ABSTRACT
The diminishing effectiveness of antimicrobials raises serious concerns for human health. While policy makers grapple to reduce the overuse of antimicrobial medicines to stem the rise of antimicrobial resistance, insufficient attention has been paid to how this applies to low-resource contexts. We provide an in-depth portrayal of antimicrobial prescribing at primary health care level in rural Chikwawa District, Malawi. Ethnographic fieldwork took place over 18 months (2018–2020). We surveyed 22 health facilities in the district, observed 1348 health worker-patient consultations, and carried out 49 in-depth interviews with staff and patients. Care was centred around provision of an antimicrobial. Amid chronic lack of essential medicines and other resources, clinic interactions were tightly scripted, providing patients little time to question or negotiate their treatment. We develop the concept of ‘antibiotic vulnerabilities’ to reveal multiple ways in which provision of antimicrobials in rural Malawi impacts care in conditions of extreme scarcity. Antibiotics are central and essential to primary care. As targets for optimal antimicrobial prescribing take a more central role in global policy, close attention is required of the ramifications for the delivery of care to ensure that efforts to stem resistance do not undermine the goal of improved health for all.
Introduction
Antibiotics are one of the most commonly used groups of medicines and form a foundation of contemporary medical and healthcare practices (Laxminarayan et al., Citation2016). The resistance of microbes to antimicrobials has been identified as a grave threat to global health and sustainable development (WHO, Citation2020). An estimated 700,000 people died due to antimicrobial resistance (AMR) in 2015, with the majority of these deaths occurring in low- and middle-income countries (LMICs) (O’Neill, Citation2016). The ineffectiveness of antimicrobials will have significant ramifications for health systems, where treatment of vulnerable patients is likely to become more challenging, as well as the treatment of everyday illness where next-line antibiotics are unavailable (Jasovský et al., Citation2016). Stemming resistance to antimicrobials – particularly antibiotics – has become a global priority as the decreasing efficacy of these previously taken-for-granted substances renders visible the vulnerability of our health, economics and security systems globally (Chandler, Citation2019). Yet, in extremely low-income contexts little work has been done to explore the implications of resistance to antibiotics. In this paper, we draw on ethnographic research to provide an in-depth portrayal of primary care practice in a very low resource setting in rural Malawi. Foregrounding social relationships between patients and prescribers we interrogate how the broader funding landscape shapes care practice to understand the role of antibiotic prescribing practices.
Antibiotic use and the Global South: Significant knowledge gaps
Broad global trends suggest that use of antibiotics is increasing across the world, with middle-and-low-income contexts accounting for much of this increase (Klein et al., Citation2018; Sulis et al., Citation2020). Data for these trends have relied principally on national level antibiotic consumption data from sales and imports (Klein et al., Citation2018). In sub-Saharan Africa, significant gaps in consumption data exist, meaning that forming an accurate picture of changing antibiotic demand and use is challenging (Van Boeckel et al., Citation2014). Within-country antibiotic use data is improving but data is mainly generated by point prevalence surveys in hospitals and systematic information on antibiotic prescribing in primary care is very sparce (Versporten et al., Citation2018). World Health Organization (WHO) guidelines recommend that within primary health care, antibiotic prescribing should make up less than 30% of outcomes from consultations (WHO, Citation2006) but this approximate figure is recognised as insensitive to the diversity of epidemiological profiles and prescribing contexts in different countries, particularly in LMICs where the burden of infectious diseases is higher (Sulis et al., Citation2020).
Response to AMR: Centrality of stewardship interventions
In policy and academic debates to address AMR, concern over lack of access to antibiotics has been voiced – notably through the rhetoric of ‘access versus excess’ (Laxminarayan et al., Citation2016). Yet the proposed framework to address AMR – the WHO’s Global Action Plan – has primarily framed AMR as a problem of excess, centring overall reduction in antibiotic use as the main goal (WHO, Citation2015). Corresponding to this framing, stewardship programmes are a central pillar to global and national action plans (Van Dijck et al., Citation2018; WHO, Citation2021). Stewardship can be defined ‘as any bundle of interventions that aims to optimise use of antibiotics’ (Davey et al., Citation2017). Initially developed in high-income hospital contexts, characterised by strong formal health systems, success is principally measured in reduction of prescriptions or duration of antibiotic courses (Davey et al., Citation2017; Wilkinson et al., Citation2018). Locating the problem of over-prescription as a matter of individual behaviour, stewardship programmes frequently use surveillance, restriction and correction to right ‘irrational’ prescribing practices (Broom et al., Citation2020; Will, Citation2018). Even in high income contexts, reducing antibiotic prescription has been extremely challenging (Broom et al., Citation2018; Tarrant et al., Citation2019) and in LMICs concerns have been raised about how to improve access but reduce unnecessary prescribing (Carlet & Pittet, Citation2013; Do et al., Citation2021; Sulis & Gandra, Citation2021). However, what has often been overlooked in these debates is the way stewardship programmes contain the implicit assumption that antibiotic prescription can be replaced with non-medicinal care, where healthcare workers possess the time, information and motivation to explain to patients why an antibiotic prescription is unnecessary and what they can do and expect instead. Critically, then, how does such stewardship of medicines translocate to settings where clinical care is constituted in extremely resource constrained contexts?
Pharmaceuticalisation of global health and the impact on health care practices
The 1990s saw the crystallisation of the field of global health alongside the increasing influence of neoliberal economics, reflected in structural adjustment programmes, support for marketisation of health services and the increasing influence of corporate and philanthropic actors in global health funding and policy making (Marstein & Babich, Citation2018). Another feature of this landscape was vertical programmes that narrowly focus on successfully treating or eradicating one or two diseases, which became the foremost intervention in global health (Birn, Pillay, & Holtz, Citation2017). Critical global health scholars use the term ‘pharmaceuticalisation’ to describe this shift from prevention of disease to pharmaceutical intervention and treatment (Bell & Figert, Citation2015; Denyer Willis & Chandler, Citation2019; Biehl, Citation2007). As a result, in countries where there is a high dependence on donor funding, clinical care has been largely stripped down to the provision of pharmaceuticals, often for a limited number of infectious diseases (Pfeiffer, Nichter, & Critical Anthropology of Global Health Special Interest, Citation2008). In practice, this narrow pharmaceuticalised form of care has been embedded within the infrastructures of frontline care through the enactment of simple case management algorithms that can be taught in a relatively short time to staff including to non-clinical professionals (Dixon & Chandler, Citation2019). Creating models of care that centre the distribution of pharmaceuticals leaves little space for other forms of care, including clinical attentiveness (Denyer Willis & Chandler, Citation2019). Additionally, ‘essential’ medicines – those that are considered ‘basic, indispensable and necessary for the health of populations’ (WHO, Citation1977) – are often unevenly funded (Khuluza & Haefele-Abah, Citation2019). The predominance of vertical programmes in combination with the uneven funding or altogether absence of essential medicines can create specific vulnerabilities for people living in the Global South that are very different from those in the Global North. Yet, the same toolkits are being drawn upon to address AMR.
Over time, AMR discourse has come to consider not only people themselves as vulnerable to resistant infections but antibiotics too as vulnerable resources. As such, the expansion of stewardship programmes that aim to protect medicines foregrounds certain forms of vulnerability. An exploration of the intersection of the assumed and enacted vulnerabilities of both people and medicines has the potential to steer the course of stewardship, especially if these vulnerabilities can be traced as dynamic, emergent of systemic and programmatic features and amplified in contexts of scarcity. In this paper, we highlight the contours of what we refer to as antibiotic vulnerabilities, exploring what constitutes care within primary health care facilities in Chikwawa District, in Malawi and how this relates to antimicrobial prescribing. By following the ways that antibiotics, and their absence, shape case management in practice, we illustrate the misfit of programmes of restriction in this context of extreme scarcity, and track paths for opportunities for stewardship in primary care.
