 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Aim: Our investigation aims to estimate the antifungal effect of propranolol hydrochloride (PNL). Methods: Oleosomes (OLs) were fabricated by thin-film hydration and evaluated for entrapment efficiency (EE%), particle size (PS), polydispersity index (PDI), zeta potential (ZP), and amount of drug released after 6 h Q6h (%). Results: The optimal OL showed a rounded shape with optimum characteristics. The ex-vivo permeation and confocal laser scanning microscopy verified the prolonged release and well deposition of PNL-loaded OLs-gel. The in-silico assessment demonstrated the good stability of PNL with OLs' ingredients. In vivo evaluations for PNL-loaded OLs-gel showed a good antifungal impact against Candida albicans with good safety. Conclusion: This work highlights the potential of PNL-loaded OLs-gel as a potential treatment for candida vaginal infection.
The work investigated in detail the impact of various processing variables on the aspects of OLs (EE%, PS, PDI, ZP, and Q6h%).
The morphology of the optimal OL using a transmission electron microscope was examined.
A compatibility study between PNL and other formulation additives using differential scanning calorimetry was included.
The effect of storage for the stored optimal OL compared with the fresh one in terms of EE%, PS, ZP, PDI, and Q6h% was investigated.
The permeation capability of optimal PNL-loaded OLs-gel compared with the PNL-gel, optimal OL, and PNL solution using vaginal rat tissues was inspected.
Visualization of the deposition of the optimal OL into the vaginal tissues was done by using confocal laser scanning microscopy.
The stability of the components of the optimal OL using an in silico study was tested.
In vivo studies were included to evaluate the capability of PNL-loaded OLs-gel for the treatment of Candida Albicans-related vaginal infections.
In vivo histopathological studies were involved to inspect the topical tolerability and safety of the fabricated formulae.
In vivo immunohistochemistry studies were done to assess the inflammatory response toward the fabricated formulae.
1. Background
Receiving approval for a novel drug is costly and may take an extended time. Drug repurposing or repositioning presents a viable possibility for lowering the time required to create a new medication. Repurposing of a drug implies the use of medicines accepted for certain indications by regulatory agencies for a new one. Due to the enormous potential of a reduced development period, many pharmaceutical companies are utilizing drug repurposing to modify some of their FDA-approved but previously unproductive pipeline compounds into innovative treatments for various illnesses [Citation1]. Further, in silico studies play a crucial role in drug repurposing efforts. In silico studies use computational methods and techniques to analyze and model biological systems and processes. These studies leverage various computational tools and databases to predict the interactions between drugs and biological targets, assess drug safety and toxicity, and understand the underlying mechanisms of drug action [Citation2].
According to their safety, azoles are the most utilized antifungal bioactives [Citation3]. Although azoles show outstanding therapeutic efficacy against molds and yeasts, the widespread use of azoles has resulted in acquired azole resistance. To address this problem, new antifungals must be developed [Citation4].
Several research papers are focused on developing repurposed antifungals. For example, statins (pitavastatin, simvastatin, pravastatin, rosuvastatin, fluvastatin, lovastatin, atorvastatin, and cerivastatin) are first recognized as lipid-lowering medications because they can restrain HMG-CoA reductase. However, they have also been shown to have a wide range of antifungal activities on Aspergillus spp., and Candida spp. In addition, antidepressant drugs such as Fluoxetine show the ability to kill azole-resistant Candida spp. in-vitro whether Fluconazole is present or not. Further, antiarrhythmic drugs, including potassium, calcium, and sodium channel antagonists, in addition to β-receptor blockers have presented antifungal activity against Candida albicans [Citation5]. A previous study confirmed that Propranolol hydrochloride (PNL) might be effective against C. albicans [Citation6].
Vaginal candidiasis, commonly known as a yeast infection, is widely recognized as the most prevalent fungal infection. Itching, dyspareunia, vulvar erythema, soreness, pruritus, and edema are the most prevalent symptoms. The primary variables that seem to cause the illness are host-related including hormonal changes, long-term antibiotics usage, and disruptions in the vaginal ecosystem [Citation7].
The treatment methods of vaginal candidiasis, that may be most effective, primarily require topical and long-term treatment, which raises the opportunity of missing the dose and augmenting the shortcomings. Hence, it is beneficial to use a formulation that decreases the frequency of administration and maintains efficacy for a prolonged time [Citation8].
Although traditional vaginal dosage forms are inexpensive and simple to fabricate, they have limited bioavailability. Consequently, developed vaginal drug-delivery systems are amended. Modern developments in vaginal drug-delivery systems rely on the drug's encapsulation in nanocarriers (NC) [Citation9]. Incorporating antifungal drugs into NC improves their pharmacological characteristics such as amphotericin B [Citation10] and miltefosine [Citation11] and enables prolonged drug release with reduced toxicity [Citation12].
It has been documented that oleic acid (OA), an unsaturated fatty acid, tends to form vesicles in the aqueous media [Citation13]. A previous study integrated OA inside phospholipid (PC) and formed oleosomes (OLs) to improve the topical deposition of sildenafil citrate inside the skin [Citation14]. It is worth noting that OA showed the ability to diminish biofilm formation and filamentation of C. albicans [Citation15]. Further, Mosalam et al. proved the synergistic effect of OA against C. albicans during their preparation of OA-enriched vesicles for topical fungal infection [Citation16].
It is worth mentioning that bioadhesive delivery systems have been fabricated to augment the duration of residence in the vagina. Among the vaginal formulae, the most utilized ones are bioadhesive gels; they are simple to produce and able to spread and adhere to the mucosa's surface to form intimate contact. Furthermore, because of their high-water amount and rheological features, they offer lubricating and hydrating effects that counteract the dryness of the vagina [Citation17].
To the extent of our knowledge, this is the first paper to inspect the potential repurposing of PNL as an antifungal medication utilizing a nanosystem. Hence, the aim was to estimate the antifungal ability of PNL incorporated in OLs-gel for sustained antifungal effect. To achieve this, multiple factors influencing OLs characteristics were examined utilizing Design Expert® software by using D-optimal design to select the optimal OL. PC amount (mg) (X1), CTAB amount (mg) (X2), and CH amount (mg) (X3) were opted as factors, whereas entrapment efficiency percentage (EE%; Y1), particle size (PS; Y2), zeta potential (ZP; Y3) and amount of drug released after 6 h (Q6h (%); Y4) were opted as responses. The optimal OL was inspected for its shape, short-term storage investigation, differential scanning calorimetry study, and mucoadhesive aspects. Using a confocal microscope, the accumulation of the fluor-labeled optimal OL within vaginal tissues was detected. To ascertain the permeation ability of the PNL solution, the optimal OL, PNL-gel, and PNL-loaded OLs-gel, ex vivo permeation investigations were conducted. Furthermore, the in silico investigation was conducted for the optimal OL ingredients to evaluate their binding stability. Finally, an in vivo antifungal investigation and histopathology study were conducted to examine the antifungal potential and safety of the prepared formulae.
2. Materials & methods
2.1. Materials
Propranolol hydrochloride (PNL) was attained from El-Kahira Pharmaceutical Co. (Cairo, Egypt). Phospholipid (PC) from egg yolk, chitosan (Mw 260,000 Da), cetyltrimethylammonium bromide (CTAB), cholesterol (CH), fluorescein diacetate (FDA), ß-estradiol-17-valerate were supplied by Sigma Aldrich Chemical Co. (MO, USA). Oleic acid (OA), methanol, and chloroform were supplied by El-Nasr Pharmaceutical Co. (Cairo, Egypt). Hydroxypropyl methylcellulose (HPMC) K4M was attained from Colorcon (Kent, UK).
2.2. Methods
2.2.1. Preparation of PNL-loaded OLs
OLs were prepared using PC, CTAB, and CH at various amounts employing a thin-film hydration technique (A). Firstly, PC and CTAB, CH and OA (60 mg) were weighted in a rounded flask and solved in 10 ml chloroform, the organic phase was vaporized below vacuum pressure for 30 min at 60°C utilizing a rotatory evaporator (Rotavapor, Heidolph VV 2000, Burladingen, Germany) at 90 r.p.m. so that a thin film was fabricated [Citation18]. The film was hydrated utilizing 10 ml distilled water comprising PNL (150 mg) and chitosan (0.6% w/w) that was previously dissolved using (0.1% glacial acetic acid) at 60°C. To ensure perfect film hydration, glass beads were employed. The OLs dispersion was maintained overnight at 4°C.
Table 1. D-optimal design for optimization of propranolol hydrochloride-loaded oleosomes (A), and the atomic composition of propranolol hydrochloride formulation complex for molecular dynamics simulation (B).
2.3. Characterization of PNL-OLs
2.3.1. Determination of entrapment efficiency percentage (EE%)
The dispersion of the fabricated OLs was centrifugated at 20,000 r.p.m. for 1 h at 4°C utilizing a cooling-centrifuge (Sigma 3K 30, Germany). The whole supernatant was diluted and PNL concentration was detected at λmax 289 nm [Citation19]. EE% was estimated utilizing the indirect method [Citation16].
2.3.2. Determination of particle size, polydispersity index & zeta potential
Particle size (PS), polydispersity index (PDI), and zeta potential (ZP) were measured for the formulated OLs by Zetasizer (Malvern Instrument Ltd., Malvern, UK). After proper dilution, the measurement was carried out. Each specimen was evaluated in triplicate [Citation16].
2.3.3. Determination of the amount of drug released after 6 h Q6h (%)
The investigation was implemented utilizing Franz's diffusion cell featuring an area of 0.785 cm2. The cellulose membrane was placed between the compartments of the donor and receptor. Precisely measured 1 ml of OLs (15 mg PNL) was set in the donor cells. The receptor compartment was loaded with 50 ml of phosphate buffer (pH 4.6) and kept at 37°C [Citation18]. At an appropriate interval, 1 ml of release media was removed, and an equivalent quantity of fresh media was inserted into the receiver cell. Specimens were withdrawn at 1, 2, 3, 4, 5 and 6 h and scanned at λmax 289 nm. Experimental runs were carried out three times.
