Abstract
The attention to rare earth oxide nanoparticles (NPs), including yttrium oxide (Y2O3), has increased in many fields due to their unique structural characteristics and functional properties. The aim of our study was to investigate the mechanisms by which bio-corona formation on Y2O3 NPs affects their environmental fate and toxicity. The Y2O3 NPs induced toxicity to freshwater filter feeder Daphnia magna at particle concentrations of 1 and 10 mg/L, regardless of particle size. Interactions between naturally excreted biomolecules (e.g. protein, lipids, and polysaccharides) derived from D. magna, and the Y2O3 NPs (30–45 nm) resulted in the formation of an eco-corona, which reduced their toxic effects toward D. magna at a particle concentration of 10 mg/L. No effects were observed at lower concentrations or for the other particle sizes investigated. Copper-zinc (Cu-Zn) superoxide dismutase, apolipophorins, and vitellogenin-1 proteins proved to be the most prominent proteins of the adsorbed corona, and possibly a reason for the reduced toxicity of the 30–45 nm Y2O3 NPs toward D. magna.
Introduction
For the latest several decades, rare earth elements (REEs) and their oxides have received attention from the research community and industry because of their unique properties. The total production of REEs was in 2021 277,100 tonnes (Government of Canada. Citationn.d.). For instance, doping of metals with REEs generally improves their physical and chemical characteristics, such as oxidation and corrosion resistance (Smirnov et al. Citation2016). The use of REE nanoparticles (NPs) in different applications has increased rapidly, especially in bioelectronics due to excellent optical, semiconductor, and magnetic properties (Liu, Hou, and Gao Citation2014). Yttrium (Y) is one of the REEs element with a global production between ∼10 000 and 14 000 tonnes in 2019 (Zinc Citation2020). Yttrium oxide (yttria, Y2O3) is used in a wide range of technical applications within electronics, optics, mechanical and metallurgical engineering, as well as in catalysis (Bondar Citation2000).
The toxicity of NPs is governed by their physicochemical properties, such as size, shape, and surface chemistry (Sukhanova et al. Citation2018), as well as by the chemical environment which determines their surface and solution speciation (Hedberg, Blomberg, and Odnevall Wallinder Citation2019). In vitro studies of Y2O3 NPs have shown increased cytotoxicity and genotoxicity of spherical-shaped NPs toward human embryonic kidney cells (Selvaraj et al. Citation2014), but no cytotoxicity to human foreskin fibroblast cells (Andelman et al. Citation2009). However, rod-like Y2O3 NPs have been shown to increase cell proliferation, and platelet-shaped Y2O3 NPs have been reported to be considerably cytotoxic (Andelman et al. Citation2009). In vivo studies have further indicated Y to be the most toxic element of the REEs (15 in total) to rainbow trout (Oncorhynchus mykiss) juveniles with an estimated 96 h LC50 of 0.7 mg/L compared to effects induced by, e.g. samarium (Sm), gadolinium (Gd), and erbium (Er) (Dubé et al. Citation2019). Accumulation of Y in soft tissue of adult zebra mussels (Dreissena polymorpha) has been reported after chronic (28 d) exposure at particle concentrations of 50 µg YCl3/L and 250 µg YCl3/L. Yttrium has also been shown able to slightly downregulate catalase and cytochrome-c-oxidase-1 in mussels after exposure to particle concentrations of 250 and 10 µg/L, respectively. Glutathione-S-transferase expression has on the other hand been shown to be upregulated upon exposure to yttrium (as YCl3, 50 µg/L) (Hanana et al. Citation2018).
Toxic effects can be caused by the metallic NPs themselves (Cronholm et al. Citation2013; Santo et al. Citation2014), by dissolved metal ions/metal complexes released from the NPs (the released fraction), or by their combination (Karlsson et al. Citation2013; Gliga et al. Citation2014; Latvala et al. Citation2016; McCarrick et al. Citation2021). The assessment of the dissolved fraction is hence an important step to enable an improved understanding of possible adverse effects induced by metallic NPs (Hedberg, Blomberg, and Odnevall Wallinder Citation2019). The metal release process for metal NPs is both electrochemically (Hedberg, Blomberg, and Odnevall Wallinder Citation2019) and chemically induced (proton-, ligand-induced), the latter in the case of metal oxides. Since the toxic response of metallic NPs is both material-, surface-, environment- and organism- and exposure route specific (Khort et al. Citation2022), no clear general mechanism have been, or can be established which could explain the underlying reason why certain metallic NPs induce toxicity.
Nanomaterials (and most surfaces), in contact with a fluid, for example, blood, cellular cytoplasm, or gastrointestinal fluid, can interact with different ligands such as biomolecules of different kind (e.g. protein, lipids, and polysaccharides), which can adsorb to their surfaces forming a biocorona (Cedervall et al. Citation2007; Nel et al. Citation2009; Monopoli et al. Citation2012). The composition of the corona is highly material-, surface, ligand-, and time dependent (Mei et al. Citation2019). The presence and composition of such a corona can impact not only the bioavailability of the NPs, but also their toxic potency (Tenzer et al. Citation2013). Adsorption of natural organic matter (NOM) and other biomolecules has recently been shown to reduce the toxic potency of some metallic NPs (Pradhan et al. Citation2018; Ekvall et al. Citation2021; Khort et al. Citation2022). Therefore, the presence of the biocorona can disguise properties of NPs and reduce the toxic potency of NPs (Tenzer et al. Citation2013). This has previously been shown in both in vivo and in vitro studies, for example for the crustacean D. magna after exposure to NPs of tungsten carbide cobalt (WC–Co) and Co (Ekvall et al. Citation2021), and for human hepatocarcinoma after exposure to ZnO NPs covered by biocorona (Yin et al. Citation2015).
The diversity of particle shapes, structures, and dissolution characteristics of Y2O3 NPs makes any evaluation of toxicity challenging. At present are (eco)toxic effects induced by Y2O3 NPs and their mechanisms not fully understood. The risk of unintended exposures of diffusely dispersed REEs-based compounds such as Y2O3 NPs and effects on aquatic organisms and humans need further exploration. In this study, we focus on assessing the environmental fate of Y2O3 NPs if dispersed into an aquatic ecosystem, using the freshwater zooplankton D. magna as a model organism. D. magna is a filter feeder and plays an important role in several food chains as a food source for various aquatic organisms (Ebert Citation2005). Taking into account the importance of the physicochemical properties of NPs on their toxic potency (Sukhanova et al. Citation2018), and that proteins, and other biomolecules and ligands generally adsorb to the surface of NPs and thereby reduce their toxic potency (Tenzer et al. Citation2013), the following questions were addressed:
Does the concentration, size, and/or shape of Y2O3 NPs affect their toxic potency toward D. magna?
Does filtration by D. magna of Y2O3 NPs and proteins released from D. magna influence the dissolution and uptake of Y?
What are the most prominent proteins present in the adsorbed biocorona on the Y2O3 NPs after filtration by D. magna?
Can the pre-filtration of Y2O3 NPs affect their toxic response to D. magna?
