Abstract
Opportunities for the exposure of pregnant women to engineered nanoparticles have been increasing with the expanding use of these materials. Therefore, there are concerns that nanoparticles could have adverse effects on the establishment and maintenance of pregnancy. The effects of nanoparticles on the mother and fetus have been evaluated from this perspective, but there is still little knowledge about the effects on placentation and function acquisition, which are essential for the successful establishment and maintenance of pregnancy. Formation of the syncytiotrophoblast is indispensable for the acquisition of placental function, and impairment of syncytialization inevitably affects pregnancy outcomes. Here, we assessed the effect of nanoparticles on placental formation by using forskolin-treated BeWo cells, a typical in vitro model of trophoblast syncytialization. Immunofluorescence staining analysis revealed that silver nanoparticles with a diameter of 10 nm (nAg10) (at 0.156 µg/mL) significantly decreased the proportion of syncytialized BeWo cells, but gold nanoparticles with a diameter of 10 nm did not. Consistently, only nAg10 (at 0.156 µg/mL) significantly suppressed forskolin-induced elevation of CGB and SDC1 mRNA expression levels and human chorionic gonadotropin β production in a dose-dependent manner; these molecules are all markers of syncytialization. Besides, nAg10 significantly decreased the expression of ERVFRD-1, which encodes proteins associated with cell fusion. Moreover, nAg10 tended to suppress the expression of sFlt-1 e15a, a placental angiogenesis marker. Collectively, our data suggest that nAg10 could suppress formation of the syncytiotrophoblast and that induce placental dysfunction and the following poor pregnancy outcomes.
1. Introduction
Nanoparticles are fine particles of less than 100 nm in diameter. Because of their innovative functions, such as tissue penetration and interfacial reactivity, compared with those of materials of conventional size (several hundreds of nanometers or more), nanoparticles are now used in many everyday items (Bajpai et al. Citation2021; Kumar et al. Citation2021; Gupta et al. Citation2022). For example, silver nanoparticles are used in medical products, antibacterial sprays, and food packaging for their antibacterial effects (Zhang et al. Citation2016; Burdușel et al. Citation2018), and gold nanoparticles, which have unique optical properties and high stability, are used not only in the industrial field but also in cosmetics and as drug delivery carriers (Talarska, Boruczkowski, and Żurawski Citation2021). However, there has been concern that, unlike conventional materials, nanoparticles could have unexpected biological effects (Yang et al. Citation2017; Malakar et al. Citation2021). There is therefore an increasingly urgent need to collect safety information on nanoparticle exposure.
A great many people, regardless of age or gender, readily use products that contain nanoparticles, and all of us may be unintentionally exposed to these materials. It is therefore essential to assess the biological effects of nanoparticles in people, such as pregnant women, who are vulnerable to chemicals. In this regard, we showed previously that a proportion of the nanoparticles to which pregnant mice were exposed could reach the placenta and induce fetal dysgenesis (Yamashita et al. Citation2011; Higashisaka et al. Citation2018). However, it remains unclear how nanoparticles affect placentation. The successful establishment of placentation is essential for the maintenance of pregnancy, leading to well-being of fetuses (Nakashima et al. Citation2021). Given that structural and functional abnormalities of the placenta lead to poor pregnancy outcomes (Wan Masliza et al. Citation2017; Berceanu et al. Citation2018), there is a need to understand the effects of nanoparticles on placentation and subsequent pregnancy.
Placental functions such as formation of the blood–placental barrier, production of hormones, and substance transportation are maintained mainly by polynuclear syncytiotrophoblast cells (Costa Citation2016a). Syncytiotrophoblast cells are formed in early pregnancy by the syncytialization process, by which mononuclear cytotrophoblast cells fuze with each other (Knöfler et al. Citation2019). As the terminally differentiated syncytiotrophoblasts directly face to maternal blood for isolating fetal blood, they are known to be exposed with multiple type of stress (Redman, Staff, and Roberts Citation2022). It is therefore possible for impairment of the syncytialization process to compromise the acquisition of placental function (Costa Citation2016b). Moreover, disruption of placental development could lead to preeclampsia (PE) which is a pregnancy specific disease characterized by high blood pressure and proteinuria (Roland et al. Citation2016). Here, as a new candidate for the environmental hazard in human placenta, we evaluated the effects of nanoparticles on syncytialization by using the human choriocarcinoma cell line BeWo, a widely used model of the forskolin-induced syncytialization process (Nampoothiri, Neelima, and Rao Citation2007; Myllynen and Vähäkangas Citation2013).
2. Materials and methods
2.1. Cell line and cell culture
The human choriocarcinoma cell line BeWo was purchased from the Japanese Collection of Research Bioresources Cell Bank (JCRB9111; Osaka, Japan). BeWo cells were cultured in 10% inactivated fetal bovine serum (Biosera, Nuaille, France) and 1% (v/v) penicillin–streptomycin–amphotericin B suspension (FUJIFILM Wako Pure Chemical, Osaka, Japan) in Ham’s F-12 nutrient mixture (Wako) and maintained under standard cell-culture conditions at 37 °C and >95% humidity under a 5% CO2 atmosphere.
2.2. Reagents
An aqueous suspensions of citrate-ligand-capped Ag nanoparticles with diameters of 10 nm (nAg10) and citrate-ligand-capped Au nanoparticles with diameters of 10 nm (nAu10) were purchased from nanoComposix (San Diego, CA, USA). We previously confirmed that the nanoparticles were smooth-surfaced spheres by transmission electron microscopy (Morishita et al. Citation2016). In addition, we measured the hydrodynamic diameter of the nanoparticles in water and in culture media by dynamic light scattering analysis. The hydrodynamic diameter of nAg10 in water was 46.72 ± 0.89 nm and that in culture media was 63.94 ± 0.59 nm. Besides, the hydrodynamic diameter of nAu10 in water was 21.60 ± 0.09 nm and that in culture media was 93.19 ± 0.60 nm. Before use, the nAg10 was mixed for 1 min in a vortex mixer with an equal volume of 20 mg/mL bovine serum albumin (Sigma-Aldrich, St Louis, MO, USA) to prevent aggregation. Forskolin and H-89 were purchased from Cayman Chemical (Ann Arbor, MI, USA). Forskolin was diluted with Ham’s F-12 to a final concentration of 50 µM, and H-89 was diluted with Ham’s F-12 to a final concentration of 10 µM.