2.3.4. D-optimal experimental design
The design was employed to estimate the impact of the independent parameters on the preparation of OLs operating Design-Expert® software (Stat-Ease Inc., Minneapolis, MN, USA). Three variables were assessed as factors: PC amount (mg) (X1), CTAB amount (mg) (X2), and CH amount (mg) (X3). As responses, EE%, PS, ZP, and Q6h (%) were selected (A).
2.3.5. Selection of the optimal PNL- OLs
The choice of the optimal OL was established based on the desirability that permitted the examination of whole dependent variables concurrently. The choice criteria were attaining OL with maximum Q6h (%), ZP, EE%, and minimum PS. The suggestion with the greatest desirability was chosen (A). The optimal OL was formulated, examined, and then compared with the predicted data to guarantee that the model was performing accurately.
2.3.6. Transmission electron microscopy
Transmission electron microscopy (TEM; Joel JEM 1230, Tokyo, Japan) operated at 80 kV was employed to discover the morphology of the optimal OL. The dyed formulation was retained on a copper-covered carbon grid and allowed to dry until TEM inspections were conducted [Citation20].
2.3.7. Differential scanning calorimetry (DSC)
The thermal evaluation of PNL and the optimal OL was done by utilizing differential scanning calorimetry (DSC-60, Shimadzu Corp., Kyoto, Japan). Almost 5 mg of specimens were put into an aluminum pan using a temperature ranging from 10 to 350°C at a rate of 5°C /min below a nitrogen flow (25 ml/min) [Citation21].
2.3.8. Muco-adhesion test
The optimal OL was assessed for the mucoadhesive properties where 1% w/v of mucin was blended with the equal amount of the optimal OL in a dropwise pattern below stirring. For an additional 5 min, the stirring was performed, and the blend was left to equilibrium at room temperature for a whole night. The mucin particles' charge and the mucin charge in the existence of mucoadhesive OL were assessed utilizing a zeta sizer [Citation22].
2.3.9. Effect of short-term storage
The impact of storage on the optimal OL was examined to track the rate of vesicle outgrowth, PNL infiltration, or any physical alternation. The optimal OL was kept for 3 months at 4°C and its stability was then assessed by evaluating the PS, PDI, EE%, ZP, and Q6h (%) of it to the freshly assembled one. Furthermore, the system was optically checked for any particle accumulation [Citation23]. Statistical significance was tested utilizing Student's t-test using SPSS® software 22.0. Based on the model-independent mathematical method of Moore and Flanner, the release of the stored optimal OL was evaluated and compared with the fresh formula. The similarity factor (f2) was computed based on the subsequent equation (EquationEquation 1(1)
(1) ):
(1)
(1)
2.4. Ex vivo studies
2.4.1. Tissue preparation
By making an abdominal opening and disarticulating the pubic symphysis, the new vaginal duct with a thickness of 3 ± 6 mm was attained from female Wistar rats. Initially, the vaginal orifice was isolated from the perineal skin that surrounds it. The vaginal duct was then taken out whole and cut into longitudinal sections, and the anterior vaginal wall was extracted. Subsequently, it was divided into proximal and distal parts by a transverse cut [Citation24].
2.4.2. Ex vivo permeation studies
The investigations were accomplished as in-vitro release studies except fresh vaginal rat tissue was placed between the compartments of the donor and receptor [Citation24]. Precisely measured 1 ml of PNL solution, the optimal OL, PNL-gel, and PNL-loaded OLs-gel (equal to 15 mg) were set in the donor cells. The receptor compartment was loaded with 50 ml of phosphate buffered solution (pH 4.6) and kept at 37°C [Citation25]. At an appropriate interval, 1 ml of permeation media was removed, and an equivalent quantity of fresh media was inserted into the receiver cell [Citation25]. The aliquots were analyzed by HPLC technique [Citation26]. Statistical significance was tested employing one-way ANOVA utilizing SPSS® software 22.0. Post hoc testing was performed utilizing Tukey's honestly significant difference (HSD) test.
2.4.3. Ex vivo confocal laser scanning microscopy studies
To determine the permeability of optimal OL through the vaginal membrane, the fluoro-labeled OL was fabricated as mentioned in the preparation procedure without the addition of the drug, and 10 mg (FDA) was added into OLs instead of PNL. The vaginal rat's tissue was handled with the above-aforementioned order as the ex vivo permeation testing. FDA-loaded OL was placed on the vaginal membrane and kept for 6 h. Utilizing a microtome (Leica Microsystems SM2400, Cambridge, UK), longitudinal sections that had been submitted to fluoro-labeled optimal OL were divided and assessed for the existence of fluorescence in the vaginal tissues employing an inverted microscope (Carl Zeiss, Oberkochen, Germany).
2.5. Preparation of PNL-loaded mucoadhesive OLs-gel
PNL solution and the optimal OL were turned into gel to improve the residence in the vagina where a certain quantity of HPMC was blended with the help of a stirrer to the optimal OL and PNL solution to fabricate an ultimate gel-concentration of 2% w/w.
2.5.1. Evaluation of PNL-gel & PNL-loaded OLs-gel
The gels (PNL-gel and the optimal PNL-loaded OLs-gel) were assessed for physical appearance by visual observations. Further, the gels' pH was inspected at room temperature utilizing a calibrated potentiometer (InolabpH720, WTW, Germany) [Citation27]. The measurements were performed in triplicates.
2.6. Rheology
The viscosity of the samples (PNL-gel, and the optimal PNL-loaded OLs-gel) was tested without further dilution using Brookfield DVIIIultraV6.0RVconeandplate rheometer (Brookfield, Inc., Middleboro, MA, USA) utilizing spindle #CPE40 at 25°C. Whole runs were conducted in triplicate. With a spindle of 30 r.p.m and a shear rate of 60 s-1, the measurements were performed in triplicates [Citation28]. The gels' rheological behavior was tested at a range of speeds (10, 20, 30, 40, and 50 r.p.m.) at 25°C with a 10 s interval between each two successive velocities. The tests were then returned in a downward sequence of velocity. The shear rate (y-axis) was plotted versus the shear stress (x-axis) to depict the gels' rheogram.
2.7. In-silico studies
2.7.1. Molecular docking-coupled dynamics simulation for PNL formulation complex
Computational investigation under vacuum conditions was performed utilizing the AutoDock package v1.2.0 (Scripps Research Institute, CA, USA). Three-dimensional structures of PNL and its combining formulation additives were constructed based on the isomeric SMILES strings adopted from the PubChem database (compound CIDs: 4946, 445639, 65167, 5997, 5974 and 71853 for PNL, OA, PC, CH, CTAB, and chitosan, respectively). Constructed compounds were transformed into 3D structures and energy was minimized at AMBER partial charges/modified forcefield before they ultimately turned into PDBQT. Files exercising the OpenBabel tool v.2.3.1 (National Supercomputer Centre, Linköping, Sweden) for later investigation [Citation29]. Molecular docking protocol proceeded via Lamarckian Genetic Algorithm-driven conformational search below AMBER Forcefield. The Genetic algorithm was implemented for forecasting the docked binding modes [Citation30]. Docking parameters were programmed at binding poses of 20, global search exhaustiveness being defined at 100, and maximum energy differences between poses of 4 Kcal/mol [Citation31]. PyMol v2.0.6 (Schrödinger, NY, USA) was employed for picturing the docking results and investigating the compound's binding interactions.
A docked PNL formulation complex was supposed as a reference structure for distinct molecular dynamics imitations utilizing GROMACS-2019 under CHARMM-General forcefield [Citation32]. The formulation complex was dissolved in a TIP3P cube-shaped box utilizing periodic boundary circumstances at a marginal distance of 10 Å (B) [Citation33]. Standard ionization at pH 8.5 was established while maintaining the entire system neutralized with adequate positive and negative ions of potassium and chloride, respectively. The system was forwarded meanwhile a steep descent-minimization stage for 5 ps [Citation34] while being then equated under NVT (303.15 K) and then NPT (303.15 K; 1 atm. Pressure) ensembles for 100 ps each. For calculating long-range electrostatic interactions, molecular dynamic simulations were performed through 10 ns under the NPT ensemble employing the Particle Mesh Ewald algorithm. LINCS at 2 fs integration time step size was used for representing whole covalent bonds. Both van der Waals and Coulomb's non-bonded interactions at 10 Å were terminated utilizing the Verlet cut-off approach. Ligand-protein binding-free energies, throughout the entire imitation experiments, were speculated utilizing MM_PBSA computations where composing energy terms were demonstrated and investigated.
2.8. In vitro antifungal activity
The antifungal action of PNL against C. albicans ATCC10231 was tested [Citation35]. The minimum inhibitory concentration (MIC) was detected utilizing the microdilution approach following the guidelines of the Clinical and Laboratory Standards Institute and as described before [Citation36,Citation37].
2.9. Effect of the optimized formulas on the antibiofilm activity of PNL
2.9.1. Biofilm inhibition assay
The antibiofilm action of the PNL-loaded OLs-gel was correlated to that of the PNL-gel. The biofilm inhibition assessment was achieved utilizing flat-bottom 96-well plates as described before [Citation22,Citation38]. C. albicans ATCC10231 was the used tested organism and Sabouraud dextrose broth was the used media. Different sub-MIC concentrations (781.25–48.83 μg/ml) of the formulas were tested. Untreated wells were used as controls (100% reference) [Citation35].
2.9.2. Biofilm detachment assay
The capability of PNL-loaded OLs-gel and PNL-gel to detach and eradicate the formerly established C. albicans ATCC10231 biofilm was investigated. The experiment was performed as described before [Citation22]. Different sub-MIC concentrations (781.25–48.83 μg/ml) of the tested formulas were added to the biofilm plate. Nothing was added to the biofilm control wells (untreated biofilm, 100% reference) [Citation35].
2.10. In vivo studies
2.10.1. Animals
All the animal approaches were confirmed by the Research Ethics Committee of the Faculty of Pharmacy, Cairo University (MI3395) following the “Guide for the Care and Use of Laboratory Animals” issued by the Institute of Laboratory Animal Research (Washington, DC, USA). 39 female Wistar rats aged 6–7 weeks were obtained from Animal House, Faculty of Pharmacy, Cairo University. The animals were given access to feed and water. They also were habituated to three per cage and maintained at 21°C and 50–55% humidity under a 12:12 h light–dark cycle. At the end of the experiments, animals were sacrificed by decapitation under anesthesia using ketamine (30 mg/kg) at the animal house of the Faculty of Pharmacy (Cairo University).