Materials and methods
Nanoparticle preparation and characterization
Dry powders of Y2O3 NPs with a purity of 99.99% (primary sizes of 10, 20–40, and 30–45 nm) were purchased from US nano (Houston, US). Before each experiment, dry powder NPs were dispersed in ultrapure water (18.2 MΩ cm) to a concentration of 1 g/L and tip-sonicated (Branson Sonifier 250, constant mode, output 2) for 5 min. This setting represents a delivered acoustic energy of 2400 J, based on the Nanogenotox dispersion protocol for NANoREG (Jensen Citation2014; Pradhan et al. Citation2016). The extent of particle agglomeration, particle size and morphology of the Y2O3 NPs were characterized using dynamic light scattering (DLS, Wyatt Technology Corp, Goleta, CA), differential centrifugal sedimentation (DCS, DC24000 UHR Disk Centrifuge, CPS Instruments Europe, Oosterhout, Netherlands), scanning electron microscopy (SEM, SEM JEOL-7800 F equipped with a Bruker X-flash silicon drift detector) at 5 kV, and transmission electron microscopy (TEM, JEOL JEM-1400 PLUS microscope, JEOL Ltd., Japan) T 100 kV. Samples for TEM were prepared by placing 2 μL of the dispersion using a pipette onto a pioloform-coated single slot grid (Ted Pella, Cu, Pelco Slot Grids). The grids were set aside to let the water evaporate before analysis. The primary particle size distribution of the Y2O3 NPs, based on the TEM images, was analyzed by using the ImageJ® software (LOCI, University of Wisconsin). Size distribution analysis was only performed when individual well-defined NPs could be determined. SEM analyses were conducted to determine the surface morphology of the Y2O3 NPs and to examine the extent of NP agglomeration upon tip sonication.
Test organism
D. magna individuals originating from the same population were used in all experiments. The culture originates from Lake Bysjön, southern Sweden (55°40′31.3″N 13°32′41.9″E) and has been kept in a laboratory environment for several hundred generations. All cultures and experiments were maintained at 18 °C under a 16:8 h light/dark photoperiod.
Toxicity test 1 – particle size and concentration effects of Y2O3 NPs to D. magna
To mimic as natural conditions as possible, 2–3 d old Daphnia were selected according to the OECD guideline. Daphnia individuals were distributed into open 50 mL Falcon tubes, freshly prepared with 25 mL of 10, 1, 0.1, 0.01, and 0.0011 mg/L Y2O3 NPs of different sizes (10, 20–40, and 30–45 nm) in tap water (n = 10 for each treatment). A control group without the addition of Y2O3 NPs was prepared in the same manner (). The survival of the D. magna was assessed twice a day (at ∼8 AM and ∼4 PM). The observation period was prolonged until all individuals were immobilized (>48 h), as the standard test duration (48 h) may underestimate the toxicity of many substances, especially of NPs (Baumann et al. Citation2014; Eltemsah and Bøhn Citation2019). D. magna individuals were not fed throughout the whole exposure period according to OECD guideline. The test medium was gently resuspended once per day using a disposable Pasteur pipette due to the possible sedimentation of Y2O3 NPs.
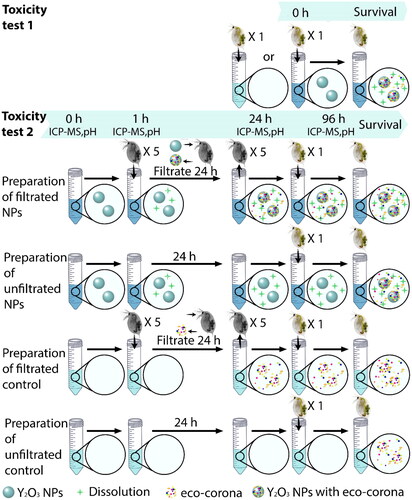
Figure 1. A Schematic representation of the different experimental setups. In total were 10 and 20 replicates used for each treatment during the toxicity test 1 and 2, respectively. There were five D. magna individuals (‘grey’ D. magna) in each tube to filtrate the water (with or without NPs), whereas there was only one D. magna individual (‘coloured’ D. magna) per tube for the toxicity tests.
NP dissolution measurements
Three replicates (in total 5 mL per replicate) for each exposure condition of the acute toxicity test of pre-filtrated NPs (Toxicity test 2), were additionally investigated for the NP dissolution measurements. The replicates and the blank sample were incubated at 18 °C. Total Y concentration in 5 mL of the stock solution was determined at the beginning of the incubation (at 0 h). From an atomic weight of Y of 89 g/mole, follows a total theoretical amount of Y in Y2O3 (atomic weight is 226 g/mole) of 78.8%. The nominal concentrations of Y in 1 and 10 mg/L of Y2O3 were hence 0.79 and 7.9 mg/L. The extent of NP dissolution was determined after 1 h after incubation with and without Daphnia. Replicate samples (for treatment and control groups) were acquired after 1, 24, and 96 h of incubation and centrifuged at the highest speed (31 510 g) at 20 °C for 10 min to remove non-dissolved Y2O3 NPs from the suspensions. To facilitate calculations, measurements of dissolved Y concentration were performed on the premise that all dissolved Y was completely converted to the most common charge Y3+, without considering any other Y species (Y2+). It is possible that Y2O3 particles smaller than 1 nm were present in the supernatant, although their concentration was likely negligible. DLS analysis of the supernatant revealed the presence of a small number of particles with a radius below 0.5 nm, or no particles were detected at all. The supernatant, as well as the pellet, were pipetted to acid-cleaned plastic tubes, and acidified using 2% HNO3 in ultrapure water (to a pH of ≈2.5). Complete particle dissolution was obtained within less than 3 d and verified using NP tracking analysis (data not shown). Total concentrations of released Y in solution were analyzed by means of inductively coupled plasma mass spectrometry (ICP-MS, Perkin Elmer 350D, Waltham, MA) in normal mode. The LOD and LOQ were 0.007 and 0.014 µg/L, respectively. Three replicates were investigated for each sample. Presented results reflect the mean concentration ± standard deviation.
Protein identification after Y2O3 NPs filtration by D. magna
D. magna were left to filtrate the Y2O3 NPs in a similar way as previously applied for polystyrene NPs (Kelpsiene et al. Citation2022). Briefly, D. magna adults were, before the incubation with the NPs, kept in fresh tap water for 24 h to clear their guts from any remaining algal cells. During the exposure, D. magna (adults, without any eggs in the brood chamber, n = 5 individuals per tube) were placed into 15 mL tubes (in total three replicates for each group) containing a total volume of 5 mL tap water with Y2O3 NPs (or without for a control group) of different size (10, 20–40, and 30–45 nm) at a particle concentration of 100 mg/L. The individuals were allowed to filter the tap water containing the NPs, or only water, for 24 h. The solutions with NPs were gently resuspended with plastic Pasteur pipettes after approximately 8 h after incubation initiation. All D. magna individuals were removed from the tubes after completed incubation. No mortality was observed during the filtration time. The particle size distribution, extent of agglomeration and morphology of the Y2O3 NPs were characterized using DCS and TEM before and after filtration by D. magna. NP–protein complexes were recovered by means of centrifugation in Eppendorf tubes at 31 510 g and 4 °C for 30 min with 1 mL total volume. After each centrifugation, 700 µL of the supernatant was gently removed in order not to disturb the pellet. More exposure media, 700 µL was added to the same tube and re-centrifuged. This procedure was repeated until the entire 5 mL of the sample had been centrifuged in the same tube. From each experimental group (treatments and control), samples were prepared by adding 20 µL of SDS-PAGE loading buffer to the particle–protein pellets, or control tubes. An aliquot of 10 µL was loaded on a 4–20% premade sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE, Bio-Rad, Hercules, CA) gel. Protein bands were visualized, using Pierce™ Silver Stain Kit (Thermo Scientific, Waltham, MA) according to the manufacturer’s protocol, and cut into 1 × 1 mm gel pieces, destained, and further analyzed.
