ABSTRACT
Many implementation efforts experience interruptions, especially in settings with developing health systems. Approaches for evaluating interruptions are needed to inform re-implementation strategies. We sought to devise an approach for evaluating interruptions by exploring the sustainability of a programme that implemented diabetes mellitus (DM) screening within tuberculosis clinics in Uganda in 2017. In 2019, we conducted nine interviews with clinic staff and observed clinic visits to determine their views and practices on providing integrated care. We mapped themes to a social ecological model with three levels derived from the Consolidated Framework for Implementation Research (CFIR): outer setting (i.e. community), inner setting (i.e. clinic), and individuals (i.e. clinicians). Respondents explained that DM screening ceased due to disruptions in the national supply chain for glucose test strips, which had cascading effects on clinics and clinicians. Lack of screening supplies in clinics limited clinicians’ opportunities to perform DM screening, which contributed to diminished self-efficacy. However, culture, compatibility and clinicians’ beliefs about DM screening sustained throughout the interruption. We propose an approach for evaluating interruptions using the CFIR and social ecological model; other programmes can adapt this approach to identify cascading effects of interruptions and target them for re-implementation.
Introduction
Implementation science and theories of quality improvement have been used to inform and assess interventions across many contexts (Theobald et al., Citation2018). The Consolidated Framework for Implementation Research (CFIR) is among the most influential of these frameworks. The CFIR was created by the Veteran Affairs Healthcare System to identify constructs that impact the successful implementation of an intervention (Damschroder et al., Citation2009). It has been applied to examine implementation of programmes ranging from evidence-based tuberculosis (TB) practices in Indonesia to child and maternal health outcomes in Kenyan hospitals (English, Citation2013; English et al., Citation2011; Lestari et al., Citation2019).
While the CFIR has been used to optimise the initial adoption and progress of interventions, it has not been used to scrutinise the sustainability of implementation efforts when health system resources or characteristics vary over time (Chambers et al., Citation2013). Variation in health system resources or characteristics is common in many low- and middle-income countries. For example, variation in availability of essential medicines and screening and management resources have impeded sustainable care for HIV, TB, reproductive health, and non-communicable diseases (Armstrong-Hough et al., Citation2018; Armstrong-Hough et al., Citation2020; Hwang et al., Citation2019; Kyei-Nimakoh et al., Citation2017; Siddharthan et al., Citation2015; Sullivan et al., Citation2017). Despite these well-reported health system barriers, no studies have applied the CFIR to analyze how variation in such settings may relate to programme interruptions or applied the CFIR to identify barriers and facilitators to re-implementation following significant interruptions.
Thus, there is critical need for an implementation science of interruption: guidance for how to prevent or mitigate interruptions, categorise their sources and targets, and design re-implementation strategies that build on the elements that tend to persist in the face of interruption. To respond to this knowledge gap, we carried out a qualitative study to examine an interruption in delivery of an evidence-based intervention to screen TB patients for diabetes mellitus (DM) in Kampala, Uganda. We conducted semi-structured interviews with clinic staff using the CFIR to identify factors that persisted and factors that diminished in the midst of a programme interruption. By reorganising these CFIR-derived factors into a social ecological model, we created an approach to guide assessments of intervention interruptions and inform re-implementation strategies.
Materials & methods
Conceptual framework
The implementation science agenda has prioritised the identification of explanatory mechanisms that describe how the context in which an intervention is being implemented impacts sustainability (Proctor et al., Citation2015). One way to categorise these mechanisms is through the use of social ecological models, which contextualise an individual’s functioning within the context of their immediate social networks, setting, and community (Bronfenbrenner, Citation1992). This theory has been applied to health programmes, whereby the programme functions within the context of its practitioners, practice setting, public policy, and population (Chambers et al., Citation2013). Factors at each ecological level can change over time and impact the compatibility and sustainability of the programme (Chambers et al., Citation2013).
Similarly, the CFIR is a multilevel framework that posits interaction among multiple domains (outer setting, inner setting, process, individual characteristics, and intervention characteristics) (Damschroder et al., Citation2009), though these levels are not explicitly organised as an ecological model. We nested CFIR constructs within a social ecological model to conceptualise how implementation factors interact between and within ecological levels during a programme interruption. By situating the CFIR more explicitly within a social ecological model, we aimed to understand the cascading effects and mechanisms of programme interruptions at each ecological level and identify targets for re-implementation.
