ABSTRACT
The burden of human papillomavirus (HPV) and HPV-related cancers and genital warts is increasing in developing countries, including Indonesia. The objective of this study was to qualitatively explore the humanistic and economic burden of these HPV-related diseases in patients in Indonesia. In 2021, in-depth interviews and focus groups were conducted with patients (N = 18) with HPV-related diseases and healthcare professionals (HCPs; N = 10) specialised in treating these patients. Interviews explored the physical, mental, social, and economic burden of HPV-related diseases. Patients emphasised the psychological and social burden of HPV-related diseases, which negatively impacted their mental state and close relationships. Treatment for HPV-related diseases was also associated with a substantial cost, which health insurance only partially alleviated. HCPs understood the physical negative impact of HPV-related diseases, but some understated patients’ social, psychological, and financial burden. This research underscores the substantial economic and humanistic burden of HPV-related diseases that could be prevented by vaccination. In addition, it highlights the need for novel interventions to reduce negative psychosocial consequences of HPV-related diseases in Indonesia. Increased HCP education of the broader humanistic impacts of HPV-related diseases may improve patient support and increase awareness for preventive strategy.
Introduction
Human papillomavirus (HPV) is a sexually transmitted infection that is the cause of almost all cervical cancer cases and is responsible for a substantial fraction of anogenital and oropharyngeal diseases and cancers (Bhatia et al., Citation2013; Centers for Disease Control and Prevention, Citation2022; Lu et al., Citation2022; National Cancer Institute, Citation2021; Okunade, Citation2020). An estimated 8% of all cancers worldwide are associated with HPV infections, equating to approximately 690,000 new cancers globally each year (de Martel et al., Citation2020). Studies show that >99% of cervical cancer cases, 90% of anal cancer cases, 90% of genital warts, 75% of vaginal cancer cases, 70% of vulvar cancer cases, 60% of penile cancer cases, and 60% of oropharyngeal cancer cases are attributable to HPV infection (Bhatia et al., Citation2013; Centers for Disease Control and Prevention, Citation2022; Lu et al., Citation2022; National Cancer Institute, Citation2021; Okunade, Citation2020).
Low- and middle-income countries (LMICs) are disproportionately affected by HPV-related diseases in comparison to high-income countries (Sung et al., Citation2021; World Health Organization, Citation2022b). Statistics from Global Cancer Observatory (GLOBOCAN) Citation2020 highlight that the incidence of cervical cancer in LMICs is disproportionately high, at 18.8 cases per 100,000 people compared to 11.3 per 100,000 people in high-income countries (World Health Organization, Citation2022b). Infection-related cancers, including those caused by HPV, are collectively responsible for approximately 30% of cancer cases in LMICs (World Health Organization, Citation2022a).
Indonesia is one such LMIC that is disproportionately affected by a high burden of HPV-related disease, with a higher prevalence in younger women than in older women (Murdiyarso et al., Citation2016). In 2020, GLOBOCAN estimated that there was a five-year all-cancer prevalence of 345.9 cases per 100,000 people in Indonesia (GLOBOCAN, Citation2020). Cervical cancer is the third leading cause of cancer deaths in females in Indonesia, and health-related quality of life patient reports demonstrate high burden of pain, discomfort, anxiety, and depression (Dwi Endarti et al., Citation2015; GLOBOCAN, Citation2020). HPV vaccination coverage in Indonesia remains relatively low (60% in 2021 according to the World Health Organization [WHO]), which may contribute to the disproportionately high burden of disease (World Health Organization, Citation2021). The introduction of HPV vaccination in other countries has resulted in reductions in disease burden, for example in the United Kingdom (UK) where genital warts diagnoses fell by 90% in 15- to 17-year-old girls and by 70% in 15- to 17-year-old boys over the first 10 years after vaccine introduction (UK Health Security Agency, Citation2018). In Scotland, pre-cancerous cervical disease has decreased in women by 71% over the same time period (UK Health Security Agency, Citation2018).
Documentation on the humanistic and economic burden of HPV-related cervical and non-cervical disease is limited in Indonesia and other LMICs. This is important to understand given the rising incidence in HPV-related cancers and diseases worldwide and increasing awareness of HPV-related cancers outside the cervix (Islami et al., Citation2016; Menezes et al., Citation2021). This study aimed to fill this gap by exploring, from both a patient and healthcare professional (HCP) perspective, the humanistic and economic burden of HPV-related diseases in Indonesia.