The gel pieces were washed twice with 500 µL 50% acetonitrile (ACN, Sigma-Aldrich, St. Louis, MO)/50 mM ammonium bicarbonate (ABC) and incubated for 30 min each time. After washing, the gel pieces were dehydrated using 100% ACN before the proteins were reduced with 25 µL 10 mM dithiothreitol (DTT) in 50 mM ABC for 30 min at 37 °C. The DTT was removed, and the gel pieces were dehydrated using 100% ACN before the proteins were alkylated with 25 µL 55 mM iodacetamide in 50 mM ABC for 30 min at dark conditions and room temperature. The gel pieces were dehydrated one last time with 100% ACN before the proteins were digested by adding 25 µL 12 ng/µL trypsin (sequence grade modified trypsin porcine, Promega, Madison, WI) in 50 mM ABC and incubated on ice for 4 h. Thereafter, 20 µL 50 mM ABC were added and the proteins were incubated overnight at 37 °C. The following day, 10% formic acid (FA) was added to a final concentration of 0.5%, to obtain a pH of 2–3 to stop the digestion, before the peptide solutions were extracted and transferred into new tubes for analysis.
Peptide separation and mass spectrometry
Peptides were subjected to a reversed phase nano-liquid chromatography (LC) source (Proxeon Biosystems, Odense, Denmark) coupled to a linear trap quadrupole (LTQ)-Orbitrap Velos Pro mass spectrometer (Thermo Fisher Scientific) equipped with a nano Easy spray ion source (Thermo Fisher Scientific). The chromatographic separation was performed using a 2 cm C18 Acclaim PepMap precolumn (75 mm i.d.) and a 15 cm C18 EASY-Spray LC Capillary Separation Column (75 mm i.d., packed with 3 µm resin, column temperature 45 °C) from Thermo Fisher. The gradient was created by solvent A (1% ACN, 0.1% FA in water) and solvent B (100% ACN, 0.1% FA). A flow rate of 300 nL/min was used throughout the whole gradient (0–30% B for 40 min, 30–50% 20 min, 50–95% for 10 min, and 95% for 10 min). One full MS scan (resolution 60 000 at 400 m/z; mass range 400–1400 m/z) was followed by MS/MS scans of the 4 most abundant ion signals. Charge state screening was enabled where singly charged and unassigned ions were rejected. The precursor ions were isolated with 3 m/z isolation width and fragmented using collision induced dissociation (CID) with the normalized collision energy set to 35%. The dynamic exclusion window was limited to 500 entries and set to 30 s. The intensity threshold for precursor ions was set to 2500. The automatic gain control was set to 10^6 for both MS and MS/MS with ion accumulation times of 100 ms.
Data analysis and protein identification
Raw files were converted to mgf-format by Mascot Distiller version 2.6 (Matrix Science) and identification of proteins was carried out using the Mascot Daemon software version 2.4 (Matrix Science). The following search settings were used: trypsin as protease, one allowed missed cleavage site, 5 ppm MS accuracy for peptides and 0.015 Da MS/MS accuracy, variable modifications: Oxidation (M). The files were searched against an in-house created database containing all Daphnia protein sequences (588 779 entries from NCBI). To be considered a true protein identification, all individual ion scores had to have a higher score than the score given when using a significant threshold of p < 0.005. The isoelectric point was calculated using the freeware https://web.expasy.org/compute_pi.
Toxicity test 2 – effects of the adsorption of proteins and other biomolecules to the Y2O3 NPs
The toxicity of Y2O3 NPs on D. magna was assessed in two exposure scenarios, as illustrated in . In the pre-filtration group, five D. magna (∼2 mm in size) were randomly allocated to 50 mL open Falcon tubes with a total volume of 25 mL. Each treatment, including the control group, comprised five D. magna which were allowed to filter tap water for 24 h in the presence and absence of Y2O3 NPs with primary particle sizes of 10, 20–40, and 30–45 nm, at concentrations of 1 and 10 mg/L. There were 20 replicates for each treatment. On the same day, in the non-filtration group, parallel experiments were initiated where tap water with or without Y2O3 NPs (control group) was left to stand for 24 h. After 24 h, in the pre-filtration group, all D. magna individuals were removed. New D. magna (one individual per tube; 2–3 d old) was added into both the pre-filtration and non-filtration groups. D. magna individuals were not fed during the exposure period according to OECD guideline. Due to the total number of samples, testing of the different particle sizes of the Y2O3 NPs was investigated on different weeks. Changes in pH were determined after 0, 1, 24, and 96 h after incubation with or without Y2O3 NPs. Four replicates were investigated for each sample. The average pH values remained stable for all treatments (Supplementary Figure 1).
Statistical analysis
Kaplan–Meier survival curves analysis was performed using the statistical computing software GraphPad Prism version 8.0.0 (224) for Windows, GraphPad Software, Inc., La Jolla, CA, www.graphpad.com. Analysis of the survival was performed using the (Log-rank) Mantel-Cox test as well as the Gehan-Breslow-Wilcox test.
Results and discussion
Nanoparticle characterization
The TEM images indicated a mean particle size of the 10 nm Y2O3 NPs of ∼13 nm (size distribution: 10–18 nm), ∼15.3 nm in width (10–18 nm), of ∼43.8 nm in length (size distribution: 30–60 nm) for the 20–40 nm Y2O3 NPs, and of ∼43.7 and ∼11.5 nm, when large and small particles were measured, respectively (size distributions: 20–80 and 6–16 nm, respectively) for the 30–45 nm Y2O3 NPs. All Y2O3 NPs revealed different shapes including spheres, rods, and sheet structures (Supplementary Figure 2).
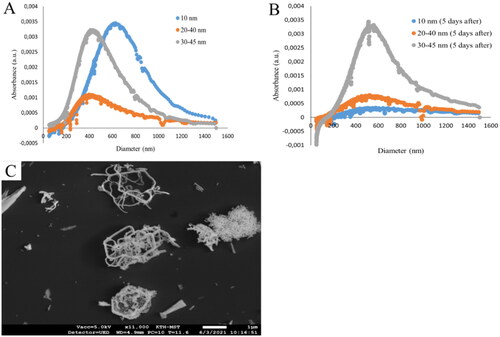
The Y2O3 NPs in tap water exhibited agglomerates with a broad range of sizes and shapes, as shown by their size distribution by using DCS of approximately 400–800, 300–600, and 400–600 nm for 10, 20–40, and 30–45 nm Y2O3 NPs, respectively (). Additionally, the particle size distribution measurements using DCS of the NP suspension after 5 d exposure to D. magna (), showed that the NPs sized 30–45 nm remained relatively stable in suspension, whereas for the 10 and 20–40 nm Y2O3 NPs the absorbance was lower after 5 d of incubation with D. magna, indicating of a loss of particles from the suspension (for example, particle sedimentation and dissolution). The formation of agglomerates was also evident from the SEM images of the 20–40 nm Y2O3 NPs (). The samples were also measured by using DLS (Supplementary Table 1). However, due to the particle aggregation with significant particle size variability, it was difficult to gather accurate data.
