ABSTRACT
Although integration of HIV and maternal health services is recommended by the World Health Organization, evidence to guide implementation is limited. We describe facility-level implementation of policies for integrating HIV care within maternal health services and explore experiences of service users and providers in rural Tanzania (Ifakara), South Africa (uMkhanyakude) and Malawi (Karonga). Policy in all countries included HIV testing during antenatal care (ANC), same-day antiretroviral therapy (ART) initiation for HIV-positive pregnant women, and postpartum referral to ART clinics, between six weeks (Malawi, South Africa) and two years after delivery (Tanzania). All facilities offered HIV testing within ANC, most commonly during the first visit. Although most women were comfortable with HIV testing, some felt that opting out would lead to sub-standard services. Some facilities conducted group post-test counselling for HIV-negative women, raising concerns of unintended HIV status disclosure. ART initiation was offered on the same day, the same room as an HIV diagnosis in >90% of facilities. Women’s worries around postpartum referral included having unknown providers, insufficient privacy and queues. Adoption and implementation of policies on integrated HIV and maternal health services varied across settings. Patients’ experiences of these policies may influence uptake and retention in care.
Background
Since 2013, initiating pregnant and postpartum women onto life-long antiretroviral treatment (ART) regardless of their immune status (Option B+) has been considered the cornerstone for the prevention of mother-to-child transmission (PMTCT) programmes in sub-Saharan African (Kim et al., Citation2015). The widespread implementation of Option B+ has dramatically impacted on treatment uptake and improved outcomes for mothers and HIV exposed infants. By 2018, in sub-Saharan Africa the proportion of HIV-infected pregnant women on ART was 92%, up from only 49% in 2010, and vertical transmission rates had reduced to less than 9%, down from 18% in 2010 (UNAIDS, Citation2019). However, the scale-up of Option B+ has contributed to increased demands for treatment initiation on already stretched health systems. Growing numbers of women on ART have subsequently increased health workers’ workloads, reduced time and space available for consultations and perpetuated supply chain challenges for drugs and commodities, potentially impacting on service quality (Sprague et al., Citation2011).
HIV service integration has been proposed as a solution to address some of the health systems challenges associated with growing client numbers, by streamlining service delivery and promoting efficiency through task-sharing (Church et al., Citation2015). Integrated HIV services have improved retention rates through various mechanisms, including by reducing the number of clinic visits during the pregnancy and postnatal periods (Joseph Davey et al., Citation2016; World Health Organization, Citation2006b), thereby better addressing client needs, leading to greater client satisfaction (Sweeney et al., Citation2012). In addition, taking the focus away from HIV and placing it on maternal and child health, integrated services can potentially reduce HIV-related stigma (Church et al., Citation2013). Furthermore, a systematic review found that integration of HIV services was broadly cost effective in sub-Saharan African settings (Sweeney et al., Citation2012).
The World Health Organization (WHO) recommends that antiretroviral therapy (ART) should be initiated and maintained in all eligible pregnant and postpartum women and infants in maternal and child healthcare settings, and the women should be referred to ongoing HIV care including ART (Fowkes et al., Citation2016; World Health Organization, Citation2010, Citation2015a). Although this is a strong recommendation with some suggestion that it may promote adherence to ART, there is limited evidence for how this integration should take place in practice (Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection, Citation2016; World Health Organization, Citation2006a). Specifically, contextual implementation research is needed to explore how long women should stay in maternal health clinics before referral to routine ART clinics and how referrals should take place.
Furthermore, there are limited data on how different countries have adopted WHO guidance on service integration within their national policies, and how these policies have been implemented at the heath facility level, and experienced by service users (Jones et al., Citation2019). In this study, we aimed to investigate differences in the adoption and implementation of policies guiding the integration of HIV and maternal health services in three African countries, and to explore women’s and health worker’s experiences and perceptions of these integrated service models.
Methods
Setting
This analysis draws on data from the study for Strengthening Health Systems for the Application of Policy to Enable Universal Test and Treat (SHAPE UTT), conducted in three health and demographic surveillance sites (HDSS) in Ifakara (Tanzania), Karonga (Malawi) and uMkhanyakude (South Africa) between 2017 and 2019, with the aim of assessing the health systems impacts of Option B+. The key characteristics of the three study settings are shown in .
Table 1. Health facility and in-depth interview participant’s characteristics.
Data collection
We analysed data from three sources: national HIV policy reviews, health facility surveys and in-depth interviews conducted with health workers, women receiving HIV services during and after their pregnancy and HIV-negative pregnant women who received ANC.
