Abstract
With ever-increasing production and use of nanoparticles (NPs), there is a necessity to evaluate the probability of consequential adverse effects in individuals exposed to these particles. It is now understood that a proportion of NPs can translocate from primary sites of exposure to a range of secondary organs, with the liver, kidneys and spleen being some of the most important. In this study, we carried out a comprehensive toxicological profiling (inflammation, changes in serum biochemistry, oxidative stress, acute phase response and histopathology) of Ag NP induced adverse effects in the three organs of interest following acute exposure of the materials at identical doses via intravenous (IV), intratracheal (IT) instillation and oral administration. The data clearly demonstrated that bioaccumulation and toxicity of the particles were most significant following the IV route of exposure, followed by IT. However, oral exposure to the NPs did not result in any changes that could be interpreted as toxicity in any of the organs of interest within the confines of this investigation. The finding of this study clearly indicates the importance of the route of exposure in secondary organ hazard assessment for NPs. Finally, we identify Connexin 32 (Cx32) as a novel biomarker of NP-mediated hepatic damage which is quantifiable both (in vitro) and in vivo following exposure of physiologically relevant doses.
Introduction
The ever-increasing utilization of engineered nanoparticles (NPs) in various products and applications has led to considerable interest in the field of nanotechnology (Kermanizadeh, Jacobsen et al. Citation2020 ; Radwan et al. Citation2021). Unfortunately, the same unique nano-specific chemical and physical characteristics which make NPs desirable could also contribute to toxicity in exposed individuals. With the inevitable rise of public and occupational exposure from increasing production and use of NPs, there is an urgent need to assess the potential health consequences of being exposed to these NPs (Jacobsen et al. Citation2017; Karakus, Bilgi, and Winkler Citation2021; Kermanizadeh et al. Citation2019). The assessment of risk to human health in a ‘nano’ context is based upon the physicochemical properties of the particle in question, dose-response relationship of adverse effects and the exposure scenarios. The exposure scenario is essential as it influences the exposure concentrations and the route of exposure; the latter being critical in governing the translocation, distribution, accumulation in a variety of target organs.
The skin, lungs and the gastrointestinal tract (GIT) are in constant contact with the external environment and are the primary exposure sites for NPs (Huang and Tang Citation2021). It is now understood that a proportion of NPs can translocate from these primary sites to a range of secondary organs with the liver, kidneys and spleen being some of the most important in terms of the accumulation of relatively large quantities of NPs (Balasubramanian et al. Citation2010; Kermanizadeh et al. Citation2015; Lee et al. Citation2013; Lipka et al. Citation2010). Importantly, with the advances over the last decade in the field of nanomedicine, there is now potential for intravenous (IV) and direct injection of NPs into the bloodstream (Kermanizadeh, Jacobsen, et al. Citation2020). The presence of NPs in the blood will result in the particles reaching a number of extra-pulmonary organs quickly and in very large concentrations (Balasubramanian et al. Citation2010; Kermanizadeh et al. Citation2015). This has been exemplified by the consensus that for particulates in the blood, the liver is key and the forefront to the xenobiotic challenge.
Silver (Ag) NPs are used in various applications which include but are not limited to additives to textiles and plastics, in water filters and disinfectants, as health supplements, health care devices as well as routinely being utilized in food preservation and packaging fundamentally due their inherent anti-microbial properties (Ahamed, Al Salhi, and Siddiqui Citation2010; Radwan et al. Citation2021). Therefore, there is very likely scenario for these particles to be ingested by consumers and reach the GIT or be inhaled during the manufacturing process (Geiser and Kreyling Citation2010; Kermanizadeh et al. Citation2015). It is believed that once in the submucosal tissue, certain particulates are able to enter the lymphatic system and the bloodstream. As touched upon nanomedicines can be deliberately introduced into the body via injection hence direct entry of NPs into the circulatory system (Kermanizadeh, Jacobsen, et al. Citation2020; Lagopati et al. Citation2021). The presence of these NPs in the blood will result in direct distribution to a wide range of target organs, including the liver, kidneys and spleen (Geraets et al. Citation2014; Hadrup and Lam Citation2014; Hadrup, Sharma, et al. Citation2020). Crucially, it is widely demonstrated that the liver has significance with regards to NPs accumulating in the organ following IV exposure compared to other organs (Kermanizadeh et al. Citation2015; Lipka et al. Citation2010). Alongside the kidneys, the liver might be responsible for the clearance of NPs from the blood (Geiser and Kreyling Citation2010; Semmler-Behnke et al. Citation2008).
In this study, we investigated the biokinetics and acute hazard potential of Ag NPs following three different routes of exposure (namely intratracheal instillation (IT), oral gavage and IV). The focus of the study was placed on three extrapulmonary organs identified for accumulating large quantities of materials. The Ag NPs was selected as a highly soluble particle. Here, we assessed a wide and comprehensive array of biomarkers/end-points of damage which included organ-specific inflammation, changes in serum biochemistry (biomarkers of general toxicity and functional and metabolic activity), anti-oxidant depletion, acute-phase response and histopathology of the liver, kidneys and the spleen. Finally, we identify a novel biomarker of NP-induced hepatic injury (connexin 32) which might prove to be useful as a means of assessing liver damage which is quantifiable both in vitro and in vivo following exposure of physiologically relevant doses. This would allow for more meaningful in vitro and in vivo data comparisons and bridges the gap between data generated from in vitro and animal models and for better linking of the two different testing strategies.
Materials and methods
Nanoparticles and AgNO3
The Ag NPs was sourced from Fraunhofer IME – Germany (NM300-K), sub-sampled under Good Laboratory Practice conditions and preserved under argon in the dark until use. AgNO3 was purchased from Sigma, UK.
Characterization of the Ag NPs
The Ag NPs used within this study were extensively characterized using a combination of analytical techniques to identify their primary physical and chemical properties important for understanding their toxicological potential. These primary physicochemical properties have been described in detail previously (Kermanizadeh, Pojana, et al. Citation2013) and reproduced below. Furthermore, the primary size as measured via TEM and the hydrodynamic size distribution of the particles in PBS (exposure medium) was determined at a concentration of 10 µg/ml by Dynamic Light Scattering (DLS) using a Malvern Zetasizer nano series – Nano ZS (USA) ().
Animals and treatments
This study used eight-week-old female C57BL/6N mice (19.5–21 g), which were obtained from The University of Edinburgh Animal Unit (UK) and from Taconic (Denmark). The mice were housed in polypropylene cages with bedding (sawdust) and enrichment at controlled environmental conditions. The mice were allowed to acclimatize and randomly divided into 12 groups of five animals. The experimental groups are summarized in . The in vivo procedures followed the guidelines for care and handling of laboratory animals according to the EC Directive 86/609/EEC, the Danish law and UK home office were approved by the local ethical committee for animal research and by the Danish Animal Experiment Inspectorate (under the Danish Ministry of Justice, permission 2015-15-0201-00465) and by both Heriot-Watt University and the University of Edinburgh.
Table 1. The outline of the experimental treatment groups – 60 female mice were randomly divided at random into 12 exposure groups.