Methods
Study setting
Medical services in Chikwawa district are extremely under-resourced, particularly for those residing in rural areas, shaped by colonial history and subsequent decades of political regimes and economic choices. British colonial rule (1891–1964) saw the introduction of biomedicine by medical missionaries, with a segregated system that delivered health services where the white population predominantly resided (Lwanda, Citation2007). The first records of antibiotics being used in Malawi was in 1952 (Palanco Lopez & Chandler, Citation2020). Following independence in 1964, Dr Hastings Kamuzu Banda’s rule (until 1993) saw him securing international donor funds to finance large scale-infrastructure projects. Today public services, including health are heavily dependent on support from international donors (Page, Citation2019). For example, with 70–88% of total health funding in districts across Malawi coming from donor support (UNICEF, Citation2020)
Malawi is classed as a low-income country, with a per capita GDP of $411 (World Bank, Citation2020). On the human development index, the United Nations Development Programme (UNDP) ranks Malawi 171 out of 184 countries (UNDP, Citation2019). During the 1980s, the funding of health systems was radically reduced by structural adjustment policies imposed by donor countries, in response to the debt crisis (Kalipeni, Citation2004). High rates of HIV placed immense pressure on health service delivery (Mwale, Citation2002). In 2013, the Malawian government faced a wide-reaching financial scandal, referred to as ‘cash-gate’, which fractured donor relationships and generated anger and distrust from Malawians (BBC News, Citation2014). Foreign donors withdrew direct budgetary support worth approximately 40% of Malawi’s annual budget, shifting to in-direct funding, making health service funding and delivery even more complex (Khuluza & Haefele-Abah, Citation2019).
Chikwawa is a rural district located in the East African Great Rift Valley, in the Southern region of Malawi. Approximately 80% of the population of Malawi resides in the rural areas (World Bank, Citation2019). Formal wage employment is low and subsistence farming is the predominant livelihood strategy. Reflecting broader trends in Malawi, where food security is a significant challenge, 82% of the Chikwawa population is classed as living in poverty and 72% of households considered to have very low food security (Chitsulo, Citation2020).
The health system in Chikwawa, like the rest of Malawi, is pluralistic. Care is sought from government facilities, private providers and faith-based organisations (Abiiro et al., Citation2014). Government health services are the only no- fee service. The health system is structured around three levels, tertiary (large referral hospitals situated in major urban centres), secondary (district hospital) and primary (health centres, community, and home-based services). Primary services are a crucial source of care, particularly for those households dependent on subsistence farming. Malaria is endemic and, annually, Chikwawa records one of the highest rates in Malawi (Ewing et al., Citation2015). In Chikwawa, there is a chronic shortage of qualified health personnel with an average 2.1 health workers per 10,000 people (Kabaghe et al., Citation2017). Responding to this critical shortage, community and primary health care provision allows for the prescription of some antimicrobials by non-clinically trained health workers (Smith et al., Citation2014). These drugs are included in Malawi’s national essential medicines list (Ministry of Health Malawi, Citation2010). Recent research at Queen Elizabeth Central Hospital in Blantyre, the nearest referral hospital for Chikwawa, at a minimum of one hour’s journey in a fast car, has found an exponential growth in drug resistant infections, placing pressure on an already fragile health system (Musicha et al., Citation2017).
Study design
The data was collected as part of the FIEBRE study, a multi-country and multidisciplinary investigation of febrile illness and antimicrobial use in Africa and Asia (Hopkins & Bassat, Citation2020). The social science component of the study utilised an ethnographic methodology that drew influence from both medical anthropology (Pfeiffer and Nichter Citation2008) and science and technology studies (STS) (Bowker & Star, Citation2000). The project began with a critical review of the history of fever case management that highlighted the need to attend to clinical guidelines and other biomedical scripts through which ‘global’ pharmaceutical imperatives come to mediate care relations locally (Dixon & Chandler, Citation2019). FIEBRE social science work in Malawi, which ran from November 2018 until March 2020, sought to bear these observations out ethnographically and followed two key objectives: to understand (1) what constituted care within primary health care services in rural Chikwawa and how this related to the scripts and practices of antimicrobial prescribing, and (2) how broader economic, political, and historical factors shaped patterns of use and care practices. The specific ethnographic methods that we detail below – participant observation, structured interviews, and in-depth interviews – were designed to capture the interconnections between people, technologies, guidelines and infrastructure constituting care in this setting. We sought to see how antibiotics are configured within these material arrangements to understand their (oft-unspoken) importance to healthcare workers and their patients and their role in health care practices.
We began the study conducting structured interviews with 22 health workers responsible for running the health facilities that provided primary care across the Chikwawa district (see supplementary file for the structured guide). This group are often referred to as ‘in-charges’ within Malawian health care context and all prescribed antimicrobials. We selected these facilities based on their provision of some services for free to patients living within the catchment area. The sample included primary health care clinics run by the government, faith-based organisations, private companies, and the outpatient department of two hospitals. The outpatient departments (OPD) were included because they were the designated primary health care facility for residents of villages close to the hospital. During the interviews, we explored what services were provided, how resources were allocated and any experiences of stockouts – when drugs that should be available were unavailable in the clinic – and how they dealt with the stockouts. Data from the structured interviews were used to select the two sites for in-depth ethnographic work based on the responses for the in-charges of both sites. We selected the OPD because it had comparatively more access to medicines and diagnostic testing in comparison to the health facility which had more limited access. Over a 9-month period, we undertook ethnographic research in two sites – a primary health facility and the district OPD. The data collection team consisted of ES, EM, AN, CP and GB. EM is white and British the rest of the team are Malawian. The team were observers as participants and the observations were conducted overtly so patients and health workers were aware their interactions were being observed (Kawulich, Citation2005). During their time at the clinic researchers would help with small jobs such as directing patients or helping move supplies into the clinic room or in the dispensary. For consultations, the patient was made aware at the start of the presence of the researcher and was always given the option to have the consultation without the observer. At the OPD at the district hospital, observations were conducted by ES, AN and CP. In the primary health care clinic observations were conducted by ES and GB. EM spent time in the clinic and with health workers, however, to minimise potential disruptions she was not present in the consultations. In all, we observed 1348 patients-health worker interactions, recording key observations on patient history taken, questions asked by patients, tests requested, drugs prescribed, and drugs received. The team observed patients throughout the care pathway including clinical interactions, malaria rapid diagnostic and the dispensing of medicines, recorded in fieldnotes throughout the research period. The ethnographic observations were vital at illuminating the system of case management that individuals can’t and won’t reveal in interviews. During the time, the research team was embedded in the two study sites interviews were conducted with frontline workers at the clinic (12) and OPD department (16). Interviews with health workers were conducted by ES, AN, CP and GB. We sampled those frontline workers who provided or supported the provision of care in the sites. These included clinical officers (only at the OPD), medical officers, health surveillance assistants, patient attendants, pharmacy assistants and laboratory technicians. Topics covered during the interviews what they considered good care, prescribing practices and how they managed patients, exploring any challenges they faced and how they managed these. A further 22 interviews were conducted by ES and GB with patients attending the facility with a fever. Interviews were conducted with patients at their homes, once they had recovered. Topics covered during these interviews included what happened before they came to the clinic, how they felt the interactions during the consultation had gone, what happened after their consultation, what they perceived as good care and what they would do next time. With consent from the participants all interviews were audio recorded. We stopped collecting data once no new information was emerging from these interviews.
