into the Fate and Effects of Tattoo Pigments in Human Skin.” Scientific Reports 7 (1): 11395. doi:10.1038/s41598-017-11721-z.
- Shi, Z., Y. Niu, Q. Wang, L. Shi, H. Guo, Y. Liu, Y. Zhu, et al. 2015. “Reduction of DNA Damage Induced by Titanium Dioxide Nanoparticles Through Nrf2 In Vitro and In Vivo.” Journal of Hazardous Materials 298: 310–319. doi:10.1016/j.jhazmat.2015.05.043.
- Shukla, R. K., A. Kumar, N. V. Vallabani, A. K. Pandey, and A. Dhawan. 2014. “Titanium Dioxide Nanoparticle-Induced Oxidative Stress Triggers DNA Damage and Hepatic Injury in Mice.” Nanomedicine (London, England) 9 (9): 1423–1434. doi:10.2217/nnm.13.100.
- Sycheva, L. P., V. S. Zhurkov, V. V. Iurchenko, N. O. Daugel-Dauge, M. A. Kovalenko, E. K. Krivtsova, and A. D. Durnev. 2011. “Investigation of Genotoxic and Cytotoxic Effects of Micro- and Nanosized Titanium Dioxide in Six Organs of Mice In Vivo.” Mutation Research 726 (1): 8–14. doi:10.1016/j.mrgentox.2011.07.010.
- Talamini, L., S. Gimondi, M. B. Violatto, F. Fiordaliso, F. Pedica, N. L. Tran, G. Sitia, et al. 2019. “Repeated Administration of the Food Additive E171 to Mice Results in Accumulation in Intestine and Liver and Promotes an Inflammatory Status.” Nanotoxicology 13 (8): 1087–1101. doi:10.1080/17435390.2019.1640910.
- Tassinari, R., F. Cubadda, G. Moracci, F. Aureli, M. D'Amato, M. Valeri, B. De Berardis, et al. 2014. “Oral, Short-Term Exposure to Titanium Dioxide Nanoparticles in Sprague-Dawley Rat: Focus on Reproductive and Endocrine Systems and Spleen.” Nanotoxicology 8 (6): 654–662. doi:10.3109/17435390.2013.822114.
- Trouiller, B., R. Reliene, A. Westbrook, P. Solaimani, and R. H. Schiestl. 2009. “Titanium Dioxide Nanoparticles Induce DNA Damage and Genetic Instability In Vivo in Mice.” Cancer Research 69 (22): 8784–8789. doi:10.1158/0008-5472.CAN-09-2496.
- Urrutia-Ortega, I. M., L. G. Garduno-Balderas, N. L. Delgado-Buenrostro, V. Freyre-Fonseca, J. O. Flores-Flores, A. Gonzalez-Robles, J. Pedraza-Chaverri, et al. 2016. “Food-Grade Titanium Dioxide Exposure Exacerbates Tumor Formation in Colitis Associated Cancer Model.” Food and Chemical Toxicology : An International Journal Published for the British Industrial Biological Research Association 93: 20–31. doi:10.1016/j.fct.2016.04.014.
- van der Zande, M., R. J. Vandebriel, M. J. Groot, E. Kramer, Z. E. Herrera Rivera, K. Rasmussen, J. S. Ossenkoppele, et al. 2014. “Sub-Chronic Toxicity Study in Rats Orally Exposed to Nanostructured Silica.” Particle and Fibre Toxicology 11: 8. doi:10.1186/1743-8977-11-8.
- Vinken, M., N. Kramer, T. E. H. Allen, Y. Hoffmans, N. Thatcher, S. Levorato, H. Traussnig, et al. 2020. “The Use of Adverse Outcome Pathways in the Safety Evaluation of Food additives.” Archives of Toxicology 94 (3): 959–966. doi:10.1007/s00204-020-02670-0.
- Wang, J., G. Zhou, C. Chen, H. Yu, T. Wang, Y. Ma, G. Jia, et al. 2007. “Acute Toxicity and Biodistribution of Different Sized Titanium Dioxide Particles in Mice after Oral Administration.” Toxicology Letters 168 (2): 176–185. doi:10.1016/j.toxlet.2006.12.001.
- Wang, Y., Z. Chen, T. Ba, J. Pu, T. Chen, Y. Song, Y. Gu, et al. 2013. “Susceptibility of Young and Adult Rats to the Oral Toxicity of Titanium Dioxide Nanoparticles.” Small (Weinheim an Der Bergstrasse, Germany) 9 (9-10): 1742–1752. doi:10.1002/smll.201201185.
- Warheit, D. B., S. C. Brown, and E. M. Donner. 2015. “Acute and Subchronic Oral Toxicity Studies in Rats with Nanoscale and Pigment Grade Titanium Dioxide Particles.” Food and Chemical Toxicology 84: 208–224. doi:10.1016/j.fct.2015.08.026.
- Weir, A., P. Westerhoff, L. Fabricius, K. Hristovski, and N. von Goetz. 2012. “Titanium Dioxide Nanoparticles in Food and Personal Care Products.” Environmental Science & Technology 46 (4): 2242–2250. doi:10.1021/es204168d.
- Yang, Y., K. Doudrick, X. Bi, K. Hristovski, P. Herckes, P. Westerhoff, and R. Kaegi. 2014. “Characterization of Food-Grade Titanium Dioxide: The Presence of Nanosized Particles.” Environmental Science & Technology 48 (11): 6391–6400. doi:10.1021/es500436x.