In this experiment, the animals were exposed to the particle or controls either via the IT, oral gavage or IV (via the lateral tail vein) routes. The IT instillation was conducted as previously described (Kyjovska et al. Citation2015). The Ag NPs were dispersed in sterile PBS (two doses of either 25 or 100 µg per animal) in a volume of 100 µl for the IT and IV exposures and 500 µl for the oral route. These experiments incorporated negative controls (PBS vehicle only and an Ag ion control (80 µg of AgNO3 per animal). The NP doses utilized within this investigation were selected based on previous acute dosing studies including those conducted as part of FP7 funded EU project ENPRA, keeping in mind the overall aim and the selection of dosage which would allow for distinction of adverse effects following different routes of exposure. The in vivo IV dose, 100 µg NPs per animal, corresponds to approximately 0.5 µg/106 hepatocytes based on previous literature regarding liver cell numbers and particle retention in the liver following IV exposure (Gaiser et al. Citation2013). The high IT doses reflect pulmonary deposition in mice equivalent to 1–10 working days of 8 hr at the Danish occupational exposure limit of 3.5 mg/m3 for materials such as carbon black (Poulsen, Saber, Mortensen, et al. Citation2015). The mice were kept under 3.5% isoflurane anesthesia during the IV and IT process. The animals were conscious within minutes after these treatments and suffered no ill effects. The mice were monitored over the entire 24-hr exposure period. Following the exposure, the mice were sacrificed by exsanguination while anesthetized (200 µl of ZRF cocktail composed of Zoletil 250 mg/ml, Rompun 20 mg/ml and Fentanyl 50 μg/ml) after collection of intracardial blood. The liver was removed, the caudate lobe (glutathione measurements) and the left lobe (biokinetics/inflammation/biomarker analysis) were snap frozen in liquid nitrogen and stored at −80 °C, while the right medial lobe was fixed in a 4% formaldehyde solution for histological and biokinetics analysis. Additionally, the spleen and kidneys were also removed and either snap-frozen or fixed in 4% formaldehyde.
Detection of Ag concentration in mouse liver, spleen and kidneys
The Ag content in the tissues was determined in the vehicle control and 100 µg/mouse dose groups by High Resolution Inductively Coupled Plasma Mass Spectrometry (HR-ICP-MS) (ELEMENT XR, Thermo Fisher Scientific, UK). In short, ∼80 mg of fixed tissue was transferred into digestion tubes. A volume of 2 ml of 60% nitric acid was added for the complete digestion of the samples. The digests were kept at 120 °C overnight. At this juncture, the samples were diluted prior to analysis by HR-ICP-MS. The Ag content was measured as 107Ag; while 109Ag was used for the control. The low-resolution mode was selected for maximal sensitivity and there was no interference for the isotopes. In our trials, external calibration with internal standard correction by rhodium as 103Rh was carried out. For quantification, the tissues from three random animals were selected from each group.
Analysis of serum biomarkers relating to toxicity
The collected intracardial blood samples were centrifuged and serum frozen at −80 °C for the analysis of biomarkers and the acute-phase response. The serum biochemistry measurements included lactate dehydrogenase (LDH), aspartate aminotransferase (AST), alanine aminotransferase (ALT), cholesterol, triglycerides and albumin. Prior to measurements, the samples were thawed, centrifuged for 10 min at 2000 g at room temperature with the analysis performed on a Cobas 8000 modular analyzer (Roche, USA).
Acute-phase response
The acute-phase protein serum amyloid A3 (SAA3) was measured in the prepared serum (described above) utilizing a commercially available mouse SAA3 ELISA kit (Abcam, UK - ab157723) according to the manufacturer’s instructions.
Tissue homogenization
The liver samples were thawed on ice and homogenized in a homogenization buffer (RIPA lysis buffer supplemented with a complete protease inhibitor mixture and a protein phosphatase inhibitor) (Abcam, UK). For tissue homogenization, pre-filled bead mill tubes (Thermo Fisher, UK) and an evolution homogenizer was used (Percellys, France). The samples were thoroughly mixed and stored on ice for 10 min before centrifugation at 10000 g for 10 min. The supernatants were transferred to a fresh tube and centrifuged for a further 10 min. The protein concentrations were measured utilizing a Coomassie Plus Bradford assay reagent (Thermo Scientific, UK) and the supernatants stored at −80 °C until use.
Liver cytokine levels
The levels of Interleukin 10 (IL10), IL6, Monocyte Chemoattractant Protein-1 (MCP-1) and keratinocyte-derived chemokine (KC) and Tumor Necrosis Factor-α (TNF-α) was determined in the cell supernatant of homogenized liver tissue using R&D Systems magnetic Luminex® Performance Assay multiplex kits (bead-based immunoassay; Bio-techne, USA) according to the manufacturers instruction. The proteins were detected via a Bio-Rad Bio-Plex MAGPIX multiplex reader. The technology is based on analyte-specific antibodies pre-coated onto magnetic microplates embedded with fluorophores at set ratios for each unique microparticle region being recognized by the MAGPIX reader.
Western blotting
The changes in the levels of the two proteins of interest (CD36 and connexin 32) was quantified by Western blotting. In short, 40 μg of denatured protein from each sample was added to a 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) gel (Thermo Fisher, UK). The electrophoresis products were transferred onto polyvinylidene difluoride (PVDF) membranes (Bio-Rad, USA), blocked with blotting-grade blocker (Abcam, UK) and 0.1% Tween 20 at 1 hr at room temperature. Subsequently, the membranes were incubated with primary anti-CD36 and anti-connexin 32 antibodies (1:1000) (Abcam, UK) over night and anti-IgG peroxidase-conjugated secondary antibody (1:5000) (Abcam, UK). In these experiments, GADPH was used as an internal control (Abcam, UK). The proteins were detected using the Clarity™ ECL Western substrate kit (Bio-Rad, USA) and visualized via a Fusion FX7 imager (Witec AG, Switzerland). Finally, immunoblotting signals were quantitated using Image Studio 4.0 (LI-COR Biotechnology, USA).
Glutathione depletion
The mouse liver and kidney samples were weighed, thawed on ice and homogenized in 2 ml of lysis buffer. The homogenized samples were incubated on ice for 10 min before being centrifuged at 10000 g for 5 min to generate lysates. Glutathione was quantified in the lysate by reaction of sulfhydryl groups with the fluorescent substrate o-phthalaldehyde (Sigma, UK) using a fluorimeter with an excitation wavelength of 350 nm and emission wavelength of 420 nm. The protocol was modified to include measurements of total glutathione by reducing oxidized glutathione dimers (GSSG) by addition of 7 µl of 10 mM sodium dithionite the samples and incubation at room temperature for 1 hr.
Histological analysis of liver, kidneys, and spleen
The tissue samples were trimmed, dehydrated and embedded in paraffin wax using a Shandon duplex tissue processor (SCE 0540). After embedding, the tissue was sectioned at a thickness of 5 μm using a BROMMA 2218 Historange Microtome (LKB). The cut sections were attached to glass microscope slides and stained using hematoxylin and eosin (H&E) before examination using a Carl Zeiss Axiovert inverted microscope (Germany). Three random animals (five slides per animal) were chosen from each group for histological analysis.
Statistical analysis
The data are expressed as mean ± standard error of the mean (SEM). For statistical analysis, the detection of significant differences was calculated using a two-way full factorial ANOVA with post hoc multiple comparisons (Tukey) and a defined significance level of p < 0.05. All statistical analysis was carried out utilizing SPSS 26.
Results
Particle characterization
The main physicochemical characteristics for the Ag NPs used within this study has been reproduced from previously published work. Additionally and importantly, the Ag NPs were also characterized in the exposure medium. The hydrodynamic size distribution and zeta potential of the materials in PBS is presented in (). Of note, no endotoxin contamination (≤0.25 EU/ml) was detected for the Ag NPs. The TEM images of the Ag NPs is provided as Supplementary information .
Figure 1. Cytokine production (a) IL1ß, (b) IL6, (c) IL10, (d) MCP-1 and (e) KC from liver of mice following IV, IT or oral exposure of 25 or 100 µg of Ag NPs for 24 hr. The values represent mean ± SEM (n = 5) with significance indicated by *p < 0.05 and **p < 0.005.