Study setting
This study took place in two public health centres with high-volume TB clinics in Kampala, Uganda that previously participated in a programme that implemented DM screening. Public clinics in Uganda provide free healthcare to patients as long as supplies are available, whereas private clinics charge patients (Nshuti et al., Citation2001). In 2017, the estimated incidence of TB in Uganda was 201 per 100,000 people, and the TB mortality rate was 26 per 100,000 people (Centers for Disease Control and Prevention Division of Global HIV & TB, Citation2019). The World Health Organization (WHO) recommends routine screening and treatment of DM among patients presenting with TB (World Health Organization, Citation2015) in response to a growing body of evidence that suggests a bidirectional relationship between DM and TB (Young et al., Citation2009). DM has been observed to increase the progression from latent to active TB three-fold and increase adverse treatment outcomes for active TB fivefold (Harries et al., Citation2016; Hayashi & Chandramohan, Citation2018). However, patients presenting at TB units with hyperglycemia in low-resource settings are rarely diagnosed or counselled on controlling their blood sugar levels: half of Ugandans living with DM and 90% living with pre-DM are left undiagnosed (Bahendeka et al., Citation2016).
Initial implementation
Both health facilities had previously participated in an internationally funded public health programme of integrated TB-DM screening and evaluation, which ended in December 2017. This pilot programme equipped staff in TB units with implementation strategies and resources, namely training, screening algorithms to support clinical decision-making, glucometers, test strips, and TB treatment registers with entries for random blood glucose and fasting blood glucose readings. Once the pilot programme ended, clinics planned to receive screening supplies for DM from the National Medical Stores, which allocate essential medicines to health centres across Uganda. However, interruptions to the supply chain led to unreliable access to key materials in clinics after the initial implementation, such as glucose test strips and functional glucometers. Consequently, delivery of DM screening in TB clinics declined once project funding ended.
Re-implementation study
In June 2019, independent of the initial pilot programme, our research team sought to assess the implementation climate for newly introducing DM care into these two TB clinics. Neither clinic had regularly offered DM evaluation since shortly after the previous pilot ended, 18 months prior. Thus, we conducted key informant interviews with health workers to identify aspects of the original DM screening programme that persisted and diminished during the interruption to create a targeted re-implementation strategy. The Consolidated Criteria for Reporting Qualitative Research (Tong et al., Citation2007) guided study reporting.
Sampling and study participants
We purposively sampled health workers and administrators from the two public health centres in August 2019. A male Ugandan researcher on the team (JG) introduced a female non-Ugandan researcher (RH) to the health workers at clinic sites. Health worker participants were recruited in person based on their involvement with delivery of care in the TB unit. Health workers were invited to participate in key informant interviews about barriers and facilitators to the re-integration of DM screening into routine TB evaluation and treatment initiation.
Data collection
We collected data from two sources: (1) ethnographic observation and (2) semi-structured interviews. First, a non-Ugandan doctoral student with six years of qualitative research experience in East Africa (RH) spent six weeks conducting ethnographic observation at clinic sites, during which she observed clinic visits and interactions between health workers and presumptive TB patients. RH took detailed fields notes on how presumptive TB patients were screened, diagnosed, and initiated on treatment, and the extent to which services were integrated for TB, HIV, and DM. RH also noted resources at the clinic that facilitated integrated TB-DM care, such as DM screening algorithms for TB patients. Then, we created semi-structured interview questions using the CFIR interview guide tool to assess health workers’ perspectives on factors related to the sustainability of TB-DM care integration (Damschroder et al., Citation2009). After creating the questions, we iteratively trialed the interview guide with a Ugandan researcher with expertise in conducting qualitative interviews in this setting (JG). Through local pre-testing, we adapted the interview questions to ensure conceptual equivalence in Uganda. All interviews were conducted at clinic sites in English by RH. Interviews lasted from 20 to 66 min and the interviewer wrote field notes after each interview. Interviews were recorded on an encrypted smart phone. Interviews were de-identified, professionally transcribed, reviewed and revised for accuracy, and uploaded into ATLAS.ti 8.
Analysis
Transcripts were double coded by a non-Ugandan researcher (RH) and the code structure was reviewed and validated by other members of the study team (MAH, JG) in two cycles using an abductive approach (Tavory & Timmermans, Citation2014). Abductive analysis, widely used in the social sciences, facilitates conversations between grounded, inductive coding and theory-derived deductive coding (Earl Rinehart, Citation2021; Meyer & Lunnay, Citation2013; Tavory & Timmermans, Citation2014; Timmermans & Tavory, Citation2012). By merging inductive and deductive codes, abductive analysis provides an opportunity to evolve existing theory in response to evidence (Earl Rinehart, Citation2021; Meyer & Lunnay, Citation2013; Tavory & Timmermans, Citation2014; Timmermans & Tavory, Citation2012). First, using the accepted CFIR inclusion and exclusion criteria for each construct (CFIR Research Team-Center for Clinical Management Research), we applied codes to passages that reflected relevant CFIR constructs and subconstructs. After this first round of directed coding, we carried out inductive content analysis using grounded theory, adding novel codes to the code tree to represent themes that emerged from the transcripts but were not strictly contained by CFIR constructs. We organised all themes by CFIR domain. We applied codes independently and allowed for co-occurrence of multiple codes. We triangulated findings from the semi-structured interviews with detailed field notes that were collected throughout ethnographic observation. Data saturation was defined as the point at which no new themes emerged from the data (Morse, Citation1995).