Policy review
A review of WHO guidance and national policies published between 2015 and 2017 was conducted, covering HIV testing and treatment, PMTCT and ANC provision. Policy documents related with HIV and ANC were retrieved online or in person from the relevant offices in each of the countries. All guidance pertaining to the delivery of HIV services for pregnant women within maternal health services was extracted and collated in an Excel spreadsheet. Policy guidance was summarised in terms of content and year of adoption, and then compiled into three indicators which captured when, where and how the delivery of HIV testing, ART initiation and ongoing HIV care for pregnant and postpartum women were stipulated in national policy. A comparative analysis was conducted to identify similarities and differences between WHO recommendations and each country’s policies as well as across each of the three countries.
Health facility survey
Surveys were conducted in 33 health facilities serving the populations of three rural HDSS in Ifakara, Tanzania (n = 11), Karonga, Malawi (n = 5), and uMkhanyakude, South Africa (n = 17) between October 2017 and September 2018. The questionnaire included four modules which covered the delivery of services for HIV testing, PMTCT, ART and ANC and has been described previously (Ambia et al., Citation2017; Church et al., Citation2015; Jones et al., Citation2019). The content was informed by the WHO service availability and readiness assessment (SARA) (World Health Organization, Citation2015b) and aligned with the information collected for the policy review. The questionnaires were conducted face-to-face in English by a trained fieldworker with staff in charge at each facility.
Health facility survey data were entered centrally into MS SQL server (Microsoft Corp, Redmond, USA) and analysed using Stata v13. Variables describing when, where and how, service delivery occurred for HIV testing, ART initiation and routine HIV care for pregnant and postpartum women were extracted for analysis, matching the selected policy indicators. Descriptive statistics were used to show the proportion of facilities implementing each policy relating to MNCH and HIV service integration per site.
In-depth Interviews
In-depth interviews were conducted with 15 women receiving ANC, 21 PMTCT users who were still in their pregnancy or postpartum period, 13 women who had disengaged from care, 17 HIV positive women who had initiated ART during PMTCT and had already transferred to routine ART clinics, 20 health workers providing PMTCT and ART services (). Two criteria were used to sample facilities: firstly, by facility level and secondly by distance from the district centre. Three facilities per site were then selected to include one hospital, one health centre from a remote area of the HDSS, and one health centre from a more urbanised area close to district headquarters. Service users and health workers were recruited from these sites for the in-depth interviews.
Sampling frames were drawn up from the PMTCT and ART registers and women attending on the days allocated to data collection were approached and invited to take part in the study. Data collection continued until data saturation was reached. Women who had disengaged from care were contacted by phone by health workers as they would have been through routine programme tracing. During these calls, women were informed about the study and invited to participate.
Data were collected by trained fieldworkers in the local vernacular and lasted approximately 45–90 min. Interviews were conducted in a private location and covered women’s experiences of their first ANC visit and the HIV testing process. For women living with HIV, the interviews also covered their experiences of treatment initiation and referral to routine care, postpartum HIV care provision, and where appropriate, reasons for care disengagement. Interviews with health workers covered their experiences of offering HIV care and maternal health services in their facilities, the training they had received and any challenges they faced.
Debriefings were held after each interview between field workers and the study coordinator, with weekly tele-conferences between the lead researchers to discuss similarities and differences in the emerging findings across the three sites. Interview summaries were written in English by the interviewers and shared with other researchers to guide the weekly discussions.
Interviews were digitally recorded, transcribed and translated to English. Data were coded with the aid of Nvivo 11 (Ifakara, Karonga) and manually (uMkhanyakude), initially according to broad thematic areas covered in the topic guides to ensure comparability, and then allowing for inductive sub-coding, led by the content of the data in each site. The lead researcher in each site kept detailed analytical memos during the coding and analysis process which were discussed during the weekly calls and shared to aid further comparative analysis which was led by the first author. The lead researchers were all locally-recruited social scientists with extensive experience in conducting community-based health research.
Ethics
Prior to the interviews, participants were provided with an information sheet that described the study aim and objectives, and were encouraged to ask questions. Written consent was sought from each participant who accepted to participate in the study. All data were kept in a data encrypted shared folder which was password protected only accessible to the lead researcher in each site. Ethical approval was obtained from the University of KwaZulu-Natal Biomedical Research Committee, South Africa (BE400/14), the National Institute of Medical Research, Tanzania (NIMR/HQ/R.8a/Vol. IX/2579), Ifakara Health Institute Tanzania (IHI/IRB/NO:14-2017), the National Health Sciences Research Committee, Malawi (17/07/1861) and from the London School of Hygiene and Tropical Medicine, UK (#13536).