Figure 2. Particle size distributions of the 10, 20–40, and 30–45 nm Y2O3 NPs in tap water at a particle concentration of 100 mg/L NPs measured by means of DCS. Measurements were taken before (A) and after (B) 5 d filtration of Y2O3 NPs by D. magna. Particle agglomeration after tip sonication is illustrated by the SEM image of the 20–40 nm Y2O3 NPs (C). the scale bar: 1 µm.
Toxicity test 1 – effects of shape, particle size, and concentration of Y2O3 NPs on the mortality of D. magna
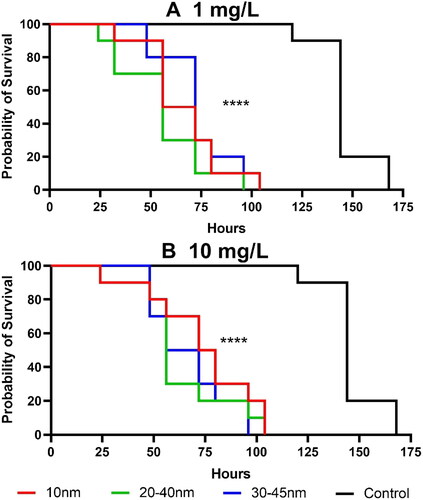
Kaplan–Meier survival analyses revealed that the survival of D. magna decreased with the duration of exposure to Y2O3 NPs, regardless of their size or shape (). Statistical analyses of the toxicity tests of Y2O3 NPs suspensions to control group are shown in Supplementary Table 2. Both particle concentrations (1 and 10 mg/L), decreased the survival time of D. magna within 7 d compared to a control group. Daphnia survived in the control group for 144 h (6 d), whereas individuals survived only for about 56–72 h when exposed to Y2O3 NPs of different sizes. These results clearly showed an overall reduction in survival time by 50–61% upon exposure to the Y2O3 NPs. The findings indicate that the survival duration did not change with increasing particle concentration (from 1 to 10 mg/L), though no significant differences were observed compared to the control group in terms of survival time when D. magna were exposed to lower Y2O3 NP concentrations (0.001, 0.01, and 0.1 mg/L) (Supplementary Figure 3). Daphnia survived at lower Y2O3 NP concentrations for 144 h (6 d), with the exception of the 30–45 nm particles at a concentration of 0.1 mg/L, where survival was observed up to 156 h. Statistical analyses are shown in Supplementary Table 2. In our study, we selected Y2O3 NPs particles of different sizes for comparison, including primary sizes of 10, 20–40, and 30–45 nm. Despite the relatively small size differences, we observed from TEM images that the sizes and shapes of these particles were not identical, suggesting that the reported size of the Y NPs in our study is not the true primary size. Although size is a well-known critical factor in nanotoxicology, other factors such as shape, dissolution, and surface area can also play a crucial role in determining toxicity. However, accurately quantifying these factors is not always straightforward. For instance, the irregular shape of the particles observed by TEM makes it difficult to accurately calculate surface area, which can impact the biological response of the particles. Effects of particle dissolution are presented below, can also make a difference. While our study did not reveal significant differences in toxicity among particles of different sizes, the influence of particle shape and surface area on the biological response cannot be ignored. These factors may have contributed to the observed toxic effects, and future studies should consider these factors when evaluating the toxicity of NPs. Furthermore, even though we did not observe any differences between various concentrations used in this study ( and Supplementary Figure 3), previously it has been observed concentration-dependent toxicity in the growth of green algae Tetraselmis suesica upon exposure to 30–50 nm Y2O3 NPs at particle concentrations ranging from 1 to 100 mg/L (Castro-Bugallo et al. Citation2014).
Figure 3. Survival of D. magna after exposure to Y2O3 NPs in particle concentrations of 1 mg/L (A) and 10 mg/L (B). the asterisks indicate significant differences between the test groups and control group ****p < 0.0001. Each treatment was replicated 10 times.
Our study revealed that Y2O3 NPs at a concentration of 10 mg/L were attached to the antennae or carapaces of D. magna. Photographic evidence of these attachments is presented in Supplementary Figure 4. Sedimentation of the NPs was visible when the concentration was high, such as at 10 or 100 mg/L. However, at lower concentrations, such as 1 mg/L or below, sedimentation was not visible.
Particle dissolution
Metallic NPs are inevitably dissolved to some extent following environmental exposure. For toxicity test 2, measurements of the released amount of Y from the Y2O3 NPs were conducted both before and after filtration to D. magna, after 0 h (dose samples), 1, 24, and 96 h (Supplementary Table 3). Fractions of dissolved Y in solution compared to the total Y content (dose samples, i.e. the total amount of Y in the added Y2O3 NPs at 0 h (i.e. 40–70% of the nominal Y content), see Supplementary Table 3) are presented in for the nominal particle concentrations of 1 and 10 mg/L.
Figure 4. Fractions of dissolved Y compared to the total amount of Y in Y2O3 added to the system (dose samples after 0 h) after for the different nominal particle concentrations; (a) 1 mg/L, and (b) 10 mg/L after 1, 24, and 96 h of the exposure with and without D. magna filtration (toxicity test 2).
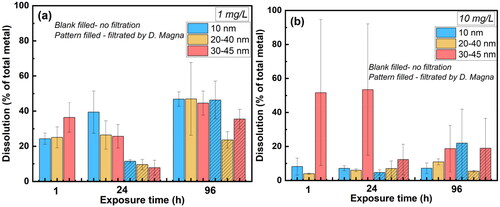
The results () showed a slightly higher dissolution of Y from the largest sized Y2O3 NPs (30–45 nm) after 1 h of exposure in tap water at the NPs concentration of 1 mg/L compared with the other two Y2O3 particle sizes. This could be an effect of a larger effective surface area of the agglomerated NPs. The dissolution of Y increased for the non-D. magna-filtrated NPs sized 10 nm, decreased for the NPs sized 30–45 nm after 24 h, whereas all NPs showed increased dissolution (∼47% of the added Y content) after 96 h. D. magna filtration after 24 h resulted in substantially reduced dissolution of Y for all NP sizes compared with non-filtrated conditions. Although the underlying reasons are not known, the factors which influence the dissolution of the Y2O3 NPs such as surface area, the extent of agglomeration, as well as ligand adsorption of NPs also influence the Daphnia. The presence of D. magna can also in turn influence the extent of NP dissolution by altering the pH or oxygen levels in the surrounding environment. The results indicate a higher extent of Y uptake. The dissolution of Y from all Y2O3 NPs increased with exposure time (up to 96 h) after the filtration by D. magna, whereas a less significant effect was observed compared to the non-filtrated condition after 24 h. This implies that the uptake of Y2O3 NPs by D. magna mainly occurred at the beginning of the filtration.