All the research team members had undertaken research in primary health care clinics in previous projects. The nine-month period (June 2019–March 2020) of participant observation allowed the team to build up rapport with the clinical staff reducing the likelihood that their behaviour changed. The study team had less time to build up rapport with the patients due to the clinical interactions being one-off. However, the team often followed patients, often over a couple of hours, which allowed the researchers to build trust. For patients they followed, they often gained more insights into the patients’ illness episodes than was given to the health workers during their consultations.
Data analysis
We used an inductive, iterative analytic approach to understand what constitutes care in Chikwawa and how this relates to antibiotic prescribing (Bernard, Citation2017). Every week the research team undertook a debriefing meeting reflecting on insights emerging and identified avenues for further enquiry in ongoing data collection. The Malawi team also met regularly with the other FIEBRE country teams (in Zimbabwe and Myanmar) to cross-pollinate ideas that were in turn fed back into the data collection, analysis and interpretation. All interviews were recorded, transcribed and translated into English by the research team. All transcripts and fieldnotes were imported into NVIVO 12 and were analysed inductively, coding line-by-line to group ideas and develop themes to explain emergent phenomena.
Ethical approval
Ethical approval was obtained from the College of Medicine Research Ethics Committee Malawi (P06/182429) and London School of Hygiene and Tropical Medicine Research Ethics Committee (14617). Permission to work in the district was provided by the district health authorities. For the in-depth ethnographic work, all staff who were observed were briefed during an initial meeting and written consent was provided. For patients, during the morning health talks, held daily at the clinic and OPD, the researchers introduced the study and the presence of the researchers at the clinic. During consultations oral consent was taken at the start of each consultation with the study explained to the patient. If patients agreed, the researchers were still careful to pick up on verbal and non-verbal cues that they were not comfortable, and if participants showed any hesitation at being observed the researcher did not observe that interaction. For patient interviews, informed written (or witnessed thumb print) was provided.
Results
Across Chikwawa district, many antibiotics on the ‘essential medicines’ list were often out of stock. Stockouts left health workers with the challenging choice to either prescribe what they had in stock, rather than prescribing according to guidelines, or asking a patient to buy medicines elsewhere, knowing that patients were frequently unable to afford to do so. Medicines were conceptualised by both health workers and patients as central to care, leading to almost all consultations observed resulting in a prescription and/or dispensing of drugs. Throughout the fieldwork period, patients told the researchers that their main motivation for attending the clinic was the desire for medicines. These dynamics, we argue, co-constitute a form of care that both responds to and creates antibiotic vulnerabilities. We expand here first on the chronic scarcity of essential antibiotics, which dictated that care became a task of management through those drugs that were available; second, that scripts of care in such chronic scarcity relied heavily on socio-material processes for recording and controlling how medicines, tests and patients are managed through the clinic space; third, that professional stability in the performance of care was upheld through particular forms – and absences – of interactions between health workers and patients during the clinical process. These dimensions of care in chronic scarcity present a scenario in which optimising antibiotic use will require not only stable availability of essential medicines but also a re-ordering of the system around provision of quality care rather than of medicines. Here, we show how practices of case management can be understood to both respond to and create antibiotic vulnerabilities.
Performing case management through available medicines
The dispensary is quiet, the outpatient opening hours over. An hour earlier the queues of patients waiting to collect their prescriptions snaked around the waiting room; the benches then full are now empty. People arriving before daybreak to ensure they were seen have left. The pharmacy assistant sits at the counter wrapping paracetamol into small packets, ready for the next day. A five other boxes of other medicines sit on the bench next to him. The antimalarial LA (the colloquial name given to artemether lumefantrine in Malawi) and a large box of cotrimoxazole are the most prominent and are waiting to be dispensed to patients. On arrival at the clinic medicines are stored in the newly refurbished container room. Donated recently by an international donor, large signs representing the donor’s logo are prominently placed both inside and outside. As we walk in through the securely lockable doors, the integrated air-conditioning is striking, both a reminder that drugs must be protected from both theft and heat. The room’s atmosphere jars uncomfortably with the dated dispensary and consulting rooms inhabited by patients and health workers.
The metal shelving reaches ambitiously almost to the celling and runs the length of the room. What medicines are in stock are stacked neatly in their boxes, assigned to labelled shelves: ART programme, Malaria, Family Planning, TB and Essential Medicines. The Essential Medicines section is running low. Today there are a few boxes of ciprofloxacin and amoxicillin. But cotrimoxazole, which sits in the ART section continues to be well stocked. A few months earlier one of the clinic health workers had explained to me that cotrimoxazole was one of the only constants, we struggle to secure so many drugs. We only have cotrimoxazole, that's all we have to give. We give it out all day because that is all we have to give.
A few months later, as I’m sat with a ministry employee talking about supply chains, I’m told that for the HIV programme aim is for ‘zero stockouts’. The electronic reporting systems, the separate and protected supply chains, and secure funding ensures they are mostly able to achieve this. I ask her about essential medicines, she shakes her head and describes how these are predominantly funded by the government, which simply does not have the funds to meet the budget requirements. (Fieldnotes 2018–2019 EM)
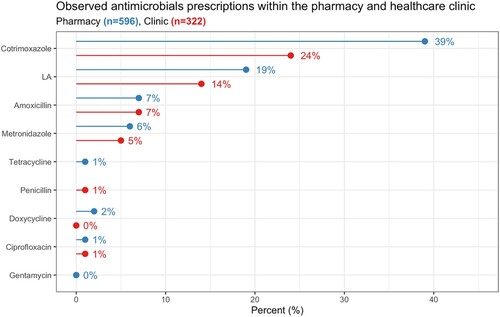
Care in the clinics was situated within, and incorporated the management of, broader contexts of uncertainty, including the unpredictable (un)availability of different medicines. shows the availability of antibiotics and antimalarials on the Malawian Essential medicines list in the 22 facilities we visited (Malawi Ministry of Health, Citation2015). Those predominantly provided by the donor programmes such as LA (malaria), cotrimoxazole (HIV) and gentamicin (sexually transmitted infections, STIs) were more frequently in stock than other essential antibiotics funded by the government. presents observational data from the pharmacy and outpatient where cotrimoxazole and lumefantrine artemether (LA) were the drugs we most frequently observed being prescribed and dispensed to patients.
Figure 1. Availability of essential antibiotics and antimalarial medications in health facilities in Chikwawa.
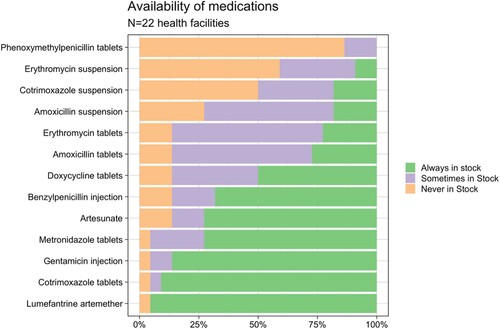
Figure 2. Prescribed antibiotics and antimalarials from observations in the consultation room and pharmacy.