Materials and methods
Study design
The study was conducted between June and September 2021. Qualitative interviews were conducted with patients experiencing, or who had experienced, HPV-related diseases, and HCPs experienced in treating HPV-related diseases.
Patient recruitment was conducted through a third-party agency specialising in healthcare research fieldwork who have affiliates based in Jakarta, Indonesia. Patients were recruited from Jakarta, Surabaya, and other areas of Java via physician referral or through communications with patient advocacy groups. All included patients were aged 18–60 years, had been diagnosed with cervical, vaginal, anal, penile, or head and neck cancer for at least 3 months or with genital warts (new [<3 months] or recurrent [≥3 months]), were able to communicate in Bahasa Indonesia and were contactable by phone. Included patients reported that their HCP told them that their disease was caused by HPV, however, given the nature of HPV, it was not possible to confirm HPV as the causative agent of disease.
HCPs were recruited through ‘snowball sampling’, whereby a small number of initial contacts who matched inclusion criteria were invited to participate and recommend other contacts who matched inclusion criteria (Charlie Parker, Citation2020). Recruitment began with initial contacts and by directly contacting members of the Ministry of Health. HCPs were recruited from government mandated primary care clinics and hospitals providing specialist care. HCPs were required to have at least 5 years of clinical practice experience as either obstetrician/gynaecologists (OBGYN), oncologists, venereologists, ear, nose, and throat (ENT) specialists, primary care physicians, or sexual health physicians.
All participants signed a written informed consent form prior to research participation. Ethical approval was obtained from the ethical committee at Muhammadiyah Purwokerto University with the registration number KEPK/UMP/15/V/2021. All participants received compensation at fair market value for taking part in this research.
Data collection and analysis
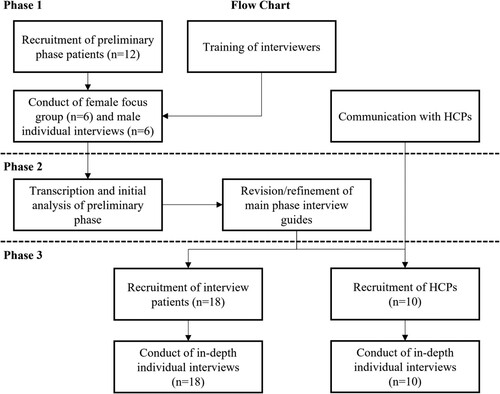
This study employed a three-phase qualitative approach (see ) to pilot interview guides (phase 1), use feedback to refine interview guides (phase 2), and conduct in-depth interviews with finalised guides (phase 3). Interviews and focus groups were conducted by telephone and lasted approximately one hour. Audio recordings were taken during phases one and three to allow translation and transcription of responses.
A purposive sampling technique was used to recruit similar numbers of patients with a variety of disease types associated with HPV; however, this was challenged by COVID-19 restrictions, and we ultimately achieved a convenience sample of participants with head and neck cancer (n = 8), anogenital cancer (n = 4), genital warts (n = 3), and cervical cancer (n = 3; total n = 18). For phase 1, there was a quota of patients with HPV-related cancer (n ≥ 4) and patients with genital warts (n ≥ 2). For phase 3, quotas were outlined to ensure that the perspectives of all types of HPV disease were captured, while considering that in Indonesia it may be difficult to guarantee a specific quota of patients for the less prevalent cancers. The quotas set were for patients with cervical cancer (n≈5), head and neck cancer (n≈5), genital warts (n≈5), and anogenital cancer (n≈2).
Phase 1: focus group and individual interviews
For phase 1, a focus group with six female patients who had an HPV-related disease and individual interviews with six male patients who had an HPV-related disease were conducted to allow for a tentative exploration of cultural sensitivities, nuances and language concerning HPV-related diseases (). The study protocol outlined that two focus groups would be conducted, including one for each sex. However, once the study had commenced and the female focus group had been conducted, male patients expressed discomfort about discussing their diseases in a group setting and therefore they were interviewed individually so that their insights were still captured. Further detail relating to patient demographics for included individuals in phase 1 can be found in .
Table 1. Demographics of patients (n = 18).
