Abstract
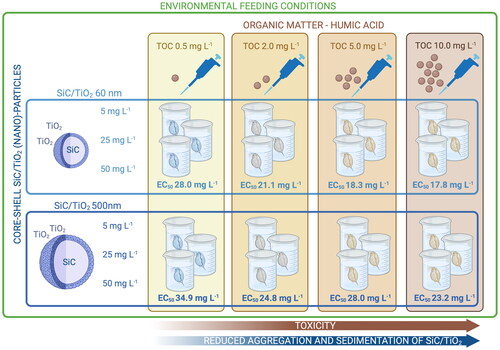
To date, research on the toxicity and potential environmental impacts of nanomaterials has predominantly focused on relatively simple and single-component materials, whilst more complex nanomaterials are currently entering commercial stages. The current study aimed to assess the long-term and size-dependent (60 and 500 nm) toxicity of a novel core–shell nanostructure consisting of a SiC core and TiO2 shell (SiC/TiO2, 5, 25, and 50 mg L−1) to the common model organism Daphnia magna. These novel core–shell nanostructures can be categorized as advanced materials. Experiments were conducted under environmentally realistic feeding rations and in the presence of a range of concentrations of humic acid (0.5, 2, 5, and 10 mg L−1 TOC). The findings show that although effect concentrations of SiC/TiO2 were several orders of magnitude lower than the current reported environmental concentrations of more abundantly used nanomaterials, humic acid can exacerbate the toxicity of SiC/TiO2 by reducing aggregation and sedimentation rates. The EC50 values (mean ± standard error) based on nominal SiC/TiO2 concentrations for the 60 nm particles were 28.0 ± 11.5 mg L−1 (TOC 0.5 mg L−1), 21.1 ± 3.7 mg L−1 (TOC 2 mg L−1), 18.3 ± 5.4 mg L−1 (TOC 5 mg L−1), and 17.8 ± 2.4 mg L−1 (TOC 10 mg L−1). For the 500 nm particles, the EC50 values were 34.9 ± 16.5 mg L−1 (TOC 0.5 mg L−1), 24.8 ± 5.6 mg L−1 (TOC 2 mg L−1), 28.0 ± 10.0 mg L−1 (TOC 5 mg L−1), and 23.2 ± 4.1 mg L−1 (TOC 10 mg L−1). We argue that fate-driven phenomena are often neglected in effect assessments, whilst environmental factors such as the presence of humic acid may significantly influence the toxicity of nanomaterials.
1. Introduction
Nanotechnology remains a rapidly growing area of research and many nanomaterials (NMs) have reached the stage of commercialization over the past years (Hansen, Hansen, and Nielsen Citation2020). As with conventional materials and chemicals, NMs are applied in a wide variety of products and processes, and inadvertent emissions to the environment resulting from their production, use and disposal cannot be entirely prevented. Over the past years, a substantial body of work has been produced which describes potential environmental impacts resulting from NM emissions (Yaqoob et al. Citation2020; Mazari et al. Citation2021). Based on this work, a number of properties and variables have been identified as primary drivers of NM toxicity, including those inherent to the NM (e.g. particle size, morphology and elemental composition) and those related to the context in which exposure takes place (e.g. medium composition and exposure time). In recognition of this, various national and international regulatory bodies have evaluated, updated and drafted guidelines which facilitate the environmental hazard and risk assessment of NMs (Rather et al. Citation2021; OECD GD 318, OECD Citation2017).
At present, our understanding of potential environmental hazards and risks of NMs mainly relates to single-element nanostructures, whilst research and development are currently progressing toward more advanced NMs, which often consist of multiple components and may exhibit novel functionalities (Bessa et al. Citation2017; Xie et al. Citation2021). A prominent example in this regard is core–shell NMs (CSNMs), of which the multicomponent design gives way to preferable properties such as high tunability and versatility in terms of applications (Camboni et al. Citation2019; Guo, Huang, and Xu Citation2021). CSNMs are materials composed of an inner core and one or more outer shells, which depending on the desired functionality may consist of various inorganic and organic materials. The design and functionalities of CSNMs can furthermore be modified by adapting the thickness ratio amongst different components and by altering their structural morphologies (e.g. size, shape, porosity, etc.) (Sabogal-Suárez, Alzate-Cardona, and Restrepo-Parra Citation2019; Zhang et al. Citation2021). To date, CSNMs have shown promising properties regarding use in, e.g. electronics, optical devices, building materials, drug delivery, and energy storage (Ghosh Chaudhuri and Paria 2011; Tsamos et al. Citation2022). Given the potential for nanoparticles to interact with biological systems in ways not yet fully understood, it is crucial to conduct testing of advanced materials like CSNMs prior to commercialization, to preemptively address any unforeseen adverse effects on the environment. Additionally, testing NMs before their release to the market admission can provide guidance for the development of safe applications and the design of safer NMs.
Based on our understanding of potential hazards and risks associated with single-component (i.e. first generation) NMs, various factors, and processes are known to constitute prominent drivers of their environmental fate and effects. Toxicity to the common model species Daphnia magna has for example been found to be dependent on particle size, being both of importance to ingestion (i.e. via size-specific filter feeding) and uptake (i.e. internalization via transfer across biological membranes) (Cui, Chae, and An Citation2017; Bacchetta et al. Citation2018). Furthermore, the composition of ecotoxicological test media has been found to influence particle fate by affecting aggregation and sedimentation rates, which consequently may alter exposure patterns and thereby toxicity. Various studies have demonstrated that the presence of organic matter (OM) can play a major role in this respect by forming a coating (i.e. eco-corona) at the particle surface which can either enhance or decrease toxicity (e.g. via mitigating bioavailability or by interfering with the gut microbiome) (Nasser, Constantinou, and Lynch Citation2020). Organic matter is naturally present in surface waters in varying compositions and may be mimicked in ecotoxicological tests by adding well-characterized sources of dissolved organic carbon (DOC) such as humic acids (HAs) or other natural organic materials depending on the type of exposure media and processes of interest. Due to potential interactions with NMs and their toxicity, nano-specific regulatory toxicity testing guidelines currently prescribe that amendment of test media with organic matter may be considered when deemed relevant, but must be clearly described and characterized to ensure interpretability and comparability across experiments (OECD GD 317, OECD GD 2021).
