ABSTRACT
Antimicrobial resistance (AMR) is a One Health problem underpinned by complex drivers and behaviours. This is particularly so in low – and middle-income countries (LMICs), where social and systemic factors fuel (mis)use and drive AMR. Behavioural change around antimicrobial use could safeguard both existing and future treatments. However, changing behaviour necessitates engaging with people to understand their experiences. This publication describes a knowledge-exchange cluster of six LMIC-based projects who co-designed and answered a series of research questions around the usage of Community Engagement (CE) within AMR. Findings suggest that CE can facilitate AMR behaviour change, specifically in LMICs, because it is a contextualised approach which supports communities to develop locally meaningful solutions. However, current CE interventions focus on human aspects, and demand-side drivers, of AMR. Our cluster suggests that broader attention should be paid to AMR as a One Health issue. The popularity of mixed methods approaches within existing CE for AMR interventions suggests there is interdisciplinary interest in the uptake of CE. Unfortunately, the specificity and context-dependency of CE can make it difficult to evaluate and scale. Nevertheless, we suggest that in synthesising learnings from CE, we can develop a collective understanding of its scope to tackle AMR across contexts.
Introduction
Across the world, antimicrobial use is excessive. It is accelerating the natural process of antimicrobial resistance (AMR) whereby bacteria and other microbes change to survive the drugs designed to control them. Because many microbes can infect humans as well as other animals, and can survive in our natural environment, AMR can spread easily and is considered a One Health problem (CDCP, Citation2017; Holmes et al., Citation2016; WHO, Citation2019). Common infections could once again become killers if they can no longer be controlled by antimicrobials (O’neill, Citation2016; WHO, Citation2015; WHO, Citation2016). This will make surgeries, and processes that reduce immune function (such as chemotherapies), much riskier. The UK Governments review of AMR (O’neill, Citation2016) shows that drug-resistant diseases already account for around 700,000 human deaths globally each year, and that without significant action, this could rise to 10 million per year by 2050. Malnutrition and food poverty will contribute to this death toll as AMR impacts the health of food-producing animals and crops (Fisher et al., Citation2018). The World Bank predicts that by 2030 up to 24 million people could be forced into extreme poverty due to AMR’s impact on health systems, food-production and the economy (CitationWorldBank). LMICs will be particularly impacted due to a complex array of factors including poor health care infrastructure, water quality and sanitation, accessibility of non-prescription antimicrobials, and limited AMR surveillance and governance. It is estimated that the cost associated with the impacts of AMR will exceed 1 trillion USD globally by 2050 if no action is taken (O'neill, Citation2014; O’neill, Citation2016; WHO, Citation2015; WHO, Citation2019).
These stark predictions have prompted the research community to prioritise funding to cross-sector AMR initiatives (Carlet et al., Citation2014; WHO, Citation2019). These include, but are not limited to, the development of new drugs, diagnostics, and vaccines, and by overhauling health services by updating prescribing practices and supplying AMR training to healthcare professionals. These product and service delivery approaches largely focus on human, and health system contexts. However, over half of the antimicrobials used globally are given to food-producing animals (Van et al., Citation2020). Widespread antimicrobial use in food-producing animals has the potential to contribute to the development of resistance in humans (WHO, Citation2000; WHO, Citation2003; WHO, Citation2005), especially given the overlap in types of antimicrobials administered in agriculture, aquaculture, veterinary and human healthcare (Tang et al., Citation2017; WHO, Citation2003). Contact between humans and animals, shared environmental resources such as water, and direct consumption can result in cross-species transmission of AMR (Holmes et al., Citation2016; Wegener, Citation2003). In food-producing animals, antimicrobials are used as prophylaxis or to promote faster growth (Yopasá-Arenas & Fostier, Citation2018), this is especially true in LMICs (Founou et al., Citation2016; Thanner et al., Citation2016). This constitutes antimicrobial misuse because most animals will not actually be suffering from an infection (Carrique-Mas et al., Citation2015) meaning the microbes in their body are thus being challenged by antimicrobials and are more likely to develop resistance. Even if the animal is unwell, it is unlikely that these methods of treatment will facilitate the use of the correct drug to treat the infecting bug (Van et al., Citation2020). Equally, many LMIC populations do not acquire their antimicrobials (for human or animal use) by prescription but instead buy drugs directly from informal providers (Sakeena et al., Citation2018) who have variable levels of training (Barman et al., Citation2021) and can be motivated by financial gain (Afari-Asiedu et al., Citation2018). This again means ‘drug-bug’ matching is unlikely to be correct, and that poorer individuals may not be able to afford a full course of treatment (Khan et al., Citation2019; Llor & Bjerrum, Citation2014; Sakeena et al., Citation2018). Financial and resource challenges can also drive self-medicating behaviours (Rodrigues, Citation2020) such as the sharing of antibiotics between family members, or re-purposing human antimicrobials for animal use (Belas et al., Citation2020; Beyene et al., Citation2018). Unfortunately, product and service delivery solutions proposed to tackle the AMR crisis tend to ignore these behaviours and contextual factors which contribute to the proliferation of AMR across healthcare, agriculture, and community settings (Charoenboon et al., Citation2019; Prestinaci et al., Citation2015; Roca et al., Citation2015).
Regardless of the new drugs developed or systems re-designed, the efficacy of antimicrobials will only be protected if they are used appropriately across the One Health sphere (human, animal, and environmental sectors). Proper usage relies on good working knowledge of antimicrobials and so interventions to ‘up-skill’ everyone from farmers to health care workers to school children have become increasingly popular avenues for funding (Bâtie et al., Citation2020; Charoenboon et al., Citation2019; Jimah et al., Citation2020; Thornber et al., Citation2019). However, recent evaluations suggest that awareness-raising and education alone are not sufficient to create meaningful, long-term behaviour change (Denyer Willis & Chandler, Citation2019 Haenssgen et al., Citation2018; Pearson & Chandler, Citation2019;). Instead, efforts are suggested to focus on what several Global Guidance documents (WHO, Citation2015; WHO, Citation2019) and National AMR Action Plans (NAPs) refer to as ‘sustained’ or ‘systematic and meaningful public engagement’ (see examples from Costa Rica, Fiji, Spain, UK). This description echoes the values and principles of Community Engagement (CE) methods which seek to develop equitable partnerships with a given community, and work toward co-designed locally meaningful, and sustainable solutions to a given challenges (King, Citationunder review; Mitchell et al., Citation2019; UNICEF, Citation2019). This understanding of CE goes beyond public engagement, awareness raising and educational approaches that are also popular within current AMR literature.
Through CE, the community take ownership of their information and co-develop solutions that are meaningful in their settings (Mitchell et al., Citation2019; King, Citationunder review). CE has been successfully applied to understand and develop local solutions to complex health issues (Decroo et al., Citation2013; Echaubard et al., Citation2020; Gupta et al., Citation2020) and based on recent research, also appears an ideal approach to tackle the challenge of AMR (Denyer Willis & Chandler, Citation2019). However, despite the growing recognition of the importance of social science approaches to tackle AMR (Chandler & Hutchinson, Citation2016; Vedadhir et al., Citation2020), the application of CE is poorly understood. If we can synthesise learnings from CE interventions, then we can develop a collective understanding of their scope to tackle AMR across contexts.