Results
Policy review
WHO guidance on the provision of HIV services for pregnant and postpartum women has included a recommendation for opt-out HIV testing at ANC since 2007 (World Health Organization, Citation2007). Furthermore same-day ART treatment initiation in maternal, new-born and child health (MNCH) settings has been included since 2013. The timing of subsequent linkage to routine ART clinics during pregnancy or following the postpartum period is not specified, with guidance stating that in generalised epidemic settings, ART should be provided to pregnant and postpartum women and infants in the maternal and child health-care settings, with linkage and referral to ongoing HIV care and ART services when appropriate. This is stated as a ‘strong recommendation’ despite weak evidence (Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection, 2016).
All countries adopted a policy on opt-out HIV testing for pregnant and postpartum women in ANC, with no variation on the timing for the first test. However, in South Africa, the policy stipulated that HIV testing should take place at every ANC visit as well as at delivery. For ART initiation, the most recent policy in all three countries indicated that pregnant women should initiate treatment through the Option B+ strategy, with variation in terms of the amount of detail provided (). In Tanzania and South Africa, the national policy specified that ART should be initiated within the maternal, neonatal and child health (MNCH) setting, whereas the location of treatment initiation was not explicitly mentioned in Malawian national policy. Finally, differences were observed in the policies for the referral of postpartum women from the MNCH setting to routine ART clinics. In Tanzania, postpartum women living with HIV are linked to routine care and treatment from MNCH settings at two years post-delivery, while in South Africa, policy stipulates that the referral should occur 18 months post-delivery, and no specific timeframe was indicated in the Malawian policy documents ( and ).
Figure 1. HIV care cascade indicators for pregnant and postpartum women; black squares denote the policy detail aligns with the specification listed.
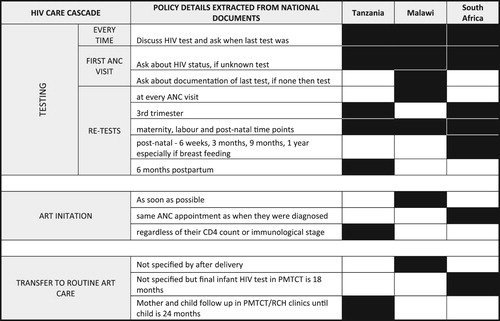
Table 2. Three countries policy analysis on pregnant or postpartum HIV testing, treatment initiation and referral to adult ART clinics.
Policy implementation, provider and user experiences
HIV testing services
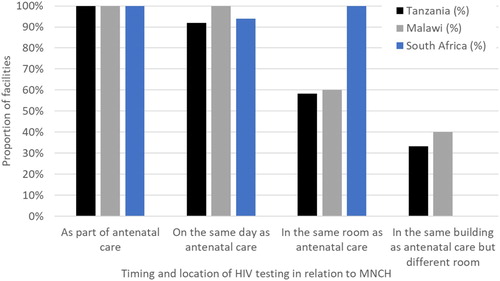
All the facilities in each of the sites offered opt-out HIV testing to women attending ANC, with the majority (>90%) of facilities offering HIV testing services on the same day as ANC (). In uMkhanyakude, all facilities reported offering HIV testing in the same room as ANC, and in Karonga and Ifakara, all facilities reported offering HIV testing in the same room or in the same building as ANC ().
Figure 2. When and where HIV testing services were offered in relation to MNCH as reported from 5 facilities in Karonga, 11 in Ifakara and 14 in uMkhanyakude.
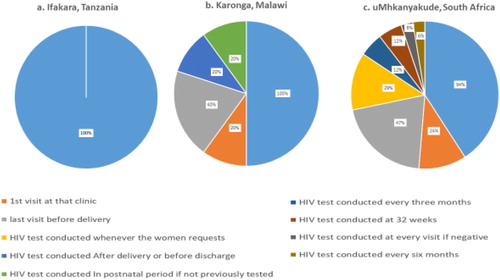
There was variation in the timing of the HIV testing offered during the pregnancy and postpartum period. Whilst >90% of facilities across all sites offered HIV testing during the first ANC visit, facilities in uMkhanyakude reported an additional six time points throughout the pregnancy and postpartum period when HIV testing was offered for women who had previously tested negative, while those in Karonga reported four different time points ().
Figure 3. Timing of HIV test in relation to pregnancy care as reported from 5 facilities in Karonga, 11 in Ifakara and 14 in uMkhanyakude.
The interview data suggested that some ANC users reported feeling comfortable with receiving these HIV tests and cited their appreciation for the health education sessions and the pre-test counselling.
I felt good, normal as it was not my first time to undergo the test, I have already had one previously. ANC user, Ifakara.
I think it’s a good rule because it helps couple to have an opportunity of testing together for HIV. ANC user Karonga.