Next to exposure-based phenomena driving bioavailability, toxicity is also influenced by factors like food availability and quality. Often, food limitation will exacerbate impacts of stressors by impacting the dynamic energy budget of organisms and subsequently affecting processes involved in detoxification (see e.g. Heugens et al., Citation2006; Ieromina et al. Citation2014). The impact of food availability on the toxicity of NMs may be particularly relevant, as a primary mode of action of particle toxicity in e.g. D. magna stems from the clogging of the digestive tract, resulting in reduced nutrient uptake (Campos et al. Citation2013). Stevenson et al. (Citation2022) for example reported substantially higher levels of toxicity of Ag nanoparticles to Daphnia pulicaria when exposed under limited but environmentally relevant feeding conditions. Similarly, Campos et al. (Citation2013) and Sun et al. (Citation2022) demonstrated that reduced feeding rates increased the toxicity of TiO2 and ZnO nanoparticles, respectively, in D. magna. These findings emphasize that ad libitum feeding, which as recommended in e.g. OECD test guidelines 202 and 211 (OECD, Citation2004 and OECD, Citation2012) constitutes approximately 400 times higher algae cell densities than commonly observed in natural waters, may result in underestimations of actual environmental impacts of NMs (Stevenson et al. Citation2017).
As more advanced and multi-component NMs are reaching early stages of commercialization, there is a need to understand to what extent their toxicity is determined by the same principles as known for single-component NMs. In the present study, we therefore assessed the long-term (21 days) and size-dependent (60 and 500 nm) toxicity of a novel multi-component NM to the common model species D. magna. The experiment was performed under realistic feeding conditions and in the presence of a range of concentrations of organic carbon, achieved by amendment of the test medium with HA. The core–shell material SiC/TiO2 was selected as a model multi-component (nano)material because of its reported promising mechanical, thermal and electrical properties, which deem it likely to enter the stage of commercial use in the near future (Liu, Liu, and Li Citation2014; Kumar, Kushwaha, and Srivastava Citation2015; Wei et al. Citation2017), including as a potential alternative to PFAS-based nonstick coating (Pizzol et al. Citation2023). We hypothesized that the toxicity of SiC/TiO2 to D. magna: (1) is more pronounced for smaller than for larger particles, (2) is reduced when the test medium is amended with HA, and (3) is exacerbated under environmentally pertinent food limitations.
2. Materials and methods
2.1. Testing materials
Core-shell SiC/TiO2 particles with reported sizes of 48–75 nm and 530–640 nm were obtained from Laurentia Technologies (Valencia, Spain, SI ). The 48–75 nm particles consisted of a 40–60 nm SiC core coated with a 8–15 nm TiO2 shell, resulting in a mass ratio of Si to Ti of 7.7. The 530–640 nm particles consisted of a 500 − 600 nm SiC core and a 30–40 nm TiO2 shell with a mass ratio of Si to Ti of 55.6. According to the producer, the SiC/TiO2 particles were produced via sol–gel synthesis, after which they were thermally treated at 400 °C to obtain a powder. The TiO2 used in the synthesis of the shell consisted of 100% anatase.
2.2. Preparation and characterization of exposure suspensions
Both particle sizes of SiC/TiO2 were tested according to a full factorial design at concentrations of 0 (control), 5, 25, and 50 mg L−1. The test medium was amended with HA (HA sodium salt, Sigma-Aldrich - St. Louis, MI, USA) at concentrations of 1.28, 5.12, 12.81, and 25.62 mg L−1 to obtain the following concentrations of total organic carbon (TOC): 0.5, 2, 5, and 10 mg L−1. HA served as a surrogate for natural organic carbon and was applied to enhance the stability of SiC/TiO2 suspensions by reducing aggregation rates. Concentrations of HA were determined based on previous studies with nano-sized TiO2 (Romanello and de Cortalezzi Citation2013; Li et al. Citation2015) and relevant environmental concentrations (Wang et al. Citation2016). Stock solutions of HA were prepared by dissolving HA in a 2 mM NaOH solution under magnetic stirring for 24 h. Next, the solution was filtered through a 0.2 μm cellulose membrane and stored at 4 °C until use. After amendment of the exposure suspensions with HA the pH was measured and adjusted to the optimum pH of 7.8 ± 0.3 for D. magna cultures, by the addition of either 1 M HNO3 or NaOH solutions.
Stock and exposure suspensions of SiC/TiO2 were prepared freshly prior to every medium renewal. Stock suspensions were prepared at a concentration of 100 mg L−1 in Milli-Q water (Waters-Millipore Corporation, Milford, MA, USA) followed by 30 minutes of ultra-sonication using a bath sonicator with a calculated energy output of 27 ± 0.2 Watt s−1 (Sonicor SC-50-22, Sonicor INC. NY, USA). Exposure suspensions were prepared in Elendt M7 medium (Elendt, Citation1990).