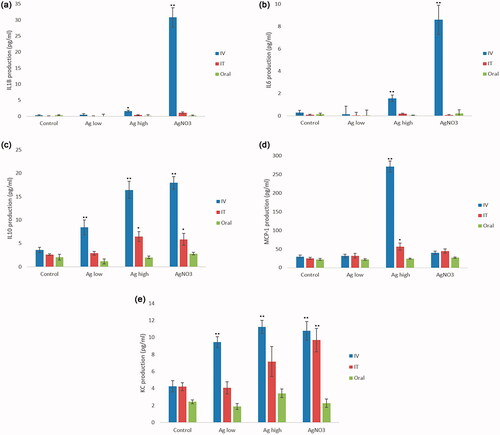
Table 2. The main primary physical and chemical properties of investigated NPs reproduced from Kermanizadeh, Pojana, et al. (Citation2013).
Bodyweight changes in the alcohol fed and/or Ag NP-exposed animals
There was no significant change in the body weight of Ag NP or AgNO3 exposed animals (data not shown). In addition, none of the animals showed any visible signs of discomfort during or subsequent to any of the treatments.
Ag biokinetics and organ accumulation following different routes of exposure
To investigate the tissue distribution and disposition of Ag NPs following exposure of the animals via the different routes of administration, the concentrations of silver in liver, kidneys and spleen were determined by HR-ICP-MS with the data presented in . Silver was below the limit of detection in the organs of mice in the control group. As expected, after a single intravenous dose of the NPs the highest concentrations of Ag was detected in the liver and spleen. In comparison, IT administration resulted in smaller quantities of Ag in all three target organs. Interestingly, very little Ag was detected in any of extra-pulmonary organs following oral exposure to the NPs.
Table 3. Silver content in the liver, kidneys and spleen determined by HR-ICP-MS following exposure to 100 µg of NP per animal via three different routes of exposure (n = 3).
Hepatic inflammation
The analysis of liver-specific inflammation demonstrated significant alterations in the levels of Interleukin 10 (IL10), Monocyte Chemoattractant Protein-1 (MCP-1) and keratinocyte-derived chemokine (KC) in tissues from mice treated with Ag NPs in a dose-dependent manner (). For these cytokines, the data demonstrated that exposure via IV route resulted in the most significant alterations as compared with the PBS treated control animals. This being said, smaller yet significant increases in levels of IL10 and MCP-1 were noted following IT exposure to the Ag NPs at the highest dose (). Moreover, a significant increase in the levels of IL6 and IL1β were noted following exposure to the NPs via the IV route (). Next, the data showed no changes in any of the cytokines investigated following oral exposure to the Ag NPs. Interestingly, one of the most notable changes in the overall hepatic cytokine response was an increase in the anti-inflammatory IL10 level following exposure to Ag NPs, suggesting an overall anti-inflammatory and immune-tolerant milieu of the healthy liver.
Acute phase response
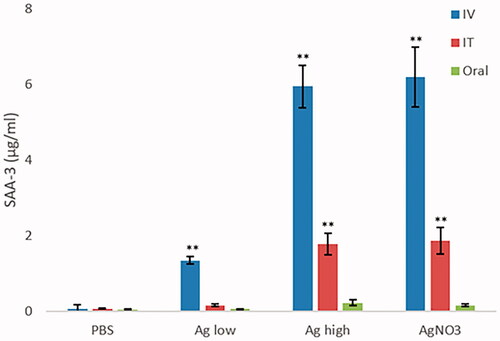
The acute-phase response is a vital systemic response to disturbances to local and/or systemic homeostasis caused by a variety of factors including infection, injury, trauma or immunological disorders. The most significant proportion of acute-phase response proteins are manufactured and secreted by the liver in response to cytokines IL1 and IL6. Here, SAA3 levels were measured as an indicator of such a response following the NP challenge. The data showed that the exposure to Ag NPs resulted in a dose-dependent increase in SAA3 levels in the appropriate animals, most notable for the IV route of exposure but also following IT exposure albeit at lower levels. There was no evidence of an SAA3 response following oral exposure at the specific doses and time-points measured ().
Glutathione depletion
As a measure of oxidative stress, the levels of reduced and total glutathione content were measured in the liver and kidney homogenates (). The data showed a clear decrease in both reduced GSH and total GSH in the livers of Ag NP-exposed animals at 24 hr at the highest dose of 100 µg per animal following IV exposure. Additionally, a small yet significant decrease in reduced GSH levels were noted following IT exposure. Finally, as one of the few positive observations relating to the oral route of exposure within the whole study, a small yet significant decrease in total GSH in the liver was noted following exposure to the highest dose of the NPs ().
Figure 3. Reduced GSH (GSH) and total glutathione (Total GSH) measured in the (a) liver and (b) kidneys of control and Ag NPs-exposed animals at 24-hr postexposure. The values depict mean ± SEM (n = 5), significance indicated by *p < 0.05 and **p < 0.005.
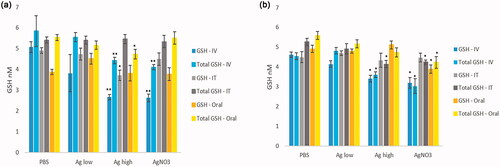
Similarly, in the kidneys, the IV route of exposure resulted in the most evident decrease in the antioxidant levels in the organs (). Moreover, the IT exposure of Ag NPs at 100 µg per animal resulted in a small decrease in total GSH in the kidneys. The oral exposure to the Ag NPs at these doses and time point did not alter kidney antioxidants.
Blood biochemistry
The analysis of blood biomarkers showed some statistically significant changes with regards to AST, ALT and LDH following the acute exposure of the Ag NPs (). Most notably the IT exposure of the Ag NPs resulted in the biggest increase in ALT, AST and LDH in serum of expose animals which is indicative of liver-specific and general cellular damage. Following exposure via the IT route, the only significant change was in LDH levels, which was evident following exposure to the NPs at the highest dose. This finding suggests that IT exposure to Ag NPs does indeed result in cell death that might not necessarily be in organs that are the focus of this study. Interestingly, the oral exposure to the NPs did not result in any significant change in any of parameters investigated including LDH. This observation is important in corroborating the findings in other endpoints all suggesting that acute exposure to a relatively toxic NP via the oral route might not be associated with any meaningful adverse effects in locations other than the gastrointestinal tract.
Table 4. Blood biomarkers of liver and systemic toxicity assessed in the serum of control and Ag NP (25 µg or 100 µg/per animal) exposed animals sacrificed 24-hr post-treatment following IV, IT and oral routes of exposure.
Liver biomarkers of damage
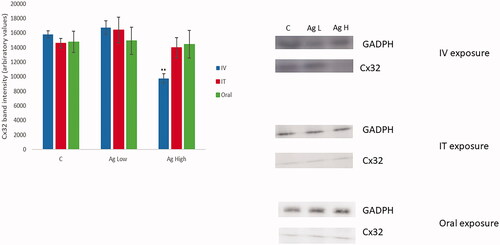
In an effort to identify potentially novel NP-induced biomarkers of liver damage, the changes in the expression of target proteins (CD36 and connexin 32) in the organ was analyzed by Western blotting () (the rationale for this is fully explained in the discussion section). The data demonstrated that Cx32 levels were decreased significantly following IV exposure to Ag NPs at the highest dose. The acute IT or oral exposure to the Ag NPs at either dose did not result in a change in the levels of the investigated proteins as compared to the PBS exposed animals. The novel finding here are further strengthened by in vitro observation of changes in Cx32 in nanoparticulate exposed quadruple cell human primary hepatic spheroids. The western blot data did not show any changes in the levels of CD36 for either of doses or routes of exposure (data not shown).
Figure 4. The alteration in the levels of liver Cx32 protein level quantified by western blot analysis of PBS or Ag NP-exposed animals sacrificed 24-hr post-treatment following IV, IT, and oral exposure. The values represent mean ± SEM with significance indicated by **p < 0.005 compared to negative control (n = 3).