Yes, I was hurt as I was there for something else, not what they told me. They [the provider] said it’s a rule, so they force you to go and do it [test for HIV] if you are pregnant. They don’t ask you nicely to go and get tested, and if that does not happen you won’t get assisted any further with regards to antenatal care, and you also need to think about how are you going to give birth. (ANC user uMkhanyakude)
Sometimes you find that someone is crying, because … it is compulsory to do HIV testing if the mother is pregnant. (Provider Karonga)
Because there were a lot of people in a group, and there was queue, so there was not enough time to ask questions. (ANC user Ifakara)
No, there wasn’t any counselling, probably because a lot of people were waiting to be tested and besides that, the time was approaching 12 o’clock and the doctor might have been rushing for lunch. (ANC user Karonga)
We are supposed to attend to the pregnant mothers who attend for the first time … If we find all are HIV negative, we provide result in a group to save time. (Provider Ifakara)
If the results happen to be negative for all, they will disclose the results openly to all at the same time. If anyone were found to be HIV positive, then they will disclose privately to one by one. I thank God that in our group we all had negative results but what if it was otherwise, like amongst us two or three peoples were infected, it would have portrayed a bad image and we would be doubting amongst us who had been infected. (ANC user Ifakara)
Integration of ART treatment initiation and other services for women
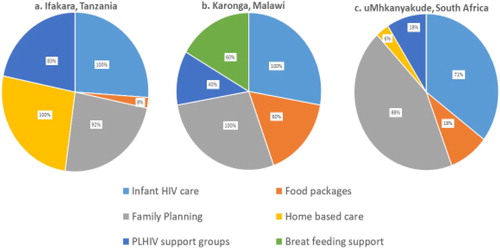
In all settings, >90% of facilities offered ART treatment on the same day as ANC consultations, and >90% of facilities offered ART in the same room as ANC. In addition to ART, facilities also offered a range of other services to women attending ANC, although these were not always provided within the MNCH clinics: 100% of facilities in Ifakara and Karonga and 70% of facilities in uMkhanyakude also provided infant HIV care, and over 80% of all facilities across all sites offered family planning services. 60% of facilities in Karonga offered breastfeeding support, while none offered this service in Ifakara and uMkhanyakude. Home-based care was offered to women living with HIV in all facilities in Ifakara, but in only 5% of facilities in uMkhanyakude and none in Karonga. Over 80% of facilities in Ifakara reported having people living with HIV (PLHIV) support groups, whilst these were available in only 40% of facilities in Karonga and less than 20% of facilities in uMkhanyakude. Food package services were more commonly offered in Karonga (60% of facilities), than Ifakara (8% of facilities) and uMkhanyakude (15% of facilities) ().
Figure 4. Additional services offered within an integrated PMTCT and MNCH service as reported from 5 facilities in Karonga, 11 in Ifakara and 14 in uMkhanyakude.
Being able to collect ART at MNCH clinics was viewed positively by service users and service providers alike. Providers reported that the integration of these services saved time for themselves as well as for their patients, and could also be less stigmatising for some women living with HIV.
PMTCT and MNCH in the same location, I see it as a good thing, because a woman is getting all the services. When she wants to go, she goes for good. Not to finish here and go there and there. Others get hungry, they may see us as troubling and wasting their time. But, when they enter here (ANC room), they get folic acid, and ARV and she puts the stuff in her pocket and goes. So, the confidentiality will be maintained. (Provider Ifakara)
I don’t understand what is going on, because we wait in the same queue until we get inside, and I also don’t understand that sometimes there’s a woman with the baby at the front, followed by a pregnant one, and there’s one with a baby again. (ANC user uMkhanyakude)
Referral and linkage to ART care
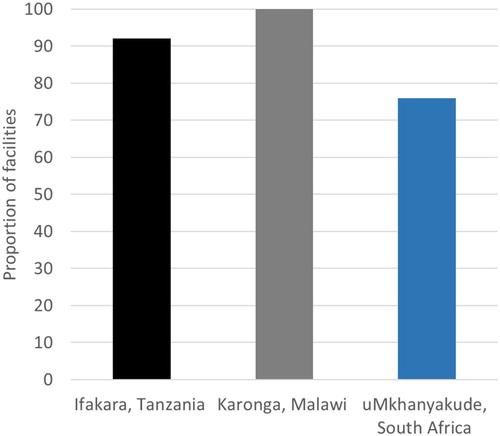
Notable differences were reported across the three sites in the timing of referral to routine ART clinics for postpartum women following the delivery of their baby. In Ifakara, over 80% of facilities transferred women two years after delivery, with the others referring as soon as a woman received an HIV diagnosis. The timing of referral varied across facilities in Karonga, with 60% referring to ART clinics following an HIV diagnosis during ANC, 20% referring after delivery, and 20% referring 6 weeks after delivery. Similarly, in uMkhanyakude, 17% of facilities reported transferring women during ANC, or as soon as they started ART, 5% reported referring women to routine ART clinics shortly after delivery, 40% referred two years after delivery, 29% reported that the referral occurred after women had finished breastfeeding and 5% reported that they never transferred the patients (). In all settings, the majority of facilities reported to conduct checks to see if a woman had successfully transferred; this ranged from 100% of facilities in Karonga, 91% in Ifakara and 75% in uMkhanyakude ().