Mass-based exposure concentrations of SiC/TiO2 were verified by measuring Ti concentrations and calculating the total particle concentration according to the mass ratios provided be the supplier. Measurements were performed in four replicates per treatment concentration and for each HA concentration by inductively coupled plasma mass spectrometry (ICP-MS, PerkinElmer NexION 300D, Perkin Elmer, Massachusetts, USA). Samples (1 mL) were collected from the center of the water column of the test vessels at 0, 1, 24, and 48 h after preparing the exposure suspensions (i.e. covering the entire timeframe between medium renewals) and digested overnight at 70 °C in 2 mL of a mixture consisting of 1:3 sulfuric acid (H2SO4 96%, Sigma-Aldrich - St. Louis, MI, USA) to nitric acid (HNO3 65% Sigma-Aldrich, St. Louis, MI, USA). Calibration curves were obtained by diluting a standard of titanium (Sigma-Aldrich, St. Louis, MI, USA) in Elendt M7 medium (concentration range 0.1 − 50 mg L−1) and reproducing the same matrix as used for the sample digestion procedure. Dissolution of SiC/TiO2 in Elendt M7 medium was assessed in two replicates per treatment concentration and for each HA concentration at time points 0, 1, 24, and 48 h, following the same procedure as described for the quantification of mass-based exposure concentrations, with the exception of an ultra-centrifuging/phase separation step immediately after sample collection (30 min at 32,000 rpm).
Sedimentation rates of SiC/TiO2 over the timeframe between medium renewals were calculated based on the percentage of nominally applied particle mass measured in suspension. Hydrodynamic sizes and zeta-potential were measured in two replicates per treatment concentration and for each HA concentration at 0, 24, 48 h after preparing the stock solutions using a Malvern Zetasizer Ultra (Malvern, Malvern, UK). The surface area of SiC/TiO2 was determined based on the assumption of a spheroidal morphology with concentric layers representing the SiC core and TiO2 shell. Given the combined radius of the core plus shell (rcore+shell), the total surface area (Atotal) was calculated using the formula for the surface area of a sphere A = 4πr2.
High-angle annular dark-field (HAADF) transmission electron microscopy (TEM) images of SiC/TiO2 were produced using a 100 kV JEOL (Tokyo, Japan) 1010 transmission electron microscope. Samples of SiC/TiO2 were dispersed onto 200 mesh carbon-coated copper TEM grids after 24 h incubation in the test medium in the presence and absence of HA.
2.3. Test organisms
Daphnia magna were obtained from an in-house culture at Leiden University which is maintained according to the conditions prescribed in OECD Test Guideline 211 (OECD, Citation2012) (climate room temperature 22 ± 1 °C, photoperiod 16:8 hours light:dark). The sensitivity of the culture to toxicant stress was assessed using K2Cr2O7 according to a D. magna Immobilization Test (OECD Test Guideline 202, OECD Citation2004) and was found to be within the recommended range prior to the start of the experiment (24h EC50: 1.8 g L−1).
2.4. Experimental design
A chronic (21 days) toxicity assay was conducted according to OECD Guideline document 211 (OECD, Citation2012), with modifications regarding food-limited conditions. Exposure suspensions were replaced every 48 h, and feeding was performed directly after every medium renewal by addition of Raphidocelis subcapitata at a density of 2.5e4 cells/mL (∼1.8e−3 mg C/Daphnid/day). Feeding rates equated an approximate 100-fold reduction of those prescribed in OECD Guideline document 211, and thereby resemble algae densities commonly observed in surface waters during summer (Stevenson et al. Citation2017).
Five replicates with five neonates each were used for each of the SiC/TiO2 and HA treatment combinations in a 100 mL glass beaker containing 50 mL of test suspension. Whereas chronic toxicity assays with D. magna commonly assess reproduction rates, it was expected, based on observations by Stevenson et al. (Citation2017) that food-limited conditions would result in energy allocation toward survival and growth rather than reproduction. Observations during the experiment confirmed this, showing mean reproduction rates of <1 neonate per mother over the experimental timeframe, and thus inhibiting an assessment of effects of SiC/TiO2 and HA on reproduction. Immobilization of D. magna was assessed every 48 h and body size was measured for 5 individuals per treatment at the end of the 21 days exposure period.
2.5. Data analysis
The contribution of HA amendments (i.e. TOC concentration) to hydrodynamic sizes and sedimentation rates of SiC/TiO2 was assessed via linear mixed models (LMMs, package: stats, function: ‘lm’) including nominal SiC/TiO2 concentration, pristine particle size, TOC concentration and time as explanatory variables. Models were assessed for relevant assumptions (i.e. linearity, normality of residuals and homoscedasticity) prior to analysis and response variables were transformed accordingly when required. Tukey adjusted estimated marginal means (EMMs, package: emmeans, function: ‘emmeans’) were calculated to assess differences between relevant contrasts.
Effects of SiC/TiO2 and HA treatment combinations on mortality over time were assessed using Quasi-Poisson-distributed generalized linear mixed models (GLMs, package: stats, function: ‘glm’) including replicate number as a random variable to account for the repeated measures design. Tukey-adjusted estimated marginal means (EMMs, package: emmeans, function: ‘emmeans’) were calculated to assess on which specific days differences between controls and treatments were present. Mortality rates after 21 days of exposure were fitted to three-parameter log-logistic models using the drm function of the ‘drc’ package (Ritz et al., Citation2015). Separate curves were fitted for each particle size and each TOC concentration using the ‘curveID’ function, using nominal and time-weighted average (TWA) particle concentrations as response variables. Differences between EC50 values derived from dose-response models for different particle sizes and NOM concentrations were identified for each dose-metric by z-tests using the compParm function.
Impacts of SiC/TiO2 and TOC treatment combinations on body size were analyzed via linear mixed models as described for models of hydrodynamic sizes and sedimentation rates. All data were analyzed using R version (R-4.2.0) and outcomes of statistical tests were considered statistically significant at p < 0.05.