2.3. Cell viability assay
BeWo cells were seeded at 1.0 × 104 cells/200 µL per well into 96-well flat plates and then treated with 50 µM forskolin with or without silver or gold nanoparticles for 48 h at 37 °C and >95% humidity under a 5% CO2 atmosphere. Cell viability was measured with MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide] (Tokyo Chemical Industry, Tokyo, Japan), in accordance with the manufacturers’ instructions.
2.4. Immunofluorescence staining
BeWo cells were seeded at 1.0 × 104 cells/200 µL per well into 96-well Black/Clear bottom plates (Thermo Fisher Scientific, Waltham, MA, USA), cultured overnight under standard cell-culture conditions, and then treated for 48 h with 50 µM forskolin containing nAg10 (0.156 µg/mL), nAu10 (2.5 µg/mL), or H-89 (10 µM.). After incubation, the BeWo were fixed with 4% paraformaldehyde phosphate buffer solution (Wako) for 15 min and then treated with 0.1% Triton X-100 (F) for 5 min. After washing with PBS, blocking was performed with 1% BSA-PBS for 30 min. The BeWo cells were then incubated with antibody against E-cadherin (1:50) (Santa Cruz Biotechnology, Dallas, Texas, USA) and human chorionic gonadotropin beta (1:200) (hCGβ; abcam, Cambridge, UK) overnight at4 °C. The cells were then incubated with goat anti-rabbit IgG (H + L) Alexa Fluor 488 conjugate or goat anti-mouse IgG (H + L) Alexa Fluor 647 conjugate (1:500) (Invitrogen, Carlsbad, CA, USA) and DAPI (4′,6-diamidino-2-phenylindole) (1: 200) for 45 minutes. Images were acquired under a confocal microscope (CellVoyager CV8000, Yokogawa, Japan). To analyze the rate of syncytialization of BeWo cells, five fields of view were randomly taken for each treatment group of cells. The nuclei were counted by using ImageJ Fiji version: 2.1.0 (National Institutes of Health, Bethesda, MD, USA) and the ratio of the number of syncytial nuclei to total nuclei was manually determined.
2.5. Real-time RT-PCR
BeWo cells were seeded at 1.5 × 105 cells cells/2 mL per well in six-well flat plates overnight and then treated for 48 h at 37 °C and >95% humidity under a 5% CO2 atmosphere with 50 µM forskolin alone or with 50 µM forskolin containing nAg10 or nAu10. Total RNA was extracted by using a FastGene RNA Kit (Nippon Genetics, Tokyo, Japan) and reverse-transcribed into cDNA by using a High-Capacity cDNA Reverse Transcription Kit (Thermo Fisher Scientific). By using the obtained cDNA as a template, reaction mixtures for quantitative PCR were prepared by using primers () (Eurofins Genomics, Tokyo, Japan) and GeneAce SYBR qPCR Mix α Low ROX (Nippon Gene Co., Ltd., Tokyo, Japan). Real-time RT-PCR was performed by using a CFX-384 Real-Time PCR Detection System (BioRad Laboratories, Hercules, CA, USA). The expression level of each gene was normalized to that of β-actin.
Table 1. Primer list.
2.6. Enzyme-linked immunosorbent assay
Concentrations of hCGβ in cell supernatants were measured by using commercial enzyme-linked immunosorbent assay kits purchased from R&D Systems (Minneapolis, MN, USA).
2.7. Statistical analysis
Statistical analyses were conducted by using Origin Pro 9.0.0 J (Origin Lab Corporation, Northampton, MA, USA) or GraphPad Prism (version 9.3.1). Data are expressed as means ± S.D. Statistical analyses were performed using Kruskal–Wallis test for or Tukey’s method for the other. p-Values lower than 0.05 were considered statistically significant.
3. Results
3.1. Silver nanoparticles suppress forskolin-induced BeWo syncytialization
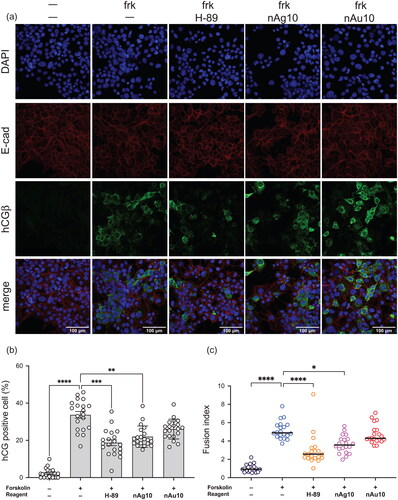
First, we assessed whether nanoparticles affect syncytialization. During placental syncytialization, E-cadherin, which is a protein involved in cell–cell adhesion at the cell boundary, disappears due to fusion with adjacent cells (Nadeem et al. Citation2014, Iwahashi et al. Citation2019). Moreover, human chorionic gonadotropin β (hCGβ, gene name; CGB), a hormone that is produced by the placenta in the first trimester of pregnancy (Kovalevskaya et al. Citationn.d.), is upregulated in a BeWo human choriocarcinoma cell model of forskolin-induced syncytialization (Leduc et al. Citation2012). We treated BeWo cells with forskolin containing silver nanoparticles with a diameter of 10 nm (nAg10), gold nanoparticles with a diameter of 10 nm (nAu10), or H-89 (a protein kinase A inhibitor used as a positive control for inhibition of the syncytialization process (Orendi et al. Citation2010)) for 48 h. Cell viability assay showed that, at a dose of 0.156 µg/mL, each type of nanoparticle in an immunofluorescence staining analysis was not toxic to BeWo cells in the absence of forskolin (). Immunofluorescence staining analysis revealed that the expression of E-cadherin at the cell boundaries was decreased by 50 µM forskolin in the absence of nanoparticles, and a forskolin-induced hCGβ increase was suppressed by nAg10 treatment (). Moreover, the ratio of hCGβ positive BeWo cells was significantly decreased in the nAg10-treated cells (). In contrast, there were no marked differences between the treatments with and without nAu10. In addition, we quantified the syncytialization rate of BeWo cells under each condition. Given that each syncytial cell has two or more nuclei, the ratio of the number of syncytial nuclei to the total number of nuclei was determined as a Fusion index (Omata et al. Citation2013). The Fusion index of the forskolin-only-treated cells was significantly greater than that of the control cells, whereas the forskolin-plus-nAg10-treated cells, but not the forskolin-plus-nAu10-treated cells, had a significantly smaller fusion index than the forskolin-only treated ones (). These results suggest that nAg10 but not nAu10 could suppress forskolin-induced BeWo syncytialization morphologically.