Phase 2: preliminary analysis and discussion guide refinement
In phase 2, findings from phase 1 were analysed and used to refine the discussion guides for phase 3. Example refinements included the addition of questions about the physical and social impact of diseases. Additionally, due to male patients’ reluctance to participate in focus groups during phase 1, questions related to disease-related stigma were added. Final interview guides were subsequently developed for patients and HCPs.
Phase 3: in-depth interviews
In phase 3, in-depth interviews were conducted with patients who had an HPV-related disease and HCPs specialised in treating HPV-related diseases. Patient interview guides explored their perceptions of the burden of their HPV-related disease. Interviews with HCPs explored their experiences with case management, knowledge, and beliefs regarding HPV-related diseases, and their perceptions of the economic, humanistic, and societal burden of HPV-related diseases.
Analysis
Audio-recorded interviews were conducted in Bahasa Indonesia for patients and HCPs, transcribed, and translated to English for analysis. Transcripts were quality checked by a bilingual researcher.
Data presented in the results are derived from the phase 3 interviews. Collection and analysis of data in this qualitative study was an iterative process (Braun & Clarke, Citation2012). Respondents were recruited until concept saturation of themes, which is the stage where no new themes emerged (Hennink et al., Citation2019). Concept saturation provided confirmation that additional participants to the sample would not provide new themes, concepts, and insights and increased confidence that diverse experiences of HPV-related diseases were captured (Hennink et al., Citation2019).
Thematic analysis allowed the identification of common themes across interviews, while providing insight into individual experiences. During analysis, the biopsychosocial model emerged as a salient framework for identifying and classifying themes (). The biopsychosocial model is an inter-disciplinary model that looks at the interconnection among biological, psychosociological, and social domains (Suls & Rothman, Citation2004). An additional economic domain was added after patient and HCP participants described financial burdens. All transcripts were single coded based on these categories using a coding framework that was iteratively developed. Analysts performed initial checks to ensure alignment on the coding process and maintained an open and ongoing consensus channel for any clarifications. Any discrepancies in interpretation were resolved by a senior analyst. Results from patients and HCPs are presented separately, however similarities and differences across narratives are highlighted.
Figure 2. Biopsychosocial and economic model describing the impacts of HPV-related diseases (adapted from: [Suls & Rothman, Citation2004]). HPV: human papillomavirus.
![Figure 2. Biopsychosocial and economic model describing the impacts of HPV-related diseases (adapted from: [Suls & Rothman, Citation2004]). HPV: human papillomavirus.](/cms/asset/2dc014a8-1cd4-4e73-b5dc-311d4dc6a3f9/rgph_a_2237096_f0002_ob.jpg)
Results
Recruitment and participant demographics
A total of 42 patients met the screening criteria and were approached, including those with head and neck cancer (n = 13), cervical cancer (n = 4), genital warts (n = 10), anal cancer (n = 9), penile cancer (n = 2), and vulvar/vaginal cancer (n = 4). There were 18 patients who agreed to complete in-depth interviews (n = 9 females and n = 9 males). Patient HPV-related disease types included: head and neck cancer (n = 8), cervical cancer (n = 3), genital warts (n = 3), anal cancer (n = 2), penile cancer (n = 1), and vaginal cancer (n = 1). Patients who declined to take part in the study mostly did so either because they were unwilling to discuss their disease or because they did not want their disease disclosed to others.
The mean age was 40 years. Most patients (n = 15/18) were married and insured (). No patients were excluded due to inability to communicate in Bahasa Indonesia.
There were 23 HCPs who met the screening criteria and were approached for participation, including four venereologists, four ENT oncologists, five primary care physicians, eight OBGYNs, and two surgeons. HCPs who declined to take part reported that they were either not interested, did not have the time, or felt that they did not have enough experience to adequately answer the questions. Ultimately, ten HCPs participated in the study, including three venereologists, one ENT oncologist, one general practitioner (GP), four OBGYNs, and one surgeon (). The number of patients with HPV-related diseases that HCPs reported that they treat per month ranged from 8 to 50 patients across HCPs. All individual interviews with patients or HCPs were approximately 60 min in length.
Table 2. HCP demographics (n = 10).