In interviews, health workers conceptualised patients not receiving prescribed medicines as a ‘failure’ of care – ‘as if we are failing our job’ [Interview 11 with Nurse; primary health centre]. This perception of failure relates both to health workers’ perceptions of not meeting patient expectations for care – ‘they expect to receive medicine when they come here’ [Interview 07 with Medical Assistant, Chikwawa District Hospital] – and to the idea of not fulfilling the caring role – ‘we … feel that we didn’t assist the patient’ [Interview 03 with Medical Assistant, Chikwawa District Hospital]. Health workers narrated the complex dilemmas that they faced when they did not have the drug they wanted to prescribe for a patient in stock. Some reported asking patients to buy them, but others said they would not prescribe because of the economic burden this placed on the household. As one in-charge narrated ‘I never ask a patient to buy drugs. They can only afford to buy a few pills not the required amount … you want to prescribe the drug, but you do not have it in stock’ [Interview 12 with Medical Assistant at a primary health care clinic].
Embedded within practices in the clinic appeared an expectation from both patients and health workers that medicines prescribed may not be successful, requiring the patient to return to the clinic. Care became ‘making do’ with medicines that might be good enough for now but may necessitate additional care-seeking in the future:
He received cotrim and aspirin again, the same medicine which he was given last time he came, and they did not help him. He is not happy with the medicine he received or by the way the doctor treated him, but he said he will still take the medicine because he is poor. [ES OPD observation notes 20, Primary Health Centre; February 2020]
Here, the patient is resigned to receiving medicines without conviction in their effectiveness, and others describe a lack of opportunity to negotiate alternative prescriptions when they have been given something they feel is not effective in their body:
To be honest I didn’t explain it to anyone … they didn’t tell me the drugs that I was going to receive. I only get to know the drugs during collection. [Interview with patient 04, primary health centre]
This is also echoed in health workers’ narratives of ‘making do’, compromising on which medicines to prescribe based on what might be available:
Because the antibiotic is always available … we just give them Bactrim [cotrimoxazole] since it assists various diseases, maybe it would help him as well … we just tell them that they should come again the following day so that we can check on the child. [Interview 02 with Clinical Officer, Chikwawa District Hospital]
The constant availability of cotrimoxazole sits against an uncertainty of its capacity to help the patient, with the embedded expectation (and in this case, recommendation) that the patient will return for future attempts to address the illness. The uncertainty of how to manage a trajectory towards recovery from illness, within this landscape of lack of constant and reliable clinical resources and medicines, becomes embedded in how care is done in the clinic, and in patients’ expectations of care-seeking.
The socio-material scripts of care in scarcity
Amid this landscape of uncertainty, and highly constrained resources, we observed the dominant role of key materials in the clinic, employed as methods to control the processes of care. We term these socio-material scripts, denoting habituated practices of care through relatively fixed configurations of people and materials (Wilson, Citation2002).
The health passport
Following increasing anthropological interest in the role of paperwork in the construction of organisational rules, ideologies and practices, we observed the prominent role of the ‘health passport’ in the construction, and delivery of care in the clinics (Hull, Citation2012). This is a physical document, carried by the patients, that acts as their medical record. It permits them access to clinical spaces, processes and drugs (Wendland, Citation2010). The passport is also used to communicate between staff in the health centre. For example, for patients referred for a malaria rapid diagnostic test (mRDT), the referral is written into the passport, then the confirmation of diagnosis and prescription of drugs:
[The] patient did not come to meet the health worker after mRDT results but the mRDT Staff brought all the health passport books for patients recommended mRDT to the health worker to prescribe medicine for the patients. [ES OPD observation notes 01, Primary Health Centre; October 2019]
With large numbers of patients attending clinics each day – often up to 200 patients per clinic – and the comparative lack of staff, time and other resources available, the health passport becomes key to managing how patients move through the clinic, and thus, receive care. This was acknowledged by a health worker when describing the process of diagnosing and prescribing for patients:
The queue of patients here is so long so it would be hard [to explain each patient’s diagnosis] and there would be congestion at the consultation room, that’s why we decided to finalise all prescriptions and write down all our findings in the health passport before returning it back to the patient. [Interview 12 with Medical Assistant, Primary Health Centre]
Here, the health passport bypassed the need to explain each patient’s diagnosis and prescription to them directly. As such, it acted as an exclusive and exclusionary material of care, whereby information about a diagnosis and prescription was confined to the health workers handling the passport within the clinic. Many patients, especially those with limited or no literacy, would only be able to make sense of their diagnosis through interpretation of the medicines they did or did not receive at the end:
I waited for the test result at the waiting area until the mRDT staff came with the books from the consultation room and gave us, but he did not tell me the test results … We were all told to go for medication at the dispensary after registration where I have received Panadol [paracetamol] therefore, I knew that I am malaria negative since I did not receive LA. [ES patient quoted in researcher’s OPD observation notes 21; Primary Health Centre; ES; October 2019]
In clinical interactions, the health passport’s socio-material role limited patients’ agency and allowed health workers to enclave knowledge about the patient’s illness and treatment. It also rationalised the delivery of care, centring it towards dispensing of medicines and constraining opportunities for negotiation or questioning of the treatment by the patients to the health workers.
Recording and monitoring resources
Paperwork in the clinic also reflected the audit functions of documents in public institutions, as instruments of accountability and efficiency (Hull, Citation2012). We saw this practice in the monitoring and recording of specific materials within the clinical space, particularly the use of drugs and diagnostic tests provided by international donors. Health workers acknowledged that these paperwork practices form part of the auditing requirements of the donor organisations providing, for example LA, and requiring evidence ‘that the medication is being handled properly’ at the clinic level [Pharmacy worker]. During regular monitoring visits coordinated by the District Health Office and mandated by the National Malaria Programme the registers and test results were reviewed to check that health workers were adhering to clinical guidelines:
They indicate the results of the patient in the lab register. And on that table over there it’s where the clerk records the disease that each patient is suffering from so if he has malaria then they indicate that he has malaria. And at the pharmacy if the patient has been given LA then they also indicate that in a register, so they make sure that the records from all the three registers match. If they don’t match then we are supposed to trace back what happened. [Interview 12 with Medical Assistant, Primary Health Centre]
This level of paperwork and monitoring was not required for the ‘essential medicines’ predominantly funded by the government, and thus medicines provided through donor-funded programme (such as for HIV, malaria and TB) were construed as distinct, and of value. The practices of monitoring and reporting the use of programme medicines could be said to confer these drugs, and their related diseases, with a particular status within the clinic, akin to the ‘exceptionalism’ bestowed upon HIV through global health programming (Benton, Citation2015). This highlights connections between the delivery of care in the clinic, and the broader landscape of provision of resources at the health system level, whereby the status of these medicines is reflected in patients’ expectations for the outcomes of their care, and how they make sense of their illness. This can be seen, for example, in patients interpreting their diagnosis as ‘not malaria’ from the medicines they were prescribed, and in dissatisfaction at not receiving LA:
I was just thinking that I was going to receive LA but then I didn’t receive LA so it made me so furious. [Interview with patient 02; primary health centre]
Avoiding disruption and disapproval
Around these mechanisms for case management within a context of very limited resources were very constrained opportunities for interaction between health workers and patients. We explain how clinic scripts avoided disruption and disapproval, which bolstered health worker professional identity and shielded them from the disappointment of patients in the face of chronic scarcity.