Figure 1. Viability of BeWo cells exposed to silver or gold nanoparticles at different concentrations. BeWo cells were treated for 48 h with (a) 0.039, 0.078, 0.156, 0.625, 1.25, or 2.5 μg/mL nAg10 or (b) 0.156, 0.625, 2.5, 10, 40, or 100 μg/mL nAu10 with or without forskolin (50 µM). Cell viability was evaluated by MTT assay. Data are expressed as means ± S.D.; (a) n = 4. (b) n = 5.
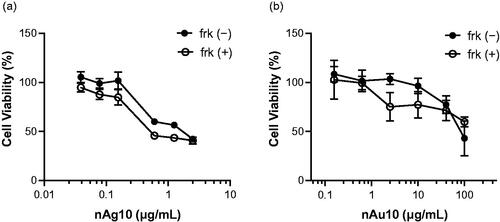
Figure 2. nAg10 suppresses forskolin-induced syncytialization in BeWo cells. (a) BeWo cells were treated with forskolin alone or with forskolin (50 µM) containing nAg10 (0.156 µg/mL), nAu10 (0.156 µg/mL), or H-89 (10 µM) for 48 h. The expression of E-cadherin and hCGβ was analyzed by immunofluorescence staining with DAPI counterstaining. Scale bars: 100 µm. (b) The rate of hCGβ positive cells to total was determined. (c) Given that each syncytial cell had two or more nuclei, the ratio of the number of syncytial nuclei to the total number of nuclei was determined as a Fusion Index. The percentage of hCGβ-positive cells and Fusion Index were quantified from images of five different fields for every two wells. The graph (b) and (c) showed that pooled result from two independent experiments. Data are expressed as means ± S.D.; *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001.
3.2. Nag10 suppresses BeWo syncytialization through downregulation of cell–cell fusion
Syndecan-1 (gene name, SDC1) is a transmembrane proteoglycan that mediates cell signaling during the placental formation process (Prakash, Suman, and Gupta Citation2011; Baston-Buest et al. Citation2017). To assess further the effect of nanoparticles on the syncytialization process, we analyzed the expression of CGB and SDC1 mRNAs. Real-time reverse transcription–polymerase chain reaction (RT-PCR) analysis showed that co-treatment with forskolin and nAg10 (0.156 µg/mL) significantly decreased the forskolin-induced increase in CGB expression level (). In contrast, there were no significant changes in CGB expression level in the forskolin-treated cells to which nAu10 were added (). Consistently, adding nAg10 (0.156 µg/mL) to forskolin-treated cells significantly downregulated SDC1 (), whereas adding nAu10 did not (). Furthermore, we assessed the concentration of hCGβ, which are increasingly secreted dependently of syncytialization, in cell culture supernatants by enzyme-linked immunosorbent assay. Adding nAg10 (0.156 µg/mL) to forskolin-treated cells significantly decreased the hCGβ concentration in the supernatant (), whereas nAu10 addition did not (). These results indicated that nAg10 inhibited the forskolin-induced syncytialization process.
Figure 3. nAg10 suppresses syncytial marker mRNA expression and protein production in BeWo cells. BeWo cells were treated for 48 h with forskolin alone or with forskolin (50 µM) containing nAg10 (0.039, 0.078, or 0.156 µg/mL), nAu10 (0.156, 0.612, or 2.5 µg/mL), or H-89 (10 µM). The expression of (a, b) CGB and (c, d) SDC1 mRNA was analyzed by real-time reverse transcription–polymerase chain reaction. The relative expression level of each gene was analyzed after normalization to that of Actin. Data are presented as means ± S.D. of three independent experiments; *p < 0.05. The concentration of hCGβ in the culture supernatant of the (e) nAg10- and (f) nAu10-treated cells was determined by enzyme-linked immunosorbent assay. Two independent experiments were performed. Dates are presented as means ± S.D.; *p < 0.05.
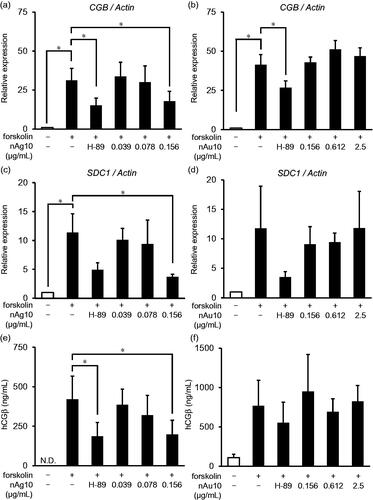
Figure 4. nAg10 decreases mRNA expression of syncytialization-promoting molecules in BeWo cells. BeWo cells were treated for 48 h with forskolin alone or with forskolin (50 µM) containing nAg10 (0.039, 0.078, or 0.156 µg/mL), nAu10 (0.156, 0.612, and 2.5 µg/mL), or H-89 (10 µM). The expression of (a, b) ERVW-1 and (c, d) ERVFRD-1 mRNA was analyzed by real-time reverse transcription–polymerase chain reaction. The relative expression level of each gene was analyzed after normalization to that of Actin. Data are presented as means ± S.D. of three independent experiments; *p < 0.05, ***p < 0.001.