Patient insights
Biological impacts
All patients experienced negative physical impacts from their disease, though patients with cancer described more severe and debilitating symptoms than patients with genital warts. For patients with cancer, general symptoms included pain, fatigue, and weakness, in addition to symptoms specific to the anatomic location of their cancer. For example, one male patient with throat cancer noted, ‘[It is] difficult to breathe, and there is a lump on my throat … I feel easily exhausted’.
Patients with genital warts commonly described feeling uncomfortable, but physical symptoms varied. One female patient with genital warts described an uncomfortable itch and soreness, ‘The itchiness comes and goes, but at times it is extremely itchy. If I scratch them [genital warts] then I will feel sore … I had a burning sensation when the lumps appeared’. However, others described feeling more discomfort related to the emotional aspect of seeing warts, as a male patient described, ‘I could see there was a bump on my genitals … Initially I felt a slight itch … . I was disturbed because there is a bump on my genitals’.
While most patients described how treatment was effective in managing physical symptoms, patients with cancer who received chemotherapy described physical and debilitating side effects of treatment. One female patient with cervical cancer described intense weakness from chemotherapy, ‘Nowadays, I mostly lie down because I still feel weak … I think it is because of the chemo. I feel that I don’t have any energy’. One male patient with anal cancer described, ‘I was given an ointment to reduce the pain [on my anus], and it worked … Every time I do chemo or radiotherapy my stamina deteriorates’.
Some patients mentioned that their treatment was impacted by the COVID-19 pandemic, resulting in a greater burden of disease following delays. A female patient with cervical cancer noted, ‘On the schedule, [radiotherapy] is twice a month but as the number of COVID [cases] was high, it was reduced to once a month’, and a male patient with anal cancer stated, ‘[The doctor] said I can recover from cancer by undergoing chemotherapy. As of now, I am told to wait because of COVID’.
Psychological health impacts
Patients’ narratives of physical symptoms often intertwined with negative psychological impacts. Patients, mostly those with cancer, commonly feared experiencing physical pain, death, and worried about how their family and finances would be impacted. A female patient with cervical cancer described: ‘I was very worried. I feel that I am going to die soon … I think to myself, how will my children manage if I die?’ In some instances, psychological health impacts reflected economic concerns, as expressed by a female patient with throat cancer, ‘I keep thinking how much the medication would cost and how much I should spend for transportation to the hospital’.
Participants with genital warts mostly described experiencing fear of having future wart outbreaks. For example, one female patient with genital warts said: ‘The wart has disappeared, but it may reappear someday … I am really afraid of this’.
Participants also described feeling embarrassed by their disease. One male patient with genital warts explained that embarrassment delayed them seeking treatment, ‘I was embarrassed because it [my genital warts diagnosis] is concerning my genitals, so I left it untreated’. These feelings were mirrored by a female patient with vaginal cancer, ‘When I went to the doctor for the very first time, I wasn’t scared, I was just embarrassed because … my problem lies on my genitals’. This embarrassment may be due to feelings of guilt for believing that their disease was caused by engaging in behaviour considered disreputable in society, such as having multiple sexual partners. One male patient with throat cancer described, ‘I am annoyed at myself … I am shocked, and I regret doing it [one-night stands]’. Similarly, one female patient with vaginal cancer said, ‘[The doctor] said it [my cancer diagnosis] is caused by my sexual behavior and having multiple partners’.
Social impacts
Feelings of embarrassment expressed by patients regarding their HPV-related disease may be a response to the social stigma of illness in Indonesian culture. One female patient with throat cancer described the cultural taboo, ‘ … Living in the village can be tough. Being sick is a taboo over here because they make it into gossip material’. Participants feared experiencing negative judgment from their peers so many chose to not disclose their illness. A female patient with oropharyngeal cancer described, ‘If someone knows about it [my cancer diagnosis], they will drift apart from me or think negatively about me … My family and friends don’t judge me because they don’t know’.
Female patients commonly described not disclosing their HPV-related disease to friends or family due to fear of being accused of engaging in socially unacceptable sexual behaviour perceived to be ‘sinful’. For example, a female patient with vaginal cancer described, ‘I haven’t told my friends … because I feel it [their cancer diagnosis] is a sin because the pain is on my genitals … People would gossip if they knew that I suffer from vaginal cancer’. Similarly, one female patient with genital warts said, ‘I felt that this disease was a sin, I didn’t want anyone to know … I know some people talk about me behind my back’.