Aims
This manuscript describes a GCRF-funded ‘challenge cluster’ project which brought together six existing LMIC-based AMR research projects. This is not a primary data collection study, all authors were Co-investigators within the cluster whose aim was to synthesise knowledge and experience regarding the current, and future uses of CE approaches to tackle AMR as a One Health problem. We reflect on our combined understanding of AMR projects utilising CE approaches and contextualise our learnings within the recent grey and published literature to discuss the current position and future potential for CE to be utilised to tackle AMR across the One Health sphere.
Methods
Known as CE4AMR: The One Health Approach, the cluster included six existing AMR research projects based in Bangladesh, Ghana, India (x2), Nepal and Vietnam (), with Co-Is based in each country and across the UK.
Table 1. Details of the projects involved within this CE4AMR: One Health Approach Challenge Cluster.
The cluster used an inductive thematic approach (Braun & Clarke, Citation2006) to synthesise various discussions on the use of Community Engagement approaches to tackle AMR. The inductive thematic approach, also known as inductive reasoning, involves looking for patterns within a dataset to build a theory. Hypotheses and theories are not applied to inductive studies at the beginning of the process. Rather the researcher uses patterns in the data to inform the study’s direction. In this cluster, a single research (JM) led all aspects of thematic analyses. Because JM was not a Co-I in any of the existing research projects, she was in a position to best maintain impartiality and thus to facilitate un-biased discussions between other Co-Is.
Cluster Co-Is engaged with the following series of tasks (). Due to COVID-19, the cluster had to work exclusively online. Although some project teams could continue face-to-face discussions within their own country settings, all international collaborations were held on the Zoom online platform. Zoom was selected as it was the most accessible platform for all Co-Is to engage with.
Table 2. The Cluster’s process of exploring how CE approaches are currently utilised in One Health AMR research.
In June-July 2020, Co-Is collectively developed a set of research questions (RQs) with the aim of capturing how CE is currently utilised within AMR research. An initial workshop between Co-Is identified key concepts around which to frame the RQs, these were: identifying communities, understanding One Health, consolidating strategy, scaling, and sustaining research, engaging stakeholders and evaluating projects. Each cluster project then worked with their individual teams to create a set of RQs around these concepts. An inductive thematic approach (Braun & Clarke, Citation2006) was used to synthesise the final set of six RQs () which were presented back to Co-Is. Each project then answered the questions with wider members of their team (not just the Cluster Co-Is). Answers were based on their experiences and fed-back to the cluster in written responses. A single researcher then synthesised answers again using an inductive thematic approach. A synthesis of answers to the Research Questions was presented in a Zoom webinar in early September 2020. This was attended by all Co-Is and several additional audience members representing funders, global health organisations and LMIC-based academics with a background in Community Engagement. Attendees reflected on the synergies and nuances within the answers to the research questions. These discussions contextualised the current scope, barriers, challenges, and future potential for CE approaches to be applied to address AMR.
Table 3. The co-designed Research Questions (RQs) which supported this synthesis and knowledge exchange exercise.
Here we present synthesised answers to these RQs, alongside relevant literature which was either identified by Co-Is during the process of refining and answering the questions, or by a light-touch, iterative literature search. This followed neither systematic nor rapid review methods because this was not the focus of the cluster project, but rather identified documents (academic or otherwise) which spoke to the key themes identified from our synthesis.
Results and discussion
Toward a shared understanding of community
We define community engagement as
“a participatory process through which equitable partnerships are developed with community stakeholders who are enabled to identify, develop and implement community-led sustainable interventions to issues that are of concern to them. This approach can result in bespoke local solutions to addressing the drivers of AMR which align with the priorities and needs of communities”. (King, Citationforthcoming)
However, our synthesis suggests that there is rarely a single definition of community. This is because individuals will belong to multiple communities at the same time, and these communities may change depending on context.
Factors often used by external stakeholders to define communities include demographic, geographic and socio-cultural dynamics, as well as shared lived experiences (Box 1). Geography is difficult to contextualise in relation to AMR. Resistance can develop in any setting due to a myriad of factors including behaviour. Also, because resistant microbes know no boundaries AMR can spread between human and animal communities across local, national, and global borders (Silva et al., Citation2020; WHO, Citation2016). As such, shared lived experiences may be more important to understanding the context of AMR than physical community boundaries. For example, using only geographic parameters to define a community could miss many people who engage in shared experiences such as visiting the same health post. Beliefs and practices may also define communities (Cislaghi et al., Citation2019) and ways of working through CE. Sections of a community may not be available to interact on faith days, whilst social norms may prohibit others from expressing their thoughts in group situations.
The parameters in Box 1 can be useful during project development as funders require a description of intended beneficiaries. However, they may shape inclusion or exclusion parameters around a community and negate the fact that even narrowly defined communities are not homogenous. This is inappropriate because CE should be inclusive and representative of the living community, no matter how complex this may be. In fact, it is reasonable to accept that within CE interventions, the academic description of a community will differ from the working definition in the field as stakeholders get to know each other and co-develop the project. Mapping a community and conducting gap analyses (Park et al., Citation2019) can be helpful to understand who is included, why, and if anyone is missing.

Understanding how far a community reaches is critical when considering the challenge of AMR. Being a One Health problem, AMR requires engagement for cross-sector stakeholders in human, animal, and environmental health (Acharya et al., Citation2019). This means CE approaches to tackling AMR need to engage beyond the immediate community. Stakeholder is a term used in relation CE (Ingabire et al., Citation2016) to describe people or organisations affected by the research topic, or who can affect it. It may not always be clear where a core community ends, and stakeholders begin. How to label, include or exclude individuals will depend upon context, and some individuals may switch between roles. For example, some community members may also act as stakeholders if they take on a gatekeeping role and provide access to hard-to-reach groups. A consensus of our synthesis was that communities and stakeholders need to be mapped early. This can clarify how different groups relate to each other and what their roles and needs are. For example, engaging Government officials early on can allow them to write letters of support for a funding application, this strengthens the relationship as stakeholders feel valued. Such nuances will be project – and context-specific which is why early mapping is crucial. The community can support mapping exercises, and CE practitioners actively encourage this approach (React, Citation2018). Co-development of stakeholder mapping begins the process of two-way knowledge exchange and develops community ownership of the project. These are both essential components of CE and set the tone for collaborative, equitable partnerships.