Histology
The histopathological examination of the mice livers, kidneys and spleens revealed that exposure via the IV route resulted in the most notable pathological changes ( and ). The exposure to the high dose of Ag NPs via the IV route resulted in comprehensive pathology in the liver manifested as numerous bi-nucleate hepatocytes, ballooning of hepatocytes, aggregation of inflammatory cells, necrotic hepatocytes, areas of necrosis, granuloma formation and the destruction of the liver plates (). The exposure via IT or oral routes resulted in very little histological change in the livers as compared to PBS control animals (). Overall, the examination of the histopathology of the kidneys showed very little change in the normal renal cortex and glomeruli structure. However, the exposure via the IV route to the highest dose of the NPs resulted in a mild influx of inflammatory cells (). The histopathological examination of the control spleen sections showed normal splenic structure with normal lymphoid follicles and sinuses with well-defined white pulp. The IV exposure to the Ag NPs at the dose of 100 µg per animal resulted in minor changes, mostly visible as low–mild distorted lymphoid structures and detection of a few giant macrophages (). The histopathological changes in the spleens of oral and IT Ag NPs exposed animals were less noticeable (). Finally, it is important to state that the damage observed was not uniform across all regions of the lobes investigated and/or all the slides examined.
Figure 5. The histopathological examination of H and E stained liver of mice exposed to (a) control (b) high dose NPs via the IV route with damage most evident by steatosis, vacuolar degeneration, inflammatory cell influx and hepatic necrosis; kidneys of mice exposed to (c) control (d) high-dose NPs via the IV route with small influx of immune cells and from spleen of mice exposed to (e) control (f) IV exposed to high-dose NPs resulting in observation in a number of giant macrophages.
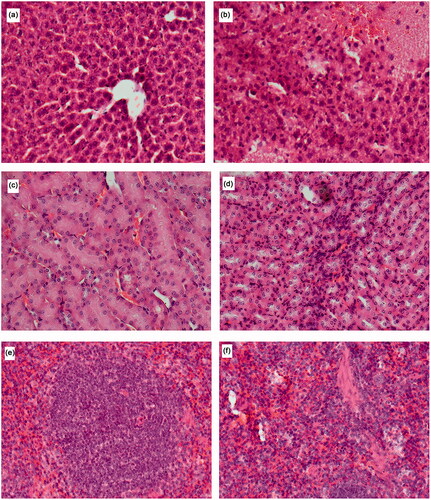
Table 5. The histological score of liver pathology from five slides from three random animals for each treatment group ranked from 0–5.
Table 6. The histological score of kidney pathology from five slides from three random animals for each treatment group ranked from 0–5.
Table 7. The histological score of spleen pathology from five slides from three random animals for each treatment group ranked from 0–5.
Discussion
It is now clear that NPs entering the blood stream will accumulate within the liver (Sadauskas et al. Citation2009), kidneys (i.e. Recordati et al. Citation2016) and the spleen (Tassinari et al. Citation2021). It has previously been shown that the Ag NPs have adverse effects on hepatic and renal cell lines/models in vitro (i.e. Gaiser et al. Citation2013; Kermanizadeh, Vranic, et al. Citation2013) and induce an acute response in the liver and kidneys in vivo (i.e. Kermanizadeh et al. Citation2017; Recordati et al. Citation2016). In this study, we demonstrate that IV administration of Ag NPs resulted in NP-induced liver-specific inflammation, anti-oxidant depletion and an acute-phase response from the organ. The histopathological examination and analysis of a novel organ specific and general blood bio-markers further corroborated NP-induced liver damage. The IV exposure at the dose of 100 µg per animal also showed mild renal and spleen toxicity in the exposed animals. Interestingly, the IT exposure of Ag NPs also resulted in significant alterations in a number of end-points investigated which will be discussed in detail in turn. However, the oral exposure of Ag NPs did not result in any changes that could be interpreted as toxicity to the examined organs.
In this study as expected, the tissue distribution and disposition of Ag NPs following acute exposure of the nanoparticles was clear in the target organs, with the liver and the spleen accumulating largest quantities of Ag. The IT administration of the NPs resulted in smaller quantities of Ag, compared to IV administration, in all three target organs (). Of note and crucial in this acute study, was the observation that very little Ag was detected in any of the extra-pulmonary organs following oral exposure to the NPs. Numerous previous studies have demonstrated accumulation of NPs in the liver and spleen following IV exposure for Ag NPs (Gaiser et al. Citation2013), Silica NPs (Tassinari et al. Citation2021) and TiO2 NPs (Kreyling et al. Citation2017). Moreover, acute IT administration of Ag NPs was shown to result in small quantities of materials reaching the liver (Gosens et al. Citation2015). In an interesting study from 2018, long-term low-dose exposure to CeO2 and TiO2 NPs for 180 days via the IT route also resulted in accumulation of materials in liver, spleen, and kidneys, while the authors did not observe any absorption from the digestive tract to other organs following oral gavage of the same materials (Modrzynska et al. Citation2018).
The analysis of hepatic organ inflammatory cytokines showed significant alterations in the levels of IL10, MCP-1 and KC in liver tissue from mice treated with Ag NPs in a dose-dependent manner (). For these cytokines, the data demonstrated that exposure via the IV route resulted in the most significant alterations compared to the negative control mice. Moreover, smaller yet significant increases in the level of IL10 and MCP-1 were noted following IT exposure of the Ag NPs at the highest dose. Next, a significant increase in the levels of IL6 and IL1ß were noted following exposure of the NPs via the IV route. Notably, the data showed no changes in any of the cytokines investigated following oral exposure to the Ag NPs.
Of note, one of the most significant changes in the overall hepatic cytokine response was increases in the anti-inflammatory IL10 levels following exposure to Ag NPs suggesting at an overall anti-inflammatory and immune-tolerant milieu of the healthy liver. It is understood that IL10 acts as an antagonist against the pro-inflammatory cytokines emphasizing that tolerance might be favored over an inflammatory response as a consequence of the acute NP challenge in vivo. Interestingly, similar observations in the fine counter-balance between anti versus pro-inflammatory hepatic cytokines have also been noted in complex multi-cellular in vitro models of the organ (Kermanizadeh et al. Citation2019). It is now understood that Kupffer cells are very important in dictating the overall liver immunity against xenobiotics including gut originated antigens and are known to contribute to a cytokine storm which can further activate hepatic T cells, in turn promoting phagocytosis and additional inflammatory response in a positive feedback loop (Kubes and Jenne Citation2018). These cells are also actively involved in progression of liver disease (Koyama and Brenner Citation2017). However, it is hypothesized that Kupffer cells in a nondiseased liver are in a constant semi-activated state and are essential in the maintenance of tolerance to food antigens in everyday life and one of the main reasons why there is not an extensive immune response to eating food (Heymann et al. Citation2015). Hence, the liver offers a unique environment in which the resident hepatic macrophages can both initiate an immune response or play an active role in retaining an immuno-tolerant state (Heymann et al. Citation2015). The data here clearly demonstrate the complexities of hepatic inflammation and reiterates the limitation of assessing this end-point in vitro where single hepatocyte cell lines and IL8 alone are often used as a surrogate and representative for inflammation in the organ in vivo.