2.11. In vivo fungal vaginal colonization model
Female Wistar rats aged 6–7 weeks (155 ± 16 gm) were used in a murine Candida vaginal colonization model as described before [Citation39,Citation40]. Estradiol (0.5 mg/kg) was intraperitoneally injected for 24 h before the fungal colonization for the induction of fungal vaginal infection by changing the hormonal levels and inducing the pseudo-estrus cycle in rats where estrogen acts in maintaining vaginal epithelium, pH, and fungal and bacterial colonization hence, augmented estrogen levels influence the vaginal environment and augment its susceptibility to vaginal infections. Dexamethasone (0.4 mg/kg) was intraperitoneally injected once daily 24 h before starting the colonization for three successive days during the induction of colonization to induce the fungal vaginal infection, to modulate the immune system and generate an immunosuppressed state in the rats and increase the susceptibility to vaginal infections. Fungal colonization was induced via vaginally inoculating the rats with C. albicans ATCC10231 suspension (7–15 × 108 CFU) for four successive days. Rats were at random divided into four groups (n = 6) and treatments were taken intra-vaginally (250 μl of the corresponding treatment utilizing a tip) 24 h post the last inoculation. One group acted as the negative control group and did not get any medication. The second group was handled with the vehicle gel to serve as the vehicle control. The third group was handled with the PNL-gel, and the fourth group was handled with the PNL- loaded-OLs gel. The vagina of every rat was rubbed with a sterile swab at three time intervals (24, 48, and 72 h). The recovered C. albicans in the swaps were serially diluted in phosphate-buffered saline and placed on Sabouraud dextrose agar for the viable count [Citation39]. The outcomes of the examined groups were analyzed and contrasted.
2.12. Histopathological safety assessment
For the histopathological safety assessment, 15 female Wistar rats were randomly distributed into five groups (n = 3) and medications were administered intra-vaginally (250 μl utilizing a soft plastic tip) once daily for 1 week. One group served as negative control (not infected and did not get any medication), the second group was infected not cured rats, the third group was handled with the vehicle gel, the fourth group was handled with the PNL-gel and the fifth group was handled with the PNL-loaded OLs-gel. At the end of the experiments, the tissues were preserved in a 10% formalin prior to preparing the sections utilizing the paraffin method [Citation20,Citation35]. The sections of the treated vaginal tissues were correlated to that of the untreated ones.
2.13. Immunohistochemistry (TNF-α expression)
Tissue sections measuring 5 μm were broken into sticky slides, deparaffinized, rehydrated, and then submitted to endogenous peroxidase blockage, heat, and protein blockage to perform the epitope retrieval method. Primary mouse monoclonal anti-TNF-α (at a dilution of 1:150) was utilized for 12 h at 4°C. Following washing, a secondary HRP-labeled antibody was utilized at room temperature for 2 h. DAB-Substrate kit was employed to prepare the color. By deletion of primary antibodies, negative slides were attained. Positive representation was measured as area % utilizing CellSens dimensions (Olympus software). Statistical testing was calculated employing the SPSS® version 22 software using analysis of variance (ANOVA) followed by Tukey's multiple comparisons [Citation41].
3. Results
3.1. Optimization of OLs using D-optimal design
The D-optimal design was utilized to prepare OLs, utilizing Design-expert®, resulting in 16 experiments without stating the non-significant dependent variable (PDI) (A). A quadratic model was chosen for EE%, PS, and ZP, respectively, and 2FI for Q6h (%). For adequate precision, all dependent variables were recorded a number higher than 4 as displayed in B. The outcomes of predicted R2 agreed in whole responses with the outcomes of adjusted R2 (B). The EE% for the formed PNL-OLs ranged from 59.18 ± 0.74 to 79.57 ± 0.76% (A). The impact of the factors: PC amount (mg) (X1), CTAB amount (mg) (X2), and CH amount (mg) (X3) on the EE% of PNL-OLs is graphically illustrated as 3D surface plots in (A & B). PC amount (mg) (X1) had a significant positive impact on EE% (p < 0.0001). The EE% for the formula comprising 120 mg PC showed the greatest values relative to other formulations that contained low amounts of PC. Further, the CTAB amount (mg) (X2) showed a significant (p < 0.0001) positive impact on EE%. For CH amount (mg) (X3), the factor revealed a nonsignificant impact on EE% with a p-value of 0.6216.
Table 2. Experimental runs, independent variables, and measured responses of the D-optimal design of propranolol hydrochloride-loaded oleosomes (A) and output data of the D-optimal analysis of oleosome formulations and predicted and observed values for the optimal oleosome (B).
Figure 1. Effect of formulation variables on EE% of PNL-OLs (A–B), PS (C–D), ZP (E–F), and Q6h (%) (G–H).
CH: Cholesterol; CTAB: Cetyltrimethylammonium bromide; EE%: Entrapment efficiency percentage; OL: Oleosome; PC: Phospholipid; PNL: Propranolol hydrochloride; PS: Particle size; Q6h (%): Amount of drug released after 6 h; ZP: Zeta potential.
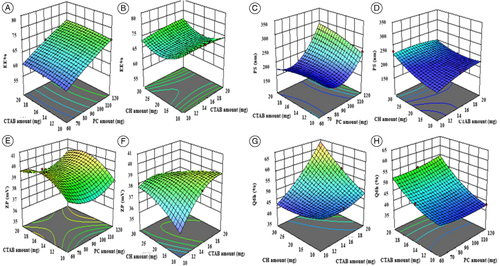
The PS for the formed PNL-OLs ranged from 170.95 ± 0.64 to 328.52 ± 1.24 nm (A). The impact of the factors: PC amount (mg) (X1), CTAB amount (mg) (X2), and CH amount (mg) (X3) on the PS of PNL-OLs is graphically illustrated as 3D surface plots in C–D. ANOVA outcomes showed that PC amount (mg) (X1) had a significant positive impact (p = 0.0002) on PS. Regarding the CTAB amount (mg) (X2), the factor exhibited a nonsignificant effect on the response with a p-value of 0.1156. Also, statistical testing manifested that the PS of the OLs augmented with the increment of CH (mg) (X3) with a p-value of 0.0014.
Regarding PDI, a zero number expresses a completely symmetric dispersion, on the contrary, a 1-number expresses a perfectly asymmetric dispersion. The model was nonsignificant hence it was removed from optimization. The PDI of the evaluated OLs ranged from 0.164 ± 0.01 to 0.638 ± 0.03 (A). The illustrated PDI is widespread in NCs prepared utilizing a thin-film hydration approach [Citation18].
The ZP values for the formed PNL-OLs ranged from 35.37 ± 2.59 to 40.88 ± 1.39 mV (A). The impact of the factors: PC amount (mg) (X1), CTAB amount (mg) (X2), and CH amount (mg) (X3) on the ZP of PNL-OLs is graphically illustrated as 3D surface plots in (E–F. Only the increase in CTAB amount (mg) (X2) had a significant positive impact on ZP (p = 0.0010).
The amount of PNL released after 6 h Q6h (%) ranged from 37.21 ± 1.57 to 64.88 ± 0.41% (A). The impact of the factors; PC amount (mg) (X1), CTAB amount (mg) (X2), and CH amount (mg) (X3) on the Q6h (%) of PNL-OLs is graphically illustrated as 3D surface plots in G–H. Regarding PC amount (mg) (X1) had a nonsignificant impact on Q6h (%) (p = 0.580). The increment in CTAB amount (mg) (X2) had a significant positive effect on Q6h (%) (p < 0.0001). In addition, the increase in CH amount (mg) (X3) had a significant positive impact on Q6h (%) (p = 0.0002) where CH at 30 mg (the highest amount) led to a significant release of PNL compared with other formulae (with lower amounts).
3.2. Determination of the optimal OL
The desirability of the optimal OL was 0.89 which is recommended to be assembled utilizing 120 mg as PC amount (X1), 20 mg as CTAB amount (X2), and 30 mg as CH amount (X3). Thus, it was formulated and assessed. As shown in B, the predicted and the observed results for the optimal OL illustrated a good correlation. Thus, the optimal OL was regarded as promising to be used in further assessment.
3.2.1. Transmission electron microscopy
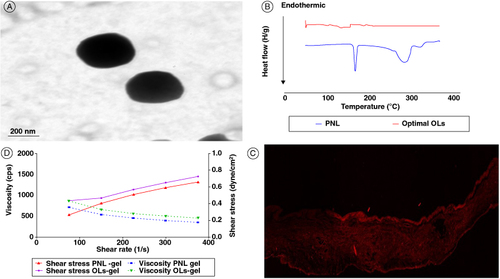
The evaluation of the optimal OL shape revealed spherical vesicles with a regular size distribution (A). The PS of the optimal OL was estimated by Zetasizer, and it was correlated perfectly with transmission electron microscopy (TEM) outcomes.
Figure 2. Transmission electron micrographs of the optimal OL (A). Differential scanning calorimetry study for the optimal OL (B). A tile scan confocal laser microscope photomicrograph of a longitudinal section in vaginal rat tissue treated with FDA-loaded OL (C), and rheological characterization of PNL-gel and PNL-loaded OLs gel (a plot of viscosity and shear stress versus shear rate). The measurements were performed at varying shear speeds (10–50 rpm) with 10 s between every two consecutive speeds (D).
FDA: Fluorescein diacetate; OL: Oleosome; PNL: Propranolol hydrochloride.
3.2.2. Differential scanning calorimetry
From B, PNL showed an endothermic peak attributed to its melting point at 166.91°C [Citation18]. The optimal OL thermogram did not reveal the melting peak for PNL.
3.2.3. Mucoadhesion test
ZP of the mucin suspension (-9.77 ± 0.53 mV) changed upon mixing with optimal OL to positive 31.43 ± 0.80 mV of mucin ZP.