The context in which community engagement (CE) approaches are currently being utilised to tackle antimicrobial resistance (AMR)
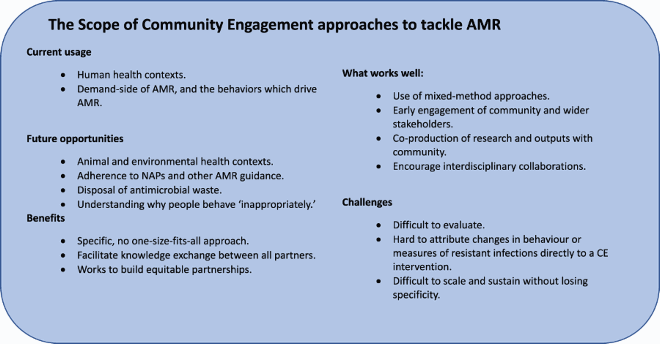
CE approaches currently tend to tackle the demand side of AMR. Interventions focus on understanding how people source antimicrobials, how and why they (mis)use them (Charoenboon et al., Citation2019; Jimah et al., Citation2020; King, Citationunder review; Rodrigues, Citation2020 Tsekleves et al., Citation2019;). As such CE is mainly employed within the context of human health. A growing number of projects are considering similar questions about the demand for agricultural antimicrobials (Bâtie et al., Citation2020; Thornber et al., Citation2019), but their rationale is often underpinned by human needs. For example, the unnecessary use of antimicrobials in food-producing animals is often framed as a negative behaviour under the rationale that it can lead to common infections developing resistance, causing mass sickness in the animals and thus reducing food supplies and economic gains for the farmer (Durso & Cook, Citation2014;; Founou et al., Citation2016 Lekshmi et al., Citation2017). However, another challenge is that antimicrobial use in agri – and aquaculture can allow antimicrobial residues to pass into the natural environment, where AMR can again develop and harm wildlife and the environment (Reverter et al., Citation2020; Van et al., Citation2020; Yopasá-Arenas & Fostier, Citation2018). Our synthesis suggests that the first scenario is more frequently engaged with because it is reflective of the need for LMIC communities to focus on immediate effects. Although environmental damage due to AMR will eventually impact on a community, it is much easier to demonstrate the direct risks of AMR impacting on food production and finances. This likely explains why there is little uptake of the CE method when considering environmental or animal health issues in isolation.
Whilst the AMR drivers considered by CE projects tend to focus on immediate human needs, there is more variability in the Strategies being utilised to understand the context in which AMR develops. Strategy can be interpreted in different ways. Firstly, there is the methodological strategy and secondly, the level of engagement of the community. Methodologically speaking, AMR research using CE tends to favour a mixed-methods approach (Gebretekle et al., Citation2018; Shallcross et al., Citation2020). This is because research teams often need to gather baseline data on an aspect, or driver, of AMR before exploring and potentially changing some of the undesirable behaviours linked to this driver. This complexity means that in terms of engagement, CE projects are often interdisciplinary and tend to address multiple layers of enquiry around their key research question. CE projects tend to begin with formative work to engage the community and stakeholders, build trust, assess their needs, and collect baseline data through surveys, interviews, or biological sampling. Projects then become more divergent, working with a community to co-design an intervention. This might be an AMR dialogue during community health meetings (King, Citationunder review), co-designed home hygiene guidance (Tsekleves et al., Citation2019), or arts-based outputs such as films (Cooke et al., Citation2020), or comics (SaS, Citation2020). An important part of creating a CE for AMR strategy is that communities know why they are providing information (such as filling in a survey or allowing a vet to take blood samples from their cattle) and see how it is being used (such as to show how few people finish a course of antibiotics, or to quantify the drug resistant infection burden in local agriculture). Mixed-method approaches facilitate knowledge exchange between the research team and the community. This helps the project become context specific and to develop a genuinely engaging process where all stakeholders can both learn and share their knowledge (Hassenforder et al., Citation2016). The use of mixed-methods approaches, and a wide variety of engagement strategies, also suggests that there is broad interdisciplinary interest in the use of CE to tackle AMR. This is promising because, considering the complexity of the AMR challenge, it is unrealistic to expect that one CE approach will fit all situations and communities.
The current usage of CE for AMR often includes co-production of outputs. For example, community members may learn about AMR through the process of creating a film on the topic, which they can then use as part of an advocacy campaign to drive change (Cooke et al., Citation2020). Co-production gives this community buy-in to the project because they have ownership of the final resource. This helps with engagement, but also develops softer skills in participants, including confidence, critical thinking, and public speaking. Our synthesis suggests that these skills are integral to tackling AMR because it is an evolving problem. The community’s needs in relation to AMR will change over time and, crucially, needs may change as knowledge increases (Gorddard et al., Citation2016). For example, once the term antimicrobial has been contextualised in the human setting the community may reveal wider needs in terms of antimicrobial usage in agriculture or animal husbandry. These needs may not have been apparent initially, but the process of CE can facilitate two-way learning between community and research team members which will capture these changing perspectives. A confident community will be able to better explain their needs and how they have changed over time (Abimbola, Citation2020). Translating these knowledges into co-produced outputs ensures that key AMR challenges within a given context are being addressed. Two-way knowledge exchange also facilitates the scalability and sustainability of the CE approach to AMR. Films, comics, training manuals and community dialogue groups can be used as both research evidence and community resources. The community keep ownership of the resource to use in meaningful ways such as lobbying for policy change, whilst the research team can use it as evidence to secure new research funding or to disseminate to their wider academic network.
The barriers and challenges in applying the CE method to AMR research
Community Engagement appears well-suited to tackling AMR, yet there are challenges to overcome. Firstly, CE hinges on the ability to create equitable partnerships (Mitchell et al., Citation2019; UNICEF, Citation2019). Whilst the community is best placed to understand their lived experience of a particular AMR problem, the biology of this issue often needs to be considered by a technical expert. This can create tension within CE for AMR projects, when a community-generated solution may need to be adapted to ensure good science is being communicated. Ensuring all stakeholders are connected from the start of the project and explaining that review stages are an intended part of project development, can make this more equitable. Remembering that CE is a process for two-way knowledge exchange is also important. ‘Experts’, for example, in environmental AMR are currently struggling to understand the dynamics of antimicrobial pollution. Thus, academics, policy makers and health care workers have a lot to gain from listening to communities on topics such as drug access, usage, and disposal. However, AMR is an invisible problem. In contrast to issues such as maternal and neonatal mortality, for example, AMR is not very easy to understand in terms of impact on people’s lives. Because people do not see AMR happening. Even after having it explained they may still wonder if it is really a problem in their community. CE can work to overcome this barrier through knowledge exchange if projects allow the community space to reflect upon and question the problem in their own lives. This knowledge-exchange process can allow AMR to be given meaning within a particular context. It then facilitates researchers to tailor their interventions more specifically to the community who will ultimately benefit.
It is important that CE does not become an extractive process (UNICEF, Citation2019). There will inevitably be a stage in each project where AMR information is ‘taken’ from the community either via discussion or sampling. The CE approach aims to find ways to return the learning from this data back to the community as soon as possible, and in a way that is meaningful. This is particularly important when gathering biological data, such as blood samples, from participants or their livestock which is often considered sensitive information (Domaradzki & Pawlikowski, Citation2019) as it relates to health and economic wellbeing. There can be mistrust of researchers who collect this type of data, but also a reluctancy of communities to engage with feedback if it is simply presented as a ‘to-do list’. CE approaches consider more inclusive ways of sharing the research findings and combining them with community knowledges, for example via a co-produced output. However, there remain challenges here, not least that co-production of films, theatre, artwork, etc. requires facilitators with specialist skillsets (Mitchell et al., Citation2019). Co-production also tends to be small-scale and this can lead to bias in the community voice that is being expressed. For example, certain community members may struggle to attend or fully engage with these types of events without support for childcare, renumeration of earnings, etc. The timing of events, along with a broader appreciation of social and cultural norms needs to be considered to ensure the outputs produced are reflective of, and applicable to, the focal community.