3. Results
3.1.1. Hydrodynamic diameter and stability of SiC/TiO2 suspension
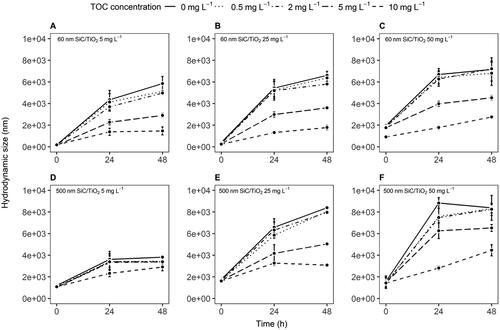
None of the collected samples from the experimental setup contained detectable quantities of dissolved Si or Ti. All SiC/TiO2 suspensions showed significant time- (F = 489.7, p < 2.2e−16) and concentration-dependent (F = 90.0, p < 2.2e−16) increases in aggregation rates (, SI , SI ). Amendment with HA resulted in partial stabilization of suspensions of both 60 and 500 nm SiC/TiO2 particles (F = 84.9, p < 2.2e−16), with significantly lower aggregation rates being observed at TOC concentrations of 5 and 10 mg L−1. The reduction in aggregation rates at higher TOC concentrations coincided with lower polydispersity indexes and more negative zeta-potentials for both SiC/TiO2 particle sizes (, SI and SI ). Collectively, these results suggest that the presence of TOC plays a role in the colloidal stability of SiC/TiO2 nanoparticles, with a higher concentration of TOC conferring a greater inhibitory effect on particle aggregation. This stabilization may be attributed to the adsorption of organic molecules on the nanoparticle surface, which can impart steric and/or electrostatic repulsion, thereby attenuating aggregation kinetics.
Figure 2. Sedimentation rates (expressed as the percentage of the nominally applied particle mass remaining in suspension, A–F, mean ± standard error) and time-weighted average (TWA) water column concentrations (G–I, mean ± standard error) of 60 and 500 nm SiC/TiO2 after 0, 24, and 48 hours measured in Elendt M7 medium in the presence of varying total organic carbon (TOC) concentrations (i.e. 0, 0.5, 2, 5, and 10 mg L−1).
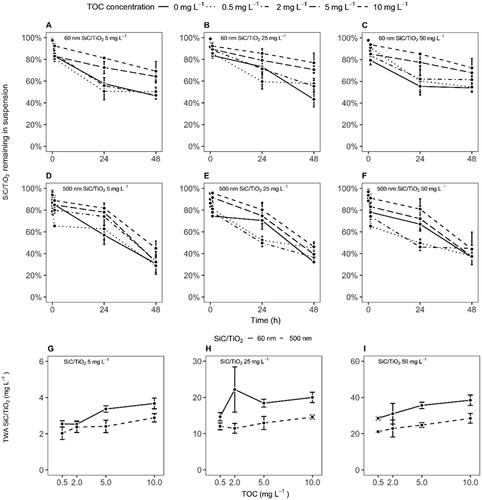
Figure 3. Correlation between time-weighted average hydrodynamic sizes and sedimentation rates (expressed as a percentage of the nominally applied particle mass remaining in suspension) of 60 and 500 nm SiC/TiO2 in Elendt M7 medium (mean ± 95% confidence interval). Values of nominal SiC/TiO2 concentrations (5, 25, and 50 mg L−1) and total organic carbon (TOC) concentrations (i.e. 0, 0.5, 2, 5, and 10 mg L−1) are aggregated.
Figure 4. Percentage of surviving D. magna (mean ± standard error) over 21 days of exposure to 60 and 500 nm SiC/TiO2 (5, 25, and 50 mg L−1) in the presence of varying total organic carbon (TOC) concentrations, i.e. 0.5 mg L−1 (A and E), 2 mg L−1 (B and F), 5 mg L−1 (C and G), and 10 mg L−1 (D and H).
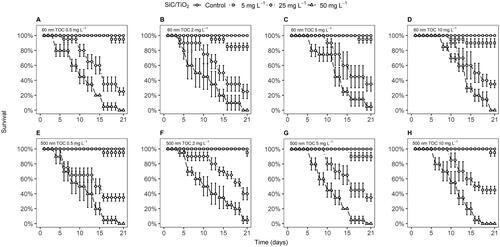
Table 1. EC50 values derived from dose–response models based on the percentage of surviving D. magna over 21 days of exposure to 60 and 500 nm SiC/TiO2 (i.e. 5, 25, and 50 mg L−1) in the presence of varying total organic carbon (TOC) concentrations (i.e. 0, 0.5, 2, 5, and 10 mg L−1) calculated using different dose metrics.
3.1.2. Mass-based concentrations and settling rates of SiC/TiO2
SiC/TiO2 concentrations measured in the water column of the test vessels showed a significant decrease over the timeframe between medium renewals (F = 964.9, p < 2.2e−16, , SI Table 2). Sedimentation was more pronounced for 500 than for 60 nm SiC/TiO2 particles (F = 75.6, p < 2.2e−16) but did not vary significantly across nominal particle concentrations (F = 1.9, p = 0.16). Similar to observations of aggregation rates, amendment with HA resulted in a concentration-dependent decrease in sedimentation rates (F = 90.2, p < 2.2e−16), with most pronounced effects being observed at TOC concentrations of 5 and 10 mg L−1. A significant correlation was observed between hydrodynamic sizes and sedimentation rates (R2 = 0.20, F = 5.5, p < 0.02, ), suggesting that the formation of an eco-corona at the particle surface of SiC/TiO2 after HA amendments resulted in reduced homo-aggregation rates, which in turn lessened gravitational forces drawing SiC/TiO2 out of suspension (i.e. consequentially, maintaining a larger fraction in suspension).