Staying quiet to avoid disruption
Data captured through structured observations in the clinics showed most patients spent one minute or less in consultation with the health worker. Patients spoke very little to the health workers, beyond a brief description of symptoms, and further dialogue and interaction were typically absent. Consultations appeared to performative, following a characteristic script, with the implicit aim of processing the patient out of the consultation space towards the receipt of medicines with minimum disruption. This script, and the related decision-making processes, such as which medicines to prescribe, was unspoken within the consultation, leaving processes of diagnosis and prescription ‘unknowable’ to the patient:
The patient came to seek medicine for himself. He explained to the Medical Assistant. ‘I am feeling body pains since yesterday afternoon’ The Medical Assistant did not ask him questions and gave him no opportunity to ask questions in case he had them. The provider did not recommend him any test, but prescribed cotrimoxazole 960 mg and paracetamol for him. [ES OPD observation notes 23, Primary Health Centre; February 2020]
Thus, opportunities for any negotiation or questioning within the clinical encounter were closed down. Our observations of consultations were at odds with formal narratives of the ‘ideal’ consultation process perceived by both health workers and patients. In interviews, health workers described ‘collecting a full proper history’ (Interview 04 with Intern Clinic Officer, Chikwawa District Hospital) from patients, undertaking a range of diagnostic tests (though more commonly described by staff at the district hospital than the health centre), and returning to the patient for further questions where necessary. However, when probed further, participants did acknowledge the practical limitations to this idealised version of care, describing necessary compromises such as prescribing based on generalised assumptions, for example following a negative mRDT:
If the patient is diagnosed malaria positive then we give him LA but if the patient was diagnosed malaria negative, we assume that it could be sepsis or some infection, so we give him antibiotics. [Interview 15 with Nurse, Primary Health Centre]
Some patients, when interviewed, presented a formal narrative of their duty to explain in detail their symptoms to the health workers to receive good care, conceptualised as the ‘right’ medicines:
The patients should not be hiding their sickness from the doctor. They should be telling the doctor everything that he or she is feeling. There is a tendency of hiding the sickness from the doctor as result patients may receive incorrect drugs. [Interview with Patient 23, Primary Health Centre]
Yet, this level of interaction was rarely observed in practice. Some patients indicated to researchers that they did not feel able to mention something they felt important in the consultation. Others described their dissatisfaction with the outcome of the clinical encounter, such as being disappointed with the type of medicines they received, and frustrated that they were not able to discuss their prescription:
The Doctor prescribed ibuprofen since she was diagnosed malaria negative, so she complained [to the researcher] that she always gets recovered with LA though she is diagnosed malaria negative. She is not happy with the medicine she received but she says she has no say. [ES observation notes 12, Primary Health Centre; November 2019]
There were a few occasions when patients did negotiate with health workers in the consultation, resisting the scripted practice. Yet in opening space for the patient’s knowledge of ‘their’ illness experiences, these types of interactions are potentially very disruptive to the efficient processing of patients, going against the expected flow of people through and out of the clinic spaces:
One patient … went out of the consultation room as if she was going to the Lab for an mRDT and came back after some minutes and told the Clinician that she had been tested for Malaria many times but she is always negative so he should just give her medicine. She cannot wait for a long time [for the lab test] because she already knows that she cannot be diagnosed Malaria positive. The provider prescribed her Ciproflaxicillin tablets after trying to convince her to go for the malaria test, but she insisted on having a prescription. [ES OPD observation notes 01, Chikwawa District Hospital; July 2019]
In the consultation, the patient is centring the prescription of an antimicrobial, demonstrating the significance of receiving medicines for patients.
Materials such as the health passport help to close down these potential disruptions, as these objects, rather than the patients, do the majority of the ‘talking’. Through this, patients are left vulnerable to a case management process that may not respond adequately to their personal situations, and to feeling disempowered within the clinic space.
Avoiding disapproval
Closing down opportunities for interaction within the care process is also shaped by the avoidance of disapproval – of patients by health workers, and in turn, of health workers by patients. As well as a lack of opportunity to speak in the consultation, some patients described staying quiet to through fear of being criticised or admonished by health workers, for example for asking a question within the consultation: ‘I feel like if I may ask [the health worker] questions maybe he wouldn’t help me’ [Interview with patient 01b; primary health centre], or questioning a prescription:
A patient said she is not happy with the treatment she received because she had been taking the same medicine for a long time but no improvement … She was not able to tell the doctor because she knew that [the doctor] would have shouted at her that she is commanding or teaching [the doctor] what to do. [ES OPD observation notes 1, Primary Health Centre; ES; October 2019]
There were a few occasions observed during the research of patients directly being disciplined by health workers, such as being reprimanded for not having the health passport open at the beginning of the consultation. Yet in many encounters in the clinic, this overt, voiced disapproval by health workers did not occur; it was the perceived threat of disapproval that appeared to keep patients quiet.
Similarly, health workers also expressed fears of being criticised by patients. These fears typically centred on the perception that patients put health workers ‘under pressure’ to receive medicines when they attend the clinic, and that to ‘[send] them home without drugs’, would leave patients dissatisfied [nurse]. Again, however, there was little indication of this kind of pressure being explicitly exerted by patients within the consultations observed. This concern among health workers also reflects a fear of doubt being cast on their professional reputation, should they have to send patients home without medicines due to stockouts. This was echoed by pharmacy workers fearing patients would be suspicious of the reasons behind a lack of medicines:
Central Medical Stores would tell you that they don’t have medicines and yet the only place where we are supposed to order medicines is from there. Then we don’t know what to do. As a result people may be rude to us and say bitter things like we don’t know how to do our job and sometimes they may be saying that we are selling drugs. [Interview with Pharmacy Worker 01, Chikwawa District Hospital]
These fears of criticism among patients and health workers contribute to the absence of interaction and dialogue within the clinical encounter. This highlights the professional vulnerabilities faced by health workers due to highly constrained resources, and of historical bureaucratic scandal (the ‘cash-gate’ incident). Within this context, case management is performed quietly and predominantly through materials rather than interaction. This typically excludes the patient from the diagnosis and prescription process, contributing further to their vulnerabilities around not receiving appropriate care and treatment.
Discussion
Globally, AMR has often been framed as either a problem of access or excess, with stewardship interventions and overall reductions the dominant intervention to ‘rationalise’ use. Insufficient attention has been paid to care relations beyond the binary of ‘too many’ or ‘too few’ – particularly in low-income contexts – and how these relations shape, and are shaped by, antibiotic prescribing. Addressing this gap, our ethnographic work provides an in-depth examination of the practices that comprise care in rural Chikwawa. In the context of scarcity, the extremely limited access to essential medicines had significant ramifications. Ensuring a stable drug supply is vital, but must be accompanied by wider changes that enable quality care to be performed and received beyond antibiotic-oriented case management. We propose programmes be designed not only to protect vulnerable antibiotics, and patients vulnerable to antibiotic resistance, but also to protect patients from systems that have come to be organised around antibiotics, resulting in case management that leaves patients vulnerable to insufficient care. We propose that considering multiple dimensions of antibiotic vulnerabilities has conceptual promise as it can at once bring to the frame the vulnerability of antibiotics, people and systems.
In Malawi, donors provide the vast majority of funding for health services, and ‘exceptional’ diseases such as HIV and malaria have secure drug supply chains (Benton, Citation2015). We found that the most frequently prescribed drugs reflected a public health model that prioritises pharmaceutical distribution to treat a narrow number of diseases (Biehl, Citation2007). This stood in direct contrast to essential medicines which were frequently out of stock, significantly constraining how patients could be treated, and requiring health workers to ‘make do’ with the medicines available. This adds to a body of social research that has demonstrated that antibiotic prescribing is not a behaviour that can be viewed in isolation from resources and relationships in clinical practice (Tompson et al., Citation2021; Will, Citation2018). Our work makes explicit the ways the broader architecture of global health, including the centring of pharmaceutical interventions for case management, created specific care relations and vulnerabilities.