One barrier that prevents CE being considered as critical to tackling AMR is that both CE as an approach (Abimbola, Citation2020; Hassenforder et al., Citation2016), and AMR as a measure (Naylor et al., Citation2020) are very difficult to evaluate. There is limited evidence to suggest that current CE interventions do impact on the AMR drivers they plan to address. AMR knowledge gains can occur following training and educational interventions. Yet, where behaviour change is the desired outcome of CE, it can be difficult to measure success (Charoenboon et al., Citation2019; Haenssgen, Citation2018; Haenssgen et al., Citation2018). This is because behaviour is complex, and any changes are difficult to attribute to a single intervention. Valuing the community voice can overcome this barrier in part because it allows the community to self-report behaviour changes. However, there can be an element of bias in terms of telling the researchers what they want to hear (Brito, Citation2017). Looking at community produced outputs can reveal deeper learnings. For example Cooke et al.’s (Citation2020) co-produced films showed communities translating their recently acquired antibiotic knowledge from human to an animal health context. Other behaviours are visible, such as good animal husbandry or soap usage (Biran et al., Citation2014). Yet observations alone don’t give us the context as to why the behaviour change has occurred or if a CE intervention is responsible. Changes in AMR, as a biological measure, are also hard to evidence. Climatic conditions, pollution, and natural evolutionary processes all impact on AMR (Deng et al., Citation2020 Macfadden et al., Citation2018;; Redshaw et al., Citation2013; Reverter et al., Citation2020; Rodríguez-Verdugo et al., Citation2020). Thus, any changes in measures of resistant infections are hard to attribute directly to a CE intervention.
Evidence regarding the ability of CE to address AMR challenges is much needed, and to develop this, definitions of success (and failure) must be agreed. Our synthesis suggests that the aims of CE projects which are communicated to funders for example ‘this project will reduce antibiotic use in a certain area’ may look very different in practice. For many projects, a large amount of work focuses on education or raising awareness of antibiotics in the first place. For some communities, the word antibiotic is not recognised but there is underlying knowledge and practices around their usage. Communities and researchers must work to get on the same page in terms of language and understand the context in which they are working (Whittaker et al., Citation2019) before the intervention can be co-designed, implemented or evaluated. By this point there may be little evidence that an AMR driver has been impacted but the community will have increased AMR awareness, confidence, and autonomy to change ingrained behaviours which contributed to the driver in the first place. How successful the intervention is deemed to have been will depend on a variety of factors, not least the methods used to evaluate it (Naylor et al., Citation2020). However, when considering the original research question in isolation there may be little evidence for success despite huge learnings being made by the community and research team.
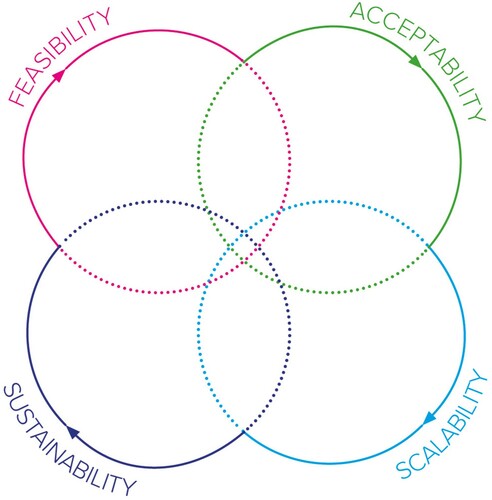
A way around this evaluative challenge is to scale and sustain CE for AMR projects across a range of contexts to better understand what success looks like and how to measure it. However, this is a somewhat academic approach which may result in a loss of specificity and thus a potentially harmful impact on communities (Zomahoun et al., Citation2019). The specificity of CE interventions and their resulting outputs can be challenging in terms of scalability. For example, although a Community Dialogue may work well in one setting, it may be poorly received in another. Equally, if outputs such as a comic book end up reaching a much wider audience will they still be specific enough to tackle the AMR challenge in that new community? Our synthesis also suggested that the terms scale and sustain may not be entirely appropriate for CE interventions, particularly those tackling AMR. Stages that pre-date the scale and sustain include ensuring that a project is feasible and accepted in each context. The relationships between acceptability, feasibility, scalability, and sustainability are not linear. This is because both communities and AMR are dynamic. Their needs and impacts change over time and in relation to external factors. As such it may be useful to reflect upon what stage a project is currently working in () and what success should look like at each of these stages. Including the community in these reflective stages is crucial to fully understand the impact an intervention is having, and where this could lead.
Gaps in AMR research which could benefit from CE interventions
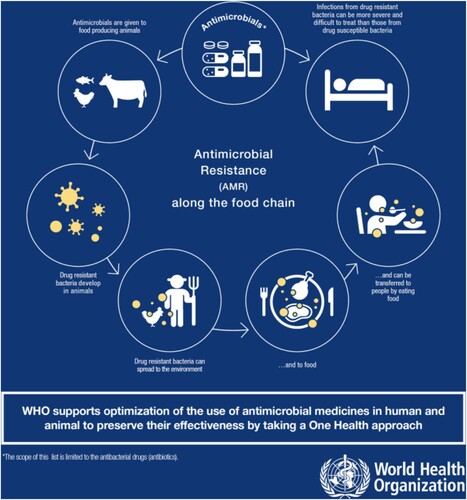
CE for AMR interventions are presently largely focused on human health and human need. Notable by their absence are environmental drivers of AMR. As discussed, this may be because they are not considered to immediately affect human health or wellbeing. This presents an area of opportunity for CE approaches to engage on broad issues of AMR. There are learnings to be gained here from the climate crisis where researchers have found meaningful ways to communicate the urgency of climate change in low resource settings, where immediate needs would usually be prioritised (Stephens & Graham, Citation2008; Wehn & Almomani, Citation2019). Attempting to prevent >1.5°C of global warming is an abstract concept for any non-specialist community. Yet breaking it down into small actions, for example using a biofuel stove (Debbi et al., Citation2014), or emphasising the need for responsible disposal of plastics (Debrah et al., Citation2021), which have other immediate benefits to health and local environments, allows the overall challenge to become achievable (Stephens & Graham, Citation2008). CE as an approach has the potential to achieve similar gains on AMR. Presenting AMR as a One Health problem as per reveals multiple points of engagement that each community may prioritise differently depending on their context. CE can engage with this context to understand which part of the One Health AMR problem is most meaningful, whilst also providing insight into other linked areas.
Figure 2. The One Health dimensions of antimicrobial resistance. Reproduced with permission of World Health Organisation from ‘Infographics: Antibiotics in the Food Chain. WHO list of critically important antimicrobials (WHO CIA list) – 5th revision’ https://www.who.int/foodsafety/areas_work/antimicrobial-resistance/AMR-food-chain-infographics/en/ Copyright © WHO (2017), all rights reserved?