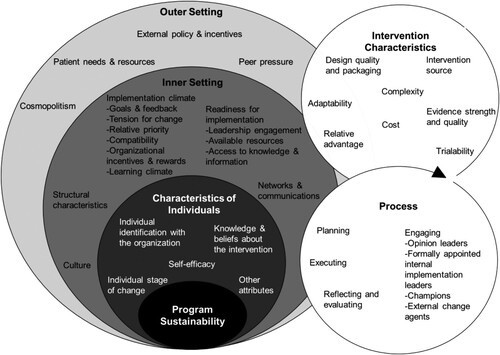
After coding, we used thematic analysis to identify how the interruption impacted other elements of the programme. To do this, we mapped the established CFIR domains, constructs, and subconstructs to a social ecological model (). The CFIR has five domains: outer setting, inner setting, characteristics of individuals involved, intervention characteristics, and process (Damschroder et al., Citation2009). The outer setting, inner setting, and individuals CFIR domains each represent their own ecological levels surrounding the programme sustainability. The outer setting domain includes community-level factors, e.g. community needs, medical supply chain, and national policy. The inner setting includes clinic-level factors, e.g. culture, goals, and resources. The individuals domain includes factors related to health workers, e.g. beliefs about diabetes screening and self-efficacy to perform screening. We included the process and intervention characteristics domains within the ecological level pertinent to the specific factor; for example, the engaging stakeholders construct within the process domain was mapped to the individuals ecological level. We then used this hybrid model to trace the factors that participants described as facilitating or hindering re-introduction of TB-DM screening. We identified relationships between factors at different ecological levels to better understand the mechanisms and cascading effects that impacted DM programme sustainability to target them for re-implementation.
Figure 1. CFIR nested within an ecological model. The outer setting, inner setting, and characteristics of individuals CFIR domains are each mapped to their appropriate ecological levels. CFIR constructs for each of these domains are included within the corresponding ecological level. The intervention characteristics and process CFIR domains and associated constructs span multiple ecological levels and are sorted to the appropriate ecological level based on the specific factor.
Human subjects and ethics approval
Each participant provided verbal consent. The study was approved by the ethics review boards of the Makerere University School of Public Health and Yale University Human Investigation Committee.
Results
Sample
All nine staff members across the two public-sector TB clinics agreed to participate in the interviews (). All key informants worked at a participating clinic during the original TB-DM implementation in 2016 and continued to work in a public facility in August 2019. Their median years of experience in their current role was 3 years (range: 2 weeks, 8 years) and median years of experience in the health field overall was 8 years (range: 3, 20 years). Most (7, 78%) were female. As all staff members across the two public-sector TB clinics participated and no new themes emerged from the data after seven interviews, sample saturation and data saturation were met, respectively.
Table 1. Participant characteristics.
Characterisation of programme interruption
Staff members explained that the delivery of DM screening in the TB clinic was interrupted by changes in the outer setting, particularly the delivery of DM screening supplies from the National Medical Stores. One TB clinic leader described:
So now if you push your order to the NMS [National Medical Stores], it can take like two weeks or two months. Nothing much I can do because I've already informed the bosses in charge of that and if it's not coming we cannot do much. Nothing.
Thematic analysis identified one factor that was diminished and two factors that were sustained at the community-level (i.e. outer setting domain) (). At the clinic-level (i.e. inner setting domain), two factors were diminished while three factors were sustained (). For factors related to the health workers (i.e. individuals domain), one was diminished and one was sustained (). The CFIR domains, constructs, descriptions, and representative quotes for each factor are presented in Tables 2–4. We mapped each of these factor-domain pairs to their respective ecological level in the hybrid model depicted in . Our adapted model describing the erosion or persistence of key factors influencing implementation of DM screening in the face of programme interruption is presented in .