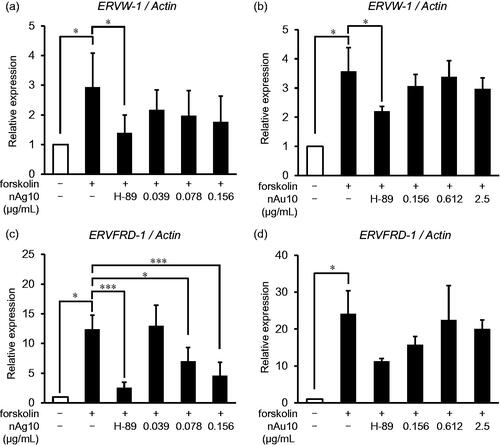
Next, we assessed the effect of nAg10 on cell–cell fusion progression. Syncytin-1 (gene name; ERVW-1 [endogenous retrovirus group W member 1]) and Syncytin-2 (gene name; ERVFRD-1 [endogenous retrovirus group FRD member 1]) play important roles in cell–cell fusion during the trophoblast syncytialization process. Syncytin-2 controls the process by which single mononuclear cells fuze with each other and Syncytin-1 promotes the fusion of formed multinuclear syncytiotrophoblast cells with mononuclear cells (Nakamura and Imakawa Citation2011; Lavialle et al. Citation2013). Real-time RT-PCR analysis showed that, when either nAg10 () or nAu10 () were added, there was no significant change in the forskolin-induced upregulation of ERVW-1 mRNA. In contrast, the ERVFRD-1 mRNA level declined significantly when nAg10 (0.078 and 0.156 µg/mL) () was added to forskolin-treated cells, but not when nAu10 () was added. These results suggest that nAg10 decreased the expression of ERVFRD-1 for trophoblast cell fusion, thus suppressing the formation of the syncytiotrophoblast.
Progression of syncytialization is regulated by various pathways centered on the cyclic adenosine monophosphate signal (Wang et al. Citation2014). In mice, oxidative phosphorylation in mitochondria is enhanced, and ATP production is increased, during early placental formation (Miyazawa et al. Citation2017). In this regard, silver nanoparticles are hazardous to multiple organelles such as mitochondria (Gurunathan et al. Citation2019), which play important roles in ATP production. As a mechanism by which nAg10 inhibits forskolin-induced syncytialization, we hypothesize that nAg10 taken up by BeWo cells are distributed in the mitochondria and cause them to malfunction in such processes as ATP synthesis. From this viewpoint, we performed an inductively coupled plasma mass spectrometry (ICP-MS) analysis to evaluate the cellular uptake in the forskolin-plus-nAg10 (0.156 μg/mL)-treated cells. ICP-MS analysis showed that nAg10 was uptaken in BeWo cells and that localized in the nucleus and mitochondria (Supplementary Figure S1). We, therefore, need to evaluate their effect on mitochondrial dysfunction by examining mitochondrial electron transfer system activity and ATP production in nanoparticle-treated cells.
3.3. Nag10 negatively affects placental vascular function
Normal placental development requires coordinated expression of angiogenic growth factors such as vascular endothelial growth factor’s fms-like tyrosine kinase 1 (Flt-1) receptor and placenta growth factor (PlGF, gene name; PGF) (Girardi et al. Citation2006). In clinical practice, an increase of soluble Flt-1 (sFlt-1), a factor that regulates maternal angiogenesis during pregnancy (Lam, Lim, and Karumanchi Citation2005), and a decrease of PlGF, pro-angiogenic factor predominantly expressed in placental trophoblast (Schiffer et al. Citation2021), was observed in circulation of pregnant woman with PE (Stepan et al. Citation2015; Chau, Hennessy, and Makris Citation2017). Thus, these placental angiogenesis markers could be associated with placental dysfunction.
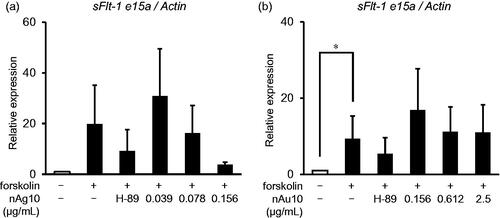
To investigate Flt-1 expression in BeWo cells treated with nAg10 or nAu10, we evaluated the mRNA expression levels of three Flt-1 splice variants (transmembrane Flt-1; tmFlt-1, sFlt-1 i13, and sFlt-1 e15a) (Sasagawa et al. Citation2018) by real-time RT-PCR. The level of sFlt-1 e15a, is primate specific and the most abundant placentally derived sFLT-1, mRNA was tended to be suppressed when nAg10 (0.078 and 0.156 µg/mL) was added to forskolin-treated cells (), but not when nAu10 () was added. However, there was no remarkable changes in the level of tmFlt-1 and sFlt-1 i13 mRNA when either nAg10 (Supplementary Figure S2a and S2c) or nAu10 (Supplementary Figure S2b and S2d) were added. Moreover, treatment with nAg10 (Supplementary Figure S2e) or nAu10 (supplementary Figure S2f) showed no changes in the level PGF mRNA. These results suggest that suppression of syncytialization by nAg10 might be related with placental impairment and the following pregnancy outcome.
Figure 5. nAg10 decreases mRNA expression of a placental angiogenesis marker in BeWo cells. BeWo cells were treated for 48 h with forskolin alone or with forskolin (50 µM) containing nAg10 (0.039, 0.078, or 0.156 µg/mL), nAu10 (0.156, 0.612, and 2.5 µg/mL), or H-89 (10 µM). The expression of sFlt-1 e15a mRNA of the (a) nAg10- and (b) nAu10-treated cells was analyzed by real-time reverse transcription–polymerase chain reaction. The relative expression level of each gene was analyzed after normalization to that of Actin. Data are presented as means ± S.D. of three independent experiments; *p < 0.05.
4. Discussion
The physicochemical properties of nanoparticles could affect their kinetics and the biological responses to them (Higashisaka et al. Citation2017). The ease of ionization of metal nanoparticles is one important determinant of their toxicity. For instance, in contrast to gold nanoparticles, which emit few ions, silver nanoparticles tend to release silver ions; this property of silver nanoparticles is an important characteristic related to their unique antibacterial action (Yin et al. Citation2020). The intracellular solubility of metal nanoparticles is another important determinant of their toxicity (Cameron, Hosseinian, and Willmore Citation2018; Attarilar et al. Citation2020). In the case of some metal nanoparticles, ionization is promoted within cells, especially under acidic conditions, such as within lysosomes (Cho et al. Citation2012), therefore it is possible that this characteristic may affect the degree of hazard posed by these materials. We consider that this difference might reflect the difference in the effects on syncytialization in the nAg10- and nAu10-treated cells. There is a need for further research into the degree to which the release of ions contributes to the inhibitory effect of silver nanoparticles on syncytialization.