The supply side of AMR, in terms of engagement with pharmacists or informal drug-sellers, is also poorly addressed by CE. Although a wealth of studies explore the knowledge, attitudes and practices (KAP) of pharmaceutical clinicians (Basu et al., Citation2020; Kotwani et al., Citation2012; Llor & Bjerrum, Citation2014; Sakeena et al., Citation2018), CE as per our definition is rarely deployed to understand the rationale behind inappropriate prescribing practices and the context in which these occur. Using CE to explore antimicrobial supply or access is an opportunity to tackle major drivers of AMR such as inappropriate usage, and non-prescription antimicrobial sales in LMICs. KAP surveys often assume that lack of knowledge is the problem and filling these knowledge gaps will address the problem. But there is frequently a disconnect between knowledge and practice. CE is better able to understand why best practice does not happen and identify alternative solutions to address this. Another grey area of understanding is the lack of enforcement of National Action Plan (NAPs) and Antibiotic Stewardship (ABS) guidance (Park et al., Citation2019; Schweitzer et al., Citation2019). The equitable and participatory nature of CE methods suggest it could be highly effective in engaging communities with Global, National or Local AMR guidance and encouraging positive behaviour change. This could link to the challenge of surveillance. Many LMICs lack an effective way to collect, analyze and synthesise AMR data from across the One Health sphere. CE methods such as the Citizen Science approach (Cohn, Citation2008) could allow communities to be part of this process and contribute vital data to inform guidance documents. Community ownership of such data could be key to driving adherence to NAPs and changing behaviours. Finally, CE tends to focus on creating positive, AMR-safe behaviour changes which is understandable considering the magnitude and urgency of this global challenge. However, CE could support the understanding of why a person or community behaves in the ‘wrong’ way (Rodrigues, Citation2020). If negative patterns of AMR behaviour are not fully understood or contextualised it may be very difficult to avoid them appearing in other situations across the local, national, or global community (Haenssgen, Citation2018) where they could continue to drive AMR.
Another gap in the CE for AMR landscape is resource and implementation support. There are significant challenges around how to define and measure the success of CE for AMR interventions. The favorability of mixed-methods approaches to tackle AMR (via CE) suggest mixed-methods evaluations should also be most appropriate. However, these can be complex to develop, implement and report upon (Haenssgen, Citation2018; Haenssgen et al., Citation2018; Hassenforder et al., Citation2016; Naylor et al., Citation2020). Community and stakeholder mapping also appeared integral to almost all group discussions which formed this synthesis. With the exception of the ReAct Groups’ recent resource (Citation2018), there is limited guidance on how to do this appropriately through an AMR lens. The One Health nature of AMR means that many situations, stakeholders beyond the research teams’ area of expertise will need to be considered. Equally this synthesis has repeatedly shared the value of involving the community in mapping exercises. This allows everyone to better understand the actors linked to AMR in each setting. Yet again, there is little guidance on how to support and safeguard communities to engage in such mapping work.
Communities need to understand why and how their involvement in mapping, as well as the other activities in a given project, will be beneficial. This leads on the need to demystify AMR. Because AMR is often considered a biological problem, it can be communicated through jargon and complex language. However, AMR relates to many everyday practices such as home and animal hygiene, food production, health seeking behaviours, and waste disposal. These are relatable themes to engage people on, and the CE approach offers many strategies to do this in locally meaningful ways. The community may have their own language around antimicrobials, drug resistance and the everyday practices mentioned above. The CE approach allows space for these factors to be discussed by the community and understood by the research team, facilitating equitable AMR interventions to develop. A ‘take home’ from this synthesis was the need to value community knowledge and let it drive the project forward, whatever aspect of AMR it is focused on.
Summary
The knowledge exchanged between this cluster (Box 2) suggests that CE can facilitate AMR behaviour change, specifically in LMICs, because it is a contextualised approach which supports communities to develop solutions that are locally meaningful (economically feasible, socially acceptable, and culturally appropriate). However, current CE interventions focus on AMR within human health, and the demand-side drivers of resistance. Additionally, the specificity of CE approaches makes them difficult to evaluate and scale. A focus on developing open access resources, particularly around evaluation, stakeholder mapping and communication could encourage the uptake of the CE approach to tackle a variety of AMR drivers across LMICs.
Acknowledgements
Authors would like to thank the wider members of each author’s host institution and projects for their contributions to designing and answering the Research Questions which form the basis of this paper. Special thanks to Dr Naomi Bull for her involvement in the early phases of this project. Thanks to Asiya Odugleh-Kolev, Lucy McDowell, Dr Sophia Latham, Jo Zaremba and Dr Madhusudan Subedi for providing insights on Community Engagement in AMR during our online workshop in September 2020. We also thank the two anonymous reviewers who provided comments and feedback on the manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Correction Statement
This article has been corrected with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- Abimbola, S. (2020). Beyond positive a priori bias: Reframing community engagement in LMICs. Health Promotion International, 35(3), 598–609. https://doi.org/10.1093/heapro/daz023
- Acharya, K. P., Subramanya, S. H., & Lopes, B. S. (2019). Combatting antimicrobial resistance in Nepal: The need for precision surveillance programmes and multi-sectoral partnership. JAC-Antimicrobial Resistance, 1(3), dlz066. https://doi.org/10.1093/jacamr/dlz066
- Afari-Asiedu, S., Kinsman, J., Boamah-Kaali, E., Abdulai, M. A., Gyapong, M., Sankoh, O., Hulscher, M., Asante, K. P., & Wertheim, H. (2018). To sell or not to sell; the differences between regulatory and community demands regarding access to antibiotics in rural Ghana. Journal of Pharmaceutical Policy and Practice, 11(1), 30. https://doi.org/10.1186/s40545-018-0158-6
- Barman, P., Thukral, T., & Chopra, S. (2021). Communication with physicians: A tool for improving appropriate antibiotic use in the absence of regulatory mechanisms. Current Treatment Options in Infectious Diseases, 13(1), 1–13. https://doi.org/10.1007/s40506-020-00241-6
- Basu, S., Bhatnagar, N., Santra, S., & Laul, A. (2020). Awareness and perspectives on paediatric dysbiosis among early-career clinicians at a tertiary care hospital in Delhi, India. JAC-Antimicrobial Resistance, 2(3), dlaa072. https://doi.org/10.1093/jacamr/dlaa072
- Bâtie, C., Kassie, D., Randravatsilavo, D. N. R. M., Baril, L., Waret Szkuta, A., & Goutard, F. L. (2020). Perception of drug vendors and Pig and Poultry farmers of imerintsiatosika, in Madagascar, toward risks related to antibiotic usage: A Q-method approach. Frontiers in Veterinary Science, 7. https://doi.org/10.3389/fvets.2020.00490
- Belas, A., Menezes, J., Gama, L. T., & Pomba, C. (2020). Sharing of clinically important Antimicrobial Resistance genes by companion animals and their human household members. Microbial Drug Resistance, 26(10), 1174–1185. https://doi.org/10.1089/mdr.2019.0380
- Beyene, K. A., Aspden, T. J., & Sheridan, J. L. (2018). A qualitative exploration of healthcare providers’ perspectives on patients’ non-recreational, prescription medicines sharing behaviours. Journal of Pharmacy Practice and Research, 48(2), 158–166. https://doi.org/10.1002/jppr.1376
- Biran, A., Schmidt, W.-P., Varadharajan, K. S., Rajaraman, D., Kumar, R., Greenland, K., Gopalan, B., Aunger, R., & Curtis, V. (2014). Effect of a behaviour-change intervention on handwashing with soap in India (SuperAmma): A cluster-randomised trial. The Lancet Global Health, 2(3), e145–e154. https://doi.org/10.1016/S2214-109X(13)70160-8
- Braun, V., & Clarke, V. (2006). Using thematic analysis in psychology. Qualitative Research in Psychology, 3(2), 77–101. https://doi.org/10.1191/1478088706qp063oa
- Brito, C. F. (2017). Demonstrating experimenter and participant bias. In J. R. Stowell & W. E. Addison (Eds.), Activities for teaching statistics and research methods: A guide for psychology instructors (pp. 94–97). American Psychological Association.