The dissolved fraction of Y was smaller for the Y2O3 NPs concentration of 10 mg/L () compared with 1 mg/L for all NP sizes, despite larger standard deviations among replicates at the higher particle concentration. However, observed differences in Y dissolution after filtration by D. magna (24 h) were less pronounced between the two particle concentrations (1 and 10 mg/L), which suggest a higher uptake of dissolved Y by D. magna at the higher NP concentration (10 mg/L) compared to the total amount of dissolved Y. The undissolved amount of Y, including both non-dissolved particles and settled Y-complexes (released Y ions forming large complexes in solution) were also filtrated by D. magna, see . The results showed an increased uptake in D. magna with particle size and larger amount (15–50%) of total Y (both dissolved and non-dissolved Y).
Figure 5. Dissolved and non-dissolved fractions of Y taken up by D. magna compared to the total amount of Y in Y2O3 added to the system (dose samples after 0 h) at particle concentrations of 1 mg/L and 10 mg/L NPs. The results are based on findings with and without D. magna filtration. Blue column: 10 nm, yellow column: 20–40 nm, pink column: 30–45 nm.
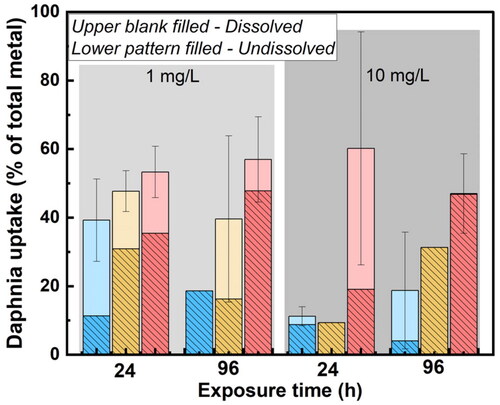
Dissolved fractions of 30% Zn from ZnO NPs in simplified M7 medium at a particle concentration of 0.5 mg/L, and 20% at a particle loading of 2 mg/L after 24 h of exposure have previously been reported (Li and Wang Citation2013). These findings show that lower particle concentrations can result in more metal dissolution compared to higher particle concentrations. This can be explained by combined effects of the characteristics of the NPs and the chemistry of the exposure medium, such as ionic strength and pH (Li and Wang Citation2013). These aspects influence e.g. the extent of particle agglomeration (effective surface area) (Hedberg, Blomberg, and Odnevall Wallinder Citation2019), and particle size (Molleman and Hiemstra Citation2017).
Proteins in the biocorona of the exposed Y2O3 NPs
D. magna releases proteins into their environment (Nasser and Lynch Citation2019, Nasser, Constantinou, and Lynch Citation2020), which consequently can be adsorbed onto surfaces of NPs (Nasser and Lynch Citation2016; Kelpsiene et al. Citation2022). Proteins in the biocorona of the Y2O3 NPs after being filtrated by D. magna for 24 h were identified using MS. Differences in particle shape and size of the Y2O3 NPs were assessed by means of DCS and TEM both before and after filtration. The DCS measurements showed relatively stable particle sizes (Supplementary Table 4), whereas the TEM images revealed clear differences in shapes of Y2O3 NPs before and after (24 h) incubation with D. magna (Supplementary Figure 5). After the D. magna incubation, the protein–NPs complexes were collected by means of centrifugation and analyzed using the SDS-PAGE loading buffer, separated by a 4–20% polyacrylamide gel and visualized by silver staining (see experimental). The results are presented in . All visible bands (Supplementary Figure 6) were cut out and analyzed by MS/MS mass spectrometry.
Figure 6. Silver-stained SDS Gels after incubation of Y2O3 NPs of varying size distribution (10 nm (4–6), 20–40 nm (7–9), and 30–45 nm (10–12)) with D. magna for 24 h compared with a control group (1–3). all measurements were made in triplicates.
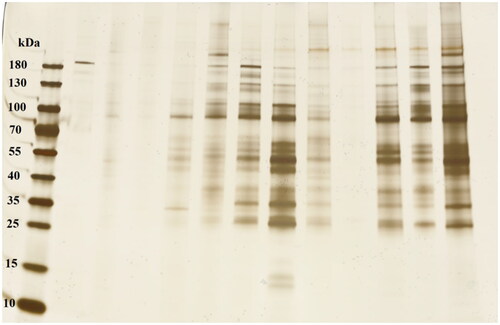
Judging from the silver-stained gel (Supplementary Figure 6), more proteins appeared to be present in the biocorona formed around 30–45 nm sized Y2O3 NPs compared to the other particle sizes. Our data show no clear relationship between primary particle size and amount of proteins adsorbed to the particle surface. The MS/MS analysis revealed the predominance of two proteins for all Y2O3 NP sizes, i.e. copper-zinc (Cu-Zn) superoxide dismutase (an antioxidant enzyme, representing the first enzymatic defense system against radical damage by oxygen (Mondola et al. Citation2016) and apolipophorins (lipoproteins, regulating lipoprotein metabolism and determine the distinctive roles for lipoproteins in lipid metabolism (Mahley et al. Citation1984) (Supplementary Table 5). These two proteins were present in all cut out gel pieces which can be due to a combination of factors as high amount of proteins, truncation of the proteins, migration of the proteins through the SDS-PAGE gel lanes and good ionization of those specific peptides in the mass spectrometer. Thus, to consider a protein to be a true protein that adsorbs on NP surfaces the two following criteria had to be fulfilled: (1) only proteins with theoretical molecular weights which agreed with the molecular weight determined from the SDS-PAGE protein ladder, and (2) only proteins with at least 2 unique peptides identified belonging to the specific protein. Based on these criteria, also vitellogenin-1 and di-domain hemoglobin proteins (Supplementary Table 5) were identified in the biocorona of all Y2O3 NPs, regardless the size.
Vitellogenin-1 has previously been identified as one of the general proteins that bind to NPs after incubation with D. magna. Examples of such NPs include Au NPs (Mattsson et al. Citation2018), differently surface-charged polystyrene NPs (Kelpsiene et al. Citation2022), and Ag NPs (Gao et al. Citation2017). Vitellogenin-1 plays an important role in oogenesis and is highly expressed in females (Hara, Hiramatsu, and Fujita Citation2016, Gao et al. Citation2017). Hemoglobin is a polyfunctional molecule that is mainly involved in oxygen binding and transport (Ahmed, Ghatge, and Safo Citation2020). Serine protease has also been identified to extensively interact with 53 nm sized PS-NH2 NPs and to some extent with 200 nm sized PS-NH2 NPs (Kelpsiene et al. Citation2022). Our previous findings show that actin, alpha skeletal muscle commonly binds to both negatively and positively charged polystyrene NPs after incubation with D. magna (Kelpsiene et al. Citation2022). Actin protein plays an important role in the structure and motio of cells. Changes of its expression can lead to toxicity (Gunning et al. Citation2015). The presence of heat shock 70 kDa protein cognate is in line with previous findings where the protein was reported to be secreted by D. magna in response to metallic NPs (Ellis and Lynch Citation2020) and to 53 nm sized PS-NH2 NPs (Kelpsiene et al. Citation2022). Results of this study show lamin-A to only be detected in the 20–40 nm sized Y2O3 NPs sample, the same protein also shown to interact only with the 200 nm sized PS-NH2 NPs (Kelpsiene et al. Citation2022).