3.2.4. Effect of short-term storage
At the end of the storage interval, there was no marked variation in the appearance of the optimal OL dispersion. The evaluated physical characteristics of the stored OL compared with the fresh one with EE% of 78.50 ± 0.005%, PS of 334.34 ± 5.89 nm, PDI of 0.630 ± 0.01, ZP 38.64 ± 0.20 mV and Q6h (%) of 65.12 ± 0.89% were statistically analyzed and displayed that there was no significant difference (p > 0.05) in the EE%, PS, PDI and Q6h (%). In addition, both fresh and stored OLs showed almost identical profiles. This result was proved by the computed value of the similarity factor (f2 = 82.62) indicating that the storage had no remarkable impact on the release of the PNL.
3.3. Ex vivo studies
3.3.1. Ex vivo permeation
The amount of PNL permeated was 1289.35 ± 31.88, 1078.97 ± 39.98, 969.01 ± 21.0,6 and 759.18 ± 15.03 from PNL solution, the optimal OL, PNL-gel, and the optimal PNL-loaded OLs-gel, respectively. The previous findings show that the amount of PNL diffused from optimal PNL-loaded OLs-gel was significantly (p < 0.5) the least compared with PNL-gel, the optimal OL, and the PNL solution. Regarding the permeation parameters, (permeation flux Jss) it was 222.66, 182.02, 169.80, and 134.15 μg/cm2/h, and for the permeation coefficient it was 0.04453, 0.03640, 0.03396, and 0.02683 for PNL solution, optimal OL, PNL-gel and optimal PNL-loaded OLs-gel, respectively.
3.3.2. Ex-vivo confocal laser scanning microscopy studies
Confocal laser scanning microscopy studies (ClSM) images (C) show intense and thoroughly distributed fluorescent signals at the upper layer of the vaginal epithelium after the topical application of mucoadhesive OLs to the surface of vaginal rats.
3.4. Evaluation of PNL-gel & PNL-loaded OLs-gel
All formulated gels displayed no signs of sedimentation or accumulation. The measured pH value for gel preparations ranged from 5.1 ± 0.02 to 5.3 ± 0.10, which is seen as appropriate for vaginal application.
3.5. Rheology
At 10 rpm, the viscosity of the PNL-gel was 711.6 cps, while it was 861.3 cps for PNL-loaded OLs-gel. As revealed in D, the rheological behavior of both PNL-gel and PNL-loaded OLs-gel displayed a shear-thinning pseudoplastic flow where the increment of the shear rates the viscosity diminished.
3.6. In silico studies
3.6.1. Molecular docking-coupled dynamics simulation for PNL formulation complex
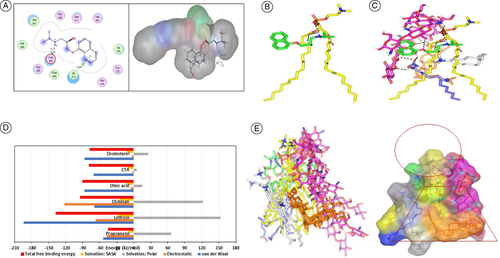
An exploratory study utilizing molecular docking was conducted to obtain further insight into the orientations, interactions, and modalities of PNL binding into the active site of lanosterol 14α-demethylase-demethylase enzyme. Molecular Operating Environment (MOE, 2010.10) was the software used to conduct the docking investigation. The x-ray crystallographic structure of lanosterol 14α-demethylase enzyme (PDB ID: 4UYL-Hargrove. 2015) obtained from the Protein Data Bank (PDB) (RCSB Protein Data Bank (RCSB PDB) UK., 2015). Generally, PNL demonstrated a comparable binding pattern in the binding site of 14α-demethylase enzyme with a predicted docking energy score of -5.1834 kcal/mol in comparison to fluconazole, lanosterol 14α-demethylase enzyme inhibitor, binding score of -6.3551. A illustrates the promising binding pattern of PNL with the essential amino acids at the lanosterol 14α-demethylase enzyme binding site. The isopropyl amine moiety interacted through a hydrogen bond with the crucial Cys463 residue in the 14α-demethylase binding site. In addition, the naphthalene scaffold demonstrated π-H interaction with Ile373 residue. It's important to note that there was a strong correlation between the findings from the biological screening and the docking investigations.
Figure 3. 2D diagram and 3D representation of propranolol hydrochloride in the 14α-demethylase binding site (A), and predicted binding poses of PNL-PC docked complex. Three-dimensional representation of PNL (green sticks) loaded on PC interface (yellow sticks), alone (B) and in combination (C) with formulation additives; chitosan (magenta sticks), OA (blue sticks), CTAB (white sticks), and CH (orange sticks). Context-described polar interactions are represented as black dashed lines. Binding free energy and configuration of simulated phospholipid, (D) MM_PBSA free binding energy calculations and dissected energy terms for formulation components, and (E) overlay of PNL-PC formulation complex across MD simulation frames (left panel) and molecular surface 3D-representation of the inverted cone micellar configuration within 100% aqueous solvation system (right panel). The Molecular surface and sticks 3D representations were illustrated in colors previously assigned for the optimized formulation components; green, yellow, magenta, blue, white, and orange colors for PNL, PC, chitosan, OA, CTAB, and CH, respectively.
CH: Cholesterol; CTAB: Cetyltrimethylammonium bromide; OA: Oleic acid; PC: Phospholipid; PNL: Propranolol hydrochloride.
3.6.2. Computational investigation for formulation binding affinity & thermodynamic stability
The drug illustrated (PNL) favored orientation with its ionizable amino propyl alcohol arm toward the negatively charged phosphate head of PC. Triple polar interactions were depicted, two were mediated by the drug's ionizable amino group (pKa = 9.45) toward the oxygen functionality of the PC, while a single hydrogen bonding was mediated by the drug's free hydroxyl group. The three polar interactions were at quite favored angles and close distances (3.3 Å/128.8°, 2.6 Å/152.8° and 2.3 Å/130.2°, respectively). On the other hand, the PNL's aromatic scaffold exhibited close contact with PC's hydrophobic extended acyl chains (B). Despite the relevant polar-mediated binding affinity between the PNL-PC complex, further stabilization was required to be observed with PNL achieving humble docking binding energy (-3.14 Kcal/mol). Investigating the PNL-PC complex in the presence of the formulation additives; chitosan, CTAB, OA, and CH, illustrated more stabilized and favored binding interactions (C). Owing to the extended conformation and the high polar potentiality of chitosan, this formulation additive depicted extended orientation around the PNL-PC binding complex mediating several favored strong polar interactions for stabilizing the constructed complex. Two successive 1→4 β-linked glucosamine monomers (M2 and M3) depicted extended polar interactions with the PC phosphate group (–OPO(O)OH). Three strong hydrogen bonds were mediated via the chitosan free C3-OH, C6-OH and C2-NH2 functionalities (hydrogen bond distance/angle; 2.3 Å/131.8°, 2.3 Å/166.3° and 2.5 Å/121.6°, respectively). On the other hand, the chitosan's penultimate glucosamine monomer (M8) furnished relevant hydrogen bond pairing with the PNL oxygen linker via its free C6-OH group (2.9 Å/124.0°). The docking of OA at the drug-PC complex was facilitated via triple polar contacts mediated by the oleic carboxylic group and chitosan's free C2-OH at M2 and C6-OH at M3 (2.8 Å/128.5° and 3.1 Å/139.6°), in addition to oxane (O) atom of M1 (2.9 Å/135.9°). Such binding interaction allowed a favored orientation of the OA's extended aliphatic chain at closer proximity toward the PC's acyl chains (∼ 4.6 Å) for mediating strong favored hydrophobic binding interactions.
The stability of CH at the drug-PC complex was mediated mainly through close-range non-polar interactions between the molecule's hydrophobic cholestane ring (logP = 8.72) and extended PC lipophilic chains. Notably, chitosan permitted further stability for the CH moiety at the PC interface where the centrally linked glucosamine monomers (M3 and M4) predicted multiple strong hydrogen bond pairs with CH's terminal OH group (1.6 Å/156.8°, 2.4 Å/144.1° and 2.1 Å/129.5°). The last formulation additive, CTAB depicted favored orientation at the drug formulation complex anchoring its polar head near the chitosan-PNL hydrophilic interface satisfying electrostatic potentiality of hydrogen bond donners and acceptors paving the way for an overwhelming non-polar energy contribution between CTAB lipophilic tail and PC acyl chains as well as CH's hydrocarbon cage. The furnished docking energy for PNL was considered favored at -7.87 Kcal/mol.
Thermodynamic stability and dispersion behavior of the PNL-PC complex within the formulation ultimate solvent (100% water) across time frames were evaluated through molecular dynamics simulations in the TIP3P water model (A). The root-mean-squared deviation (RMSD) of the simulated complex compared with its reference position illustrated minimal fluctuations across the simulated time (B). This was obvious since RMSD trajectories depicted rising across the premier times because of system relaxation followed by a leveling off around particular averages (5.08 ± 0.25 Å) for more than half the imitation run (>25 ns). The whole system was further highlighted as being relaxed and balanced by monitoring the kinetic, potential, and total energies across the entire time frames (C). The depicted minimal fluctuation across the entire simulation runs confers adequate system stability and balance. Conformational analysis across extracted simulated frames (10, 20, 30, and 40 ns) illustrated significant stability for the simulated PNL-PC formulation complex (D). PNL maintained its orientation at the PC's polar phosphate head till the end of the simulation run. Chitosan formulation additive kept coherent polar contacts with PC and PNL, in addition to OA and CH across most of the simulation time. Both OA and CTAB provided non-polar binding support for the PC acyl chains as well as the highly lipophilic steroidal nucleus of CH.
Figure 4. Propranolol hydrochloride–phospholipid formulation complex across explicit molecular dynamics simulation within a 100% aqueous solvation system. (A) Solvated PNL-PC formulation complex within TIP3P water cube and ionizable potassium and chloride atoms; (B) RMSD trajectories for simulated formulation complex versus time frames in nanoseconds (ns); (C) Plots for the system's total energy and its constituting terms (kinetics and potentials) versus the simulated time frames (ns); (D) Conformation alterations-time evolution of PNL-PC-formulation additive heterocomplex. Thermodynamic movements formulation components: PNL (green sticks), PC interface (yellow sticks), chitosan (magenta sticks), OA (blue sticks), CTAB (white sticks), and CH (orange sticks) were monitored over MD simulation trajectories being captured at different snapshots ① 10 ns, ② 20 ns, ③ 30 ns, and ④ 40 ns. Polar interactions, discussed within context, are depicted as black dashed lines.