3.2.1. Mortality and growth of Daphnia magna exposed to SiC/TiO2
Survival rates of D. magna exposed to both 60 and 500 nm particle sizes of SiC/TiO2 declined in a concentration-dependent manner (F = 2947, p < 2.2e−16 and F = 4413, p < 2.2e−16, respectively, ). A slightly more pronounced effect of SiC/TiO2 was observed in D. magna exposed to 60 nm in comparison to 500 nm particle sizes (F = 6.6, p = 9.9e−3). HA predominantly affected impacts of SiC/TiO2 by shortening the exposure time upon which reductions in survival rates were observed and did not exert any impacts in the absence of SiC/TiO2 at any of the tested concentrations (, SI Table 4), showing an overall increase in toxicity at higher TOC concentrations (F = 41.0, p = 1.8e−10). A similar trend was observed for effect concentrations (ECs) calculated based on survival rates after 21 days of exposure. These effect concentrations displayed a small decline upon increasing TOC concentrations, although statistically significant differences between ECs were absent (, SI Table 5). Whilst the previously discussed contribution of increasing TOC concentrations to reductions in settling rates of SiC/TiO2 may have contributed to the observed differences in toxicity, it should be noted that when TWA water column concentrations of SiC/TiO2 were used as the dose-metric in the overall models for survival rates over time, TOC remained to display a significant contribution to observed mortality rates (F = 4.02, p = 0.03). Similarly, when ECs were calculated based on TWA water column concentrations of SiC/TiO2, a concentration-dependent trend in toxicity across TOC concentrations remained present (, ).
Figure 5. Dose–response curves (A and B) based on mortality rates and body sizes (C and D) of D. magna after 21 days of exposure to 60 and 500 nm SiC/TiO2 (5, 25, and 50 mg L−1) in the presence of varying total organic carbon (TOC) concentrations (i.e. 0.5, 2.0, 5, and 10 mg L−1). Dose–response curves were calculated using nominal and time-weighted average (TWA) treatment concentrations. Error bars in dose-response curves represent standard errors.
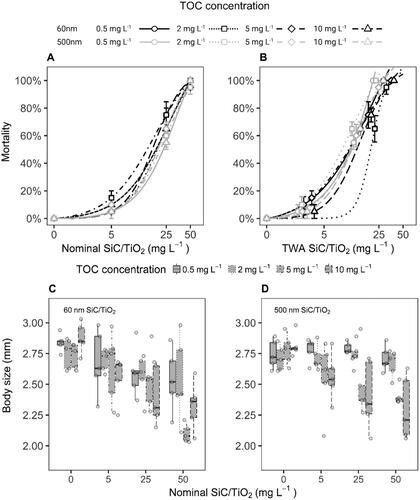
In line with observations for survival rates, a concentration-dependent reduction in body size of D. magna was observed after 21 days of exposure to both 60 and 500 nm particle sizes of SiC/TiO2 (F = 40.7, p = 1.2e−8 and F = 18.0, p = 6.1e−5, respectively; and , SI Figure 5). Here, however, the pristine particle size was found not to be a significant predictor of impacts (F = 2.9, p = 0.09), although a slightly stronger response was observed for 60 nm SiC/TiO2. Increasing TOC concentrations exacerbated the effects of SiC/TiO2 (F = 22.1, p = 5.8e−6), as demonstrated by the presence of most pronounced differences in body sizes from controls at TOC concentrations > 2 mg L−1 (SI Table 6).
4. Discussion
The current study aimed to assess whether 60 and 500 nm pristine particle sizes of SiC/TiO2 differ in their chronic toxicity to D. magna under environmental realistic food conditions and to which extent amendment with HA may affect their fate and toxicity. Both (pristine) 60 and 500 nm particles of SiC/TiO2 were found to aggregate and sediment in a time- and concentration-dependent manner after being applied to the test medium. The kinetics of aggregation and subsequent sedimentation were reduced by amendment with HA. Aggregation of particles in suspension as observed in the current study is a well-described phenomenon in ecotoxicity testing of NMs. As for instance, as reported by Cupi et al. (Citation2015), Farner et al. (Citation2019), and Lu et al. (Citation2021), aggregation can alter the bioavailable fraction of the tested material and hence the subsequent toxicity. Various studies have additionally demonstrated that the stability of exposure suspensions in terms of both aggregation and sedimentation rates may be enhanced by amendment with HA or other sources of dissolved organic matter (DOM). This is generally ascribed to eco-corona formation which may result in the alteration of particle surface charges, thereby reducing attractive interactions between particles (see e.g. Lin et al. Citation2012; Lu et al. Citation2021). Importantly, however, these processes have been shown to vary depending on the composition of the DOM, as well as across NMs with different surface charges (Cupi et al., Citation2015).
In the current study, measures of toxicity of SiC/TiO2 particles were found to be within the same order of magnitude despite a near 10-fold difference in pristine particle sizes. This is particularly notable considering that pristine 60 nm SiC/TiO2 particles would have been too small to be captured in the filtering apparatus of D. magna, which exhibits a mesh size in the range of 0.4–0.7 μm (Gophen and Geller Citation1984). As actual hydrodynamic sizes of both pristine 60 and 500 nm SiC/TiO2 particles measured in suspension were in the range within which D. magna is able to filter, it is likely that aggregation mitigated most of the effect of primary particle size on bioavailability via filter-feeding in the current experiment. HA was found to enhance the toxicity of SiC/TiO2 in a concentration-dependent manner, which could partly be attributed to reduced aggregation and sedimentation rates. Nevertheless, measures of toxicity calculated based on actual water column concentrations of SiC/TiO2 over the experimental timeframe remained to show concentration-dependent differences across levels of HA amendments. As such, additional factors relating to eco-corona formation, such as enhancement of bioavailability at the cellular level or longer depuration times from the gastro-intestinal tract, may have contributed to the observed increase in toxicity across amendments of HA (Nasser, Constantinou, and Lynch Citation2020). Interestingly, several studies have reported observations of reduced toxicity as a result of eco-corona formation in D. magna exposed to NMs in the presence of organic matter (see e.g. Nasser and Lynch Citation2016 and Ekvall et al. Citation2021), whilst others have reported increased toxicity (see e.g. González-Andrés et al., Citation2017 and Yu et al. Citation2022). This is likely due to the diversity in interactions taking place between NMs and sources of organic matter, which may in some cases result in e.g. reduced production of reactive oxygen species by photocatalytic NMs, and in other cases (such as in the current study) increase exposure concentrations and bioavailability. The interactions between NMs and organic matter are thus complex and variable and depend on the particle characteristics, such as surface properties as well as the composition of the source of organic matter. We therefore argue that in order for hazard assessments of (advanced) nanomaterials to adequately represent natural conditions, such variables should be considered when performing and interpreting ecotoxicological tests.