Construction of the syncytiotrophoblast via the cell fusion process is essential to the placental formation process. The endogenous retrovirus genes ERVW-1 and ERVFRD-1 play an important role in facilitating this fusion process (Orendi et al. Citation2010). Both ERVW-1 and ERVFRD-1 have cell-fusion activity, but there are differences in their expression patterns in placental cells. The ERVW-1 is expressed in not only syncytiotrophoblast cells but also mononuclear cytotrophoblast cells, whereas ERVFRD-1 is expressed only in mononuclear cytotrophoblast cells (Malassiné et al. Citation2007; Holder et al. Citation2012). Because of this difference, it is considered that ERVFRD-1 promotes fusion between mononuclear cells and ERVW-1 is involved in fusion between syncytiotrophoblast cells and mononuclear cytotrophoblast cells (Nakamura and Imakawa Citation2011). Moreover, it is reported that reduced expression of Syncytin-1 and Syncytin-2 correlates with severity of preeclampsia and impaired cell fusion and differentiation in placentae from patients with intrauterine growth restriction. Furthermore, Syncytin-2 knockdown BeWo cells more suppressed syncytialization compared to Syncytin-1 knockdown BeWo cells (Vargas et al. Citation2009) indicating that Syncytin-2 could play a critical role in syncytinization. Present study showed that nAg10 suppressed the expression of ERVFRD-1 mRNA. Thus, it is considered that nAg10 could be involved in placental hypoplasia by suppressing the expression of ERVFRD-1.
In the syncytiotrophoblast formation process, it is essential that fusion proteins such as Syncytin-2 are correctly recognized by cell-surface receptors (Sugimoto et al. Citation2013). Major facilitator superfamily domain-containing protein 2 (MFSD2) is a receptor for Syncytin-2 and is expressed particularly highly on placental syncytiotrophoblast cells (Liang et al. Citation2010). MFSD2 is regulated by the master transcription factor Glial cells missing (GCM), which is also upstream of Syncytin-2. Given that nAg10 suppress the fusion process by targeting mononuclear trophoblast cells, nAg10 might also affect the activity of MFSD2 and GCM. Therefore, there is a need to pay attention to the activity of MFSD2 and GCM to clarify the mechanism by which nAg10 suppresses the syncytialization process.
Given the complexity of the intracellular dynamics of silver nanoparticles and the diversity of syncytialization-promoting signals, we can expect the mechanisms of hazard expression by silver nanoparticles to be diverse. It has been reported that the syncytialization process is promoted by an epigenetic mechanism (Milano-Foster et al. Citation2019). In this regard, as we have previously found that silver nanoparticles induce DNA hypomethylation in human lung adenocarcinoma cells (Maki et al. Citation2020), we intend to continue to elucidate the mechanism of the biological effects of nAg10 on syncytialization-promoting signals from an epigenetic perspective.
Maintaining placental construction and function through proper syncytialization is important for a continued, healthy pregnancy. This is because syncytiotrophoblast cells need constantly to fuze with cytotrophoblast cells to maintain their function (Reiter et al. Citation2013). The hypothesis that poor pregnancy outcomes are due to abnormal syncytialization has been widely proposed. In fact, it has been reported that the placentas of patients with preeclampsia have lower levels of production of Syncytin—especially Syncytin-2—than the placenta of normal pregnancies (Vargas et al. Citation2011). Another study has shown that placental weight is reduced, and Syncytin production decreased, in intrauterine growth retardation (Ruebner et al. Citation2010). Furthermore, abnormal expression of Flt-1 induces poor pregnancy outcomes since Flt-1 control placental angiogenic growth (Stepan, Hund, and Andraczek Citation2020). In this regard, our previous study showed that silica nanoparticles reduced sFlt-1 levels in the plasma of pregnant mice on day 18.5 of gestation and induced intrauterine growth retardation (Yamashita et al. Citation2011; Higashisaka Citation2022). Besides, present study showed that nAg10 (0.078 and 0.156 µg/mL) tended to decrease the level of sFlt-1 e15a mRNA. Thus, there is need further analysis to clarify whether nAg10-induced abnormal syncytialization could lead to poor pregnancy outcomes and delayed fetal growth.
Collectively, we found that nAg10 suppresses the syncytialization process of BeWo cells and decrease the expression of molecules involved in cell–cell fusion during syncytialization, suggesting that these nanoparticles could inhibit the syncytialization process during the placental formation period. Our results indicate the need to accelerate assessments of the placental toxicity of nanoparticles in cellular models and in vivo.
Author contributions
Y.S. and K.H. designed the study; R.Is., R.Iz., J.S., A.F., and A.Y-U. performed experiments and analyzed data; Y.S. and K.H. wrote the manuscript; H.T., Y.H., and A.N. provided technical support and conceptual advice; Y.T. supervised all projects. All authors discussed the results and commented on the manuscript.
Supplemental Material
Download MS Power Point (118.3 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
All data generated or analyzed during this study are included in this published article and its supplementary information files.
Additional information
Funding
References
- Attarilar, S., J. Yang, M. Ebrahimi, Q. Wang, J. Liu, Y. Tang, and J. Yang. 2020. “The Toxicity Phenomenon and the Related Occurrence in Metal and Metal Oxide Nanoparticles: A Brief Review From the Biomedical Perspective.” Frontiers in Bioengineering and Biotechnology 8: 822. doi:10.3389/fbioe.2020.00822.
- Bajpai, S., S. K. Tiwary, M. Sonker, A. Joshi, V. Gupta, Y. Kumar, N. Shreyash, and S. Biswas. 2021. “Recent Advances in Nanoparticle-Based Cancer Treatment: A Review.” ACS Applied Nano Materials 4 (7): 6441–6470. doi:10.1021/acsanm.1c00779.
- Baston-Buest, D. M., O. Altergot-Ahmad, S. J. Pour, J. S. Krüssel, U. R. Markert, T. N. Fehm, and A. P. Bielfeld. 2017. “Syndecan-1 Acts as an Important Regulator of CXCL1 Expression and Cellular Interaction of Human Endometrial Stromal and Trophoblast Cells.” Mediators of Inflammation 2017: 8379256. doi:10.1155/2017/8379256.