Figure 2. Mechanisms that facilitated and hindered sustainability of the TB-DM programme. Dashed arrows highlight the ecological dynamics contributing to the sustainability of TB-DM care during the intervention interruption. Solid arrows highlight the ecological dynamics contributing to the unsustainability of TB-DM care during the intervention interruption.
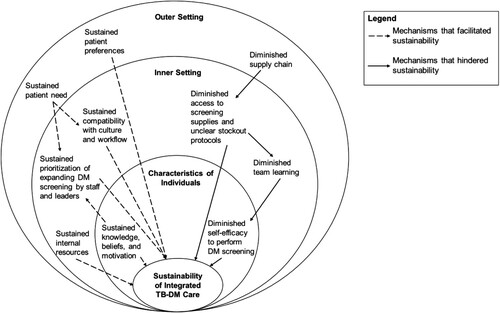
Table 2. Outer setting factors that were sustained or diminished during study interruption.
Table 3. Inner setting factors that were sustained or diminished during study interruption.
Table 4. Characteristics of individuals that were sustained or diminished during study interruption.
During the programme interruption, key informants reported that community-level factors, including community needs and patient preferences, sustained (). For example, one community health worker described:
Our patients are poor. They can't afford testing from a private clinic. Whenever I tell them go, and test for this, it’s just 5,000 [shillings], but they can't afford it. So, at the end of the day, this patient is not helped, they're not healing. So, I think there is that need for them to test from the clinic, if we get the chances of getting the [testing] kits. Because you would help this person know their diabetic status and if they are diabetic, we are able to start treatment.
At the clinic-level, many factors facilitating DM screening persisted. These included the compatibility of DM screening with the culture and workflow of the clinic, prioritisation of DM screening by staff and leaders, and internal resources, such as physical space, workforce, and available time to carry out DM screening (). For example, one facility in-charge commented:
DM screening is very positive for the client's sake and also for the health workers because when you diagnose the DM in a TB client that it is good because both are deadly diseases.
However, over time, the lack of DM testing kits and equipment from the outer setting diminished available screening resources and team learning in the clinic. One TB nurse described what happened when the clinic faced material shortages that reduced opportunities to practice screening:
Even if you had a training before but you didn't practice, so that will be old. So you really need constant trainings so that you're up to date.
At the individual-level, health workers demonstrated sustained knowledge, beliefs, and motivation to screen for DM in the TB clinic long after the interruption began (). One community health worker said:
I feel bad that we are not screening DM because I know that TB can move with diabetes. We are dealing with the co-infected patients probably, they can have diabetes. If we have not screened them, they will go minus that service, so I feel bad.
However, health workers reported that the interruption hindered their ability to reinforce and improve their DM clinical skills, leading to diminished self-efficacy. One community health worker described:
We are taking a full year since my last doing it [DM screening]. So, somethings I may now be forgetting.
Cascading effects of programme interruption
There were several mechanisms by which factors related to the community (i.e. outer setting domain), clinic (i.e. inner setting domain), and health workers (i.e. individuals domain) interacted during the service interruption. First, we identified a cascading effect by which the inconsistent supply chain in the outer setting inhibited the availability of resources to screen for DM in the clinic (, solid arrows). Without access to screening supplies, team members could not conduct ongoing training and reinforce their skills through clinical practice. The lack of team learning in the clinic, in turn, trickled down to diminish health workers’ self-efficacy to perform DM screening.
Notably, several factors sustained during the 18-month programme interruption, primarily through institutional memory of the TB-DM programme’s goals and outcomes (, dashed arrows). For example, key informants reported sustained patient need and preferences for DM screening in the community (i.e. outer setting domain). Patient needs and preferences interacted with the clinic’s culture of altruism and reinforced their high prioritisation of DM screening among staff and clinic leadership. At the individual provider level, memory and positive associations of the programme led to sustained knowledge, beliefs, and motivation to screen for DM in the TB clinic. The sustained knowledge, beliefs, and motivation related to DM screening contributed to continuing high prioritisation of DM screening within clinics, even when no patients had been screened for DM for over a year. The institutional memory of the DM screening programme also sustained providers’ and administrators’ perceptions of the programme’s compatibility with the clinic workflow, physical space, workforce, and available time for DM screening. Providers and administrators retained positive perceptions of the intervention characteristics long after daily delivery of DM screening had ceased. The persistence of factors associated with sustainability within the community and clinic and among health workers suggests a favourable re-implementation climate.