- Carlet, J., Pulcini, C., & Piddock, L. J. V. (2014). Antibiotic resistance: A geopolitical issue. Clinical Microbiology and Infection, 20(10), 949–953. https://doi.org/10.1111/1469-0691.12767
- Carrique-Mas, J. J., Trung, N. V., Hoa, N. T., Mai, H. H., Thanh, T. H., Campbell, J. I., Wagenaar, J. A., Hardon, A., Hieu, T. Q., & Schultsz, C. (2015). Antimicrobial usage in chicken production in the Mekong delta of Vietnam. Zoonoses and Public Health, 62(s1), 70–78. https://doi.org/10.1111/zph.12165
- CDCP. (2017). OneHealth fact sheet [Online] [Online]. https://www.cdc.gov/onehealth/pdfs/OneHealth-FactSheet-FINAL.pdf.
- Chandler, C., & Hutchinson, E. (2016). Addressing Antimicrobial Resistance through social theory: An anthropologically oriented report. Technical Report. London School of Hygiene & Tropical Medicine.
- Charoenboon, N., Haenssgen, M. J., Warapikuptanun, P., Xayavong, T., & Khine Zaw, Y. (2019). Translating antimicrobial resistance: A case study of context and consequences of antibiotic-related communication in three northern Thai villages. Palgrave Communications, 5(1), 23. https://doi.org/10.1057/s41599-019-0226-9
- Cislaghi, B., Denny, E. K., Cissé, M., Gueye, P., Shrestha, B., Shrestha, P. N., Ferguson, G., Hughes, C., & Clark, C. J. (2019). Changing Social Norms: the Importance of “Organized Diffusion” for Scaling Up Community Health Promotion and Women Empowerment Interventions. Prevention Science, 20, 936–946. https://doi.org/10.1007/s11121-019-00998-3
- Cohn, J. P. (2008). Citizen science: Can volunteers do real research? BioScience, 58(3), 192–197. https://doi.org/10.1641/B580303
- Cooke, P., Shrestha, A., Aryjal, A., Giri, R., Jones, N., King, R., Mitchell, J., Tait, C., Soria-Donlan, I., & Baral, S. (2020). What is antimicrobial resistance’ and why should anyone make films about it? Using ‘participatory video’ to Advocate for Community-led Change in Public Health. New Cinemas, 17(1), 85–107. https://doi.org/10.1386/ncin_00006_1
- Debbi, S., Elisa, P., Nigel, B., Dan, P., & Eva, R. (2014). Factors influencing household uptake of improved solid fuel stoves in low- and middle-income countries: A qualitative systematic review. International Journal of Environmental Research and Public Health, 11(8), 8228–8250. https://doi.org/10.3390/ijerph110808228
- Debrah, J. K., Vidal, D. G., & Dinis, M. A. P. (2021). Innovative use of plastic for a clean and sustainable environmental management: Learning cases from Ghana, africa. Urban Science, 5(1), 12. https://doi.org/10.3390/urbansci5010012
- Decroo, T., Rasschaert, F., Telfer, B., Remartinez, D., Laga, M., & Ford, N. (2013). Community-based antiretroviral therapy programs can overcome barriers to retention of patients and decongest health services in sub-saharan Africa: A systematic review. International Health, 5(3), 169–179. https://doi.org/10.1093/inthealth/iht016
- Deng, W., Zhang, A., Chen, S., He, X., Jin, L., Yu, X., Yang, S., Li, B., Fan, L., Ji, L., Pan, X., & Zou, L. (2020). Heavy metals, antibiotics and nutrients affect the bacterial community and resistance genes in chicken manure composting and fertilized soil. Journal of Environmental Management, 257, 109980. https://doi.org/10.1016/j.jenvman.2019.109980
- Denyer Willis, L., & Chandler, C. (2019). Quick fix for care, productivity, hygiene and inequality: Reframing the entrenched problem of antibiotic overuse. BMJ Global Health, 4(4), e001590. https://doi.org/10.1136/bmjgh-2019-001590
- Domaradzki, J., & Pawlikowski, J. (2019). Public attitudes toward biobanking of human biological material for research purposes: A literature review. International Journal of Environmental Research and Public Health, 16(12), 2209. https://doi.org/10.3390/ijerph16122209
- Durso, L. M., & Cook, K. L. (2014). Impacts of antibiotic use in agriculture: What are the benefits and risks? Current Opinion in Microbiology, 19, 37–44. https://doi.org/10.1016/j.mib.2014.05.019
- Echaubard, P., Thy, C., Sokha, S., Srun, S., Nieto-Sanchez, C., Grietens, K. P., Juban, N. R., Mier-Alpano, J., Deacosta, S., Sami, M., Braack, L., Ramirez, B., & Hii, J. (2020). Fostering social innovation and building adaptive capacity for dengue control in Cambodia: A case study. Infectious Diseases of Poverty, 9(1), 126. https://doi.org/10.1186/s40249-020-00734-y
- Fisher, M. C., Hawkins, N. J., Sanglard, D., & Gurr, S. J. (2018). Worldwide emergence of resistance to antifungal drugs challenges human health and food security. Science, 360(6390), 739–742. https://doi.org/10.1126/science.aap7999
- Founou, L. L., Founou, R. C., & Essack, S. Y. (2016). Antibiotic resistance in the food chain: A developing country-perspective. Frontiers in Microbiology, 7. https://doi.org/10.3389/fmicb.2016.01881
- Gebretekle, G. B., Mariam, D. H., Workeabeba, T., Amogne, W., Tenna, A., Fenta, T. G., Libman, M., Yansouni, C. P., Semret, M., & Figueras, A. (2018). Opportunities and barriers to implementing antibiotic stewardship in low and middle-income countries: Lessons from a mixed-methods study in a tertiary care hospital in Ethiopia. PLoS ONE, 13(12), e0208447. https://doi.org/10.1371/journal.pone.0208447
- Gorddard, R., Colloff, M. J., Wise, R. M., Ware, D., & Dunlop, M. (2016). Values, rules and knowledge: Adaptation as change in the decision context. Environmental Science & Policy, 57, 60–69. https://doi.org/10.1016/j.envsci.2015.12.004
- Gupta, M., Rahman, A., Dutta, N. C., Nambiar, D., Ivers, R., & Jagnoor, J. (2020). Opportunities for gender transformative approaches in a community-based drowning reduction program in Bangladesh. International Journal for Equity in Health, 19(1), 108. https://doi.org/10.1186/s12939-020-01226-z
- Haenssgen, M. J. (2018). New impulses from international development for more comprehensive and balanced public engagement evaluation. Global Health Action, 12(sup1). https://doi.org/10.1080/16549716.2019.1680067
- Haenssgen, M. J. X. T., Charoenboon, N., Warapikuptanun, P., & Khine Zaw, Y. (2018). The consequences of AMR education and awareness raising: Outputs, outcomes, and behavioural impacts of an antibiotic-related educational activity in Lao PDR. Antibiotics, 7. https://doi.org/10.3390/antibiotics7040095
- Hassenforder, E., Pittock, J., Barreteau, O., Daniell, K. A., & Ferrand, N. (2016). The MEPPP framework: A framework for monitoring and evaluating participatory planning processes. Environmental Management, 57(1), 79–96. https://doi.org/10.1007/s00267-015-0599-5