In Chikwawa, forty years after international agreement on the necessity of access to essential medicines (Greene, Citation2010), access remains uncertain and often negligible. Cotrimoxazole is a broad-spectrum antibiotic considered an essential medicine, that should be widely accessible (WHO, Citation2019), and is, in Chikwawa, as a result of donor programmes. However, when other essential medicines are lacking, depending on an extremely limited number of antibiotics in primary care raises questions about resistance patterns and how this may drive AMR (Marwa et al., Citation2015). These findings speak to the urgent need to fund more antibiotics, as well as more consistent provision of a range of essential medicines.
While ensuring more consistent provision of medicines is crucial, we also show how constrained care has become even when antibiotics were present. High patient numbers and low human resources meant that patients interactions were extremely short. Case management involved the use of materials such as the health passport and hierarchies of power kept patients silent during consultations. Clinical attentiveness, such as taking an extensive patient history or allowing patients to discuss their illness or ask questions were mostly absent from consultations. This reflects other research which highlights the issue of short consultation times in low resource settings, with implications for the quality of communication with patients and appropriateness of prescriptions (Irving et al., Citation2017; Manderson, Citation2020). The organisation and procedures in the clinic were indelibly marked by international donor priorities and international algorithms of case management that foregrounded access to drugs for a limited number of diseases. By interrogating what constitutes care in primary health care we see how the blueprint of the global health architecture shapes models of care and ultimately what prescriptions of medicines are made (Dixon & Chandler, Citation2019). This highlights that strengthening health systems including financing, human resources and access to medicines (Kruk et al., Citation2018), as well as adapting models of care to build in better models of communication (Østergaard, Citation2015), may be required if the trend to reduce care to the provision of medicines is to be reversed.
In September 1978, the Alma-Ata Declaration set out a vision for nations to restructure their health systems around the primary health care for all, ensuring services meet the needs of local communities (Packard, Citation2016). Forty years later, universal health coverage (UHC), particularly now it is part of the Sustainable Development Framework, has brought attention back to the need for strong primary health care systems that respond to the needs of the population (Prince, Citation2020). International policy actors have had intense discussions around what UHC represents and how it will be implemented (Abadía-Barrero & Bugbee, Citation2019). Prince (Citation2020) notes that quality of care has received far less discussion than coverage of services, and our research highlights the challenges patients face when care is reduced to the provision of medicines. Abadía-Barrero & Bugbee, Citation2019 argue that coverage in UHC is not a neutral term and reflects terminology pushed by financial institutions for market-based health care reform reflecting a neoliberal agenda. These debates get to the heart of tensions within global health and the push against neoliberal reforms to health care. If momentum around UHC to ensure better models of care could be harnessed, this could provide important avenues for intervening on AMR that moves beyond individual behaviour change and reduces broader vulnerabilities that are brought to light by the current concerns around antibiotic provision.
Drawing on our analysis we suggest that multiple dimensions of antibiotic vulnerabilities are addressed. Recognising vulnerability often provokes a need to protect. Antibiotics are often portrayed as vulnerable, for example in awareness campaigns (Langdridge et al., Citation2019), which call on publics and professionals to protect these medicines. Patients are also vulnerable in the face of drug resistance, but moreover, we show that by tracing out the ways in which antibiotics are deployed in case management in resource constrained settings, patients are also vulnerable to receiving sub-standard care that is provided through an unhelpful antibiotic. Here, we show that both patients and health workers police the boundaries of expected scripts of ‘care’, delivered without explanation, bolstering a scenario that perpetuates patient vulnerabilities. Our analysis suggests that stewardship interventions that aim to reduce and restrict antibiotic prescribing are a misfit for low-income contexts and could have significant unintended consequences for patients and health workers (Chandler, Citation2019). The continuing inaccessibility of many essential medicines here, as in other low resource settings, may require that targets for optimal antimicrobial prescribing are taken to the provincial level as a means to ensure adequate provision and use. Addressing antibiotic vulnerabilities, however, means going further to expand expectations from case management and re-envision ‘care’. Here, stewardship should mean a holistic policy that moves beyond restrictions to ensure not only better access to medicines but better care.
Limitations of the study
The structured interviews were conducted at a single time point and medicine stocks at that time may have shaped the responses provided by those in-charge of the public health clinics. During the ethnographic work, the research team were able to build longer-term relationships with health workers, however, interactions with patients were briefer and overt which could have shaped the ways patients interacted with the health worker and study team and challenge that is difficult to overcome with such short interactions. However, it is important to note, that patients often give the researchers more information about their illness to the researchers than they did to the health workers. In-depth interviews conducted later at participants homes also provided the study team with an opportunity to build greater rapport with patients. We developed the concept of antibiotic vulnerabilities through the analysis of our data. We believe the conceptualisation could apply to other low-income contexts; however, further empirical work should be undertaken in other low-income countries to confirm this.
Conclusion
In low resource settings, such as primary care in Malawi, antimicrobial prescription is embedded in complex systems that often reflect international donor priorities over local realities. This creates specific vulnerabilities for patients and health workers, as well as for antimicrobials, which go far beyond the simplified ‘access’ or ‘excess’ narrative of essential medicines and AMR agendas in global health. Addressing AMR in the Global South requires us to look beyond restrictive stewardship interventions towards developing better models of care and better resourced health systems, to limit – rather than (re)produce - vulnerabilities of people and resources for health.
Supplemental Material
Download MS Word (27.6 KB)Acknowledgements
We are grateful to all study participants who took part in this study and to the FIEBRE and DRUM Consortiums. We would like to acknowledge Grace Bongololo (GB) for her support in conducting the interviews and participant observations.
Disclosure statement
No potential conflict of interest was reported by the author(s ).
Additional information
Funding
References
- Abadía-Barrero, C. E., & Bugbee, M. (2019). Primary health care for universal health coverage? Contributions for a critical anthropological agenda. Medical Anthropology, 38(5), 427–435. https://doi.org/10.1080/01459740.2019.1620744
- Abiiro, G. A., Mbera, G. B., & De Allegri, M. (2014). Gaps in universal health coverage in Malawi: A qualitative study in rural communities. BMC Health Services Research, 14(1), 234. https://doi.org/10.1186/1472-6963-14-234
- BBC News. (2014). ‘Cashgate’ – Malawi’s murky tale of shooting and corruption. BBC News. https://www.bbc.com/news/world-africa-25912652.
- Bell, S. E., & Figert, A. E. (2015). Moving sideways and forging ahead. Reimagining "-Izations" in the twenty-first century. In S. E. Bell & A. E. Figert (Eds.), Reimagining (bio)medicalization, pharmaceuticals and genetics (pp. 19–40). Routledge.
- Benton, A. (2015). HIV exceptionalism: Development through disease in Sierra Leone. U of Minnesota Press.
- Bernard, H. R. (2017). Research methods in anthropology: Qualitative and quantitative approaches. Rowman & Littlefield.
- Biehl, J. (2007). Pharmaceuticalization: AIDS treatment and global health politics. Anthropological Quarterly, 80(4), 1083–1126. https://doi.org/10.1353/anq.2007.0056
- Birn, A.-E, Pillay, Y., & Holtz, T. H. (2017). Textbook of global health. Oxford University Press.
- Bowker, G., & Star, S. L. (2000). Sorting things out: Classification and its consequences. MIT Press.