Acute-phase proteins are a range of blood proteins predominately produced in the liver (Jain, Gautam, and Naseem Citation2011). The acute-phase response is a vital component of the innate immune system, which can be triggered by different stress stimuli including but not limited to infection, inflammation and physical trauma. To date, over 200 acute-phase proteins have been identified (Eklund, Niemi, and Kovanen Citation2012). The biological activities of these proteins are extremely important and varied and described previously in detail (Gruys et al. Citation2005). Serum amyloid A is a highly conserved protein produced by the liver. The plasma SAA concentration begins to increase 3–6 hr after an inflammatory stimulus, peaks on day 3, and returns to baseline levels after day 4 (this states that the peak of this response was not detected in this study). During an acute inflammatory response, the liver dictates a significant proportion of its synthesis capacity into producing SAA, which in mice comprised 2% of the total hepatic protein production (Eklund, Niemi, and Kovanen Citation2012). Important to this study is recognition that SAA is extremely immunologically active and plays a vital role in the recruitment of macrophages and neutrophils (Saber et al. Citation2014). In our experiments, the exposure to Ag NPs resulted in a dose-dependent increase in SAA3 levels most notable for the IV route of exposure but also following IT at lower levels. There was no evidence of a response following oral exposure at the specific doses and time-point. Similar observations have been observed following acute IV exposure to a different Ag NPs (Kermanizadeh et al. Citation2017) and IT exposure of multi-walled carbon nanotubes (Poulsen, Saber, Mortensen, et al. Citation2015).
As a means of assessing NP-induced oxidative stress, the levels of reduced and total glutathione content were measured in liver and kidney homogenates (). The data in this study, showed a decrease in both reduced GSH and total GSH in the livers of Ag NP-exposed animals at 24 hr at the highest dose of Ag NPs after IV exposure. Next, a small yet significant decrease in reduced GSH levels were noted following IT exposure. Finally, the oral route of exposure resulted in a small yet significant decrease in total GSH in the liver following exposure to the highest dose of the NPs. The anti-oxidant depletion data are very much in line with other end-points in establishing the ranking of the route of NP exposure being vital in the adverse effects observed in extra-pulmonary organs. In a healthy human adult, hepatocytes contain about 10% of the total human body pool of GSH (Loguercio and Federico Citation2003); therefore, in theory, the assessment of GSH could be a useful tool in assessing xenobiotic oxidative stress induced in the organ. This being said, it is important to state that the preservation of the redox balance is extremely complex and constantly is flux; hence, it is very difficult to identify an optimal time point for these measurements. It is conceivable that the assessment of GSH levels in the liver and kidneys a few hours before or after the 24-hr time point used within this study would have resulted in different outcomes. As a means of corroborating this point previous literature shows disparities in patterns of GSH depletion in the liver. As an example, the examination of anti-oxidant levels in the liver post IV administration of Ag NPs in rats had no significant effect in hepatic GSH levels 24 hr after exposure (Gaiser et al. Citation2013). However, the IT exposure to Ag NP resulted in a dose-dependent decrease in GSH levels in the liver of a mouse model (Gosens et al. Citation2015). Similarly, in the kidneys, the IV route of exposure resulted in the most evident decrease in the antioxidant levels in the organs. Moreover, the IT exposure to Ag NPs resulted in a small decrease in total GSH in the kidneys. The oral exposure to the Ag NPs at these doses and time-point did not alter kidney GSH levels. In a previous study on IV exposure of rats to 34 mg of AlCl3/kg for 24 hr resulted in a clear decrease in reduced GSH in the kidneys in the exposed animals (Al Kahtani Citation2010). Elsewhere, acute 24 hr in vitro exposure of a kidney cell line to varying NPs have also resulted in decreased GSH levels in the exposed cells (Enea et al. Citation2020; Kermanizadeh, Vranic, et al. Citation2013).
In summary of blood biochemistry, the data showed statistically significant increases in the levels of AST, ALT and LDH following the acute exposure to the Ag NPs most notable for the IV route of exposure but also significant alterations in LDH levels following s the IT route. Collectively, these biomarkers are indicative of Ag NP-induced cell death with AST and ALT being liver specific and LDH as a general systemic indicator of cell damage. Interestingly, there was no changes in any of the functional and metabolic biomarkers (albumin, triglycerides and cholesterol). Once again, these observations strongly suggest that the route of exposure is critical in governing extra-pulmonary NP-induced adverse effects. Previous IV exposure to two differently sized PEG modified gold NPs at a dose of 4 mg per animal over 28 days, resulted in changes in blood biochemistry indicative of liver damage (Zhang et al. Citation2011). Elsewhere, 5 day repeated oral exposure of mice at a dose of 1000 mg/kg of amorphous silica NPs did not cause changes in blood chemistry (Cabellos et al. Citation2020). As another example, the IT exposure to 800 µg/kg of ZnO NPs four times over a week did not result in changes in AST/ALT levels in the serum of exposed animals (Wang et al. Citation2017). In a subacute study in which rats were intravenously co-administered with Au and Ag NPs, no changes in blood chemistry (Lee et al. Citation2018) were found. The variations between the data presented here and those observed by Lee and colleagues could be explained by the different species used in the studies, and the important consideration that acute transient changes in biochemistry might or might not manifest or be detectable in organ toxicity at a single time point following long-term exposure to NPs.
It is generally accepted that it is not always possible to make direct or meaningful comparisons between in vitro and in vivo hepatic toxicological responses. This statement is equally valid for all xenobiotics including chemicals, drugs and particulates (discussed in detail in Kermanizadeh, Powell, and Stone Citation2020). Amongst numerous reasons for such differences is the clear absence of appropriate organ-specific biomarkers and toxicological end points that can be measured both in vitro and in vivo. In an attempt to address this issue, in this study the expression of two target proteins (CD36 and Cx32) were investigated in the homogenates of the Ag NP-exposed animals (these proteins were identified from a pilot study looking at a total of 15 potential candidate biomarkers (data not shown)).
Gap junctions are intercellular channels consisting of connexin proteins that directly connect the cytosol of coupled cells and allow rapid communication of cellular signals and act as a unique route for amplification of innate immunity. The hepatic gap junction Cx32, is readily expressed throughout the organ and is understood to be most highly distributed in the pericentral region. Several previous studies have demonstrated the crucial role of Cx32 gap junctions in injury in various models of liver disease (Guerra, Hadjihambi, and Jalan Citation2019; Luther et al. Citation2018; Willebrords et al. Citation2017). In this study, we show that Cx32 levels were decreased significantly following IV exposure to Ag NPs at the highest dose. The acute IT or oral exposure to the Ag NPs at either dose did not result in a change in the levels of the investigated proteins as compared to the PBS exposed animals. The novel findings here are further strengthened by in vitro observation of changes in Cx32 in nanoparticulate exposed quadruple cell human primary hepatic spheroids. Overall, the data within this study demonstrated Cx32 to be a promising candidate as a meaningful biomarker of NP-mediated liver damage which is quantifiable both in vitro and in vivo following exposure to physiologically relevant doses. In time, this will hopefully allow for more meaningful in vitro and in vivo data comparisons to be made which in turn will bridge the vast gap between data generated from in vitro and animal models and for better linking of the two different testing strategies. It is very important to state that despite early promise this biomarker still requires comprehensive validation in order to better understand the nature of the response, its mechanism and consequences. In order to address this, we have now analyzed over 200 pieces of liver tissue from animals exposed to a panel of NPs acutely and chronically via varying route of exposure at a range of doses (manuscript in preparation).
CD36 is a member of the class B scavenger receptor family with the ability to bind oxidized low-density lipoprotein (LDL). CD36 expression is relatively low in normal hepatocytes, but has been shown to increase in lipid-rich diets, hepatic steatosis, and nonalcoholic fatty liver disease (NAFLD) in animal models (Wilson et al. Citation2016). The western blot data here did not show any changes in the levels of CD36 for either Ag NP doses or any of the routes of exposure. Despite some encouraging in vitro observations, the reliability, and/or suitability of this protein as a meaningful biomarker of NP-induced hepatic injury is questionable (as mentioned above further investigations are in progress).