Of the 18 participants, 17 (n = 9 males and n = 8 females) disclosed their illness to at least one person, most frequently their partner. These participants faced a variety of negative social repercussions, like negative judgment, strained relations, and social isolation. One male patient with throat cancer recalled, ‘The attitude of my relatives towards me changed. They were disappointed … so they drifted apart’. Relationships between spouses were also impacted. For example, a male patient with laryngeal cancer described, ‘People who were close to me became distant. My wife really loved me but now she looks down on me’. Blame of one’s partner for acquiring the disease was a main contributor to relationship strains. For example, a female patient with throat cancer described,
I feel really angry at my husband because there is a chance that he cheated and brought the disease to me … I also feel mad because I have the fear of my husband hooking up with other women because I am unwell.
Self-isolation was also caused by negative physical symptoms: a male patient with anal cancer said, ‘I rarely meet my friends because I don’t feel well. I can’t even sit properly … I feel uncomfortable’. One female patient with oropharynx cancer similarly noted, ‘I reduce the frequency of hanging out with my friends because of my disease. Doctor told me to rest more’.
Economic burden
Most patients (89%) reported having insurance that alleviated much of the economic burden associated with their treatment. Of the patients who were insured, most (75%) were insured by the Badan Penyelenggara Jaminan Sosial (BPJS), which is the social insurance administration organisation in Indonesia ().
All patients reported at least one out-of-pocket cost, often related to medication or transportation for treatment. For example, one female patient with throat cancer explained how insurance only covered half of her required medication, ‘The medicine costs around Rp. 750,000–2,000,000. The medicine is covered by BPJS but only half of it is covered, I need to buy the rest on my own’. Transportation costs were often described as substantial economic expenses related to their disease. A male patient with anal cancer described, ‘I spend [money] on transportation because I don’t usually go to that area. My expenses have increased … approximately 30% … I think [I spend] above Rp. 1,000,000 … for transportation and food [bought while receiving treatment]’. A female patient with oropharyngeal cancer noted
I have to go to the hospital back and forth, if I am feeling dizzy then I will order [a taxi service] because it is impossible to drive a motorbike … the transportation cost in total is Rp. 220,000–240,000.
In total I received 39 sessions of radiotherapy … I pay for medication every month; it is around Rp. 100,000–150,000 … I have to pay Emergency Room (ER) expense on my own … I generally spend around Rp. 200,000–300,000 in the ER … I take out costs for transportation as well … Yes. [I spend the most on transportation]
When I am having fever, I don’t go to work, and the company policy says that there will be pay cut if I don’t come in for work … . My focus to work and concentrate has deteriorated. In one week, I can take up to two days off.
Changes in employment resulted in anxiety, changes in social roles, and economic instability. A male patient with throat cancer described, ‘The biggest obstacle [in relation to my disease] for me is financial problems … My parents are paying for [my treatment] and I owe them … Expenses for medication are quite high’.
Patients’ decreased ability to work also led to changes in their usual social role within families, often described by males in relation to their inability to be the primary financial provider for their family. A male patient with throat cancer said,
I used to fully support my child financially but now my ex-wife does this. I used to give my parents money for house expenses, now I don’t, I use all the money I earn for my own expenses … I used to be the primary earner but now my parents are the primary earners.
As a result of economic issues, several patients reported substantial economic hardship that entailed borrowing money, selling belongings, and/or using savings. One female patient with cervical cancer stated, ‘Yes, I have no more savings, I have used them all’. One male patient said, ‘I took a loan from online applications … I also sold my wife’s ring … [and] my wife’s necklace’.
HCP insights
Biological impact
HCPs mostly emphasised the biological and physical health burdens of disease treatment, particularly for patients with cancer. Descriptions of the physical burden of treatment by HCPs usually included how each patient’s symptoms negatively impacts their ability to work and complete household chores. One OBGYN stated, ‘Patients feel weak because of the chemo … Some are still doing their daily chores at home, but their [ability to do the same] workload has decreased. Many patients quit their job because they don’t have the energy to work anymore’. Another OBGYN stated, ‘Radiation has its own side effect … Patients can’t do their activities, not even the activities they do in their own house’. HCPs also discussed the negative physical impact of genital warts. A venereologist explained, ‘[Patients] feel pain because of the wound caused by warts … This area can easily bleed’.