- Broom, A., Kenny, K., Prainsack, B., & Broom, J. (2020). Antimicrobial resistance as a problem of values? Views from three continents. Critical Public Health, 31(4), 451-463. https://doi.org/10.1080/09581596.2020.1725444
- Broom, J. K., Broom, A. F., Kirby, E. R., & Post, J. J. (2018). How do professional relationships influence surgical antibiotic prophylaxis decision making? A qualitative study. American Journal of Infection Control, 46(3), 311–315. https://doi.org/10.1016/j.ajic.2017.09.004
- Carlet, J., & Pittet, D. (2013). Access to antibiotics: A safety and equity challenge for the next decade. Antimicrobial Resistance and Infection Control, 2(1), 1–4. https://doi.org/10.1186/2047-2994-2-1
- Chandler, C. I. R. (2019). Current accounts of antimicrobial resistance: Stabilisation, individualisation and antibiotics as infrastructure. Palgrave Communications, 5(1), 53. https://doi.org/10.1057/s41599-019-0263-4
- Chitsulo, L. (2020, March 6). Malawi is most hungry nation. The Nation, 1.
- Davey, P., Marwick, C. A., Scott, C. L., Charani, E., McNeil, K., Brown, E., Gould, I. M., Ramsay, C. R., & Michie, S. (2017). Interventions to improve antibiotic prescribing practices for hospital inpatients. Cochrane Database of Systematic Reviews, (2), CD003543. https://doi.org/10.1002/14651858.CD003543.pub4
- Denyer Willis, L., & Chandler, C. (2019). Quick fix for care, productivity, hygiene and inequality: Reframing the entrenched problem of antibiotic overuse. BMJ Global Health, 4(4), e001590. https://doi.org/10.1136/bmjgh-2019-001590
- Dixon, J., & Chandler, C. (2019). Opening up ‘fever’, closing down medicines: Algorithms as blueprints for global health in an era of antimicrobial resistance. Medicine Anthropology Theory | An Open-Access Journal in the Anthropology of Health, Illness, and Medicine, 6(4), 53–79. https://doi.org/10.17157/mat.6.4.676
- Do, N. T., Vu, H. T., Nguyen, C. T., Punpuing, S., Khan, W. A., Gyapong, M., Asante, K. P., Munguambe, K., Gómez-Olivé, F. X., & John-Langba, J. (2021). Community-based antibiotic access and use in six low-income and middle-income countries: A mixed-method approach. The Lancet Global Health, 9(5), e610–e619. https://doi.org/10.1016/S2214-109X(21)00024-3
- Ewing, V. L., Tolhurst, R., Kapinda, A., SanJoaquin, M., Terlouw, D. J., Richards, E., & Lalloo, D. G. (2015). Understanding interpretations of and responses to childhood fever in the Chikhwawa District of Malawi. PLoS One, 10(6), e0125439. https://doi.org/10.1371/journal.pone.0125439
- Greene, J. A. (2010). When did medicines become essential? Bulletin of the World Health Organization, 88(7), 481. https://doi.org/10.2471/BLT.10.079970
- Hopkins, H., & Bassat, Q. (2020). Febrile illness evaluation in a broad range of endemicities (FIEBRE): Protocol for a multisite prospective observational study of the causes of fever in Africa and Asia. BMJ Open, 10(8), e035632. https://doi.org/10.1136/bmjopen-2019-035632corr1
- Hull, E. (2012). Paperwork and the contradictions of accountability in a South African hospital. The Journal of the Royal Anthropological Institute, 18(3), 613–632. https://doi.org/10.1111/j.1467-9655.2012.01779.x
- Hull, M. S. (2012). Documents and bureaucracy. Annual Review of Anthropology, 41(1), 251–267. https://doi.org/10.1146/annurev.anthro.012809.104953
- Irving, G., Neves, A. L., Dambha-Miller, H., Oishi, A., Tagashira, H., Verho, A., & Holden, J. (2017). International variations in primary care physician consultation time: A systematic review of 67 countries. BMJ Open, 7(10), e017902. https://doi.org/10.1136/bmjopen-2017-017902
- Jasovský, D., Littmann, J., Zorzet, A., & Cars, O. (2016). Antimicrobial resistance – A threat to the world’s sustainable development. Upsala Journal of Medical Sciences, 121(3), 159–164. https://doi.org/10.1080/03009734.2016.1195900
- Kabaghe, A. N., Phiri, M. D., Phiri, K. S., & van Vugt, M. (2017). Challenges in implementing uncomplicated malaria treatment in children: A health facility survey in rural Malawi. Malaria Journal, 16(1), 419. https://doi.org/10.1186/s12936-017-2066-7
- Kalipeni, E. (2004). Structural adjustment and the health-care crisis in Malawi. Proteus-Shippensburg, 21(1), 23–30.
- Kawulich, B. B. (2005). Participant observation as a data collection method. Forum: Qualitative Social Research, 6(2). https://doi.org/10.17169/fqs-6.2.466
- Khuluza, F., & Haefele-Abah, C. (2019). The availability, prices and affordability of essential medicines in Malawi: A cross-sectional study. PLoS One, 14(2), e0212125. https://doi.org/10.1371/journal.pone.0212125
- Klein, E. Y., Van Boeckel, T. P., Martinez, E. M., Pant, S., Gandra, S., Levin, S. A., Goossens, H., & Laxminarayan, R. (2018). Global increase and geographic convergence in antibiotic consumption between 2000 and 2015. Proceedings of the National Academy of Sciences, 115(15), E3463–E3470. https://doi.org/10.1073/pnas.1717295115
- Kruk, M. E., Gage, A. D., Arsenault, C., Jordan, K., Leslie, H. H., Roder-DeWan, S., Adeyi, O., Barker, P., Daelmans, B., & Doubova, S. V. (2018). High-quality health systems in the sustainable development goals era: Time for a revolution. The Lancet Global Health, 6(11), e1196–e1252. https://doi.org/10.1016/S2214-109X(18)30386-3
- Langdridge, D., Davis, M., Gozdzielewska, L., McParland, J., Williams, L., Young, M., Smith, F., MacDonald, J., Price, L., & Flowers, P. (2019). A visual affective analysis of mass media interventions to increase antimicrobial stewardship amongst the public. British Journal of Health Psychology, 24(1), 66–87. https://doi.org/10.1111/bjhp.12339
- Laxminarayan, R., Matsoso, P., Pant, S., Brower, C., Røttingen, J.-A., Klugman, K., & Davies, S. (2016). Access to effective antimicrobials: A worldwide challenge. The Lancet, 387(10014), 168–175. https://doi.org/10.1016/S0140-6736(15)00474-2
- Lwanda, J. (2007). Scotland, Malawi and medicine: Livingstone’s legacy, I presume? An historical perspective. Scottish Medical Journal, 52(3), 36–44. https://doi.org/10.1258/rsmsmj.52.3.36
- Malawi Ministry of Health. (2015). Malawi standard treatment guidelines (MSTG): Incorporating Malawi essential medicines list.
- Manderson, L. (2020). Prescribing, care and resistance: Antibiotic use in urban South Africa. Humanities and Social Sciences Communications, 7(1), 1–10. https://doi.org/10.1057/s41599-020-0492-6
- Marstein, E., & Babich, S. M. (2018). The corporatization of global health: The impact of neoliberalism. South Eastern European Journal of Public Health (SEEJPH). https://doi.org/10.4119/seejph-1874
- Marwa, K. J., Mushi, M. F., Konje, E., Alele, P. E., Kidola, J., & Mirambo, M. M. (2015). Resistance to cotrimoxazole and other antimicrobials among isolates from HIV/AIDS and non-HIV/AIDS patients at Bugando Medical Centre, Mwanza, Tanzania. AIDS Research and Treatment. https://doi.org/10.1155/2015/103874
- Ministry of Health Malawi. (2010). Malawi: Essential medicines list.