Finally, the histopathological examination of the mice livers, kidneys, and spleens revealed that exposure via the IV route resulted in the most notable pathological changes. The exposure to the high dose of Ag NPs via the IV route resulted in comprehensive hepatic pathology manifested most notably as necrosis, changes in the structure of the organ and inflammatory cell infiltration. The degenerative changes in the Ag NP treated groups were observed in all zones of the hepatic lobules, but were not uniform across all regions of the lobes investigated and slices examined. The exposure via IT or oral rotes resulted in almost no histopathological change in the livers as compared to PBS control mice. Overall, the examination of the histopathology of the kidneys only showed a mild influx of inflammatory cells following IV administration of NPs. In the spleen, the IV exposure of the Ag NPs at the highest dose resulted in low distortion in the lymphoid structure as well the detection of a number of giant macrophages. There were no histopathological changes from the controls in the spleens of oral and IT Ag NP-exposed animals. In the past, Ag NP exposure in mice has also shown varying degrees of similar hepatic histological pathologies (Ansari et al. Citation2016; Patlolla, Hackett, and Tchounwou Citation2015; Recordati et al. Citation2016). Elsewhere, the oral daily treatment of Ag NPs for three weeks at a dose of 50 mg/kg in rats resulted in increased number of abnormal glomeruli and necrotic tubules (Abdel-Wahhab et al. Citation2019). In a 2016 study, a single IV exposure to Ag NPs at a dose of 10 mg/kg resulted in severe hyperemia of the red pulp in the spleen of exposed animals (Recordati et al. Citation2016).
Ag NPs dispersed in any aqueous medium will release a degree of soluble Ag ions. Therefore, it might be important to distinguish between the toxic effects of Ag NPs and the dissolved Ag ion content. In this study, we included a AgNO3 exposure group which was equivalent to 80% of NP dissolution. This being said, it is important to mention that it is almost impossible to state unequivocally what aspects of adverse effect observed in the organs of interest were attributed to the Ag ions, the Ag NPs or a contribution from both (which is most likely scenario at the 24-hr end-point).
Despite the many insights offered in this study and the comprehensive toxicological assessment in the extra-pulmonary organs, there is a major limitation in the study design that needs to be mentioned and considered in the interpretation of findings. In all reality, any realistic NP-induced adverse effects to extra-pulmonary organs in man would only occur following long-term exposure (with the exception of intentional IV administration of nanomedicines). Hence in an ideal in vivo hazard assessment, studies should to be carried out with low intermittent repeated dosing and to incorporate recovery periods to allow for the assessment of clearance of NPs, manifestation of adverse effects and potential for organ recovery to be identified. That being said, this study is valuable in allowing for a direct comparison to be made between the different routes of toxicity and how this important variable affects the toxicity observed extra-pulmonary tissues. Any upcoming studies might also investigate a wider range of time points post material exposure to identify the optimum peak for certain endpoints (e.g. acute-phase response, cytokine production). It is also important to state that this is the first of two independent studies investigating NP-induced extra-pulmonary toxicity following different routes of exposure. In this paper, Ag NPs was selected as a high solubility material, while TiO2 is utilized in the second study as a low solubility NP (manuscript in preparation).
Conclusions
In this study, we carried out a comprehensive toxicity profiling of Ag NP-induced adverse effects in liver, kidneys, and spleen following acute exposure of the materials at identical doses via IV, IT and oral administration. The data clearly demonstrated that bioaccumulation and toxicity of the particles were most significant following IV, followed by IT. However, the oral exposure of the nanoparticles did not result in any changes that could be interpreted as toxicity in any of the organs of interest within confines of this investigation. The finding of this study clearly indicates the importance of the route of exposure in hazard assessment for NPs. Finally, we identify CX32 as a novel biomarker of NP-mediated hepatic damage which is quantifiable both (in vitro) and in vivo following exposure of physiologically relevant doses.
Supplemental Material
Download TIFF Image (803.4 KB)Acknowledgements
The authors are grateful to colleagues University of Derby, National Research Centre for Working Environment and Heriot Watt University. A special mention for Michael Guldbrandsen for assisting with animal experiments.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Abdel-Wahhab, M. A., H. M. S. Ahmed, A. A. El-Nekeety, S. H. Abdel-Aziem, H. A. Sharaf, M. S. Abdel-Aziz, M. F. Sallam, and F. A. Mannaa. 2019. “Chenopodium Murale Essential Oil Alleviates the Genotoxicity and Oxidative Stress of Silver Nanoparticles in the Rat Kidney.” Egyptian Journal of Chemistry 0 (0): 0–2646. doi:https://doi.org/10.21608/ejchem.2019.18341.2128.
- Ahamed, M., M. S. Al Salhi, and M. K. J. Siddiqui. 2010. “Silver Nanoparticle Applications and Human Health.” Clinica Chimica Acta; International Journal of Clinical Chemistry 411 (23–24): 1841–1848. doi:https://doi.org/10.1016/j.cca.2010.08.016.
- Al Kahtani, M. A. 2010. “Renal Damage Mediated by Oxidative Stress in Mice Treated with Aluminium Chloride: Protective Effects of Taurine.” Journal of Biological Sciences 10 (7): 584–595. doi:https://doi.org/10.3923/jbs.2010.584.595.
- Ansari, M. A., A. K. Shukla, M. Oves, and H. M. Khan. 2016. “Electron Microscopic Ultrastructural Study on the Toxicological Effects of AgNPs on the Liver, Kidney and Spleen Tissues of Albino Mice.” Environmental Toxicology and Pharmacology 44: 30–43. doi:https://doi.org/10.1016/j.etap.2016.04.007.
- Balasubramanian, S. K., J. Jittiwat, J. Manikandan, C. N. Ong, L. E. Yu, and W. Y. Ong. 2010. “Biodistribution of Gold Nanoparticles and Gene Expression Changes in the Liver and Spleen after Intravenous Administration in Rats.” Biomaterials 31 (8): 2034–2042. doi:https://doi.org/10.1016/j.biomaterials.2009.11.079.
- Cabellos, J., I. Gimeno-Benito, J. Catalan, H. K. Lindberg, G. Vales, E. Fernandez-Rosas, R. Ghemis, et al. 2020. “Short-Term Oral Administration of Non-Porous and Mesoporous Silica Did Not Induce Local or Systemic Toxicity in Mice.” Nanotoxicology 14 (10): 1324–1341. doi:https://doi.org/10.1080/17435390.2020.1818325.
- Eklund, K. K., K. Niemi, and P. T. Kovanen. 2012. “Immune Functions of Serum Amyloid A.” Critical Reviews in Immunology 32 (4): 335–348. doi:https://doi.org/10.1615/critrevimmunol.v32.i4.40.
- Enea, M., E. Pereira, M. P. de Almeida, A. M. Araujo, M. D. Bastos, and H. Carmo. 2020. “Gold Nanoparticles Induce Oxidative Stress and Apoptosis in Human Kidney Cells.” Nanomaterials 10 (5): 995. doi:https://doi.org/10.3390/nano10050995.
- Gaiser, B. K., S. Hirn, A. Kermanizadeh, N. Kanase, K. Fytianos, A. Wenk, N. Haberl, A. Brunelli, W. G. Kreyling, and V. Stone. 2013. “Effects of Silver Nanoparticles on the Liver and Hepatocytes in Vitro.” Toxicological Sciences 131 (2): 537–547. doi:https://doi.org/10.1093/toxsci/kfs306.
- Geiser, M., and W. G. Kreyling. 2010. “Deposition and Biokinetics of Inhaled nanoparticles.” Particle and Fibre Toxicology 7: 2. doi:https://doi.org/10.1186/1743-8977-7-2.
- Geraets, L., A. G. Oomen, P. Krystek, N. R. Jacobsen, H. Wallin, M. Laurentie, H. W. Verharen, E. F. Brandon, and W. H. de Jong. 2014. “Tissue Distribution and Elimination after Oral and Intravenous Administration of Different Titanium Dioxide Nanoparticles in Rats.” Particle and Fibre Toxicology 11: 30. doi:https://doi.org/10.1186/1743-8977-11-30.