Toxicity test 2 – effects of the adsorption of proteins and other biomolecules to the Y2O3 NPs
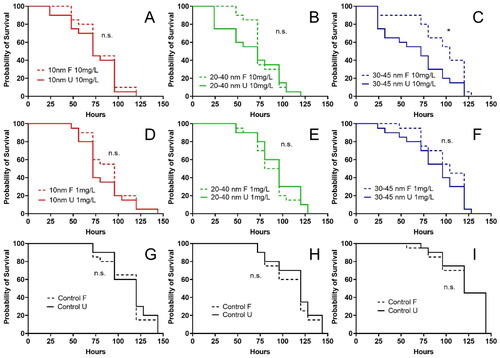
The importance of adsorbed biomolecules on the NPs forming a biocorona on their toxic potency was assessed by comparing effects of pre-filtrated Y2O3 NPs to D. magna with direct non-filtrated Y2O3 NPs exposure (). Results showed a significantly reduced survival duration of D. magna after exposure to pre-filtrated 35–45 nm Y2O3 NPs compared to non-filtrated NPs at concentration of 10 mg/L (*p < 0.05, ). However, this effect of pre-filtration of Y2O3 NPs on the toxic potency was not observed for neither the 10 nm nor the 20–40 nm Y2O3 NPs (, Supplementary Table 6). Similar to our results Ekvall et al. (Citation2021) showed the toxic effects of NPs of WCCo and Co of different concentrations (0.05–10 mg/L) on D. magna to be reduced in the presence and adsorption of natural biological degradation products from D. magna (eco-corona biomolecules). In contrast, the formation of a biocorona consisting of adsorbed proteins secreted by D. magna on carboxylated and aminated polystyrene NPs have also been shown to result in lower EC50 values compared to uncoated NPs (Nasser and Lynch Citation2016). Similarly, to naturally derived biomolecules, NOM can also adsorb on the surfaces of NPs and influence the behavior, risk, and fate of NPs. Adsorption of NOM, which is a heterogeneous mixture of naturally occurring organic compounds, onto Cu and Zn NPs has been shown to reduce the toxic potency toward aquatic organisms, such as Ceriodaphnia cf dubia and D. pulex (Hyne et al. Citation2005; Clifford and McGeer Citation2009). Khort and coauthors (Khort et al. Citation2022) have also recently shown reduced cytotoxicity for O. mykiss gills Waterloo 1 (RTgill-W1) cells of tin (Sn) NPs in the presence of NOM.
Figure 7. Survival of D. magna after exposure to pre-filtrated (F) and un-filtrated (U) Y2O3 NPs of different sizes (10, 20–40, and 30–45 nm) at two particle concentrations (1 and 10 mg/L). Each treatment was replicated 20 times. The asterisk indicates a significant difference between pre-filtrated and un-filtrated in 30–45 nm 10 mg/L groups, *p < 0.05.
Conclusions
In this study, we aimed to answer several questions. First, we wanted to identify if the concentration, size, and/or shape of Y2O3 NPs affect their toxic potency toward D. magna. Generated results show a reduced longevity in D. magna upon filtration of Y2O3 NPs, independent the particle concentration at 1 and 10 mg/L, whereas lower concentrations (0.001, 0.01, and 0.1 mg/L) did not induce any significant toxicity in comparison with a control group. Furthermore, we observed that the toxicity was independent on particle size and shape. Secondly, we aimed to answer if the filtration by D. magna of Y2O3 NPs influence the dissolution and uptake of Y. D. magna filtration of Y2O3 NPs for 24 h resulted in substantially reduced dissolution of Y for all NP sizes compared with non- filtrated conditions, and a higher extent of NP uptake (15–50%), both as dissolved and non-dissolved Y2O3. The D. magna uptake of the non-dissolved fraction increased with particle size. Furthermore, we investigated which are the most prominent proteins present in the adsorbed biocorona on the surfaces of Y2O3 NPs after being filtrated by D. magna. We show that Cu-Zn superoxide dismutase, apolipophorins, and vitellogenin-1 mainly bound to the surfaces in a biocorona of the Y2O3 NPs of different primary sizes. However, there were no clear differences in protein interactions between the different particle sizes of the Y2O3 NPs could be identified after their pre-filtration with D. magna. Yet, a higher number of proteins in a biocorona were observed for 30–45 nm Y2O3 NPs. Finally, we investigated if the pre-filtration of Y2O3 NPs affects their toxic response to D. magna. We show that a short (24 h) pre-filtration by D. magna of the 30–45 nm sized Y2O3 NPs at a concentration of 10 mg/L resulted in significantly reduced toxic effects to D. magna, whereas prefiltration of the other particle sizes (10 and 20–40 nm) had no effect on the duration of survival.
The obtained results highlight that the same type of particles can, depending on their physicochemical properties such as size, induce different toxic effects on aquatic organisms. It is hence of large importance to provide a mechanistic understanding on interactions between metallic NPs of different characteristics and natural biomolecules in order to assess the environmental fate and possible adverse effects on aquatic organisms induced by diffusely dispersed engineered NPs such as Y2O3.
Author contributions
Egle Kelpsiene: investigation, methodology, writing – original draft, review & editing. Tingru Chang: investigation, writing – review & editing. Alexander Khort: investigation, writing – review & editing. Katja Bernfur: formal analysis, writing – review & editing. Inger Odnevall: conceptualization, funding acquisition, writing – review and editing. Tommy Cedervall: conceptualization, funding acquisition, methodology, supervision, writing – writing – review & editing. Jing Hua: investigation, methodology, writing – review and editing.
Supplemental Material
Download MS Word (4.1 MB)Acknowledgments
The authors also thank Dr. Ola Gustafsson at the Department of Biology, Lund University, for his dedicated help with TEM. Frida Domeij at Department of Occupational and Environmental Medicine, Linköping University Hospital, for the help with ICP-MS analysis.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Correction Statement
This article has been corrected with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- Ahmed, M. H., M. S. Ghatge, and M. K. Safo. 2020. “Hemoglobin: Structure, Function and Allostery.” In Vertebrate and Invertebrate Respiratory Proteins, Lipoproteins and Other Body Fluid Proteins, edited by Hoeger Ulrich and Jeffrey R Harris, 345–382. Berlin, Germany: Springer International Publishing.
- Andelman, T., S. Gordonov, G. Busto, P. V. Moghe, and R. E. Riman. 2009. “Synthesis and Cytotoxicity of Y2O3 Nanoparticles of Various Morphologies.” Nanoscale Research Letters 5 (2): 263–273. doi: 10.1007/s11671-009-9445-0.
- Baumann, J., Y. Sakka, C. Bertrand, J. Köser, and J. Filser. 2014. “Adaptation of the Daphnia sp. acute Toxicity Test: Miniaturization and Prolongation for the Testing of Nanomaterials.” Environmental Science and Pollution Research International 21 (3): 2201–2213. doi: 10.1007/s11356-013-2094-y.
- Bondar, V. 2000. “Structure and Luminescence Properties of Individual and Multi-Layer Thin-Film Systems Based on Oxide Phosphors.” Materials Science and Engineering: B 69-70: 505–509. doi: 10.1016/S0921-5107(99)00310-4.