- Berceanu, C., A. Victor Tetileanu, A. Ofiţeru, E. Brătilă, C. Mehedinţu, N. Loredana Voicu, F. Adrian Szasz, S. Berceanu, S. Vlădăreanu, and D. Navolan. 2018. “Morphological and Ultrasound Findings in the Placenta of Diabetic Pregnancy.” Revue Roumaine de Morphologie et Embryologie [Romanian Journal of Morphology and Embryology] 59 (1): 175–186.
- Burdușel, A. C., O. Gherasim, A. M. Grumezescu, L. Mogoantă, A. Ficai, and E. Andronescu. 2018. “Biomedical Applications of Silver Nanoparticles: An up-to-Date Overview.” Nanomaterials 8 (9): 681–625. doi:10.3390/nano8090681.
- Cameron, S. J., F. Hosseinian, and W. G. Willmore. 2018. “A Current Overview of the Biological and Cellular Effects of Nanosilver.” International Journal of Molecular Sciences 19 (7): 2030–2040. doi:10.3390/ijms19072030.
- Chau, K., A. Hennessy, and A. Makris. 2017. “Placental Growth Factor and Pre-Eclampsia.” Journal of Human Hypertension 31 (12): 782–786. doi:10.1038/jhh.2017.61.
- Cho, W. S., R. Duffin, F. Thielbeer, M. Bradley, I. L. Megson, W. MacNee, C. A. Poland, C. L. Tran, and K. Donaldson. 2012. “Zeta Potential and Solubility to Toxic Ions as Mechanisms of Lung Inflammation Caused by Metal/Metal Oxide Nanoparticles.” Toxicological Sciences 126 (2): 469–477. doi:10.1093/toxsci/kfs006.
- Costa, M. A. 2016a. “The Endocrine Function of Human Placenta: An Overview.” Reproductive Biomedicine Online 32 (1): 14–43. doi:10.1016/j.rbmo.2015.10.005.
- Costa, M. A. 2016b. “Scrutinising the Regulators of Syncytialization and Their Expression in Pregnancy-Related Conditions.” Molecular and Cellular Endocrinology 420: 180–193. doi:10.1016/j.mce.2015.11.010.
- Girardi, G., D. Yarilin, J. M. Thurman, V. M. Holers, and J. E. Salmon. 2006. “Complement Activation Induces Dysregulation of Angiogenic Factors and Causes Fetal Rejection and Growth Restriction.” The Journal of Experimental Medicine 203 (9): 2165–2175. doi:10.1084/jem.20061022.
- Gupta, V., S. Mohapatra, H. Mishra, U. Farooq, K. Kumar, M. J. Ansari, M. F. Aldawsari, A. S. Alalaiwe, M. A. Mirza, and Z. Iqbal. 2022. “Nanotechnology in Cosmetics and Cosmeceuticals—a Review of Latest Advancements.” Gels 8 (3): 173. doi:10.3390/gels8030173.
- Gurunathan, S., M. Jeyaraj, M. H. Kang, and J. H. Kim. 2019. “Mitochondrial Peptide Human in Protects Silver Nanoparticles-Induced Neurotoxicity in Human Neuroblastoma Cancer Cells (SH-SY5Y).” International Journal of Molecular Sciences 20 (18): 4439. doi:10.3390/ijms20184439.
- Higashisaka, K. 2022. “Health Effects and Safety Assurance of Nanoparticles in Vulnerable Generations.” Biological & Pharmaceutical Bulletin 45 (7): 806–812. doi:10.1248/bpb.b22-00277.
- Higashisaka, K., K. Nagano, Y. Yoshioka, and Y. Tsutsumi. 2017. “Review Nano-Safety Research: Examining the Associations among the Biological Effects of Nanoparticles and Their Physicochemical Properties and Kinetics.” Biological & Pharmaceutical Bulletin 40 (3): 243–248. doi:10.1248/bpb.b16-00854.
- Higashisaka, K., A. Nakashima, Y. Iwahara, A. Aoki, M. Nakayama, I. Yanagihara, Y. Lin, et al. 2018. “Neutrophil Depletion Exacerbates Pregnancy Complications, Including Placental Damage, Induced by Silica Nanoparticles in Mice.” Frontiers in Immunology 9: 1850. doi:10.3389/fimmu.2018.01850.
- Holder, B. S., C. L. Tower, V. M. Abrahams, and J. D. Aplin. 2012. “Syncytin 1 in the Human Placenta.” Placenta 33 (6): 460–466. doi:10.1016/j.placenta.2012.02.012.
- Iwahashi, N., M. Ikezaki, I. Matsuzaki, M. Yamamoto, S. Toujima, S. Ichi Murata, Y. Ihara, and K. Ino, 2019. “Calreticulin Regulates Syncytialization through Control of the Synthesis and Transportation of E-Cadherin in BeWo Cells.” Endocrinology 160 (2): 359–374. doi:10.1210/en.2018-00868.
- Knöfler, M., S. Haider, L. Saleh, J. Pollheimer, T. K. J. B. Gamage, and J. James. 2019. “Human Placenta and Trophoblast Development: Key Molecular Mechanisms and Model Systems.” Cellular and Molecular Life Sciences 76 (18): 3479–3496. doi:10.1007/s00018-019-03104-6.
- Kovalevskaya, G., O. Genbacev, S. J. Fisher, E. Caceres, and J. F. O'Connor. n.d. “Trophoblast Origin of hCG Isoforms: cytotrophoblasts Are the Primary Source of Choriocarcinoma-like hCG.” Molecular and Cellular Endocrinology 194 (1–2): 147–155. doi:10.1016/S0303-7207(02)00135-1.
- Kumar, A., A. Choudhary, H. Kaur, S. Mehta, and A. Husen. 2021. “Metal-Based Nanoparticles, Sensors, and Their Multifaceted Application in Food Packaging.” Journal of Nanobiotechnology 19 (1): 256. doi:10.1186/s12951-021-00996-0.
- Lam, C., K. H. Lim, and S. A. Karumanchi. 2005. “Circulating Angiogenic Factors in the Pathogenesis and Prediction of Preeclampsia.” Hypertension 46 (5): 1077–1085. doi:10.1161/01.HYP.0000187899.34379.b0.