Interestingly, many HCPs described symptoms related to odour that were not captured in patient accounts. This may be due to reluctance of patients to discuss this symptom associated with HPV-related disease due to embarrassment. One OBGYN oncologist said, ‘The other problem they [patients] face is bad odour, when their spouse approach them, they can smell the odour’. Similarly, a venerologist stated, ‘Symptoms include a bad smell … a severe, strong, smell’.
Psychological health impacts
HCPs differed in their perceptions towards psychological impacts of HPV-related diseases. Some HCPs (n = 4/10) reported that HPV-related diseases have a profound negative emotional impact on patients, which mirrored the narratives of participants who reported depression and anxiety. One OBGYN oncologist stated, ‘The patient feels like there is no reason to live anymore’. Another OBGYN oncologist said, ‘The patient must be depressed’. Of note, one surgeon reported referring patients experiencing severe mental health challenges to mental health professionals: ‘Some suffer from depression … their anxiety level increases … We refer them to psychiatrists’.
Most HCPs (n = 6/10) perceived little to no psychological impact associated with HPV-related diseases, which contrasted with patient experiences. One venerologist described, ‘No, this kind of disease doesn’t affect [patients’] moods … Some can accept the reality, some cannot … If the person can accept reality, accept the education, and undergo treatment, there is no effect’. An OBGYN oncologist echoed a similar sentiment: ‘[Patients] are surprised because of the cancer diagnosis and if the stage is late. Even though they are surprised, normally just for a few moments after that they start to accept their condition’.
Some HCPs offered an explanation as to why, generally, HCPs might not consider HPV-related diseases as emotionally burdensome. It was noted that patients may already understand their disease when they visit their HCP for a formal diagnosis. For example, a venereologist stated: ‘[Patients] react normally because most of them have searched for information via Google. They just come to me to confirm their diagnosis’. Similarly, a GP said, ‘There are patients who have been experiencing pain [prior to visiting their HCP] so they [patients with HPV-related diseases] have already guessed their condition … mostly they are not shocked’.
Social impacts
Most HCPs described social impacts of HPV-related diseases, in particular strain on romantic relationships. One OBGYN oncologist explained, ‘Most of my patients think that HPV is always related to irresponsible sexual behavior’. Venereologists and OBGYNs described experiencing tension between spouses, often due to one partner blaming another for acquiring the disease. One venereologist said, ‘Some blame their spouse for the disease. In this case I educate the patient … .Some [couples argue]’. Another OBGYN described, ‘One mistake in our words in front of the couple can cause fights and divorce. So, we must be extra careful. They [patients] can blame us for their marriage condition’. One OBGYN describes the social consequences for families: ‘Yes, [patients] are impacted because their husband and children won’t get close to them’.
HCPs were also aware of societal stigma towards patients living with HPV-related diseases and how, as a result, many patients would not disclose their diagnosis. A venereologist stated: ‘People who have genital warts would not announce it to anyone … so they can exercise their normal social life’. Another venereologist echoed, ‘They keep it to themselves because it is considered a taboo … People wouldn’t want to have sexual intercourse with someone who is suffering from HPV’. Similar to patients’ accounts of self-isolating due to embarrassment, one OBGYN noted, ‘Yes, they close themselves [off]. Maybe they used to speak to their neighbours or hang out with their family, now no more’. One GP also identified that patients may not disclose their HPV-related disease to their employer due to general illness-related stigma: ‘Maybe [if the disease affects patients] in their workplace, they will receive a warning from [Human Resources] and management because it would give a bad image to the company’.
Of note, two HCPs did not believe patients experienced any social impacts, which contrasts with patient narratives. One ENT described, ‘I think they [patients] are treated normally. They are welcomed in the society because people know how to avoid getting infected with this virus. People are more knowledgeable’. Similarly, an OBGYN stated, ‘[Society] thinks [illness] is written in people’s destiny, it is something that they need to deal with. Society reacts very well’. Moreover, these two HCPs did not perceive any psychological impacts as a consequence of HPV-related disease for their patients.