- Castro-Bugallo, A., Á. González-Fernández, C. Guisande, and A. Barreiro. 2014. “Comparative Responses to Metal Oxide Nanoparticles in Marine Phytoplankton.” Archives of Environmental Contamination and Toxicology 67 (4): 483–493. doi: 10.1007/s00244-014-0044-4
- Cedervall, T., I. Lynch, S. Lindman, T. Berggård, E. Thulin, H. Nilsson, K. A. Dawson, and S. Linse. 2007. “Understanding the Nanoparticle–Protein Corona Using Methods to Quantify Exchange Rates and Affinities of Proteins for Nanoparticles.” Proceedings of the National Academy of Sciences of the United States of America 104 (7): 2050–2055. doi: 10.1073/pnas.0608582104
- Clifford, M., and J. C. McGeer. 2009. “Development of a Biotic Ligand Model for the Acute Toxicity of Zinc to Daphnia pulex in Soft Waters.” Aquatic Toxicology (Amsterdam, Netherlands) 91 (1): 26–32. doi: 10.1016/j.aquatox.2008.09.016
- Cronholm, P., H. L. Karlsson, J. Hedberg, T. A. Lowe, L. Winnberg, K. Elihn, I. O. Wallinder, and L. Möller. 2013. “Intracellular Uptake and Toxicity of Ag and CuO Nanoparticles: A Comparison between Nanoparticles and Their Corresponding Metal Ions.” Small (Weinheim an Der Bergstrasse, Germany) 9 (7): 970–982. doi: 10.1002/smll.201201069
- Dubé, M., J. Auclair, H. Hanana, P. Turcotte, C. Gagnon, and F. Gagné. 2019. “Gene Expression Changes and Toxicity of Selected Rare Earth Elements in Rainbow Trout Juveniles.” Comparative Biochemistry and Physiology Toxicology & Pharmacology 223: 88–95. doi: 10.1016/j.cbpc.2019.05.009
- Ebert, D. 2005. “Introduction to Daphnia Biology.” In Ecology, Epidemiology and Evolution of Parasitism in Daphnia, 5–18. Bethesda, MD: National Center for Biotechnology Information (US).
- Ekvall, M. T., J. Hedberg, I. O. Wallinder, A. Malmendal, L. A. Hansson, and T. Cedervall. 2021. “Adsorption of Bio-Organic Eco-Corona Molecules Reduces the Toxic Response to Metallic Nanoparticles in Daphnia Magna.” Scientific Reports 11 (1): 1–11. doi: 10.1038/s41598-021-90053-5
- Ellis, L. J. A., and I. Lynch. 2020. “Mechanistic Insights into Toxicity Pathways Induced by Nanomaterials in Daphnia Magna from Analysis of the Composition of the Acquired Protein Corona.” Environmental Science: Nano 7 (11): 3343–3359. doi: 10.1039/D0EN00625D
- Eltemsah, Y. S., and T. Bøhn. 2019. “Acute and Chronic Effects of Polystyrene Microplastics on Juvenile and Adult Daphnia Magna.” Environmental Pollution (Barking, Essex: 1987) 254 (Pt A): 112919. doi: 10.1016/j.envpol.2019.07.087
- Gao, J., L. Lin, A. Wei, and M. S. Sepúlveda. 2017. “Protein Corona Analysis of Silver Nanoparticles Exposed to Fish Plasma.” Environmental Science & Technology Letters 4 (5): 174–179. doi: 10.1021/acs.estlett.7b00074
- Gliga, A. R., S. Skoglund, I. Odnevall Wallinder, B. Fadeel, and H. L. Karlsson. 2014. “Size-Dependent Cytotoxicity of Silver Nanoparticles in Human Lung Cells: The Role of Cellular Uptake, Agglomeration and Ag Release.” Particle and Fibre Toxicology 11 (1): 11. doi: 10.1186/1743-8977-11-11
- Government of Canada. n.d. Rare earth elements facts. https://natural-resources.canada.ca/our-natural-resources/minerals-mining/minerals-metals-facts/rare-earth-elements-facts/20522
- Gunning, P. W., U. Ghoshdastider, S. Whitaker, D. Popp, and R. C. Robinson. 2015. “The Evolution of Compositionally and Functionally Distinct Actin Filaments.” Journal of Cell Science 128 (11): 2009–2019. doi: 10.1242/jcs.165563
- Hanana, H., P. Turcotte, M. Dubé, C. Gagnon, and F. Gagné. 2018. “Response of the Freshwater Mussel, Dreissena Polymorpha to Sub-Lethal Concentrations of Samarium and Yttrium after Chronic Exposure.” Ecotoxicology and Environmental Safety 165: 662–670. doi: 10.1016/j.ecoenv.2018.09.047
- Hara, A., N. Hiramatsu, and T. Fujita. 2016. “Vitellogenesis and Choriogenesis in Fishes.” Fisheries Science 82 (2): 187–202. doi: 10.1007/s12562-015-0957-5
- Hedberg, J., E. Blomberg, and I. Odnevall Wallinder. 2019. “In the Search for Nanospecific Effects of Dissolution of Metallic Nanoparticles at Freshwater-Like Conditions: A Critical Review.” Environmental Science & Technology 53 (8): 4030–4044. doi: 10.1021/acs.est.8b05012
- Hyne, R. V., F. Pablo, M. Julli, and S. J. Markich. 2005. “Influence of Water Chemistry on the Acute Toxicity of Copper and Zinc to the Cladoceran Ceriodaphnia cf Dubia.” Environmental Toxicology and Chemistry 24 (7): 1667–1675. doi: 10.1897/04-497r.1
- Jensen, K. 2014. The NANOGENOTOX Dispersion Protocol for NANoREG, 01. Vol. 21. European Union Grant Agreement.