- Lavialle, C., G. Cornelis, A. Dupressoir, C. Esnault, O. Heidmann, C. Vernochet, and T. Heidmann. 2013. “Paleovirology of ‘Syncytins’, Retroviral Env Genes Exapted for a Role in Placentation.” Philosophical Transactions of the Royal Society B: Biological Sciences 368 (1626): 20120507. doi:10.1098/rstb.2012.0507.
- Leduc, K., V. Bourassa, É. Asselin, P. Leclerc, J. Lafond, and C. Reyes-Moreno. 2012. “Leukemia Inhibitory Factor Regulates Differentiation of Trophoblastlike BeWo Cells through the Activation of JAK/STAT and MAPK3/1 MAP Kinase-Signaling Pathways.” Biology of Reproduction 86 (2): 54. doi:10.1095/biolreprod.111.094334.
- Liang, C. Y., L. J. Wang, C. P. Chen, L. F. Chen, Y. H. Chen, and H. Chen. 2010. “GCM1 Regulation of the Expression of Syncytin 2 and Its Cognate Receptor MFSD2A in Human Placenta.” Biology of Reproduction 83 (3): 387–395. doi:10.1095/biolreprod.110.083915.
- Maki, A., Y. Lin, M. Aoyama, K. Sato, J.-Q. Gao, H. Tsujino, K. Nagano, K. Higashisaka, and Y. Tsutsumi. 2020. “Silver Nanoparticles Induce DNA Hypomethylation through Proteasome-Mediated Degradation of DNA Methyltransferase 1.” Biological & Pharmaceutical Bulletin 43 (12): 1924–1930. doi:10.1248/bpb.b20-00631.
- Malakar, A., S. R. Kanel, C. Ray, D. D. Snow, and M. N. Nadagouda. 2021. “Nanomaterials in the Environment, Human Exposure Pathway, and Health Effects: A Review.” The Science of the Total Environment 759: 143470. doi:10.1016/j.scitotenv.2020.143470.
- Malassiné, A., S. Blaise, K. Handschuh, H. Lalucque, A. Dupressoir, D. Evain-Brion, and T. Heidmann. 2007. “Expression of the Fusogenic HERV-FRD Env Glycoprotein (Syncytin 2) in Human Placenta is Restricted to Villous Cytotrophoblastic Cells.” Placenta 28 (2–3): 185–191. doi:10.1016/j.placenta.2006.03.001.
- Milano-Foster, J., S. Ray, P. Home, A. Ganguly, B. Bhattacharya, S. Bajpai, A. Pal, C. W. Mason, and S. Paul. 2019. “Regulation of Human Trophoblast Syncytialization by Histone Demethylase LSD1.” The Journal of Biological Chemistry 294 (46): 17301–17313. doi:10.1074/jbc.RA119.010518.
- Miyazawa, H., Y. Yamaguchi, Y. Sugiura, K. Honda, K. Kondo, F. Matsuda, T. Yamamoto, M. Suematsu, and M. Miura. 2017. “Rewiring of Embryonic Glucose Metabolism via Suppression of PFK-1 and Aldolase during Mouse Chorioallantoic Branching.” Development 144 (1): 63–73. doi:10.1242/dev.138545.
- Morishita, Y., Y. Yoshioka, Y. Takimura, Y. Shimizu, Y. Namba, N. Nojiri, T. Ishizaka, et al. 2016. “Distribution of Silver Nanoparticles to Breast Milk and Their Biological Effects on Breast-Fed Offspring Mice.” ACS Nano 10 (9): 8180–8191. doi:10.1021/acsnano.6b01782.
- Myllynen, P., and K. Vähäkangas. 2013. “Placental Transfer and Metabolism: An Overview of the Experimental Models Utilizing Human Placental Tissue.” Toxicology in Vitro 27 (1): 507–512. doi:10.1016/j.tiv.2012.08.027.
- Nadeem, U., G. Ye, M. Salem, and C. Peng. 2014. “MicroRNA-378a-5p Targets Cyclin G2 to Inhibit Fusion and Differentiation in BeWo Cells.” Biology of Reproduction 91 (3): 76. doi:10.1095/biolreprod.114.119065.
- Nakamura, Y., and K. Imakawa. 2011. “Retroviral Endogenization and Its Role in the Genital Tract during Mammalian Evolution.” Journal of Mammalian Ova Research 28 (4): 203–218. doi:10.1274/jmor.28.203.
- Nakashima, A., T. Shima, A. Aoki, M. Kawaguchi, I. Yasuda, S. Tsuda, S. Yoneda, et al. 2021. “Molecular and Immunological Developments in Placentas.” Human Immunology 82 (5): 317–324. doi:10.1016/j.humimm.2021.01.012.
- Nampoothiri, L. P., P. S. Neelima, and A. J. Rao. 2007. “Proteomic Profiling of Forskolin-Induced Differentiated BeWo Cells: An in-Vitro Model of Cytotrophoblast Differentiation.” Reproductive Biomedicine Online 14 (4): 477–487. doi:10.1016/s1472-6483(10)60896-6.
- Omata, W., I. V. W. E. Ackerman, D. D. Vandre, and J. M. Robinson. 2013. “Trophoblast Cell Fusion and Differentiation Are Mediated by Both the Protein Kinase C and a Pathways.” PLOS One 8 (11): e81003. doi:10.1371/journal.pone.0081003.
- Orendi, K., M. Gauster, G. Moser, H. Meiri, and B. Huppertz. 2010. “The Choriocarcinoma Cell Line BeWo: Syncytial Fusion and Expression of Syncytium-Specific Proteins.” Reproduction 140 (5): 759–766. doi:10.1530/REP-10-0221.
- Prakash, G. J., P. Suman, and S. K. Gupta. 2011. “Relevance of Syndecan-1 in the Trophoblastic BeWo Cell Syncytialization.” American Journal of Reproductive Immunology 66 (5): 385–393. doi:10.1111/j.1600-0897.2011.01017.x.
- Redman, C. W. G., A. C. Staff, and J. M. Roberts. 2022. “Syncytiotrophoblast Stress in Preeclampsia: The Convergence Point for Multiple Pathways.” American Journal of Obstetrics and Gynecology 226 (2S): S907–S927. doi:10.1016/j.ajog.2020.09.047.