- Holmes, A. H., Moore, L. S. P., Sundsfjord, A., Steinbakk, M., Regmi, S., Karkey, A., Guerin, P. J., & Piddock, L. J. V. (2016). Understanding the mechanisms and drivers of antimicrobial resistance. The Lancet, 387(10014), 176–187. https://doi.org/10.1016/S0140-6736(15)00473-0
- Ingabire, C. M., Hakizimana, E., Kateera, F., Rulisa, A., Van den Borne, B., Nieuwold, I., Muvunyi, C., Koenraadt, C. J. M., Van Vugt, M., Mutesa, L., & Alaii, J. (2016). Using an intervention mapping approach for planning, implementing and assessing a community-led project towards malaria elimination in the eastern province of Rwanda. Malaria Journal, 15(1), 594. https://doi.org/10.1186/s12936-016-1645-3
- Jimah, T., Fenny, A. P., & Ogunseitan, O. A. (2020). Antibiotics stewardship in Ghana: A cross-sectional study of public knowledge, attitudes, and practices among communities. One Health Outlook, 2(1), 12. https://doi.org/10.1186/s42522-020-00021-8
- Khan, M. S., Durrance-Bagale, A., Legido-Quigley, H., Mateus, A., Hasan, R., Spencer, J., & Hanefeld, J. (2019). ‘LMICs as reservoirs of AMR’: A comparative analysis of policy discourse on antimicrobial resistance with reference to Pakistan. Health Policy and Planning, 34(3), 178–187. https://doi.org/10.1093/heapol/czz022
- King, R., Cooke, P., Aryjal, A., Baral, S., Das, M., Fieroze, F., Giri, R., Hamade, P., Hicks, J. P., Sophia, L., Rassi, C., Shafique, M., Shrestha, A., Soria-Donlan, I., Tait, C., Huque, R. (forthcoming). Community-Led Solutions to Antimicrobial Resistance.
- King, R., Hicks, J., Rassi, C., Shafique, M., Barua, D., Bhowmik, P., Das, M., Elsey, H., Questa, K., Fieroze, F., Hamade, P., Huque, S., Newell, J., & Huque, R. (under review). Community dialogue approach for addressing the drivers of antibiotic resistance in Bangladesh: A mixed methods study on developing a sustainable and scalable approach to community engagement. BMC Public Health. https://doi.org/10.21203/rs.3.rs-15638/v2
- Kotwani, A., Wattal, C., Joshi, P. C., & Holloway, K. (2012). Irrational use of antibiotics and role of the pharmacist: An insight from a qualitative study in New Delhi, India. Journal of Clinical Pharmacy and Therapeutics, 37(3), 308–312. https://doi.org/10.1111/j.1365-2710.2011.01293.x
- Lekshmi, M., Ammini, P., Kumar, S., & Varela, M. F. (2017). The food production environment and the development of antimicrobial resistance in human pathogens of animal origin. Microorganisms, 5(1), 11. https://doi.org/10.3390/microorganisms5010011
- Llor, C., & Bjerrum, L. (2014). Antimicrobial resistance: Risk associated with antibiotic overuse and initiatives to reduce the problem. Therapeutic Advances in Drug Safety, 5(6), 229–241. https://doi.org/10.1177/2042098614554919
- Macfadden, D. R., Mcgough, S. F., Fisman, D., Santillana, M., & Brownstein, J. S. (2018). Antibiotic resistance increases with local temperature. Nature Climate Change, 8(6), 510–514. https://doi.org/10.1038/s41558-018-0161-6
- Mitchell, J., Cooke, P., Baral, S., Bull, N., Stones, C., Tsekleves, E., Verdezoto, N., Arjyal, A., Giri, R, Shrestha, A, & King, R. (2019). The values and principles underpinning community engagement approaches to tackling antimicrobial resistance (AMR). Global Health Action, 12(sup1). https://doi.org/10.1080/16549716.2020.1837484
- Naylor, N. R., Lines, J., Waage, J., Wieland, B., & Knight, G. M. (2020). Quantitatively evaluating the cross-sectoral and One Health impact of interventions: A scoping review and case study of antimicrobial resistance. One Health, 11, 100194. https://doi.org/10.1016/j.onehlt.2020.100194
- O'neill, J. (2014). Antimicrobial Resistance: Tackling a crisis for the health and wealth of nations. O'Neill Report Wellcome Trust. Review on Antimicrobial Resistance. London.
- O’neill, J. (2016). Tackling drug-resistant infections globally: FInal report and recommendations. The review on antimicrobial resistance.
- Park, S., Kang, J. E., Choi, H. J., Kim, C.-J., Chung, E. K., Kim, S. A., & Rhie, S. J. (2019). Antimicrobial stewardship programs in community health systems perceived by physicians and pharmacists: A qualitative study with Gap analysis. Antibiotics, 8(4), 252. https://doi.org/10.3390/antibiotics8040252
- Pearson, M., & Chandler, C. (2019). Knowing antimicrobial resistance in practice: A multi-country qualitative study with human and animal healthcare professionals. Global Health Action, 12(sup1), 1599560. https://doi.org/10.1080/16549716.2019.1599560
- Prestinaci, F., Pezzotti, P., & Pantosti, A. (2015). Antimicrobial resistance: A global multifaceted phenomenon. Pathogens and Global Health, 109(7), 309–318. https://doi.org/10.1179/2047773215Y.0000000030
- React, W. (2018). A global mapping of stakeholders working with antimicrobial resistance.
- Redshaw, C. H., Stahl-Timmins, W. M., Fleming, L. E., Davidson, I., & Depledge, M. H. (2013). Potential changes in disease patterns and pharmaceutical use in response to climate change. Journal of Toxicology and Environmental Health, Part B, 16(5), 285–320. https://doi.org/10.1080/10937404.2013.802265
- Reverter, M., Sarter, S., Caruso, D., Avarre, J.-C., Combe, M., Pepey, E., Pouyaud, L., Vega-Heredía, S., de Verdal, H., & Gozlan, R. E. (2020). Aquaculture at the crossroads of global warming and antimicrobial resistance. Nature Communications, 11(1), 1870. https://doi.org/10.1038/s41467-020-15735-6
- Roca, I., Akova, M., Baquero, F., Carlet, J., Cavaleri, M., Coenen, S., Cohen, J., Findlay, D., Gyssens, I., Heure, O. E., Kahlmeter, G., Kruse, H., Laxminarayan, R., Liébana, E., López-Cerero, L., Macgowan, A., Martins, M., Rodríguez-Baño, J., Rolain, J. M., … Vila, J. (2015). The global threat of antimicrobial resistance: Science for intervention. New Microbes and New Infections, 6, 22–29. https://doi.org/10.1016/j.nmni.2015.02.007
- Rodríguez-Verdugo, A., Lozano-Huntelman, N., Cruz-Loya, M., Savage, V., & Yeh, P. (2020). Compounding effects of climate warming and antibiotic resistance. iScience, 23(4), 101024. https://doi.org/10.1016/j.isci.2020.101024
- Rodrigues, C. F. (2020). Self-medication with antibiotics in Maputo, Mozambique: Practices, rationales and relationships. Palgrave Communications, 6(1), 6. https://doi.org/10.1057/s41599-019-0385-8
- Sakeena, M. H. F., Bennett, A. A., & Mclachlan, A. J. (2018). Non-prescription sales of antimicrobial agents at community pharmacies in developing countries: A systematic review. International Journal of Antimicrobial Agents, 52(6), 771–782. https://doi.org/10.1016/j.ijantimicag.2018.09.022
- SAS, S. A. S. (2020). superheroesagainstsuperbugs.com/superheroes-against-superbugs-2/ [Online].