- Gosens, I., A. Kermanizadeh, N. R. Jacobsen, A. G. Lenz, B. Bokkers, W. H. de Jong, P. Krystek, et al. 2015. “Comparative Hazard Identification by a Single Dose Lung Exposure of Zinc Oxide and Silver Nanomaterials in Mice.” PLOS One 10 (5): e0126934. doi:https://doi.org/10.1371/journal.pone.0126934.
- Gruys, E., M. J. M. Toussaint, T. A. Niewold, and S. J. Koopmans. 2005. “Acute Phase Reaction and Acute Phase Proteins.” Journal of Zhejiang University. Science. B 6 (11): 1045–1056. doi:https://doi.org/10.1631/jzus.2005.B1045.
- Guerra, M. H., A. Hadjihambi, and R. Jalan. 2019. “Gap Junctions in Liver Disease: Implications for Pathogenesis and Therapy.” Journal of Hepatology 70 (4): 759–772.” doi:https://doi.org/10.1016/j.jhep.2018.12.023.
- Hadrup, N., and H. R. Lam. 2014. “ Oral Toxicity of Silver Ions, Silver Nanoparticles and Colloidal Silver-A Review.” Regulatory Toxicology and Pharmacology 68 (1): 1–7. doi:https://doi.org/10.1016/j.yrtph.2013.11.002.
- Hadrup, N., A. K. Sharma, K. Loeschner, and N. R. Jacobsen. 2020. “Pulmonary Toxicity of Silver Vapours, Nanoparticles and Fine Dusts: A Review.” Regulatory Toxicology and Pharmacology 115: 104690.
- Hadrup, N., V. Zhernovkov, N. R. Jacobsen, C. Voss, M. Strunz, M. Ansari, H. B. Schiller, et al. 2020. “Acute Phase Response as a Biological Mechanism-of-Action of (Nano)Particle-Induced Cardiovascular Disease.” Small 16(21): e1907476. doi:https://doi.org/10.1002/smll.201907476.
- Heymann, F., J. Peusquens, I. Ludwig-Portugall, M. Kohlhepp, C. Ergen, P. Niemietz, C. Martin, et al. 2015. “Liver Inflammation Abrogates Immunological Tolerance Induced by Kupffer cells.” Hepatology 62 (1): 279–291. doi:https://doi.org/10.1002/hep.27793.
- Huang, X. Q., and M. Tang. 2021. “Review of Gut Nanotoxicology in Mammals: exposure, Transformation, Distribution and Toxicity.” Science of the Total Environment 773: 145078. doi:https://doi.org/10.1016/j.scitotenv.2021.145078.
- Jacobsen, N. R., P. Moller, P. A. Clausen, A. T. Saber, C. Micheletti, K. A. Jensen, H. Wallin, and U. Vogel. 2017. ”Biodistribution of Carbon Nanotubes in Animal Models.” Basic and Clinical Pharmacology and Toxicology 121: 30–43. doi:https://doi.org/10.1111/bcpt.12705.
- Jain, S., V. Gautam, and S. Naseem. 2011. “Acute-Phase Proteins: As Diagnostic Tool.” Journal of Pharmacy and Bioallied Sciences 3 (1): 118–127. doi:https://doi.org/10.4103/0975-7406.76489.
- Karakus, C. O., E. Bilgi, and D. A. Winkler. 2021. “Biomedical Nanomaterials: Applications, Toxicological Concerns, and Regulatory Needs.” Nanotoxicology 15(3): 331–351. doi:https://doi.org/10.1080/17435390.2020.1860265.
- Kermanizadeh, A., D. Balharry, H. Wallin, S. Loft, and P. Møller. 2015. “Nanomaterial Translocation-the Biokinetics, Tissue Accumulation, Toxicity and Fate of Materials in Secondary Organs-A Review.” Critical Reviews in Toxicology 45(10): 837–872. doi:https://doi.org/10.3109/10408444.2015.1058747.
- Kermanizadeh, A., T. Berthing, E. Guzniczak, M. Wheeldon, G. Whyte, U. Vogel, W. Moritz, and V. Stone. 2019. “Assessment of Nanomaterial-induced Hepatotoxicity using a 3D Human Primary Multi-cellular Microtissue Exposed Repeatedly Over 21 Days – The Suitability of the In vitro System as an In vivo Surrogate.” Particle and Fibre Toxicology 16 (1): 42. doi:https://doi.org/10.1186/s12989-019-0326-0.
- Kermanizadeh, A., N. R. Jacobsen, F. Murphy, L. Powell, L. Parry, H. Zhang, and P. Moller. 2020. “A Review of the Current State of Nanomedicines for Targeting and Treatment of Cancers – Achievements and Future Challenges.” Advanced Therapeutics 4 (2): 2000186. doi:https://doi.org/10.1002/adtp.202000186.
- Kermanizadeh, A., N. R. Jacobsen, M. Roursgaard, S. Loft, and P. Møller. 2017. “Hepatic Hazard Assessment of Silver Nanoparticle Exposure in Healthy and Chronically Alcohol Fed Mice.” Toxicological Sciences 158 (1): 176–187. doi:https://doi.org/10.1093/toxsci/kfx080.
- Kermanizadeh, A., G. Pojana, B. K. Gaiser, R. Birkedal, D. Bilanicˇová, H. Wallin, K. A. Jensen, et al. 2013. “In Vitro Assessment of Engineered Nanomaterials Using C3A Cells: Cytotoxicity, Pro-Inflammatory Cytokines and Function Markers.” Nanotoxicology 7 (3): 301–313. doi:https://doi.org/10.3109/17435390.2011.653416.
- Kermanizadeh, A., L. G. Powell, and V. Stone. 2020. “A Review of Hepatic Nanotoxicology - Summation of Recent Findings and Considerations for the Next Generation of Study Designs.” Journal of Toxicology and Environmental Health, Part B 23 (4): 137–176. doi:https://doi.org/10.1080/10937404.2020.1751756.
- Kermanizadeh, A., S. Vranic, S. Boland, K. Moreau, A. B. Squiban, B. K. Gaiser, L. A. Andrzejczuk, and V. Stone. 2013. “An in Vitro Assessment of Panel of Engineered Nanomaterials Using a Human Renal Cell Line: Cytotoxicity, Pro-Inflammatory Response, Oxidative Stress and Genotoxicity.” BMC Nephrology 14 (1): 96. doi:https://doi.org/10.1186/1471-2369-14-96.
- Koyama, Y., and D. A. Brenner. 2017. “Liver Inflammation and Fibrosis.” The Journal of Clinical Investigation 127 (1): 55–64. doi:https://doi.org/10.1172/JCI88881.
- Kreyling, W. G., U. Holzwarth, N. Haberl, J. Kozempel, S. Hirn, A. Wenk, C. Schleh, et al. 2017. “Quantitative Biokinetics of Titanium Dioxide Nanoparticles after Intravenous Injection in Rats: Part 1.” Nanotoxicology 11 (4): 434–442. doi:https://doi.org/10.1080/17435390.2017.1306892.
- Kubes, P., and C. Jenne. 2018. “Immune Responses in the Liver.” Annual Review of Immunology 36: 247–277. doi:https://doi.org/10.1146/annurev-immunol-051116-052415.
- Kyjovska, Z. O., N. R. Jacobsen, A. T. Saber, S. Bengtson, P. Jackson, H. Wallin, and U. Vogel. 2015. “DNA Strand Breaks, Acute Phase Response and Inflammation following Pulmonary Exposure by Instillation to the Diesel Exhaust Particle NIST1650b in Mice.” Mutagenesis 30 (4): 499–507. doi:https://doi.org/10.1093/mutage/gev009.