CH: Cholesterol; CTAB: Cetyltrimethylammonium bromide; OA: Oleic acid; PC: Phospholipid; PNL: Propranolol hydrochloride.
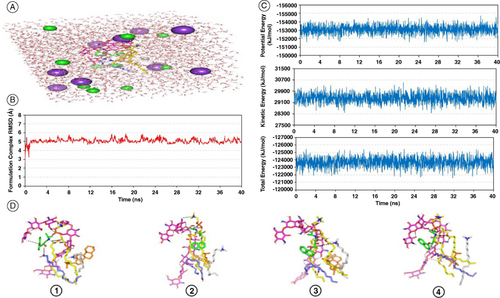
To further explore the nature of binding among the PNL formulation complex, the MM_PBSA equation was employed for estimating the free binding energies in addition to the contributing energy terms. Interestingly, Van der Waal potentials showed superior energy contributions over Coulomb's electrostatic interactions for all formulation components (D). The highest total energy contribution was assigned for PC (-137.52 ± 30.59 kJ/mol) followed by chitosan (-94.70 ± 37.89 kJ/mol) serving as the backbone and binding platforms for all PNL formulation complexes. Electrostatic potentials were of the highest value for chitosan (-122.06 ± 16.66 kJ/mol) the thing that is highly reasoned for its role in mediating polar contacts via its large number of hydrogen bond donors and acceptors. On the contrary, minimal electrostatic energy contributions were assigned for OA, CTAB, and CH (-2.65 to -5.69 kJ/mol) owing to their preferential orientation at the PC's acyl chains. Both PC and chitosan depicted high solvent accessible surface area (SASA) non-polar solvation energies (-28.65 ± 4.50 and -25.29 ± 2.91 kJ/mol, respectively), being correlated to their extended conformations and large structures as compared with other formulation components. Finally, these two formulation additives again showed high polar solvation penalties against binding (152.88 ± 90.80 and 122.05 ± 47.69 kJ/mol, respectively). Regarding the locative conformation of PC lipophilic chains, important observations were depicted. The preserved polar interactions near the phosphate present in the PC carrier as well as the maintained strong hydrogen bond pairing between the PC acyl group and chitosan allowed for the hydrophobic acyl tails of the PC to be dragged apart from one another. The ultimate dynamic behavior provided an open-compass conformation for the PC elongated tails, that might augment the hydrophobic chain volume. Conversely, the limited surface area was maintained throughout the imitation trial because the PNL-chitosan-PC complex furnished several robust compact hydrogen bond interactions near the phosphate polar head (E).
3.7. In-vitro antifungal activity
PNL demonstrated promising antifungal activity against C. albicans ATCC10231 with MIC of 1.5625 ± 0 mg/ml.
3.8. Effect of the tested formula on the biofilm activity
The anti-biofilm potency of PNL-loaded OLs-gel and PNL-gel against C. albicans ATCC10231 was tested. PNL-loaded OLs-gel and PNL-gel significantly inhibited C. albicans ATCC10231 biofilm formation at all the tested concentrations (p < 0.05). PNL-loaded OLs-gel showed significantly greater biofilm inhibition activity against C. albicans ATCC10231 than PNL-gel at concentrations MIC/2, MIC/4 and MIC/8 (781.25–195.3125 μg/ml) (p < 0.05). PNL-loaded OLs-gel and PNL-gel significantly detached the formerly formed C. albicans ATCC10231 biofilm at all the tested concentrations (p < 0.05). Both PNL-loaded OLs-gel and PNL-gel showed non-significant differences in the detachment activity against C. albicans ATCC10231 biofilm at all the tested concentrations (p > 0.05).
3.9. In vivo studies
3.9.1. In vivo fungal vaginal colonization model
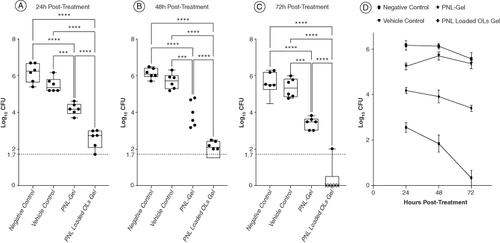
The antifungal potency of the PNL was investigated using a murine model of fungal vaginal infection. Four groups of female Wistar rats (n = 6) were vaginally infected with C. albicans ATCC10231 suspension. The application of the PNL-loaded OLs-gel significantly diminished the fungal count retrieved from the infected-vagina relative to the vehicle control, and negative control groups at all tested time intervals (p < 0.0001) (). The PNL-loaded OLs-gel significantly enhanced the in vivo antifungal activity of PNL where the antifungal activity of the PNL-loaded OLs-gel was significantly greater than that of PNL-gel (p < 0.0001) (A). The fungal load recovered from the PNL-loaded OLs gel-treated group on the last day of the experiment was 5.014 and 5.242 logs less than that of the vehicle control and negative control groups, respectively. The fungal load recovered from the PNL-gel-handled group on the last day of the experiment was 1.952 and 2.18 logs less than that of the vehicle control and negative control groups, respectively. Interestingly the application of the PNL-loaded OLs-gel completely eradicated the colonized fungi from the vagina of rats by the last day of the experiment (72 h post-treatment) (C). There was no significant variation between the fungal loads retrieved from the vehicle control group and the negative control (no treatment) group at all the tested time intervals (p > 0.05) (D).
Figure 5. Efficacy of propranolol hydrochloride-loaded oleosomes gel in an in vivo murine model of fungal vaginal infection. 24 Wistar rats were divided into four groups (n = 6). One group served as the negative control group and did not receive any treatment. The second group was treated with the empty gel to serve as the vehicle control. The two other groups were treated with either drug gel or nanogel. Each data point in the figure represents a rat. Results are expressed as box plots of fungal loads recovered from the vaginal lumen at 24 h (A), 48 h (B), and 72 h (C) post-treatment. The whiskers span the difference between the minimum and maximum readings and the horizontal bar represents the median. The horizontal line represents the limit of detection of fungal loads. **, ***, and **** indicate that the difference is significant at p < 0.01, <0.001, and <0.0001, respectively (one-way ANOVA, Tukey's post-hoc test). X means no colonies were detected in the sample.
OL: Oleosome; PNL: Propranolol hydrochloride.
3.9.2. Histopathological safety assessment
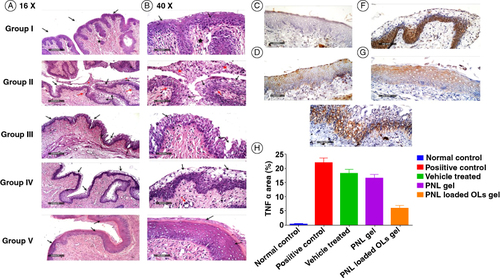
Microscopic examination (A–B) of rat vaginal samples showed for the normal control group normally organized histological structures of the vaginal wall including apparent intact covering stratified squamous epithelial layers (black arrow), intact submucosal connective tissue layers without abnormal infiltrates (star) as well as intact outer muscular coat. Positive control (infected samples not treated) demonstrated marked disruption of covering epithelial layer with significant thickening accompanied by vacuolar degenerative changes of luminal layers (black arrow) with luminal exudate formed of exfoliated cells mixed with inflammatory cells (red star). Moderate submucosal mixed inflammatory infiltrates were observed (red arrow). Regarding the vehicle control treated group, it showed degenerative and disrupted changes of covering epithelial layers (black arrow) with minimal exfoliation of epithelial cells into the vaginal lumen, as well as mild submucosal inflammatory cells, infiltrates. In addition, the PNL-gel-treated group showed mild persistent submucosal inflammatory infiltrates in all examined samples (red arrow). However, persistent records of degenerative changes of covering epithelial layers in mid and luminal layers with intact basal layers were shown (black arrow). PNL-loaded OLs-gel treated group showed significant protective efficacy with more organized morphological features of the vaginal wall including apparent intact covering epithelium without abnormal microscopic alterations (black arrow) with intact submucosal layers without abnormal infiltrates (black star).
Figure 6. Light microscope photomicrographs showing histopathological sections (hematoxylin and eosin stained) of rat vagina normal control (group I), positive control (group II), vehicle-treated group (III), rat vagina treated with PNL gel (group IV) and rat vagina treated with PNL-loaded OLs gel (group V) with a magnification power of 16× to illustrate all vagina layers (A) (Left side) and magnification power of 40× (B) (Right side). Microscopic images of vaginal tissues, immunostaining of TNF-α. (C) Normal control, (D) positive control, (E) vehicle-treated group, (F) PNL-gel, (G) PNL-loaded Ols gel, and (H) immune expression of TNF-α (area%).
OL: Oleosome; PNL: Propranolol hydrochloride.
3.10. Immunohistochemistry (TNF-α-expression)
As displayed in (C–H), the greatest value of TNF-α was proved in a positive control group (D). PNL-gel (F) and PNL-loaded OLs groups (G) displayed a significant diminish in TNF-α relative to the positive control. The highest significant (p < 0.05) diminish in TNF-α expression was proved in PNL loaded OLs gel-treated group supporting that it might be able to lessen the inflammatory response upon vaginal application.
4. Discussion
The stepwise screening of the components for the preparation of NC is required to set the effective ranges of the factor [Citation42]. The approach of response surface relies on discovering the optimum characteristics for a specific response aim with the least number of experiments. Regarding EE% the increase in PC amount (mg) (X1) could improve EE% due to the development of strong cohesive layers surrounding OLs and thus might minimize the infiltration of PNL [Citation43]. Further, previous literature showed similar outcomes during their preparation of PC-containing vesicles [Citation44]. Moreover, the increase in EE% with the increase in the CTAB amount (mg) (X2) could be explained by CTAB's capability to improve the inclusion of the drug inside the PC bilayer [Citation45]. In addition, the inclusion of positively charged lipids (CTAB) in high amounts can increase the hydrophobicity of vesicles and lead to an increase in EE% [Citation46].