Figure 5. Timing of transfer to routine ART care and checks carried out to ensure transfer has taken place as reported from 5 facilities in Karonga, 11 in Ifakara and 14 in uMkhanyakude.
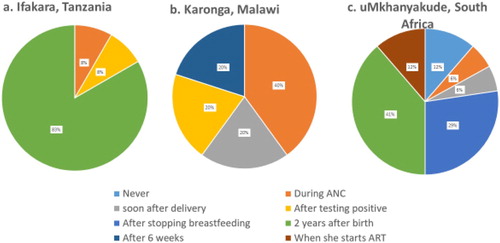
Figure 6. Facilities reporting to conduct checks to see if a woman has successfully transferred from PMTCT to routine ART care as reported from 5 facilities in Karonga, 11 in Ifakara and 14 in uMkhanyakude.
Some providers in Ifakara and Karonga reported resistance when referring women from PMTCT to routine ART clinics, and some women also expressed concerns over the transfer process. Whilst the timing of transfer varied across and within sites, concerns cited by service users and providers in Karonga and Ifakara were similar, and included fears that their HIV status would be deduced in routine ART clinics where services were no longer integrated, as well as worries about long queues, and building rapport with a new provider.
I felt ashamed because people were looking at me entering in a room showing that I will get drugs hence they knew my HIV positive status. (ANC user Karonga)
The problem is to reveal a secret like this to a person, it requires courage. If another person knows your situation [status] like this, even if she/he will keep the secret to herself, you will still feel uncomfortable. (ANC user Ifakara)
Most of these women continue receiving treatment, but obviously a handful of them may not continue because they will be still in denial, and they are comfortable receiving their medicine from here [MNCH clinics] because this place is somehow discreet. But as soon as they leave this place to go that side [ART clinic], they don’t feel comfortable. Some of them then decide to quit treatment and then it becomes our job to follow them up. (Provider Karonga)
When they were receiving treatment from this department, it was difficult for anybody to know that they are HIV positive. So when you tell them to go that side [ART clinic], there is some kind of resistance. (Provider Ifakara)
Transferring the patient from this department to routine HIV care, is difficult as she refuses to be transferred, so we get piled up with patients, and if you force her to move, they sometimes get pregnant again, despite their current child still being young. (Provider Ifakara)
I don’t know but what I can say is that I still have not seen any change here at the clinic as I have delivered now. everything is still the same even now, meaning that if I am at the clinic, I still get the service that I need. (ANC user uMkhanyakude)
Discussion
This mixed methods case study highlighted differences and similarities in the implementation of HIV policies for pregnant and postpartum women in rural settings in three countries. Overall, the facilities in each country closely followed the national policy for the integration of HIV services within MNCH in their given country. Policy guiding ART initiation within ANC was similar in all countries and subsequently meant the facilities also had similar implementation of ‘same day’ initiation of ART and provision in the same room or building as ANC. However, the policy review highlighted key differences across the countries which manifested in differences in the frequency of HIV testing and the time of transfer for routine care. The in-depth interviews highlighted that despite impressive efforts to integrate HIV care and treatment within MNCH, various unintended consequences impacted on women’s experiences of care. Some women reported feeling forced into testing, confused over what service they were and should be receiving and exhibited resistance to transferring their HIV care out of ANC to routine HIV care post-delivery.
The study found that implementation of HIV testing policies to all pregnant women during their first ANC visit was near universal, although the way in which the testing process took place varied. Group counselling was permitted in national policy in all countries and reportedly helped to address some health systems constraints in terms of a lack of personnel and inadequate infrastructure, perpetuated by the requirement to conduct multiple HIV tests at the same time as offering comprehensive ANC. However, in some instances providers also used the same approach to provide negative results in a group setting. This increased anxiety and is inconsistent with the ‘confidentiality’ required within WHO’s ‘5Cs’ approach to HIV testing (World Health Organisation (WHO), Citation2013). In line with other studies, our findings call for increased efforts to balance the need to conduct HIV tests for multiple pregnant women during a busy ANC clinic with that of each women’s individual needs to reflect, process and prepare for a potentially HIV positive result (McLean et al., Citation2017; Ngangue et al., Citation2017).