Discussion
Many implementation efforts experience cycles of interruption, especially in settings with developing health systems (Ssengooba et al., Citation2012; Zakumumpa et al., Citation2018). Implementation programmes must adapt to the dynamic context in which they function to maximise sustainable delivery (Chambers et al., Citation2013; Scheirer & Dearing, Citation2011; Schell et al., Citation2013; Stirman et al., Citation2012). However, little guidance is available for understanding or responding to interruptions when they do occur. This study adds to the literature by organising key domains and constructs of the CFIR within a social ecological model to systematically evaluate and respond to programme interruptions. Using an empirical case study of an interruption to delivery of DM screening in Uganda, we identified implementation factors that persisted long after the screening programme ceased, as well as implementation factors that faltered in response to the interruption. Most importantly, we described and theorised a cascading effect whereby changes in the outer setting first imperil constructs in the clinic that once facilitated implementation, and through these clinic changes eventually begin to erode clinician characteristics that once facilitated implementation. By integrating the CFIR with social ecological theory, we propose an approach for systematically evaluating and responding to programme interruptions.
This approach to understanding the causes, consequences, and remedies available following programme interruptions is particularly relevant to other non-communicable disease programmes in low-income countries. Low-income countries in sub-Saharan Africa increasingly face a double burden of disease, with high rates of infectious diseases compounded with increasing rates of non-communicable diseases (Addo et al., Citation2007; Mbanya et al., Citation2010; Nyirenda, Citation2016). However, health systems in sub-Saharan Africa have been tailored to address infectious diseases, namely HIV, rather than non-communicable diseases (Pastakia et al., Citation2018; Schwartz et al., Citation2015). Variation over time in the outer setting (e.g. supply chain for essential medicines), inner setting (e.g. availability of functional glucometers), and characteristics of individuals (e.g. community health worker knowledge of diabetes) must be considered when developing, implementing, and re-implementing interventions for non-communicable diseases.
For example, our study found that lack of DM screening supplies at the clinic was the main reason that screening initially declined. Shortages of technologies and tools that the WHO deems as essential for non-communicable diseases are common in East Africa (Peck et al., Citation2014; Rogers et al., Citation2018). A study including 53 health centres in Uganda found that only 62% had a glucometer and 13% had urine testing strips to screen for DM. Further, only 40% had a standard blood pressure cuff and 43% had an automated blood pressure machine, which are necessary for assessing hypertension (Rogers et al., Citation2018). Although most labs had random blood glucose capabilities (92%), only 9% could measure hemoglobin A1c and 28% could conduct a lipid profile, which are necessary for diagnosing and monitoring DM and hyperlipidemia, respectively. Lastly, all health centres reported that they experienced at least one stockout of a non-communicable disease essential medicine within the past year. While a project that provides these technologies and resources might be effective to screen and treat non-communicable diseases in the short term, interventions may not be sustainable without targeting the domestic supply chains themselves. Given the far-reaching implications of these supply chain disruptions, future research is warranted to improve our understanding of supply chains using appropriate frameworks, such as Tanahashi’s model for health system bottlenecks (Kiwanuka Henriksson et al., Citation2017; Tanahashi, Citation1978).
Nesting CFIR constructs within a social ecological model can facilitate the evaluation of interruptions and identify opportunities for re-implementation. In our study, we found that uncertainty in the supply chain hindered team learning in the clinic and ultimately diminished the self-efficacy of practitioners to deliver routine DM screening. These cascading failures could be addressed as part of a targeted re-implementation strategy by creating and disseminating tailored refresher training for key stakeholders. For example, providing targeted training to health teams in clinics would likely disrupt the cascading effect that decreased team learning had on health worker self-efficacy. This training should also include the introduction of standardised protocols for dealing with stockouts in clinics, given the lack of consensus among participants about who to contact and how to procure DM screening supplies when they run out. We also found that the screening programme in our study continued to be seen as compatible with community needs, culture, and priorities of the clinic, as well as with motivations of practitioners. A targeted re-implementation strategy could draw on the elements that sustained during the programme interruption to facilitate rapid buy-in and uptake from the community, clinic leadership, and practitioners.
Strengths and limitations
Our study was strengthened by its engagement with health workers through both ethnographic and interview methods. This approach enabled triangulation, enhancing the internal validity of findings. Another strength was our use of the CFIR from the earliest planning stages through the final analysis. We adapted the interview guide from the standard CFIR guide and carried out directed content analysis using pre-defined CFIR construct codes. We also conducted an additional round of coding using inductive content analysis to create open codes, which ultimately generated insights on cascading effects of the interruption. Our use of the CFIR and social ecological model also enabled us to generate findings that are transferrable to other settings that experience disruptions in the supply chain, for example in other areas of Uganda and East Africa (Peck et al., Citation2014; Rogers et al., Citation2018). Ugandan members of our research team reviewed qualitative data and provided feedback throughout analysis to improve the validity of our interpretations. Finally, this study was strengthened by reflecting on a two-year timeframe, which enabled key informants to comment on sustainability.