5. Conclusions
The application of core–shell (nano)materials is likely to increase across various sectors in the coming years (see e.g. Kumar et al. Citation2020 and Dhiman et al. Citation2021), and the current study is one of the first to describe the fate and toxicity of SiC/TiO2 particles to an aquatic organism. Our findings show that overall effect concentrations were several orders of magnitude higher than reported environmental concentrations of high-production volume NMs such as TiO2, despite the test being performed under realistic feeding conditions that exacerbate sensitivity. Impacts of HA on particle toxicity, as observed in the current experiment, have also been shown to occur in single-component NM ecotoxicity assays. As such, we emphasize actual exposure conditions in relation to water chemistry and food levels constitute important considerations for realistic effect predictions of both single- and (advanced) multi-component nanomaterials.
Author contributions
K.S.: conceptualization; investigation; methodology; data curation; formal analysis; visualization; writing—original draft; T.N.: methodology; data curation; formal analysis; visualization; writing—review and editing; W.P.: conceptualization; supervision; resources; writing-review and editing; M.V.: conceptualization, supervision, resources, funding acquisition, project administration, writing—review and editing.
Supplemental Material
Download Zip (4.9 MB)Acknowledgements
We thank Frank Senden, who was involved in practical work for his traineeship. Kornelia Serwatowska, Tom Nederstigt and Martina Vijver are supported by the ERC-consolidator grant granted to Martina Vijver entitled EcoWizard (101002123) within the European Union’s Horizon 2020 Framework Programme. Part of this work was performed within the framework of the European Union’s Horizon 2020 research and innovation program “SUNSHINE” (Grant agreement number 952924). The graphical abstract was created with BioRender.com.
Data availability statement
The data that support the findings of this study are available from the corresponding author, KS, upon reasonable request.
References
- Bacchetta, R., N. Santo, I. Valenti, D. Maggioni, M. Longhi, and P. Tremolada. 2018. “Comparative Toxicity of Three Differently Shaped Carbon Nanomaterials on Daphnia magna: Does a Shape Effect Exist?” Nanotoxicology 12 (3): 201–223. https://doi.org/10.1080/17435390.2018.1430258
- Bessa, M. J., C. Costa, J. Reinosa, C. Pereira, S. Fraga, J. Fernández, M. A. Bañares, and J. P. Teixeira. 2017. “Moving into Advanced Nanomaterials. Toxicity of Rutile TiO2 Nanoparticles Immobilized in Nanokaolin Nanocomposites on HepG2 Cell Line.” Toxicology and Applied Pharmacology 316: 114–122. https://doi.org/10.1016/j.taap.2016.12.018
- Camboni, M., P. Floyd, J. Hanlon, and P. G. Rodrigo. 2019. “A State of Play Study of the Market for so Called “Next Generation” Nanomaterials.” European Chemicals Agency https://doi.org/10.2823/242422.
- Campos, B., C. Rivetti, P. Rosenkranz, J. M. Navas, and C. Barata. 2013. “Effects of Nanoparticles of TiO2 on Food Depletion and Life-History Responses of Daphnia magna.” Aquatic Toxicology (Amsterdam, Netherlands) 130-131: 174–183. https://doi.org/10.1016/j.aquatox.2013.01.005
- Cui, R., Y. Chae, and Y. J. An. 2017. “Dimension-Dependent Toxicity of Silver Nanomaterials on the Cladocerans Daphnia magna and Daphnia galeata.” Chemosphere 185: 205–212. https://doi.org/10.1016/j.chemosphere.2017.07.011
- Cupi, D., N. B. Hartmann, and A. Baun. 2015. “The Influence of Natural Organic Matter and Aging on Suspension Stability in Guideline Toxicity Testing of Silver, Zinc Oxide, and Titanium Dioxide Nanoparticles with Daphnia Magna.” Environmental Toxicology and Chemistry 34 (3): 497–506. https://doi.org/10.1002/etc.2855. 25546145
- Dhiman, S., A. Yadav, N. Debnath, and S. Das. 2021. “Application of Core/Shell Nanoparticles in Smart Farming: A Paradigm Shift for Making the Agriculture Sector More Sustainable.” Journal of Agricultural and Food Chemistry 69 (11): 3267–3283. https://doi.org/10.1021/acs.jafc.0c05403
- Ekvall, M. T., J. Hedberg, I. Odnevall Wallinder, A. Malmendal, L. A. Hansson, and T. Cedervall. 2021. “Adsorption of Bio-Organic Eco-Corona Molecules Reduces the Toxic Response to Metallic Nanoparticles in Daphnia magna.” Scientific Reports 11 (1): 10784. https://doi.org/10.1038/s41598-021-90053-5
- Elendt, B.-P. 1990. “Selenium Deficiency in Crustacea.” Protoplasma 154 (1): 25–33. https://doi.org/10.1007/BF01349532