- Reiter, R. J., S. A. Rosales-Corral, L. C. Manchester, and D. X. Tan. 2013. “Peripheral Reproductive Organ Health and Melatonin: Ready for Prime Time.” International Journal of Molecular Sciences 14 (4): 7231–7272. doi:10.3390/ijms14047231.
- Roland, C. S., J. Hu, C. E. Ren, H. Chen, J. Li, M. S. Varvoutis, L. W. Leaphart, D. B. Byck, X. Zhu, and S. W. Jiang. 2016. “Morphological Changes of Placental Syncytium and Their Implications for the Pathogenesis of Preeclampsia.” Cellular and Molecular Life Sciences 73 (2): 365–376. doi:10.1007/s00018-015-2069-x.
- Ruebner, M., P. L. Strissel, M. Langbein, F. Fahlbusch, D. L. Wachter, F. Faschingbauer, M. W. Beckmann, and R. Strick. 2010. “Impaired Cell Fusion and Differentiation in Placentae from Patients with Intrauterine Growth Restriction Correlate with Reduced Levels of HERV Envelope Genes.” Journal of Molecular Medicine 88 (11): 1143–1156. doi:10.1007/s00109-010-0656-8.
- Sasagawa, T., T. Nagamatsu, K. Morita, N. Mimura, T. Iriyama, T. Fujii, and M. Shibuya. 2018. “HIF-2α, but Not HIF-1α, Mediates Hypoxia-Induced up-Regulation of Flt-1 Gene Expression in Placental Trophoblasts.” Scientific Reports 8 (1): 17375. doi:10.1038/s41598-018-35745-1.
- Schiffer, V. M. M. M., C. W. J. Borghans, N. Arts, J. A. P. Bons, C. A. H. Severens-Rijvers, S. M. J. van Kuijk, M. E. A. Spaanderman, and S. Al-Nasiry. 2021. “The Association between First Trimester Placental Biomarkers and Placental Lesions of Maternal Vascular Malperfusion.” Placenta 103: 206–213. doi:10.1016/j.placenta.2020.10.035.
- Stepan, H., I. Herraiz, D. Schlembach, S. Verlohren, S. Brennecke, F. Chantraine, E. Klein, et al. 2015. “Implementation of the sFlt-1/PlGF Ratio for Prediction and Diagnosis of Pre-Eclampsia in Singleton Pregnancy: Implications for Clinical Practice.” Ultrasound in Obstetrics & Gynecology 45 (3): 241–246. doi:10.1002/uog.14799.
- Stepan, H., M. Hund, and T. Andraczek. 2020. “Combining Biomarkers to Predict Pregnancy Complications and Redefine Preeclampsia the Angiogenic-Placental Syndrome.” Hypertension 75 (4): 918–926. doi:10.1161/HYPERTENSIONAHA.119.13763.
- Sugimoto, J., M. Sugimoto, H. Bernstein, Y. Jinno, and D. Schust. 2013. “A Novel Human Endogenous Retroviral Protein Inhibits Cell-Cell Fusion.” Scientific Reports 3: 1462. doi:10.1038/srep01462.
- Talarska, P., M. Boruczkowski, and J. Żurawski. 2021. “Current Knowledge of Silver and Gold Nanoparticles in Laboratory Research—Application, Toxicity, Cellular Uptake.” Nanomaterials 11 (9): 2454. doi:10.3390/nano11092454.
- Vargas, A., J. Moreau, S. Landry, F. LeBellego, C. Toufaily, É. Rassart, J. Lafond, and B. Barbeau. 2009. “Syncytin-2 Plays an Important Role in the Fusion of Human Trophoblast Cells.” Journal of Molecular Biology 392 (2): 301–318. doi:10.1016/j.jmb.2009.07.025.
- Vargas, A., C. Toufaily, F. Lebellego, É. Rassart, J. Lafond, and B. Barbeau. 2011. “Reduced Expression of Both Syncytin 1 and Syncytin 2 Correlates with Severity of Preeclampsia.” Reproductive Sciences 18 (11): 1085–1091. doi:10.1177/1933719111404608.
- Wan Masliza, W. D., M. Y. Bajuri, M. R. Hassan, N. M. Naim, A. Shuhaila, and S. Das. 2017. “Sonographically Abnormal Placenta: An Association with an Increased Risk Poor Pregnancy Outcomes.” La Clinica Terapeutica 168 (5): e283–e289. doi:10.7417/T.2017.2021.
- Wang, H., Z. Zhou, R. Wang, X. Yang, X. Y. Lu, Q. Zhang, Y. L. Wang, H. Wang, C. Zhu, and H. Y. Lin. 2014. “The cAMP-Responsive Element Binding Protein (CREB) Transcription Factor Regulates Furin Expression during Human Trophoblast Syncytialization.” Placenta 35 (11): 907–918. doi:10.1016/j.placenta.2014.07.017.
- Yamashita, K., Y. Yoshioka, K. Higashisaka, K. Mimura, Y. Morishita, M. Nozaki, T. Yoshida, et al. 2011. “Silica and Titanium Dioxide Nanoparticles Cause Pregnancy Complications in Mice.” Nature Nanotechnology 6 (5): 321–328. doi:10.1038/nnano.2011.41.
- Yang, Y., Z. Qin, W. Zeng, T. Yang, Y. Cao, C. Mei, and Y. Kuang. 2017. “Toxicity Assessment of Nanoparticles in Various Systems and Organs.” Nanotechnology Reviews 6 (3): 279–289. doi:10.1515/ntrev-2016-0047.
- Yin, I. X., J. Zhang, I. S. Zhao, M. L. Mei, Q. Li, and C. H. Chu. 2020. “The Antibacterial Mechanism of Silver Nanoparticles and Its Application in Dentistry.” International Journal of Nanomedicine 15: 2555–2562. doi:10.2147/IJN.S246764.
- Zhang, X. F., Z. G. Liu, W. Shen, and S. Gurunathan. 2016. “Silver Nanoparticles: Synthesis, Characterization, Properties, Applications, and Therapeutic Approaches.” International Journal of Molecular Sciences 17 (9): 1534. doi:10.3390/ijms17091534.