However, this study had some limitations. First, a non-Ugandan conducted ethnographic observation and semi-structured interviews, which could have influenced how health workers conducted themselves during observation and what they were willing to say during the interviews. Because we used purposive sampling from two public clinics in Kampala, the study setting and participants may not be representative of all public TB clinics in Kampala that participated in the earlier DM screening programme. Nonetheless, many health workers reported having previously worked at other public clinics in Kampala at the time of the implementation and spoke about their collective experiences. Additionally, this study could be at risk for recall bias given the two-year period between initial programme implementation and interviews. Furthermore, this study could be at risk for social desirability bias insofar that clinic staff could have reported perspectives that they perceived as agreeable to the research team’s mission. Lastly, this study only captures the perspectives of nine clinic staff; thus, future studies are needed that explore interruptions in programmes from the perspective of these and other health system stakeholders.
Conclusion
We critically analyzed the sustainability of a DM screening programme in the face of supply chain instability. We used this empirical study to generate an approach for understanding the ecological dynamics of sustainability, drawing on the CFIR and social ecological model. The resulting hybrid approach can be used to systematically assess factors that persisted and diminished during programme interruptions at each ecological level, including the community-level (i.e. outer setting CFIR domain), clinic-level (i.e. inner setting domain), and health worker-level (i.e. individuals domain). This approach can clarify the cascading effects of programme interruptions and inform re-implementation strategies. Future empirical studies are needed to apply, assess, and adapt this approach and further develop the study of interruption in implementation science.
Acknowledgements
We would like to acknowledge the participation of all the health workers and administrators who participated in our study and provided their insights.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Addo, J., Smeeth, L., & Leon, D. A. (2007). Hypertension in sub-saharan Africa: A systematic review. Hypertension, 50(6), 1012–1018. https://doi.org/10.1161/HYPERTENSIONAHA.107.093336
- Armstrong-Hough, M., Kishore, S. P., Byakika, S., Mutungi, G., Nunez-Smith, M., & Schwartz, J. I. (2018). Disparities in availability of essential medicines to treat non-communicable diseases in Uganda: A Poisson analysis using the service availability and readiness assessment. PLoS ONE, 13(2), e0192332. https://doi.org/10.1371/journal.pone.0192332
- Armstrong-Hough, M., Sharma, S., Kishore, S. P., Akiteng, A. R., & Schwartz, J. I. (2020). Variation in the availability and cost of essential medicines for non-communicable diseases in Uganda: A descriptive time series analysis. PLoS ONE, 15(12), e0241555. https://doi.org/10.1371/journal.pone.0241555
- Bahendeka, S., Wesonga, R., Mutungi, G., Muwonge, J., Neema, S., & Guwatudde, D. (2016). Prevalence and correlates of diabetes mellitus in Uganda: A population-based national survey. Tropical Medicine & International Health, 21(3), 405–416. https://doi.org/10.1111/tmi.12663
- Bronfenbrenner, U. (1992). Ecological systems theory. Jessica Kingsley Publishers.
- Centers for Disease Control and Prevention Division of Global HIV & TB. (2019). Uganda Country Profile. CFIR Research Team-Center for Clinical Management Research. Consolidated Framework for Implementation Research. 2020, from cfirguide.org.