- Musicha, P., Feasey, N. A., Cain, A. K., Kallonen, T., Chaguza, C., Peno, C., Khonga, M., Thompson, S., Gray, K. J., Mather, A. E., Heyderman, R. S., Everett, D. B., Thomson, N. R., & Msefula, C. L. (2017). Genomic landscape of extended-spectrum β-lactamase resistance in Escherichia coli from an urban African setting. The Journal of Antimicrobial Chemotherapy, 72(6), 1602–1609. PubMed. https://doi.org/10.1093/jac/dkx058
- Mwale, B. (2002). HIV/AIDS in Malawi. Malawi Medical Journal : The Journal of Medical Association of Malawi, 14(2), 2–3.
- O’neill, J. (2016). Tackling drug-resistant infections globally: Final report and recommendations. Review on Antimicrobial Resistance.
- Østergaard, L. R. (2015). Trust matters: A narrative literature review of the role of trust in health care systems in sub-Saharan Africa. Global Public Health, 10(9), 1046–1059. https://doi.org/10.1080/17441692.2015.1019538
- Packard, R. M. (2016). A history of global health: Interventions into the lives of other peoples. Baltimore.
- Page, S. (2019). The development aid situation in Malawi. In, Development, sexual cultural practices and HIV/AIDS in Africa (pp. 43–60). Springer.
- Palanco Lopez, P., & Chandler, C. I. R. (2020). Histories of antibiotics: A one health account of the arrival of antimicrobial drugs to Zimbabwe, Malawi and Uganda. [Report for the Improving Human Health Programme, Agriculture for Nutrition and Health, CGIAR].
- Pfeiffer, J., Nichter, M., & Critical Anthropology of Global Health Special Interest. (2008). What can critical medical anthropology contribute to global health? A health systems perspective. Medical Anthropology Quarterly, 22(4), 410–415.
- Prince, R. J. (n.d.). Utopian aspirations in a dystopian world: “Health for all” and the Universal Health Coverage agenda – An introduction. 2020.
- Smith, S., Deveridge, A., Berman, J., Negin, J., Mwambene, N., Chingaipe, E., Ritchie, L. M. P., & Martiniuk, A. (2014). Task-shifting and prioritization: A situational analysis examining the role and experiences of community health workers in Malawi. Human Resources for Health, 12(1), 1–13. https://doi.org/10.1186/1478-4491-12-24
- Sulis, G., Adam, P., Nafade, V., Gore, G., Daniels, B., Daftary, A., Das, J., Gandra, S., & Pai, M. (2020). Antibiotic prescription practices in primary care in low- and middle-income countries: A systematic review and meta-analysis. PLoS Medicine, 17(6), e1003139. https://doi.org/10.1371/journal.pmed.1003139
- Sulis, G., & Gandra, S. (2021). Access to antibiotics: Not a problem in some LMICs. The Lancet Global Health, 9(5), e561–e562. https://doi.org/10.1016/S2214-109X(21)00085-1
- Tarrant, C., Colman, A. M., Chattoe-Brown, E., Jenkins, D. R., Mehtar, S., Perera, N., & Krockow, E. M. (2019). Optimizing antibiotic prescribing: Collective approaches to managing a common-pool resource. Clinical Microbiology and Infection, 25(11), 1356–1363. https://doi.org/10.1016/j.cmi.2019.03.008
- Tompson, A., Manderson, L., & Chandler, C. (2021). Understanding antibiotic use: Practices, structures and networks. JAC-Antimicrobial Resistance, 3(4), 4. dlab150. https://doi.org/10.1093/jacamr/dlab150
- UNDP. (2019). Human development report 2019: Briefing note for countries on the 2019 human development report. http://hdr.undp.org/sites/all/themes/hdr_theme/country-notes/MWI.pdf.
- UNICEF. (2020). 2019/20 Health budget brief: Towards full implementation of the essential health package: Achieving SDG 3 in Malawi. UNICEF Malawi. https://www.unicef.org/esa/media/6186/file/UNICEF-Malawi-2019-2020-Health-Budget-Brief.pdf.
- Van Boeckel, T. P., Gandra, S., Ashok, A., Caudron, Q., Grenfell, B. T., Levin, S. A., & Laxminarayan, R. (2014). Global antibiotic consumption 2000 to 2010: An analysis of national pharmaceutical sales data. The Lancet Infectious Diseases, 14(8), 742–750. https://doi.org/10.1016/S1473-3099(14)70780-7
- Van Dijck, C., Vlieghe, E., & Cox, J. A. (2018). Antibiotic stewardship interventions in hospitals in low-and middle-income countries: A systematic review. Bulletin of the World Health Organization, 96(4), 266–280. https://doi.org/10.2471/BLT.17.203448
- Versporten, A., Zarb, P., Caniaux, I., Gros, M.-F., Drapier, N., Miller, M., Jarlier, V., Nathwani, D., Goossens, H., & Koraqi, A. (2018). Antimicrobial consumption and resistance in adult hospital inpatients in 53 countries: Results of an internet-based global point prevalence survey. The Lancet Global Health, 6(6), e619–e629. https://doi.org/10.1016/S2214-109X(18)30186-4
- Wendland, C. L. (2010). A heart for the work: Journeys through an African medical school. University of Chicago Press.
- WHO. (1977, October 17-21). The selection of essential drugs: Report of a WHO expert committee, Geneva.
- WHO. (2006). Using indicators to measure country pharmaceutical situations: Fact book on WHO level I and level II monitoring indicators. In Using indicators to measure country pharmaceutical situations: Fact book on WHO level I and level II monitoring indicators (pp. xviii–84).
- WHO. (2015). WHO: Global action plan on antimicrobial resistance. http://www.who.int/antimicrobial-resistance/publications/global-action-plan/en/.
- WHO. (2019). WHO | WHO releases the 2019 AWaRe classification antibiotics. http://www.who.int/medicines/news/2019/WHO_releases2019AWaRe_classification_antibiotics/en/.
- WHO. (2020). Antibiotic resistance: Key facts. https://www.who.int/news-room/fact-sheets/detail/antibiotic-resistance.
- WHO. (2021). Monitoring global progress on antimicrobial resistance: Tripartite AMR country self-assessment survey (TrACSS) 2019–2020: Global analysis report.
- Wilkinson, A., Ebata, A., & MacGregor, H. (2018). Interventions to reduce antibiotic prescribing in LMICs: A scoping review of evidence from human and animal health systems. Antibiotics, 8(1), 2. https://doi.org/10.3390/antibiotics8010002
- Will, C. M. (2018). Beyond behavior? Institutions, interactions and inequalities in the response to antimicrobial resistance. Sociology of Health & Illness, 40(3), E1–E9. https://doi.org/10.1111/1467-9566.12735
- Wilson, M. (2002). Making nursing visible? Gender, technology and the care plan as script. Information Technology & People, 15(2).
- World Bank. (2019). Rural population (% of total population) Malawi. https://data.worldbank.org/indicator/SP.RUR.TOTL.ZS?locations=MW.
- World Bank. (2020). GDP per capita (current US$) – Malawi | Data. https://data.worldbank.org/indicator/NY.GDP.PCAP.CD?locations=MW.