WHO recommends re-testing HIV-negative pregnant women in settings with HIV prevalence >5%, with a first test during the first ANC consultation and a second test during the third trimester. Catch-up testing is proposed during labour or delivery or in the postpartum period or if the first test or rested are missed or delayed (World Health Organization, Citation2019). The decision as to how often testing should occur during pregnancy presents a trade-off between reducing missed opportunities to diagnose new cases and the costs of using limited public health resources on unnecessary testing (Drake et al., Citation2019). We found variations in when and how often facilities undertook re-testing across our study settings, with Ifakara having fewer testing time-points than uMkhanyakude. It is possible that the cost burden to conduct retesting during pregnancy and the postpartum period has led to these differences between the WHO recommendation (World Health Organization, Citation2019) and national policy and subsequent implementation.
The differences in policy implementation within the three sites could in part be influenced by the differences in how the policy guidance is phrased. The guidance in Malawi and South Africa pertaining to when postpartum women living with HIV should transfer to routine HIV care was less explicit than in Tanzania. This potentially led to more flexibility at the facility level, enabling room to tailor services to the needs of women and moving care towards a more patient-cantered care approach (World Health Organization, Citation2017). It is possible that the flexibility on when women can transfer in South Africa led to less resistance over this process, with women transferring when they were ready rather than when they had to. However, the same was not seen in Karonga, where despite women being given more options as to when to transfer, and the way in which PMTCT care is embedded within all HIV care, resistance to receiving HIV care in routine vis a vis ANC was noted. Our study findings resonate with others in similar settings which suggest that as well as flexibility in deciding when transfers should take place, the perceived quality of the service they are being transferred to, and stigma perpetuated resistance (Clouse et al., Citation2014; Pellowski et al., Citation2020; T. K. Phillips et al., Citation2020). We support others in suggesting that overall quality improvements and supportive transfer processes could serve to address this resistance (Srivasta et al., Citation2018).
Other studies have reported a need to consider the individual characteristics of women and tailor transfer procedures according to their needs (T. K. Phillips et al., Citation2018). Pregnant and postpartum women who discover their HIV status for the first time at ANC face extra challenges. In addition to their recent introduction to ART care they may also face, for example a lack of partner support, worry of child’s HIV status (McLean et al., Citation2017) and fears for their own health and safety during their delivery (T. K. Phillips et al., Citation2018; T. Phillips et al., Citation2015). Our study supports the growing evidence that differentiated service delivery mechanisms for the continued delivery of ART could be rolled out to include postpartum women who need to transfer out of PMTCT (Srivasta et al., Citation2018; World Health Organization, Citation2017). Additional qualitative research is needed to understand if such options would be attractive to this population and quantitatively to assess the potential effectiveness of such strategies on patient retention and treatment outcomes. As increasing numbers of people are initiated onto lifelong ART, countries must continue to develop context specific and flexible models of differentiated care to meet local needs.
To our knowledge this is the first mixed methods study to explore the implementation of policies on the integration of HIV care into routine pregnancy and postpartum care. The findings provided an in-depth case study of the continuum from WHO recommendations to adoption in national policy, through to facility-level implementation, and explored the experiences of service providers and users in three African settings with generalised HIV epidemics.
However, various limitations should be considered when interpreting the study findings. Firstly, the study was conducted in a small sample of health facilities serving the populations of HDSS sites in each country, and should not be considered as nationally representative (Slaymaker et al., Citation2014). However, no additional services were being offered within these facilities as part of the HDSS and all these facilities operated under typical national directives, and thus the implementation of policies and the experiences of users and providers should be fairly typical of what would occur in other rural areas. Secondly, the completion of the survey by facility managers may have introduced a reporting bias, leading to an over-optimistic picture of service delivery. Whilst in person observations on the day by the trained field workers were conducted, this would not confirm the real availability or existence of the service at other times. However, our triangulation with the in-depth interviews from both the provider and the client perspective provided opportunities to deepen our understanding of processes and delivery. Thirdly the timing of the facility surveys differed between countries, making some interpretations challenging.
Conclusion
In conclusion, the adoption and implementation of policies on integrated HIV and MNCH services was similar across the three settings in terms of the location of HIV testing, but varied in terms of when and how women were transferred into routine ART care after their pregnancy. Flexibility within policies and health systems strengthening to reduce pressures on health workers may enable a more patient-centered approach to service delivery. Enabling women to choose when and how to receive their post-pregnancy HIV care may serve to reduce resistance to change and ultimately promote improved retention in long-term ART care.
Acknowledgements
We would like to acknowledge Kathryn Church and Aisha N. Z. Dasgupta for their work in the first two rounds of policy reviews and facility surveys. This work was supported by the Medical Research Council, through a Health Systems Research Initiative grant [grant number MR/ P014313/1]. JR receives funding support from DELTA/THRIVE under DEL15-011/07742/Z/15/Z. Study participants from three HDSS settings for willingness to participate in the study.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection. (2016). Geneva: World Health Organisation.