- Chambers, D. A., Glasgow, R. E., & Stange, K. C. (2013). The dynamic sustainability framework: Addressing the paradox of sustainment amid ongoing change. Implementation Science, 8(1), 117. https://doi.org/10.1186/1748-5908-8-117
- Damschroder, L. J., Aron, D. C., Keith, R. E., Kirsh, S. R., Alexander, J. A., & Lowery, J. C. (2009). Fostering implementation of health services research findings into practice: A consolidated framework for advancing implementation science. Implementation Science, 4(1), 886. https://doi.org/10.1186/1748-5908-4-50
- Earl Rinehart, K. (2021). Abductive analysis in qualitative inquiry. Qualitative Inquiry, 27(2), 303–311. https://doi.org/10.1177/1077800420935912
- English, M. (2013). Designing a theory-informed, contextually appropriate intervention strategy to improve delivery of paediatric services in Kenyan hospitals. Implementation Science, 8(1), 39. https://doi.org/10.1186/1748-5908-8-39
- English, M., Nzinga, J., Mbindyo, P., Ayieko, P., Irimu, G., & Mbaabu, L. (2011). Explaining the effects of a multifaceted intervention to improve inpatient care in rural Kenyan hospitals–interpretation based on retrospective examination of data from participant observation, quantitative and qualitative studies. Implementation Science, 6(1), 124. https://doi.org/10.1186/1748-5908-6-124
- Harries, A. D., Kumar, A. M. V., Satyanarayana, S., Lin, Y., Zachariah, R., Lönnroth, K., & Kapur, A. (2016). Addressing diabetes mellitus as part of the strategy for ending TB. Transactions of The Royal Society of Tropical Medicine and Hygiene, 110(3), 173–179. https://doi.org/10.1093/trstmh/trv111
- Hayashi, S., & Chandramohan, D. (2018). Risk of active tuberculosis among people with diabetes mellitus: Systematic review and meta-analysis. Tropical Medicine & International Health, 23(10), 1058–1070. https://doi.org/10.1111/tmi.13133
- Hwang, B., Shroufi, A., Gils, T., Steele, S. J., Grimsrud, A., Boulle, A., Yawa, A., Stevenson, S., Jankelowitz, L., Versteeg-Mojanaga, M., Govender, I., Stephens, J., Hill, J., Duncan, K., & Cutsem, G. V. (2019). Stock-outs of antiretroviral and tuberculosis medicines in South Africa: A national cross-sectional survey. PLoS ONE, 14(3), e0212405. https://doi.org/10.1371/journal.pone.0212405
- Kiwanuka Henriksson, D., Fredriksson, M., Waiswa, P., Selling, K., & Swartling Peterson, S. (2017). Bottleneck analysis at district level to illustrate gaps within the district health system in Uganda. Global Health Action, 10(1), 1327256. https://doi.org/10.1080/16549716.2017.1327256
- Kyei-Nimakoh, M., Carolan-Olah, M., & McCann, T. V. (2017). Access barriers to obstetric care at health facilities in sub-Saharan Africa—A systematic review. Systematic Reviews, 6(1), 110. https://doi.org/10.1186/s13643-017-0503-x
- Lestari, T., Graham, S., vandenBoogard, C., Triasih, R., Poespoprodjo, J. R., Ubra, R. R., Kenangalem, E., Mahendradhata, Y., Anstey, N. M., & Bailie, R. S. (2019). Bridging the knowledge-practice gap in tuberculosis contact management in a high-burden setting: A mixed-methods protocol for a multicenter health system strengthening study. Implementation Science, 14(1), 31. https://doi.org/10.1186/s13012-019-0870-x
- Mbanya, J. C., Motala, A. A., Sobngwi, E., Assah, F. K., & Enoru, S. T. (2010). Diabetes in sub-Saharan Africa. The Lancet, 375(9733), 2254–2266. https://doi.org/10.1016/S0140-6736(10)60550-8
- Meyer, S. B., & Lunnay, B. (2013). The application of abductive and retroductive inference for the design and analysis of theory-driven sociological research. Sociological Research Online, 18(1), 86–96. https://doi.org/10.5153/sro.2819
- Morse, J. M. (1995). The significance of saturation. Sage.
- Nshuti, L., Neuhauser, D., Johnson, J. L., Adatu, F., & Whalen, C. C. (2001). Public and private providers’ quality of care for tuberculosis patients in Kampala, Uganda. The international Journal of Tuberculosis and Lung Disease, 5(11), 1006–1012.