Economic burden
Impact on income, ability to work, and presenteeism were identified by most HCPs as HPV-related economic burdens. While patients (both with genital warts and cancer) described that insurance covered most treatment costs, all venereologists separately highlighted that insurance in Indonesia does not cover costs associated with genital warts, ‘Insurance doesn’t cover genital warts … most of my patients pay on their own. I don’t think it is covered by BPJS. In Indonesia, all genital diseases are not covered by insurance because they assume it is self-created’. Similar to patient narratives, a few HCPs described a significant economic burden related to the travel for treatment. This burden is higher for patients who lived further away from treatment centres. An OBGYN described,
A patient coming from a faraway city … They need to pay for transport, accommodation. Lab check, X-ray, medications are all paid for. So, they do not need to spend a dime for treatment cost. They need to spend for the non-medical expenses.
A few HCPs described patients experiencing devasting economic consequences as a result of their reduced employment, which is in line with patient narratives. An OBGYN oncologist described, ‘[Patients’] savings will also reduce … There are patients who sell their belongings but pass away after receiving medication. Patients need to spend on transport, so some of them have to sacrifice. There are cases [of bankruptcy]’. A surgeon also explained, ‘Yes, some [patients sell their assets and] even sell their house … Not only bankrupt, but some are also left without shelters’.
In contrast, four HCPs felt that patients experienced little economic burden. Four HCPs explained that it is not customary to ask about patient finances, which may explain their lack of awareness of financial challenges. For example, one venereologist stated, ‘My patients know that they need to pay the doctor, I don’t know about them borrowing money’. Additionally, a GP described being unaware of any financial burden, ‘I’m sure [patients] have to pay for transport to come to the hospital … In the hospital [they pay for] consultation, medicines and supporting check-ups. We do not know regarding their transportation cost and other costs’.
Discussion
Discussion of main study findings
Data from patients and HCPs in this study revealed substantial biological and physical, psychological, social, and financial burdens of HPV-related diseases among adult men and women in Indonesia. Many HCPs were aware of the burden of HPV beyond physical symptoms, but others were not aware of any psychological, social, or financial burden experienced by their patients. Findings have important implications for strategies to better support people with HPV-related diseases in Indonesia, which may be generalisable for other LMICs.
Patients in this study experienced psychological and social burdens that stemmed from stigma, which is the construction of deviation from some ideal or expectation (Alonzo & Reynolds, Citation1995), related to HPV specifically and cancer in general. Researchers identify three types of stigma – public, self- and institutional – which were all described by participants in this study (Corrigan, Citation2014). Public stigma involves the negative attitudes that others have about an illness, self-stigma refers to the negative attitudes that people have about their own condition, and institutional stigma involving policies of government and organisations that limit opportunities for people with an illness (Corrigan, Citation2014). Stigma has been shown to have negative impacts on population health by worsening or impeding social relationships, resource availability, stress, psychological and behavioural responses, and exacerbating poor health (Hatzenbuehler et al., Citation2013). While the stigma within Indonesia can be understood within the context of a conservative culture strongly influenced by religious beliefs (Himawan, Citation2020), stigma towards HPV and cancer have been reported across countries (Ginjupalli et al., Citation2022; Lebel & Devins, Citation2008; Nyblade et al., Citation2017). Previous research has reported that in India it is often thought that cervical cancer is contagious or that it manifests as a punishment, resulting in the social isolation of patients (Nyblade et al., Citation2017). In Kenya, HPV-positive individuals may be viewed as promiscuous or unfaithful to their partners and this discrimination may result in delayed diagnosis or treatment avoidance (Ginjupalli et al., Citation2022; Scott et al., Citation2015). These findings highlight the need for both local and global initiatives to reduce stigma towards HPV and HPV-related diseases (Ginjupalli et al., Citation2022; Lebel & Devins, Citation2008; Nyblade et al., Citation2017).
The financial burden experienced by patients was also substantial, particularly with regards to out-of-pocket costs associated with transportation and food, and loss of income due to symptoms impacting employment. The cost of private transportation to a hospital appointment was reportedly Rp. 220,000–240,000 for one patient. For reference, this is comparable to the cost of a short visit to a private doctor (∼Rp. 240,493) and is greater than the cost of a monthly public transport ticket (∼Rp. 201,035; 2022 prices) (Expatistan, Citation2022). Of the patients who reported out-of-pocket costs (n = 6), two noted that their options for transportation were limited by their symptoms. In both instances, dizziness and/or discomfort caused by their condition meant that they were unable to drive, meaning they had to spend money on alternative transportation. Of note, some HCPs in this study were unaware of financial challenges experienced by patients. Educating HCPs on the financial burdens of HPV-related diseases may improve HCPs’ ability to support patients.