- Farner, J. M., R. S. Cheong, E. Mahé, H. Anand, and N. Tufenkji. 2019. “Comparing TiO2 Nanoparticle Formulations: Stability and Photoreactivity Are Key Factors in Acute Toxicity to Daphnia magna.” Environmental Science: Nano 6 (8): 2532–2543. https://doi.org/10.1039/C9EN00666D
- Ghosh Chaudhuri, R., and S. Paria. 2012. “Core/Shell Nanoparticles: classes, Properties, Synthesis Mechanisms, Characterization, and Applications.” Chemical Reviews 112 (4): 2373–2433. https://doi.org/10.1021/cr100449n
- González-Andrés, V., M. Diez-Ortiz, C. Delpivo, G. Janer, A. Fritzsche, and S. Vázquez-Campos. 2017. “Acute Ecotoxicity of Coated Colloidal Goethite Nanoparticles on Daphnia Magna: Evaluating the Influence of Exposure Approaches.” The Science of the Total Environment 609: 172–179. https://doi.org/10.1016/j.scitotenv.2017.07.047. 28738199
- Gophen, M., and W. Geller. 1984. “Filter Mesh Size and Food Particle Uptake by Daphnia.” Oecologia 64 (3): 408–412. https://doi.org/10.1007/bf00379140
- Guo, M., K. Huang, and W. Xu. 2021. “Third Generation Whole-Cell Sensing Systems: synthetic Biology Inside, Nanomaterial outside.” Trends in Biotechnology 39 (6): 550–559. https://doi.org/10.1016/j.tibtech.2020.10.002
- Hansen, S. F., O. F. H. Hansen, and M. B. Nielsen. 2020. “Advances and Challenges Towards Consumerization of Nanomaterials.” Nature Nanotechnology 15 (12): 964–965. https://doi.org/10.1038/s41565-020-00819-7
- Heugens, E. H., L. T. Tokkie, M. H. Kraak, A. J. Hendriks, N. M Van Straalen, and W. Admiraal. 2006. “Population Growth of Daphnia Magna under Multiple Stress Conditions: joint Effects of Temperature, Food, and Cadmium.” Environmental Toxicology and Chemistry 25 (5): 1399–1407. https://doi.org/10.1897/05-294r.1
- Ieromina, O., W. J. Peijnenburg, G. de Snoo, J. Müller, T. P. Knepper, and M. G. Vijver. 2014. “Impact of Imidacloprid on Daphnia magna Under Different Food Quality Regimes.” Environmental Toxicology and Chemistry 33 (3): 621–631. https://doi.org/10.1002/etc.2472
- Kumar, R., A. S. Kushwaha, and S. K. Srivastava. 2015. “One-Dimensional Nano Layered SiC/TiO2 Based Photonic Band Gap Materials as Temperature Sensor.” Optik 126 (14): 1324–1330. https://doi.org/10.1016/j.ijleo.2015.04.012
- Kumar, R., K. Mondal, P. K. Panda, A. Kaushik, R. Abolhassani, R. Ahuja, H. G. Rubahn, and Y. K. Mishra. 2020. “Core–Shell Nanostructures: Perspectives Towards Drug Delivery Applications.” Journal of Materials Chemistry. B 8 (39): 8992–9027. https://doi.org/10.1039/D0TB01559H
- Li, Y., C. Yang, X. Guo, Z. Dang, X. Li, and Q. Zhang. 2015. “Effects of Humic Acids on the Aggregation and Sorption of Nano-TiO2.” Chemosphere 119: 171–176. https://doi.org/10.1016/j.chemosphere.2014.05.002
- Lin, D., J. Ji, Z. Long, K. Yang, and F. Wu. 2012. “The Influence of Dissolved and Surface-Bound Humic Acid on the Toxicity of TiO2 Nanoparticles to Chlorella Sp.” Water Research 46 (14): 4477–4487. https://doi.org/10.1016/j.watres.2012.05.035
- Liu, H., Y. Liu, and J. Li. 2014. “Preparation of SiC/TiO2 Ceramic Microspheres.” Advances in Applied Ceramics. 113 (7): 438–443. https://doi.org/10.1179/1743676114Y.0000000182
- Lu, Y., H. Zhang, H. Wang, N. Ma, T. Sun, and B. Cui. 2021. “Humic Acid Mediated Toxicity of Faceted TiO2 Nanocrystals to Daphnia magna.” Journal of Hazardous Materials 416: 126112. https://doi.org/10.1016/j.jhazmat.2021.126112
- Mazari, S. A., E. Ali, R. Abro, F. S. A. Khan, I. Ahmed, M. Ahmed, S. Nizamuddin, et al. 2021. “Nanomaterials: Applications, Waste-Handling, Environmental Toxicities, and Future Challenges–A Review.” Journal of Environmental Chemical Engineering. 9 (2): 105028. https://doi.org/10.1016/j.jece.2021.105028
- Nasser, F., and I. Lynch. 2016. “Secreted Protein Eco-Corona Mediates Uptake and Impacts of Polystyrene Nanoparticles on Daphnia magna.” Journal of Proteomics 137: 45–51. https://doi.org/10.1016/j.jprot.2015.09.005
- Nasser, F., J. Constantinou, and I. Lynch. 2020. “Nanomaterials in the Environment Acquire an “Eco‐Corona” Impacting Their Toxicity to Daphnia magna—a Call for Updating Toxicity Testing Policies.” Proteomics 20 (9): e1800412. https://doi.org/10.1002/pmic.201800412
- OECD. 2012. Test No. 211: Daphnia Magna Reproduction Test, OECD Guidelines for the Testing of Chemicals, Section 2. OECD Publishing, Paris. https://doi.org/10.1787/9789264185203-en
- OECD 2022. Guidance Document No. 317, Guidance Document on Aquatic and Sediment Toxicological Testing of Nanomaterials, Series on Testing and Assessment. OECD Publishing, Paris.