- Karlsson, H. L., P. Cronholm, Y. Hedberg, M. Tornberg, L. De Battice, S. Svedhem, and I. O. Wallinder. 2013. “Cell Membrane Damage and Protein Interaction Induced by Copper Containing Nanoparticles—Importance of the Metal Release Process.” Toxicology 313 (1): 59–69. doi: 10.1016/j.tox.2013.07.012
- Kelpsiene, E., I. Brandts, K. Bernfur, M. T. Ekvall, M. Lundqvist, M. Teles, and T. Cedervall. 2022. “Protein Binding on Acutely Toxic and Non-Toxic Polystyrene Nanoparticles during Filtration by Daphnia Magna.” Environmental Science: Nano 9 (7): 2500–2509. doi: 10.1039/D2EN00125J
- Khort, A., M. Brookman-Amissah, J. Hedberg, T. Chang, N. Mei, A. Lundberg, J. Sturve, E. Blomberg, and I. Odnevall. 2022. “Influence of Natural Organic Matter on the Transformation of Metal and Metal Oxide Nanoparticles and Their Ecotoxic Potency in Vitro.” NanoImpact 25: 100386. doi: 10.1016/j.impact.2022.100386
- Latvala, S., J. Hedberg, S. Di Bucchianico, L. Möller, I. Odnevall Wallinder, K. Elihn, and H. L. Karlsson. 2016. “Nickel Release, ROS Generation and Toxicity of Ni and NiO Micro-and Nanoparticles.” PLoS One 11 (7): e0159684. doi: 10.1371/journal.pone.0159684
- Li, W. M., and W. X. Wang. 2013. “Distinct Biokinetic Behavior of ZnO Nanoparticles in Daphnia Magna Quantified by Synthesizing 65Zn Tracer.” Water Research 47 (2): 895–902. doi: 10.1016/j.watres.2012.11.018
- Liu, C., Y. Hou, and M. Gao. 2014. “Are Rare-Earth Nanoparticles Suitable for in Vivo Applications.” Advanced Materials (Deerfield Beach, FL) 26 (40): 6922–6932. doi: 10.1002/adma.201305535
- Mahley, R. W., T. L. Innerarity, S. C. Rall, and K. H. Weisgraber. 1984. “Plasma Lipoproteins: Apolipoprotein Structure and Function.” Journal of Lipid Research 25 (12): 1277–1294. doi: 10.1016/S0022-2275(20)34443-6
- Mattsson, K., R. Aguilar, O. Torstensson, D. Perry, K. Bernfur, S. Linse, L.-A. Hansson, K. S. Åkerfeldt, and T. Cedervall. 2018. “Disaggregation of Gold Nanoparticles by Daphnia Magna.” Nanotoxicology 12 (8): 885–900. doi: 10.1080/17435390.2018.1485982
- McCarrick, S., V. Romanovski, Z. Wei, E. M. Westin, K.-A. Persson, K. Trydell, R. Wagner, I. Odnevall, Y. S. Hedberg, and H. L. Karlsson. 2021. “Genotoxicity and Inflammatory Potential of Stainless Steel Welding Fume Particles: An in Vitro Study on Standard vs Cr(VI)-Reduced Flux-Cored Wires and the Role of Released Metals.” Archives of Toxicology 95 (9): 2961–2975. doi: 10.1007/s00204-021-03116-x
- Mei, N., J. Hedberg, I. Odnevall Wallinder, and E. Blomberg. 2019. “Influence of Biocorona Formation on the Transformation and Dissolution of Cobalt Nanoparticles under Physiological Conditions.” ACS Omega 4 (26): 21778–21791. doi: 10.1021/acsomega.9b02641
- Molleman, B., and T. Hiemstra. 2017. “Time, pH, and Size Dependency of Silver Nanoparticle Dissolution: The Road to Equilibrium.” Environmental Science: Nano 4 (6): 1314–1327. doi: 10.1039/C6EN00564K
- Mondola, P., S. Damiano, A. Sasso, and M. Santillo. 2016. “The Cu, Zn Superoxide Dismutase: Not Only a Dismutase Enzyme.” Frontiers in Physiology 7: 594. doi: 10.3389/fphys.2016.00594
- Monopoli, M. P., C. Aberg, A. Salvati, and K. A. Dawson. 2012. “Biomolecular Coronas Provide the Biological Identity of Nanosized Materials.” Nature Nanotechnology 7 (12): 779–786. doi: 10.1038/nnano.2012.207
- Nasser, F., and I. Lynch. 2016. “Secreted Protein Eco-Corona Mediates Uptake and Impacts of Polystyrene Nanoparticles on Daphnia Magna.” Journal of Proteomics 137: 45–51. doi: 10.1016/j.jprot.2015.09.005
- Nasser, F., and I. Lynch. 2019. “Updating Traditional Regulatory Tests for Use with Novel Materials: Nanomaterial Toxicity Testing with Daphnia Magna.” Safety Science 118: 497–504. doi: 10.1016/j.ssci.2019.05.045
- Nasser, F., J. Constantinou, and I. Lynch. 2020. “Nanomaterials in the Environment Acquire an “Eco-Corona” Impacting Their Toxicity to Daphnia Magna—a Call for Updating Toxicity Testing Policies.” Proteomics 20 (9): 1800412. doi: 10.1002/pmic.201800412
- Nel, A. E., L. Mädler, D. Velegol, T. Xia, E. M. Hoek, P. Somasundaran, F. Klaessig, V. Castranova, and M. Thompson. 2009. “Understanding Biophysicochemical Interactions at the Nano-Bio Interface.” Nature Materials 8 (7): 543–557. doi: 10.1038/nmat2442
- Pradhan, S., J. Hedberg, E. Blomberg, S. Wold, and I. Odnevall Wallinder. 2016. “Effect of Sonication on Particle Dispersion, Administered Dose and Metal Release of Non-Functionalized, Non-Inert Metal Nanoparticles.” Journal of Nanoparticle Research: An Interdisciplinary Forum for Nanoscale Science and Technology 18 (9): 285. doi: 10.1007/s11051-016-3597-5
- Pradhan, S., J. Hedberg, J. Rosenqvist, C. M. Jonsson, S. Wold, E. Blomberg, and I. Odnevall Wallinder. 2018. “Influence of Humic Acid and Dihydroxy Benzoic Acid on the Agglomeration, Adsorption, Sedimentation and Dissolution of Copper, Manganese, Aluminum and Silica Nanoparticles–a Tentative Exposure Scenario.” PLoS One 13 (2): e0192553. doi: 10.1371/journal.pone.0192553
- Santo, N., U. Fascio, F. Torres, N. Guazzoni, P. Tremolada, R. Bettinetti, P. Mantecca, and R. Bacchetta. 2014. “Toxic Effects and Ultrastructural Damages to Daphnia Magna of Two Differently Sized ZnO Nanoparticles: Does Size Matter?” Water Research 53: 339–350. doi: 10.1016/j.watres.2014.01.036
- Selvaraj, V., S. Bodapati, E. Murray, K. M. Rice, N. Winston, T. Shokuhfar, Y. Zhao, and E. Blough. 2014. “Cytotoxicity and Genotoxicity Caused by Yttrium Oxide Nanoparticles in HEK293 Cells.” International Journal of Nanomedicine 9: 1379–1391. doi: 10.2147/IJN.S52625
- Smirnov, L., V. Rovnushkin, A. Oryshchenko, G. Y. Kalinin, and V. Milyuts. 2016. “Modification of Steel and Alloys with Rare-Earth Elements. Part 1.” Metallurgist 59 (11–12): 1053–1061. doi: 10.1007/s11015-016-0214-x
- Sukhanova, A., S. Bozrova, P. Sokolov, M. Berestovoy, A. Karaulov, and I. Nabiev. 2018. “Dependence of Nanoparticle Toxicity on Their Physical and Chemical Properties.” Nanoscale Research Letters 13 (1): 44–44. doi: 10.1186/s11671-018-2457-x
- Tenzer, S., D. Docter, J. Kuharev, A. Musyanovych, V. Fetz, R. Hecht, F. Schlenk, et al. 2013. “Rapid Formation of Plasma Protein Corona Critically Affects Nanoparticle Pathophysiology.” Nature Nanotechnology 8 (10): 772–781. doi: 10.1038/nnano.2013.181
- Yin, H., R. Chen, P. S. Casey, P. C. Ke, T. P. Davis, and C. Chen. 2015. “Reducing the Cytotoxicity of ZnO Nanoparticles by a Pre-Formed Protein Corona in a Supplemented Cell Culture Medium.” RSC Advances 5 (90): 73963–73973. doi: 10.1039/C5RA14870G
- Zinc, U. 2020. US Geological Survey, Mineral Commodity Summaries. January 2020.