Considering PS results, previous literature stated that displaying PS of the prepared OLs range might enhance vaginal tissue uptake and retention [Citation47]. When the PC amount (mg) X1 was augmented the viscosity of the internal phase subsequently increased, which furthermore produced an increment in surface tension, hence, resulting in the development of OLs with larger PS [Citation48]. It is worth noting that the increase in PS by increasing PC amount is correlated with EE% results as previous literature reported a direct positive relation between PS and EE% [Citation49]. Also, with the increment of CH (mg) (X3), the PS augmented the previous findings might be attributed to the bulkiness of CH that led to the fabrication of larger OLs -PS [Citation50].
For ZP, surface cationic modification, which depends on the electrostatic interaction with negatively charged mucin in mucus and mucosal cells, is one of the most favorable mucoadhesive approaches. Cationic functionalization for the vaginal formulation was reported to significantly prolong the intravaginal drug residence and improve mucosal infiltration [Citation51]. It is worth noting that the positive charges, brought by the cationic head group of CTAB and the positive amino group of chitosan, gradually neutralize the negative charges of OA and PC, resulting in a charge reversal and ultimately a positive charge on the OLs [Citation48]. The increase in CTAB amount (mg) (X2) increases ZP the previous outcomes agreed with the previous literature [Citation52]. This could be related to the increase of the cationic group of CTAB as it is composed of a tetrasubstituted ammonium cationic surfactant with positively charged quaternary nitrogen and alkyl-chain of C16 [Citation53].
For the release profile of the drug, the primary burst release of a drug is useful because it helps to attain therapeutic concentration in a negligible time frame after that it maintains a steady release to keep up a managed and controlled concentration of medicine [Citation54], thus was observed in the release pattern of the formed OLs. The increment in CTAB amount (mg) (X2) increased Q6h (%) which could be because the vesicles became smaller, augmenting the accessible surface area for drug release [Citation55]. In addition, the increase in CH amount (mg) (X3) increased Q6h, this might be because CH might disorganize the uniform structure of the vesicle membrane, hence favoring PNL leakage from vesicles [Citation56].
The optimal OL thermal analysis manifested that PNL was in an amorphous form, and it was completely entrapped into the OL [Citation57]. Further, the optimal OL showed good mucoadhesive aspects, as a positive sign was attained from the complexes formed between mucin and OL, where the mucin negative sign might be neutralized with the positive OL sign. It was previously stated that the adhesion of the polymer could modify the surface charge of the mucin if the polymer has a mucoadhesive aspect [Citation58]. On the other hand, prolonged contact of the drug-containing formulation with the vaginal surface is necessary for the successful topical vaginal treatment. To obtain a local distribution to vaginal tissue, permeation into/through the mucus, steady drug delivery into the tissue, and high concentration of the medication are demanded. A mucus is an adhesive barrier that adheres to most particles and blocks their penetration into the epithelium surface [Citation59]. Chitosan, when utilized as a coating, allows vesicles to permeate through the vaginal tissues by the adhesive interactions among the nanoparticles and mucus. The combined effect between the aspects of OLs as a protecting carrier for PNL and the mucoadhesive aspects of chitosan available on OLs surface permits the evolution of a vaginal drug-delivery system offering the controlled drug release near the vaginal epithelium. Furthermore, the presence of chitosan aids in forming more stable OLs because of the steric stabilization impact produced by chitosan coating present in OLs structure [Citation60].
Regarding ex-vivo permeation studies, previous literature stated that the mucoadhesive drug-delivery system improves the residence time at the demanded site and extends adjacent contact between the dosage form and the desired tissue. This could affect the bioavailability and increase drug penetration [Citation61]. Abdellatif et al. suggested for vaginal candidiasis management, it is necessary to restrict the systemic contact of the patients to the antifungal medication. Hence, when minimal medication crosses via the vaginal mucosa, vaginal candidiasis management becomes more efficient with minimal systemic impacts [Citation25]. The significantly lower permeation from the gels (OLs-loaded gel and PNL-gel) might be attributed to the presence of HPMC k4m as a polymer which increases the viscosity of the formulation producing a controlled release profile [Citation62]. Moreover, the significant (p < 0.05) lower permeation of optimal PNL-loaded OLs-gel relative to whole formulae, and the lower permeability profile from the optimal OL relative to PNL solution was in addition attributed to the presence of lipids (PC, CH, and OA) and polymers (chitosan coating) that might successfully encapsulate PNL and provide sustained release, protection and controlled release profiles. Furthermore, previous investigations suggested that the presence of OA served as a depot for the slow and controlled release of the active substance [Citation63]. It is worth bearing in mind that the amphoteric characteristic of PNL rationalized its higher permeability from PNL solution relative to the other formulations [Citation18].
Ex vivo CLSM confirmed the adhesion of the mucoadhesive OLs to the vaginal epithelium which is due to the mucoadhesive features associated with such systems. Further, the fluorescence present throughout the vaginal cross-section confirmed the author's speculation about the ability of mucoadhesive OLs to successfully diffuse throughout the vaginal mucosa and retain within the vaginal tissues exerting the anticipated pharmacological impact.
Besides, because the vaginal cavity is small, just a small amount of gel needs to be administered. Therefore, for improved localization and detention for a medication, the designed gel should be smoothly instilled and maintain its viscosity in the vagina. Generally, polymeric systems, such as hydrophilic gels, present non-Newtonian, pseudoplastic behavior, which participates in their spreadability when applied on a surface. Although gels should spread smoothly through the vagina, this characteristic might cause leakage. Typical vaginal gels should display a premium capability to distribute through the mucosa to completely cover the vaginal surface, coupled with appropriate retention aspects that permit the maintenance of the formulation [Citation64]. Moreover, according to earlier research, a gel's desired viscosity for the vaginal route needs to be between 200 and 5000 cp favoring both gels with perfect viscosity [Citation18]. Fortunately, as stated in the results section the rheological behavior of both PNL-gel and PNL-loaded OLs-gel displayed a desired viscosity, shear-thinning pseudoplastic flow where the increment of the shear rates the viscosity diminished.
Considering the in silico study results the magnitude and nature of interaction for PNL with the adopted formulation additives were investigated through a molecular docking study. Throughout the in silico investigation, the nature of PNL–PC interaction, in the absence of other formulation additives, was predicted to be dominated by Coulomb's electrostatic potentials. Both demonstrated PNL-PC binding mode and preferential orientation near the phosphate head were consistent with several reported studies investigating the binding affinity of drug-like small molecules such as spironolactone, rosuvastatin, levocetirizine, or metformin toward the PC molecule [Citation65–68]. Interestingly, the chitosan formulation additive served as a supportive/connective platform allowing favored anchoring of the other two formulation additives: OA and CH, within the drug-PC complex. It came to our delight that a more stabilized PNL-PC complex was depicted following the introduction of the four formulation components where an extended network of both electrostatic and hydrophobic potentials was illustrated. These docking observations governed to illustrate the improved formulation parameters post the insertion of chitosan formulation additive where it is controlled to act as a carrier agent intermediating the PNL loading on the PC molecule for optimized drug solubilization.
To further validate our furnished docking results, thermodynamic stability and dispersion behavior of the PNL-PC complex within the formulation ultimate solvent (100% water) across time frames were evaluated through molecular dynamics simulations. Generally, molecular dynamics simulation studies are considered effective for investigating the dynamic nature of compound-target complexes as well as their respective relative stabilities. Furthermore, molecular dynamics simulations are particularly special to be applied for exploring the compound-target conformational space through a more efficient approach that is more beneficial over the static image provided by molecular docking and even the most sophisticated mechanics energy minimization approaches and flexible docking protocols. Within the simulated TIP3P water model, the formulation complex demonstrated low RMSD trajectories with minimal fluctuations across the simulated time. Typically, low-value RMSDs have been correlated with minimal conformational alteration and high stability profiles for the simulated complex. Dominant electrostatic potential was illustrated for the whole complex yet with a couple of formulation additives; PC and chitosan, exhibited high polar solvation energies. These formulation additives were suggested to compromise the total formulation binding free energy since the binding process is considered a solvent displacement process. However, the higher van der Waal hydrophobic energy contributions depicted with PC managed to partially compensate the solvation enthalpy the thing that translated into non-compromised total binding energy as compared with chitosan. The PNL formulation further highlighted an open-compass conformation for the PC elongated tails. This kind of packing permitted the drug-PC complex to acquire an inverted cone structure with the preserved micellar configuration being formerly mentioned with multiple drugs like small molecules [Citation67,Citation68]. Based on the above data, all formulation additives provided thermodynamic stability for PNL at the PC interface permitting relevant drug solubilization the thing that was also confirmed through measured pharmaceutical parameters. Finally, the in vivo antifungal study showed significant superiority of the PNL-loaded OLs-gel group overall groups that might be due to the synergetic effect of chitosan as it can be used as an antifungal agent against different types of fungi [Citation69] in addition to the existence of the synergistic effect of OA as previously stated.
5. Conclusion
Chitosan-coated OLs loaded with PNL were prepared to employ a thin-film approach using a D-optimal design. The optimal OL exhibited spherical vesicles with a suitable EE%, PS, PDI, ZP, and Q6h (%). Moreover, the optimal OL was stable for 90 days and displayed perfect mucoadhesive aspects by utilizing the mucin test. Furthermore, ex-vivo investigations proved the sustained impact of PNL-loaded OLs-gel. The confocal-laser-scanning-microscopy proved the perfect accumulation of the optimal OL in the vaginal tissues. The in silico investigation outcomes pointed to the potential perfect stability of PNL upon including with the other formulation constituents. The in vivo investigations confirmed the capability of PNL versus C. albicans. Histopathological outcomes proved the tolerability of using PNL-loaded OLs-gel. These promising outcomes proved that PNL-loaded OLs-gel could be utilized as a possible antifungal against C. albicans-related vaginal infections.
Author contributions
Conceptualization, M Eltabeeb, MA El-Nabarawi, M Hassan and MM Abdellatif; Formal analysis, M Eltabeeb, MA El-Nabarawi, MH Teaima, M Hassan, KM Darwish, MIA Hamed and MM Abdellatif; Investigation, M Eltabeeb, MA El-Nabarawi, MH Teaima, M Hassan, KM Darwish, MIA Hamed, AME Hamdan, and MM Abdellatif; Resources, M Eltabeeb, M Hassan, MIA Hamed, RR Hamed, and MM Abdellatif; Writing—original draft preparation, M Eltabeeb; Writing—review and editing, M Eltabeeb, RR Hamed, MA El-Nabarawi, MH Teaima, MIA Hamed, KM Darwish, MIA Hamed, AME Hamdan, and MM Abdellatif; Supervision, MM Abdellatif, MH Teaima, and MA Eltabeeb. All authors have read and agreed to the published version of the manuscript.