- Nyirenda, M. J. (2016). Non-communicable diseases in sub-Saharan Africa: Understanding the drivers of the epidemic to inform intervention strategies. International Health, 8(3), 157–158. https://doi.org/10.1093/inthealth/ihw021
- Pastakia, S. D., Tran, D. N., Manji, I., Wells, C., Kinderknecht, K., & Ferris, R. (2018). Building reliable supply chains for noncommunicable disease commodities: Lessons learned from HIV and evidence needs. AIDS, 32(Suppl. 1), S55–S61. https://doi.org/10.1097/QAD.0000000000001878
- Peck, R., Mghamba, J., Vanobberghen, F., Kavishe, B., Rugarabamu, V., Smeeth, L., Hayes, R., Grosskurth, H., & Kapiga, S. (2014). Preparedness of Tanzanian health facilities for outpatient primary care of hypertension and diabetes: A cross-sectional survey. The Lancet Global Health, 2(5), e285–e292. https://doi.org/10.1016/S2214-109X(14)70033-6
- Proctor, E., Luke, D., Calhoun, A., McMillen, C., Brownson, R., McCrary, S., & Padek, M. (2015). Sustainability of evidence-based healthcare: Research agenda, methodological advances, and infrastructure support. Implementation Science, 10(1), 88. https://doi.org/10.1186/s13012-015-0274-5
- Rogers, H. E., Akiteng, A. R., Mutungi, G., Ettinger, A. S., & Schwartz, J. I. (2018). Capacity of Ugandan public sector health facilities to prevent and control non-communicable diseases: An assessment based upon WHO-PEN standards. BMC Health Services Research, 18(1), 606. https://doi.org/10.1186/s12913-018-3426-x
- Scheirer, M. A., & Dearing, J. W. (2011). An agenda for research on the sustainability of public health programs. American Journal of Public Health, 101(11), 2059–2067. https://doi.org/10.2105/AJPH.2011.300193
- Schell, S. F., Luke, D. A., Schooley, M. W., Elliott, M. B., Herbers, S. H., Mueller, N. B., & Bunger, A. C. (2013). Public health program capacity for sustainability: A new framework. Implementation Science, 8(1). https://doi.org/10.1186/1748-5908-8-15
- Schwartz, J. I., Dunkle, A., Akiteng, A. R., Birabwa-Male, D., Kagimu, R., Mondo, C. K., Mutungi, G., Rabin, T. L., Skonieczny, M., Sykes, J., & Mayanja-Kizza, H. (2015). Towards reframing health service delivery in Uganda: The Uganda initiative for integrated management of non-communicable diseases. Global Health Action, 8(1), 26537. https://doi.org/10.3402/gha.v8.26537
- Siddharthan, T., Ramaiya, K., Yonga, G., Mutungi, G. N., Rabin, T. L., List, J. M., Kishore, S. P., & Schwartz, J. I. (2015). Noncommunicable diseases in East Africa: Assessing the gaps in care and identifying opportunities for improvement. Health Affairs, 34(9), 1506–1513. https://doi.org/10.1377/hlthaff.2015.0382
- Ssengooba, F., McPake, B., & Palmer, N. (2012). Why performance-based contracting failed in Uganda – An “open-box” evaluation of a complex health system intervention. Social Science & Medicine, 75(2), 377–383. https://doi.org/10.1016/j.socscimed.2012.02.050
- Stirman, S. W., Kimberly, J., Cook, N., Calloway, A., Castro, F., & Charns, M. (2012). The sustainability of new programs and innovations: A review of the empirical literature and recommendations for future research. Implementation Science, 7(17). https://doi.org/10.1186/1748-5908-7-17
- Sullivan, B. J., Esmaili, B. E., & Cunningham, C. K. (2017). Barriers to initiating tuberculosis treatment in sub-Saharan Africa: A systematic review focused on children and youth. Global Health Action, 10(1), 1290317. https://doi.org/10.1080/16549716.2017.1290317
- Tanahashi, T. (1978). Health service coverage and its evaluation. Bulletin of the World Health Organization, 56(2), 295.
- Tavory, I., & Timmermans, S. (2014). Abductive analysis: Theorizing qualitative research. University of Chicago Press.
- Theobald, S., Brandes, N., Gyapong, M., El-Saharty, S., Proctor, E., Diaz, T., Wanji, S., Elloker, S., Raven, J., Elsey, H., Bharal, S., Pelletier, D., & Peters, D. H. (2018). Implementation research: New imperatives and opportunities in global health. The Lancet, 392(10160), 2214–2228. https://doi.org/10.1016/S0140-6736(18)32205-0
- Timmermans, S., & Tavory, I. (2012). Theory construction in qualitative research: From grounded theory to abductive analysis. Sociological Theory, 30(3), 167–186. https://doi.org/10.1177/0735275112457914
- Tong, A., Sainsbury, P., & Craig, J. (2007). Consolidated criteria for reporting qualitative research (COREQ): A 32-item checklist for interviews and focus groups. International Journal for Quality in Health Care, 19(6), 349–357. https://doi.org/10.1093/intqhc/mzm042
- World Health Organization. (2015). Collaborative framework for care and control of tuberculosis and diabetes.
- Young, F., Critchley, J. A., Johnstone, L. K., & Unwin, N. C. (2009). A review of co-morbidity between infectious and chronic disease in Sub Saharan Africa: TB and diabetes mellitus, HIV and metabolic syndrome, and the impact of globalization. Globalization and Health, 5(1), 9. https://doi.org/10.1186/1744-8603-5-9
- Zakumumpa, H., Dube, N., Damian, R. S., & Rutebemberwa, E. (2018). Understanding the dynamic interactions driving the sustainability of ART scale-up implementation in Uganda. Global Health Research and Policy, 3(1), 23. https://doi.org/10.1186/s41256-018-0079-6