- Ambia, J., Renju, J., Wringe, A., Todd, J., Geubbels, E., Nakiyingi-Miiro, J., Urassa, M., Lutalo, T., Crampin, A. C., Kwaro, D., Kyobutungi, C., Chimbindi, N., Gomez-Olive, F. X., Tlhajoane, M., Njamwea, B., Zaba, B., & Mee, P. (2017). From policy to practice: Exploring the implementation of antiretroviral therapy access and retention policies between 2013 and 2016 in six sub-Saharan African countries. BMC Health Services Research, 17(1), https://doi.org/10.1186/s12913-017-2678-1
- Church, K., Wringe, A., Fakudze, P., Kikuvi, J., Simelane, D., Mayhew, S. H., & Initiative, I. (2013). Are integrated HIV services less stigmatizing than stand-alone models of care? A comparative case study from Swaziland. Journal of the International AIDS Society, 16(1), 17981. https://doi.org/10.7448/IAS.16.1.17981
- Church, K., Wringe, A., Lewin, S., Ploubidis, G. B., Fakudze, P., Initiative, I., & Mayhew, S. H. (2015). Exploring the feasibility of service integration in a low-income setting: A mixed methods investigation into different models of reproductive health and HIV care in Swaziland. PloS One, 10(5), e0126144–e0126144. https://doi.org/10.1371/journal.pone.0126144
- Clouse, K., Schwartz, S., Van Rie, A., Bassett, J., Yende, N., & Pettifor, A. (2014). What they wanted was to give birth; Nothing else. JAIDS Journal of Acquired Immune Deficiency Syndromes, 67(1), e12–e18. https://doi.org/10.1097/QAI.0000000000000263
- Drake, A. L., Thomson, K. A., Quinn, C., Newman Owiredu, M., Nuwagira, I. B., Chitembo, L., Essajee, S., Baggaley, R., & Johnson, C. C. (2019). Retest and treat: A review of national HIV retesting guidelines to inform elimination of mother-to-child HIV transmission (EMTCT) efforts. Journal of the International AIDS Society, 22, e25271. https://doi.org/10.1002/jia2.25271
- Fowkes, F. J. I., Draper, B. L., Hellard, M., & Stoové, M. (2016). Achieving development goals for HIV, tuberculosis and malaria in sub-Saharan Africa through integrated antenatal care: Barriers and challenges. BMC Medicine, 14(1), 0–10. https://doi.org/10.1186/s12916-016-0753-9
- Jones, H., Wringe, A., Todd, J., Songo, J., Gómez-Olivé, F. X., Moshabela, M., Geubbels, E., Nyamhagatta, M., Kalua, T., Urassa, M., Zaba, B., & Renju, J. (2019). Implementing prevention policies for mother-to-child transmission of HIV in rural Malawi, South Africa and United Republic of Tanzania, 2013–2016. Bulletin of the World Health Organization, 97(3), 200–212. https://doi.org/10.2471/BLT.18.217471
- Joseph Davey, D., Myer, L., Bukusi, E., Ramogola-Masire, D., Kilembe, W., & & Klausner, J. D. (2016). Integrating human immunodeficiency virus and reproductive, maternal and child, and tuberculosis health services within national health systems. Current HIV/AIDS Reports, 13(3), 170–176. https://doi.org/10.1007/s11904-016-0316-x
- Kim, M. H., Ahmed, S., Hosseinipour, M. C., Giordano, T. P., Chiao, E. Y., Yu, X., Nguyen, C., Chimbwandira, F., Kazembe, P. N., & Abrams, E. J. (2015). Implementation and operational research: The impact of option B+ on the antenatal PMTCT cascade in Lilongwe, Malawi. Journal of Acquired Immune Deficiency Syndromes, 68(5), e77–e83. https://doi.org/10.1097/QAI.0000000000000517
- McLean, E., Renju, J., Wamoyi, J., Bukenya, D., Ddaaki, W., Church, K., Zaba, B., & Wringe, A. (2017). “I wanted to safeguard the baby”: A qualitative study to understand the experiences of Option B+ for pregnant women and the potential implications for “test-and-treat” in four sub-Saharan African settings. Sexually Transmitted Infections, 93(Suppl. 3), https://doi.org/10.1136/sextrans-2016-052972
- Ngangue, P., Gagnon, M.-P., & Bedard, E. (2017). Challenges in the delivery of public HIV testing and counselling (HTC) in Douala, Cameroon: Providers perspectives and implications on quality of HTC services. BMC International Health and Human Rights, 17(1), 9. https://doi.org/10.1186/s12914-017-0118-2
- Pellowski, J. A., Weber, A. Z., Phillips, T. K., Brittain, K., Zerbe, A., Abrams, E. J., & Myer, L. (2020). “You must leave but I didn’t want to leave”: Qualitative evaluation of the integration of ART into postnatal maternal and child health services in Cape Town, South Africa. AIDS Care, 32(4), 480–485. https://doi.org/10.1080/09540121.2019.1659913