- Lagopati, N., K. Evangelou, P. Falaras, E.-P. C. Tsilibary, P. V. S. Vasileiou, S. Havaki, A. Angelopoulou, E. A. Pavlatou, and V. G. Gorgoulis. 2021. “Nanomedicine: Photo-Activated Nanostructured Titanium Dioxide, as a Promising Anticancer Agent.” Pharmacology and Therapeutics 222: 107795. doi:https://doi.org/10.1016/j.pharmthera.2020.107795.
- Lee, J. H., M. Gulumian, E. M. Faustman, T. Workman, K. Jeon, and I. J. Yu. 2018. “Blood Biochemical and Hematological Study after Subacute Intravenous Injection of Gold and Silver Nanoparticles and Coadministered Gold and Silver Nanoparticles of Similar Sizes.” Biomed Research International 2018: 1–10. doi:https://doi.org/10.1155/2018/8460910.
- Lee, J. H., Y. S. Kim, K. S. Song, H. R. Ryu, J. H. Sung, H. M. Park, N. W. Song, et al. 2013. “Biopersistence of Silver Nanoparticles in Tissues from Sprague-Dawley Rats.” Particle and Fibre Toxicology 10: 36. doi:https://doi.org/10.1186/1743-8977-10-36.
- Lipka, J., M. Semmler-Behnke, R. A. Sperling, A. Wenk, S. Takenaka, C. Schleh, T. Kissel, W. J. Parak, and W. G. Kreyling. 2010. “Biodistribution of PEG-Modified Gold Nanoparticles following Intratracheal Instillation and Intravenous Injection.” Biomaterials 31(25): 6574–6581. doi:https://doi.org/10.1016/j.biomaterials.2010.05.009.
- Loguercio, C., and A. Federico. 2003. “Oxidative Stress in Viral and Alcoholic Hepatitis.” Free Radical Biology and Medicine 34 (1): 1–10. doi:https://doi.org/10.1016/S0891-5849(02)01167-X.
- Luther, J., M. K. Gala, N. Borren, R. Masia, R. P. Goodman, I. H. Moeller, E. Di Giacomo, et al. 2018. “Hepatic Connexin 32 Associates with Nonalcoholic Fatty Liver Disease Severity.” Hepatology Communications 2 (7): 786–797. doi:https://doi.org/10.1002/hep4.1179.
- Modrzynska, J., T. Berthing, G. Ravn-Haren, K. Kling, A. Mortensen, R. R. Rasmussen, E. H. Larsen, A. T. Saber, U. Vogel, and K. Loeschner. 2018. “In Vivo-Induced Size Transformation of Cerium Oxide Nanoparticles in Both Lung and Liver Does Not Affect Long-Term Hepatic Accumulation following Pulmonary Exposure.” PLOS One 13 (8): e0202477. doi:https://doi.org/10.1371/journal.pone.0202477.
- Patlolla, A. K., D. Hackett, and P. B. Tchounwou. 2015. “Silver Nanoparticle-Induced Oxidative Stress-Dependent Toxicity in Sprague-Dawley Rats.” Molecular and Cellular Biochemistry 399 (1–2): 257–268. doi:https://doi.org/10.1007/s11010-014-2252-7.
- Poulsen, S. S., A. T. Saber, A. Mortensen, J. Szarek, D. Wu, A. Williams, O. Andersen, et al. 2015. “Changes in Cholesterol Homeostasis and Acute Phase Response Link Pulmonary Exposure to Multi-Walled Carbon Nanotubes to Risk of Cardiovascular Disease.” Toxicology and Applied Pharmacology 283 (3): 210–222. doi:https://doi.org/10.1016/j.taap.2015.01.011.
- Poulsen, S. S., A. T. Saber, A. Williams, A. Williams, O. Andersen, C. Købler, R. Atluri, et al. 2015. “MWCNTs of Different Physicochemical Properties Cause Similar Inflammatory Responses, but Differences in Transcriptional and Histological Markers of Fibrosis in Mouse Lungs.” Toxicology and Applied Pharmacology 284 (1): 16–32. doi:https://doi.org/10.1016/j.taap.2014.12.011.
- Radwan, I. M., P. M. Potter, D. D. Dionysiou, and S. R. Al-Abed. 2021. “Silver Nanoparticle Interactions with Surfactant Based Household Surface Cleaners.” Environmental Engineering Science 38(6): 481–488. doi:https://doi.org/10.1089/ees.2020.0160.
- Recordati, C., M. De Maglie, S. Bianchessi, S. Argentiere, C. Cella, S. Mattiello, F. Cubadda, et al. 2016. “Tissue Distribution and Acute Toxicity of Silver after Single Intravenous Administration in Mice: Nano-Specific and Size-Dependent Effects.” Particle and Fibre Toxicology 13: 12. doi:https://doi.org/10.1186/s12989-016-0124-x.
- Saber, A. T., N. R. Jacobsen, P. Jackson, S. S. Poulsen, Z. O. Kyjovska, S. Halappanavar, C. L. Yauk, H. Wallin, and U. Vogel. 2014. “Particle-Induced Pulmonary Acute Phase Response May be the Causal Link Between Particle Inhalation and Cardiovascular Disease.” Wiley Interdisciplinary Reviews. Nanomedicine and Nanobiotechnology 6 (6): 517–531. doi:https://doi.org/10.1002/wnan.1279.
- Sadauskas, E., N. R. Jacobsen, G. Danscher, M. Stoltenberg, U. Vogel, A. Larsen, W. Kreyling, and H. Wallin. 2009. “Bio-Distribution of Gold Nanoparticles in Mouse Lung Following Intratracheal Instillation.” Chemistry Central Journal 3: 16.
- Semmler-Behnke, M., K. G. Wolfgang, J. Lipka, S. Fertsch, A. Wenk, S. Takeneka, G. Schmid, and W. Brandau. 2008. “Biodistribution of 1.4- and 18-nm Gold Particles in Rats.” Small 4(12):2108–2111. doi:https://doi.org/10.1002/smll.200800922.
- Tassinari, R., A. Martinelli, M. Valeri, and F. Maranghi. 2021. “Amorphous Silica Nanoparticles Induced Spleen and Liver Toxicity after Acute Intravenous Exposure in Male and Female Rats.” Toxicology and Industrial Health 37 (6): 328–335. doi:https://doi.org/10.1177/07482337211010579.
- Wang, D. J., H. B. Li, Z. H. Liu, J. Y. Zhou, and T. L. Zhang. 2017. “Acute Toxicological Effects of Zinc Oxide Nanoparticles in Mice after Intratracheal Instillation.” International Journal of Occupational and Environmental Health 23 (1): 11–19. doi:https://doi.org/10.1080/10773525.2016.1278510.
- Willebrords, J., B. Cogliati, I. V. A. Pereira, T. C. da Silva, S. Crespo Yanguas, M. Maes, V. M. Govoni, et al. 2017. “Inhibition of Connexin Hemichannels Alleviates Non-Alcoholic Steatohepatitis in Mice.” Scientific Reports 7 (1): 8268. doi:https://doi.org/10.1038/s41598-017-08583-w.
- Wilson, C. G., J. L. Tran, D. M. Erion, N. B. Vera, M. Febbraio, and E. J. Weiss. 2016. “Hepatocyte-Specific Disruption of CD36 Attenuates Fatty Liver and Improves Insulin Sensitivity in HFD-Fed Mice.” Endocrinology 157 (2): 570–585. doi:https://doi.org/10.1210/en.2015-1866.
- Zhang, X. D., D. Wu, X. Shen, P. X. Liu, N. Yang, B. Zhao, H. Zhang, Y. M. Sun, L. A. Zhang, and F. Y. Fan. 2011. “Size-Dependent in Vivo Toxicity of PEG-Coated Gold Nanoparticles.” International Journal of Nanomedicine 6: 2071–2081. doi:https://doi.org/10.2147/IJN.S21657.