Financial disclosure
The authors have no financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Writing disclosure
No writing assistance was utilized in the production of this manuscript.
Ethical conduct of research
All the ex vivo and in vivo tests were approved by REC-FOPCU (approval no. MI3395) which followed the Guide for Care and Use of Laboratory Animals declared by the US National Institute of Health (NIH Publication No. 85–23, revised 2011).
Competing interests disclosure
The authors have no competing interests or relevant affiliations with any organization or entity with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
References
- Parvathaneni V, Kulkarni NS, Muth A, et al. Drug repurposing: a promising tool to accelerate the drug discovery process. Drug Discov Today. 2019;24(10):2076–2085. doi:10.1016/j.drudis.2019.06.014
- Montes-Grajales D, Puerta-Guardo H, Espinosa DA, et al. In silico drug repurposing for the identification of potential candidate molecules against arboviruses infection. Antiviral Res. 2020;173:104668. doi:10.1016/j.antiviral.2019.104668
- Shafiei M, Peyton L, Hashemzadeh M, et al. History of the development of antifungal azoles: a review on structures, SAR, and mechanism of action. Bioorg Chem. 2020;104:104240. doi:10.1016/j.bioorg.2020.104240
- Assress HA, Selvarajan R, Nyoni H, et al. Antifungal azoles and azole resistance in the environment: current status and future perspectives – a review. Rev Environ Sci Biotechnol. 2021;20:1011–1041. doi:10.1007/s11157-021-09594-w
- Zhang Q, Liu F, Zeng M, et al. Drug repurposing strategies in the development of potential antifungal agents. Appl Microbiol Biotechnol. 2021;105(13):5259–5279. doi:10.1007/s00253-021-11407-7
- Mayandi V, Kang W, Shu D, et al. Propranolol ameliorates the antifungal activity of azoles in invasive candidiasis. Pharmaceutics. 2023;15(4):1–15. doi:10.3390/pharmaceutics15041044
- Alkhanjaf AAM, Athar T, Ullah Z, et al. In vitro and in vivo evaluation of a nano-tool appended oil mix (clove and tea tree oil) thermosensitive gel for vaginal candidiasis. J Funct Biomater. 2022;13(4):1–13. doi:10.3390/jfb13040203
- de Cássia Orlandi Sardi J, Romário Silva D, Cristina Anibal P, et al. Vulvovaginal candidiasis: epidemiology and risk factors, pathogenesis, resistance, and new therapeutic options. Curr Fungal Infect Rep. 2021;15:32–40. doi:10.1007/s12281-021-00415-9
- Pandey M, Choudhury H, Abdul-Aziz A, et al. Promising drug delivery approaches to treat microbial infections in the vagina: a recent update. Polymers. 2020;13(1):1–65. doi:10.3390/polym13010026
- Marcelino HR, Solgadi A, Chéron M, et al. Exploring the permeability of Amphotericin B through serum albumin dispersions and lipid nanocarriers for oral delivery. Int J Pharm. 2023;646:123444. doi:10.1016/j.ijpharm.2023.123444
- Khan AS, ud Din F, Ali Z, et al. Development, in vitro and in vivo evaluation of miltefosine loaded nanostructured lipid carriers for the treatment of cutaneous leishmaniasis. Int J Pharm. 2021;593:120109. doi:10.1016/j.ijpharm.2020.120109
- Walt F, De Bastiani S, Spadari CDC, et al. Nanocarriers provide sustained antifungal activity for Amphotericin B and Miltefosine in the topical treatment of murine vaginal candidiasis. Front Microbiol. 2020;10:1–9. doi:10.3389/fmicb.2019.02976
- Atef B, Ishak RAH, Badawy SS, et al. Exploring the potential of oleic acid in nanotechnology-mediated dermal drug delivery: an up-to-date review. J Drug Deliv Sci Technol. 2022;67:103032. doi:10.1016/j.jddst.2021.103032
- Abdelalim LR, Elnaggar YSR, Abdallah OY. Oleosomes encapsulating sildenafil citrate as potential topical nanotherapy for palmar plantar erythrodysesthesia with high ex vivo permeation and deposition. AAPS PharmSciTech. 2020;21(8):310–319. doi:10.1208/s12249-020-01862-2
- Yang T, Hsieh C, Meng L, et al. Oleic acid-based self-micro-emulsifying delivery system for enhancing antifungal activities of clotrimazole. Pharmaceutics. 2022;14(3):1–13. doi:10.3390/pharmaceutics14030478
- Mosallam S, Ragaie MH, Moftah NH, et al. Use of novasomes as a vesicular carrier for improving the topical delivery of terconazole: in vitro characterization, in vivo assessment and exploratory clinical experimentation. Int J Nanomed. 2021;16:119–132. doi:10.2147/IJN.S287383
- Osmałek T, Froelich A, Jadach B, et al. Recent advances in polymer-based vaginal drug delivery systems. Pharmaceutics. 2021;13(6):884. doi:10.3390/pharmaceutics13060884
- Teaima MH, Eltabeeb MA, El-nabarawi MA, et al. Utilization of propranolol hydrochloride mucoadhesive invasomes as a locally acting evaluation. Drug Deliv. 2022;29(1):2549–2560. doi:10.1080/10717544.2022.2100514
- Giordani T, Dose J, Kuskoski Y, et al. Photocatalytic degradation of propranolol hydrochloride using Nd–TiO2 nanoparticles under UV and visible light. J Mater Res. 2021;36(7):1584–1599. doi:10.1557/s43578-021-00207-4
- El-naggar MM, El-nabarawi MA, Teaima MH, et al. Integration of terpesomes loaded Levocetrizine dihydrochloride gel as a repurposed cure for methicillin-resistant Staphylococcus aureus and in vivo studies. Int J Pharm. 2023;633:122621. doi:10.1016/j.ijpharm.2023.122621
- Albash R, El-Nabarawi MA, Refai H, et al. Tailoring of PEGylated bilosomes for promoting the transdermal delivery of olmesartan medoxomil: in vitro characterization, ex-vivo permeation, and in-vivo assessment. Int J Nanomed. 2019;14:6555–6574. doi:10.2147/IJN.S213613
- Albash R, Abdellatif MM, Hassan M, et al. Tailoring terpesomes and leciplex for the effective ocular conveyance of moxifloxacin hydrochloride (comparative assessment): in-vitro, ex-vivo, and in-vivo evaluation. Int J Nanomed. 2021;16:5247–5263. doi:10.2147/IJN.S316326
- Elmahboub Y, Albash R, Magdy William M, et al. Metformin loaded zein polymeric nanoparticles to augment antitumor activity against Ehrlich carcinoma via activation of AMPK pathway: d-optimal design optimization, in vitro characterization, and in vivo study. Molecules. 2024;29(7):1614. doi:10.3390/molecules29071614
- Albash R, Elmahboub Y, Baraka K, et al. Ultra-deformable liposomes containing terpenes (terpesomes) loaded fenticonazole nitrate for treatment of vaginal candidiasis: box-Behnken design optimization, comparative ex vivo and in vivo studies. Drug Deliv. 2020;27(1):1514–1523. doi:10.1080/10717544.2020.1837295
- Abdellatif MM, Josef M, El-nabarawi MA, et al. Sertaconazole-nitrate-loaded leciplex for treating keratomycosis: optimization using D-optimal design and in vitro, ex vivo, and in vivo studies. Pharmaceutics. 2022;14(10):2215. doi:10.3390/pharmaceutics14102215
- Czopek A, Żmudzki P, Dąbrowska M, et al. Reversed-phase thin-layer chromatography and ultra-performance liquid chromatography/mass spectrometry to estimate the drug-likeness of phosphodiesterase 10A inhibitors with phthalimide core. JPC-J Planar Chromat. 2024;37:1–10. doi:10.1007/s00764-024-00298-9
- Arafa MG, Girgis GNS, El-Dahan MS. Chitosan-coated PLGA nanoparticles for enhanced ocular anti-inflammatory efficacy of atorvastatin calcium. Int J Nanomed. 2020;15:1335–1347. doi:10.2147/IJN.S237314
- Abdellatif MM, Elakkad YE, Elwakeel AA, et al. Formulation and characterization of propolis and tea tree oil nanoemulsion loaded with clindamycin hydrochloride for wound healing: in-vitro and in-vivo wound healing assessment. Saudi Pharm J. 2021;29(11):1238–1249. doi:10.1016/j.jsps.2021.10.004
- Yang X, Tang Y, Wang M, et al. Co-delivery of methotrexate and nicotinamide by cerosomes for topical psoriasis treatment with enhanced efficacy. Int J Pharm. 2021;605:120826. doi:10.1016/j.ijpharm.2021.120826
- Eberhardt J, Santos-Martins D, Tillack AF, et al. Autodock Vina 1.2.0: new docking methods, expanded force field, and python bindings. J Chem Inf Model. 2021;61(8):3891–3898. doi:10.1021/acs.jcim.1c00203
- Xue Q, Liu X, Russell P, et al. Ecotoxicology and environmental safety evaluation of the binding performance of flavonoids to estrogen receptor alpha by Autodock, Autodock Vina, and Surflex-Dock. Ecotoxicol Environ Saf. 2022;233:113323. doi:10.1016/j.ecoenv.2022.113323
- Elhady SS, Abdelhameed RFA, Malatani RT, et al. Molecular docking and dynamics simulation study of hyrtios erectus isolated scalarane sesterterpenes as potential SARS-CoV-2 dual-target inhibitors. Biology. 2021;10(5):1–35. doi:10.3390/biology10050389
- Saleh AH, Abdelwaly A, Darwish KM, et al. Deciphering the molecular basis of the kappa opioid receptor selectivity: a molecular dynamics study. J Mol Graph Model. 2021;106:107940. doi:10.1016/j.jmgm.2021.107940