- OECD 2017. Test No. 318: Dispersion Stability of Nanomaterials in Simulated Environmental Media, OECD Guidelines for the Testing of Chemicals, Section 3. OECD Publishing, Paris https://doi.org/10.1787/9789264284142-en
- OECD 2004. Test No. 202: Daphnia Sp. Acute Immobilisation Test, OECD Guidelines for the Testing of Chemicals, Section 2, OECD Publishing, Paris, https://doi.org/10.1787/9789264069947-en
- Pizzol, L., A. Livieri, B. Salieri, L. Farcal, L. G. Soeteman-Hernández, H. Rauscher, A. Zabeo, et al. 2023. “Screening Level Approach to Support Companies in Making Safe and Sustainable by Design Decisions at the Early Stages of Innovation.” Cleaner Environmental Systems 10: 100132. https://doi.org/10.1016/j.cesys.2023.100132
- Rather, G. A., M. Z. Gul, M. Riyaz, A. Chakravorty, M. H. Khan, A. Nanda, and M. Y. Bhat. 2021. “Toxicity and Risk Assessment of Nanomaterials.” In Handbook of Research on Nano-Strategies for Combatting Antimicrobial Resistance and Cancer (pp. 391–416). Pennsylvania: IGI Global. https://doi.org/10.4018/978-1-7998-5049-6
- Ritz, C., F. Baty, J. C. Streibig, and D. Gerhard. 2015. “Dose-Response Analysis Using R.” PloS One 10 (12): e0146021. https://doi.org/10.1371/journal.pone.0146021. PMC: 26717316
- Romanello, M. B., and M. M. F. de Cortalezzi. 2013. “An Experimental Study on the Aggregation of TiO2 Nanoparticles under Environmentally Relevant Conditions.” Water Research 47 (12): 3887–3898. https://doi.org/10.1016/j.watres.2012.11.061
- Sabogal-Suárez, D., J. D. Alzate-Cardona, and E. Restrepo-Parra. 2019. “Influence of the Shape on Exchange Bias in Core/Shell Nanoparticles.” Journal of Magnetism and Magnetic Materials. 482: 120–124. https://doi.org/10.1016/j.jmmm.2019.03.037
- Stevenson, L. M., K. E. Krattenmaker, E. Johnson, A. J. Bowers, A. S. Adeleye, E. McCauley, and R. M. Nisbet. 2017. “Standardized Toxicity Testing May Underestimate Ecotoxicity: Environmentally Relevant Food Rations Increase the Toxicity of Silver Nanoparticles to Daphnia.” Environmental Toxicology and Chemistry 36 (11): 3008–3018. https://doi.org/10.1002/etc.3869
- Stevenson, L. M., K. E. Krattenmaker, E. McCauley, and R. M. Nisbet. 2022. “Extrapolating Contaminant Effects from Individuals to Populations: A Case Study on Nanoparticle Toxicity to Daphnia Fed Environmentally Relevant Food levelsArch.” Archives of Environmental Contamination and Toxicology 83 (4): 361–375. https://doi.org/10.1007/s00244-022-00950-7
- Sun, Y., Q. Liu, J. Huang, D. Li, Y. Huang, K. Lyu, and Z. Yang. 2022. “Food Abundance Mediates the Harmful Effects of ZnO Nanoparticles on Development and Early Reproductive Performance of Daphnia magna.” Ecotoxicology and Environmental Safety 236: 113475. https://doi.org/10.1016/j.ecoenv.2022.113475
- Tsamos, D., A. Krestou, M. Papagiannaki, and S. Maropoulos. 2022. “An Overview of the Production of Magnetic Core-Shell Nanoparticles and Their Biomedical Applications.” Metals 12 (4): 605. https://doi.org/10.3390/met12040605
- Wang, Z., L. Zhang, J. Zhao, and B. Xing. 2016. “Environmental Processes and Toxicity of Metallic Nanoparticles in Aquatic Systems as Affected by Natural Organic Matter.” Environmental Science: Nano 3 (2): 240–255. https://doi.org/10.1039/C5EN00230C
- Wei, B., C. Zou, X. Yuan, and X. Li. 2017. “Thermo-Physical Property Evaluation of Diathermic Oil Based Hybrid Nanofluids for Heat Transfer Applications.” International Journal of Heat and Mass Transfer 107: 281–287. https://doi.org/10.1016/j.ijheatmasstransfer.2016.11.044
- Xie, P., W. Yuan, X. Liu, Y. Peng, Y. Yin, Y. Li, and Z. Wu. 2021. “Advanced Carbon Nanomaterials for State-of-the-Art Flexible Supercapacitors.” Energy Storage Materials 36: 56–76. https://doi.org/10.1016/j.ensm.2020.12.011
- Yaqoob, A. A., T. Parveen, K. Umar, and M. N. Mohamad Ibrahim. 2020. “Role of Nanomaterials in the Treatment of Wastewater: A Review.” Water 12 (2): 495. https://doi.org/10.3390/w12020495
- Yu, Q., Z. Wang, G. Wang, W. J. Peijnenburg, and M. G. Vijver. 2022. “Effects of Natural Organic Matter on the Joint Toxicity and Accumulation of Cu Nanoparticles and ZnO Nanoparticles in Daphnia magna.” Environmental Pollution (Barking, Essex: 1987) 292 (Pt B): 118413. https://doi.org/10.1016/j.envpol.2021.118413
- Zhang, Y. J., P. M. Radjenovic, X. S. Zhou, H. Zhang, J. L. Yao, and J. F. Li. 2021. “Plasmonic Core–Shell Nanomaterials and Their Applications in Spectroscopies.” Advanced Materials (Deerfield Beach, Fla.) 33 (50): e2005900. https://doi.org/10.1002/adma.202005900