- Phillips, T. K., Clouse, K., Zerbe, A., Orrell, C., Abrams, E. J., & Myer, L. (2018). Linkage to care, mobility and retention of HIV-positive postpartum women in antiretroviral therapy services in South Africa. Journal of the International AIDS Society, https://doi.org/10.1002/jia2.25114
- Phillips, T., McNairy, M. L., Zerbe, A., Myer, L., & Abrams, E. J. (2015). Postpartum transfer of care among HIV-infected women initiating antiretroviral therapy during pregnancy. Journal of Acquired Immune Deficiency Syndromes, https://doi.org/10.1097/QAI.0000000000000771
- Phillips, T. K., Mogoba, P., Brittain, K., Gomba, Y., Zerbe, A., Myer, L., & Abrams, E. J. (2020). Long-term outcomes of HIV-infected women receiving antiretroviral therapy after transferring out of an integrated maternal and child health service in South Africa. JAIDS Journal of Acquired Immune Deficiency Syndromes, 83(3), https://journals.lww.com/jaids/Fulltext/2020/03010/Long_Term_Outcomes_of_HIV_Infected_Women_Receiving.2.aspx. https://doi.org/10.1097/QAI.0000000000002236
- Slaymaker, E., Todd, J., Marston, M., Calvert, C., Michael, D., Nakiyingi-Miiro, J., Crampin, A., Lutalo, T., Herbst, K., & Zaba, B. (2014). How have ART treatment programmes changed the patterns of excess mortality in people living with HIV? Estimates from four countries in East and Southern Africa. Global Health Action, 7(1), 22789. https://doi.org/10.3402/gha.v7.22789
- Sprague, C., Chersich, M. F., & Black, V. (2011). Health system weaknesses constrain access to PMTCT and maternal HIV services in South Africa: A qualitative enquiry. AIDS Research and Therapy, 8(1), 10. https://doi.org/10.1186/1742-6405-8-10
- Srivasta, M., Sullivan, D., Ryan Phelps, B., Modi, S., & Broyles, L. N. (2018). Boosting ART uptake and retention among HIV-infected pregnant and breastfeeding women and their infants: The promise of innovative service delivery models. Journal of the International AIDS Society, 2016–2019, https://doi.org/10.1002/jia2.25053
- Sweeney, S., Obure, C. D., Maier, C. B., Greener, R., Dehne, K., & Vassall, A. (2012). Costs and efficiency of integrating HIV/AIDS services with other health services: A systematic review of evidence and experience. Sexually Transmitted Infections, 88(2), 85–99. https://doi.org/10.1136/sextrans-2011-050199
- UNAIDS. (2019). UNAIDS data 2019.www.unaids.org/sites/default/files/media_asset/2019-UNAIDS-data_en.pdf
- World Health Organisation (WHO). (2013). Consolidated ARV guidelines. https://www.who.int/hiv/pub/guidelines/arv2013/clinical/box5_1/en/
- World Health Organization (WHO). (2006a). Antiretroviral drugs for treating pregnant women and preventing HIV infections in infants: Towards universal access. https://www.who.int/hiv/pub/guidelines/pmtct/en/.
- World Health Organization (WHO). (2006b). HIV/AIDS programme – ARV drugs for treating pregnant women and preventing HIV in infants in resource-limited settings. https://www.who.int/hiv/pub/mtct/antiretroviral/en/.
- World Health Organization (WHO). (2007). HIV/AIDS programme guidance on provider-initiated HIV testing and counselling in health facilities. www.who.int/hiv/pub/vct/pitc2007/en/.
- World Health Organization (WHO). (2010, April). Programmatic update: Use of antiretroviral drugs for treating pregnant women and preventing HIV INfection in infants, 1–117. https://doi.org/WHO/HIV/2012.6
- World Health Organization (WHO). (2015a). Guidelines on when to start antiretroviral therapy and on pre-exposure prophylaxis for HIV. https://doi.org/978-92-4-150956-5
- World Health Organization (WHO). (2015b). Service availability and readiness assessment (SARA). https://www.who.int/healthinfo/systems/sara_reference_manual/en/
- World Health Organization (WHO). (2017). Key considerations for differentiated antiretroviral therapy delivery for specific populations. /tz.usembassy.gov/wp-content/uploads/sites/258/2017/08/WHO-HIV-2017.34-eng.pdf.
- World Health Organization (WHO). (2019). Consolidated guidelines on HIV testing services for a changing epidemic. www.paho.org/en/node/72097.