- Schweitzer, V. A., Van Heijl, I., Van Werkhoven, C. H., Islam, J., Hendriks-Spoor, K. D., Bielicki, J., Bonten, M. J. M., Walker, A. S., Llewelyn, M. J., Harbarth, S., Huttner, B., Little, P., Rodriguez-Baño, J., Savoldi, A., Van Smeden, M., Tacconelli, E., Timsit, J. F., & Wolkewitz, M. (2019). The quality of studies evaluating antimicrobial stewardship interventions: A systematic review. Clinical Microbiology and Infection, 25(5), 555–561. https://doi.org/10.1016/j.cmi.2018.11.002
- Shallcross, L., Lorencatto, F., Fuller, C., Tarrant, C., West, J., Traina, R., Smith, C., Forbes, G., Crayton, E., Rockenschaub, P., Dutey-Magni, P., Richardson, E., Fragaszy, E., Michie, S., Hayward, A., & Group, P. R. (2020). An interdisciplinary mixed-methods approach to developing antimicrobial stewardship interventions: Protocol for the preserving antibiotics through safe stewardship (PASS) research programme. Wellcome Open Research, 5, 8–8. https://doi.org/10.12688/wellcomeopenres.15554.1
- Silva, V., Correia, S., Pereira, J. E., Igrejas, G., & Poeta, P. (2020). Surveillance and environmental risk assessment of antibiotics and AMR/ARGs Related with MRSA: One Health perspective. In M. Z. Hashmi (Ed.), Antibiotics and Antimicrobial Resistance genes: Environmental occurrence and treatment technologies (pp. 271–295). Springer International Publishing.
- Stephens, J. C., & Graham, A. C. (2008). Climate science to citizen action: Energizing nonformal climate science education. Eos, Transactions American Geophysical Union, 89(22), 204–205. https://doi.org/10.1029/2008EO220010
- Tang, K. L., Caffrey, N. P., Nóbrega, D. B., Cork, S. C., Ronksley, P. E., Barkema, H. W., Polachek, A. J., Ganshorn, H., Sharma, N., Kellner, J. D., & Ghali, W. A. (2017). Restricting the use of antibiotics in food-producing animals and its associations with antibiotic resistance in food-producing animals and human beings: A systematic review and meta-analysis. The Lancet Planetary Health, 1(8), e316–e327. https://doi.org/10.1016/S2542-5196(17)30141-9
- Thanner, S., Drissner, D., & Walsh, F. (2016). Antimicrobial resistance in agriculture. mBio, 7(2), e02227–15. https://doi.org/10.1128/mBio.02227-15
- Thornber, K., Huso, D., Rahman, M. M., Biswas, H., Rahman, M. H., Brum, E., & Tyler, C. R. (2019). Raising awareness of antimicrobial resistance in rural aquaculture practice in Bangladesh through digital communications: A pilot study. Global Health Action, 12(sup1), 1734735. https://doi.org/10.1080/16549716.2020.1734735
- Tsekleves, E., Darby, A., Ahorlu, C., de Souza, D., Pickup, R., & Boakye, D. (2019). Combining design research with microbiology to tackle drug-resistant infections in different home environments in Ghana: Challenging the boundaries of design thinking. The Design Journal, 22(sup1), 347–358. https://doi.org/10.1080/14606925.2019.1595424
- UNICEF. (2019). Minimum quality standards and indicators for community engagement.
- Van, T. T. H., Yidana, Z., Smooker, P. M., & Coloe, P. J. (2020). Antibiotic use in food animals worldwide, with a focus on Africa: Pluses and minuses. Journal of Global Antimicrobial Resistance, 20, 170–177. https://doi.org/10.1016/j.jgar.2019.07.031
- Vedadhir, A. A., Rodrigues, C., & Lambert, H. (2020). Social science research contributions to antimicrobial resistance: Protocol for a scoping review. Systematic Reviews, 9(1), 24. https://doi.org/10.1186/s13643-020-1279-y
- Wegener, H. C. (2003). Antibiotics in animal feed and their role in resistance development. Current Opinion in Microbiology, 6(5), 439–445. https://doi.org/10.1016/j.mib.2003.09.009
- Wehn, U., & Almomani, A. (2019). Incentives and barriers for participation in community-based environmental monitoring and information systems: A critical analysis and integration of the literature. Environmental Science & Policy, 101, 341–357. https://doi.org/10.1016/j.envsci.2019.09.002
- Whittaker, A., Lohm, D., Lemoh, C., Cheng, A. C., & Davis, M. (2019). Investigating understandings of antibiotics and antimicrobial resistance in diverse ethnic communities in Australia: Findings from a qualitative study. Antibiotics, 8(3), 135. https://doi.org/10.3390/antibiotics8030135
- WHO. (2000). WHO Global principles for the containment of antimicrobial resistance in animals intended for food: report of a WHO consultation with the participation of the Food and Agriculture Organization of the United Nations and the Office International des Epizooties. In: ORGANISATION, W. H. (ed.). Geneva.
- WHO. (2003). Joint FAO/OIE/WHO expert workshop on non-human antimicrobial usage and antimicrobial resistance: scientific asssessment. In: ORGANISATION, W. H. (ed.). Geneva.
- WHO. (2005). Critically important antibacterial agents for human medicine for risk management strategies of non-human use: report of a WHO working group consultation. In: ORGANISATION, W. H. (ed.). Canberra.
- WHO. (2015). Global action plan on antibiotic resistance.
- WHO. (2016). Superbugs: We need action now.
- WHO. (2019). No time to wait: Securing the future from drug-resistant infections.
- WORLDBANK. Drug-resistant infections.
- Yopasá-Arenas, A., & Fostier, A. H. (2018). Exposure of Brazilian soil and groundwater to pollution by coccidiostats and antimicrobial agents used as growth promoters. Science of The Total Environment, 644, 112–121. https://doi.org/10.1016/j.scitotenv.2018.06.338
- Zomahoun, H. T. V., Ben Charif, A., Freitas, A., Garvelink, M. M., Menear, M., Dugas, M., Adekpedjou, R., & Légaré, F. (2019). The pitfalls of scaling up evidence-based interventions in health. Global Health Action, 12(1), 1670449. https://doi.org/10.1080/16549716.2019.1670449