- Zaki AA, Ashour A, Elhady SS, et al. Calendulaglycoside showing potential activity against SARS-CoV-2 main protease: molecular docking, molecular dynamics, and SAR studies. J Tradit Complement Med. 2022;12(1):16–34. doi:10.1016/j.jtcme.2021.05.001
- Al-mahallawi AM, Ahmed D, Hassan M, et al. Enhanced ocular delivery of clotrimazole via loading into mucoadhesive microemulsion system: in vitro characterization and in vivo assessment. J Drug Deliv Sci Technol. 2021;64:102561. doi:10.1016/j.jddst.2021.102561
- Muhammad A, Hassan M, El-setouhy DA, et al. Voriconazole ternary micellar systems for the treatment of ocular mycosis: statistical optimization and in vivo evaluation. J Pharm Sci. 2021;110(5):2130–2138. doi:10.1016/j.xphs.2020.12.013
- Albash R, Al-mahallawi AM, Hassan M, et al. Development and optimization of terpene-enriched vesicles (terpesomes) for effective ocular delivery of Fenticonazole Nitrate: in vitro characterization and in vivo assessment. Int J Nanomed. 2021;16:609–621. doi:10.2147/IJN.S274290
- Salem MA, El-Shiekh RA, Hashem RA, et al. In vivo, antibacterial activity of star anise (Illicium verum hook.) extract using murine MRSA skin infection model in relation to its metabolite profile. Infect Drug Resist. 2021;14:33–48. doi:10.2147/IDR.S285940
- Ali NB, El-Shiekh RA, Ashour RM, et al. In vitro and in vivo antibiofilm activity of red onion scales: molecules. 2023;28(1):1–11. doi:10.3390/molecules28010355
- De Toledo LG, Aparecido M, Silva B, et al. Improved in vitro and in vivo anti-Candida albicans activity of Cymbopogon nardus essential oil by its incorporation into a microemulsion system. Int J Nanomed. 2020;15:10481–10497. doi:10.2147/IJN.S275258
- El-ezz DA, Abdel-rahman LH, Al-farhan BS, et al. Enhanced in vivo wound healing efficacy of a novel hydrogel loaded with Copper (II) Schiff Base Quinoline Complex (CuSQ) solid lipid nanoparticles. Pharmaceuticals. 2022;15(8):1–21. doi:10.3390/ph15080978
- Ho HMK, Craig DQM, Day RM. Design of experiment approach to modeling the effects of formulation and drug loading on the structure and properties of therapeutic nanogels. Mol Pharm. 2022;19(2):602–615. doi:10.1021/acs.molpharmaceut.1c00699
- Ammar HO, Tadros MI, Salama NM, et al. Ethosome-derived invasomes as a potential transdermal delivery system for vardenafil hydrochloride: development, optimization, and application of physiologically based pharmacokinetic modeling in adults and geriatrics. Int J Nanomed. 2020;15:5671–5685. doi:10.2147/IJN.S261764
- Abdallah MH, Elghamry HA, Khalifa NE, et al. Development and optimization of erythromycin-loaded transethosomes cinnamon oil-based emulgel for antimicrobial efficiency. Gels. 2023;9(2):137. doi:10.3390/gels9020137
- Salama A, Badran M, Elmowafy M, et al. Spironolactone-loaded leciplexes as potential topical delivery systems for female acne: in vitro appraisal and ex vivo skin permeability studies. Pharmaceutics. 2019;12(1):1–17. doi:10.3390/pharmaceutics12010025
- Chaikul P, Khat-udomkiri N, Iangthanarat K, et al. Characteristics and in vitro anti-skin aging activity of gallic acid loaded in cationic CTAB niosome. Eur J Pharm Sci. 2019;131:39–49. doi:10.1016/j.ejps.2019.02.008
- Awaad A, Nakamura M. Size-dependent biodistribution of thiol – organosilica nanoparticles and F4/80 protein expression in the genital tract of female mice after intravaginal administration. Histochem Cell Biol. 2021;155(6):683–698. doi:10.1007/s00418-021-01974-1
- Rathore C, Kumar N, Sharma A, et al. Phospholipid nanoformulation of thymoquinone with enhanced bioavailability: development, characterization and anti-inflammatory activity. J Drug Deliv Sci Technol. 2019;52:316–324. doi:10.1016/j.jddst.2019.04.041
- Albash R, Abdelbary AA, Refai H, et al. Use of transethosomes for enhancing the transdermal delivery of olmesartan medoxomil: in vitro, ex vivo, and in vivo evaluation. Int J Nanomed. 2019;14:1953–1968. doi:10.2147/IJN.S196771
- Talebi V, Ghanbarzadeh B, Hamishehkar H, et al. Effects of different stabilizers on colloidal properties and encapsulation efficiency of vitamin D3 loaded nano-niosomes. J Drug Deliv Sci Technol. 2021;61:101284. doi:10.1016/j.jddst.2019.101284
- Valamla B, Thakor P, Phuse R, et al. Engineering drug delivery systems to overcome the vaginal mucosal barrier: current understanding and research agenda of mucoadhesive formulations of vaginal delivery. J Drug Deliv Sci Technol. 2022:70:103162. doi:10.1016/j.jddst.2022.103162
- Fu Z, Li L, Wang Y, et al. Direct preparation of drug-loaded mesoporous silica nanoparticles by sequential flash nanoprecipitation. Chem Eng J. 2020;382:122905. doi:10.1016/j.cej.2019.122905
- Das S, Bououdina M, Manoharan C. The influence of cationic surfactant CTAB on optical, dielectric, and magnetic properties of cobalt ferrite nanoparticles. Ceram Int. 2020;46(8):11705–11716. doi:10.1016/j.ceramint.2020.01.202
- Tawfik MA, Tadros MI, Mohamed MI. Polyamidoamine (PAMAM) dendrimers as potential release modulators and oral bioavailability enhancers of vardenafil hydrochloride. Pharm Dev Technol. 2019;24(3):293–302. doi:10.1080/10837450.2018.1472611
- Abdellatif MM, Ahmed SM, El-nabarawi MA, et al. Oral bioavailability enhancement of vancomycin hydrochloride with cationic nanocarrier (leciplex): optimization, in vitro, ex vivo, and in vivo studies. Sci Pharm. 2023;91(1):1–19. doi:10.3390/scipharm91010001
- Maritim S, Boulas P, Lin Y. Comprehensive analysis of liposome formulation parameters and their influence on encapsulation, stability and drug release in glibenclamide liposomes. Int J Pharm. 2021;592:120051. doi:10.1016/j.ijpharm.2020.120051
- Tawfik MA, Eltaweel MM, Farag MM, et al. Sonophoresis-assisted transdermal delivery of antimigraine-loaded nanolipomers: radio-tracking, histopathological assessment and in-vivo biodistribution study. Int J Pharm. 2023;644:123338. doi:10.1016/j.ijpharm.2023.123338
- Pham QD, Nöjd S, Edman M, et al. Mucoadhesion: mucin-polymer molecular interactions. Int J Pharm. 2021;610:121245. doi:10.1016/j.ijpharm.2021.121245
- Liu L, Tian C, Dong B, et al. Models to evaluate the barrier properties of mucus during drug diffusion. Int J Pharm. 2021;599:120415. doi:10.1016/j.ijpharm.2021.120415
- Ali MS, Metwally AA, Fahmy RH, et al. Chitosan-coated nanodiamonds: a mucoadhesive platform for intravesical delivery of doxorubicin. Carbohydr Polym. 2020;245:116528. doi:10.1016/j.carbpol.2020.116528
- Kanojiya PS, Ghodake PN, Wadetwar RN. Design and optimization of liquisolid compact based vaginal sustained release tablet of antifungal agent for vaginal candidiasis. J Dispers Sci Technol. 2022;1–16. doi:10.1080/01932691.2022.2158854
- Ghayas S, Shoaib MH, Qazi F, et al. Influence of different viscosity grade cellulose-based polymers on the development of valsartan controlled-release tablets. Polym Bull. 2020;77:1281–1306. doi:10.1007/s00289-019-02802-2
- Yu Y, Ngo HV, Jin G, et al. Double-controlled release of poorly water-soluble paliperidone palmitate from self-assembled albumin-oleic acid nanoparticles in PLGA in situ forming implant. Int J Nanomed. 2021;16:2819–2831. doi:10.2147/IJN.S302514
- Permana AD, Utomo E, Pratama MR, et al. Bioadhesive-thermosensitive in situ vaginal gel of the gel flake-solid dispersion of Itraconazole for enhanced antifungal activity in the treatment of vaginal candidiasis. ACS Appl Mater Interfaces. 2021;13(15):18128–18141. doi:10.1021/acsami.1c03422
- Farag MM, Abd NS, Malak E, et al. Hyaluronic acid conjugated Metformin- phospholipid sonocomplex: a biphasic complexation approach to correct hypoxic tumour microenvironment. Int J Nanomed. 2021;16:1005–1019. doi:10.2147/IJN.S297634
- Albash R, Fahmy AM, Hamed MIA, et al. Spironolactone hyaluronic acid enriched cerosomes (HAECs) for topical management of hirsutism: in silico studies, statistical optimization, ex vivo, and in vivo studies. Drug Deliv. 2021;28(1):2289–2300. doi:10.1080/10717544.2021.1989089
- Albash R, Badawi NM, Hamed MIA, et al. Exploring the synergistic effect of bergamot essential oil with spironolactone loaded nano-phytosomes for treatment of acne vulgaris: in vitro optimization, in silico studies, and clinical evaluation. Pharmaceuticals. 2023;16(1):128. doi:10.3390/ph16010128
- Albash R, El-dahmy RM, Hamed MIA, et al. Repurposing levocetirizine hydrochloride loaded into cationic ceramide/phospholipid composite (CCPCs) for management of alopecia: central composite design optimization, in-silico and in-vivo studies. Drug Deliv. 2022;29(1):2784–2795. doi:10.1080/10717544.2022.2108939
- Poznanski P, Hameed A, Orczyk W. Chitosan and chitosan nanoparticles: parameters enhancing antifungal activity. Molecules. 2023;28(7):2996. doi:10.3390/molecules28072996