The economic burden of disease was influenced by the level of insurance coverage held by patients. Insurance did not always cover HPV-related diseases and associated treatment costs in their entirety, and therefore patient experience may depend on their insurance provider. Insurance providers often cover preventative measures such as cervical cancer screening, which likely created a disparity in the health services accessible to those with insurance coverage compared to the patients without coverage in our study (n = 16 versus n = 2) (Wahidin, Citation2018). Previous studies have demonstrated that disruptions to insurance coverage led to reduced utilisation of screening services and receipt of treatment, often resulting in later cancer stages at diagnosis and therefore poorer prognoses (Suk et al., Citation2022; Yabroff et al. Citation2020; Zhao et al., Citation2018). Reduced use of screening services in cases of insurance coverage disruption has been particularly prevalent in low-income populations, indicating that insurance coverage may play a significant role in the experiences of patients in LMICs like Indonesia (Yabroff et al. Citation2020).
To our knowledge, this is the only study that explores the burden of HPV-related diseases from the perspective of patients and HCPs, allowing a comparison of narratives. Overall, many HCPs underestimated the psychological, social, and financial burdens experienced by patients. This gap in understanding may prevent HCPs from being able to recommend patients to services that provide the required mental, social, and financial support. HCPs may therefore benefit from education to increase awareness and understanding, and it may be beneficial to facilitate a culture of open communication between patients and their HCP team to allow patients to speak openly on topics beyond their clinical symptoms.
HPV vaccination has the potential to prevent the acquisition of HPV-related diseases. In 2016, an HPV demonstration programme was initiated in five Indonesian provinces and an HPV vaccine was provided at no cost to all female students in years 5 and 6 through school-based vaccination programmes (Wijayanti et al., Citation2021). Vaccine uptake within these programmes and parental acceptance was estimated to be >90% (D. Endarti et al. Citation2018; Kompas.com, Citation2019). In April 2022, the HPV vaccine was added to the list of routine immunisations funded by the government (no cost for patients) for administration across Indonesia (Linda Rae Bennett, Citation2022). An HPV vaccination policy in Indonesia has been shown to be a cost-effective strategy, and existing research has highlighted that the budget required to implement this is affordable for Indonesia (Setiawan et al., Citation2020).
Findings from our study highlight the need for complementary interventions such as targeted educational programmes to reduce HPV stigma and support the expansion of the vaccination programme for both men and women. Educational programmes may be used to increase the awareness of HPV transmission (Joo et al., Citation2019). Furthermore, it has been shown that receipt of HPV vaccination is associated with increased knowledge about HPV among adolescent girls in Indonesia, highlighting that increased vaccination coverage may result in greater understanding of the associated diseases (Prayudi et al., Citation2016).
Further measures may be useful in preventing or reducing HPV-related disease burdens. Screening for cervical cancer is an important tool in countries with high population coverage, however the logistical complexities have impeded screening programmes reaching high population coverage in LMICs (Casas et al., Citation2022). These logistical barriers may be reduced with the use of self-sampling, where individuals can carry out the procedure to detect for the presence of HPV themselves (Ma'som et al., Citation2016). Self-sampling has proven a reliable and cost-effective alternative to conventional screening, particularly in LMICs (Arrossi et al., Citation2016; Ma'som et al., Citation2016; Östensson et al., Citation2013; Citation2016; Vahabi & Lofters, Citation2016), and may be useful for reducing cervical cancer prevalence. An additional disease prevention strategy is to target behavioural risk factors that exacerbate HPV-related diseases, such as tobacco-use (Holipah et al., Citation2020). Tobacco-use is a major independent risk factor for oral cancer (Petersen, Citation2009). Tobacco-use also increases HPV susceptibility by weaking the immune system (Castle, Citation2008), and negatively impacts treatment response and survival of HPV-related cancers (Elhalawani et al., Citation2020; Mayadev et al., Citation2018). Given the high smoking prevalence in Indonesia (Holipah et al., Citation2020), the use of evidence-based tobacco prevention and cessation programmes are critical for reducing disease burden.
Findings must be interpreted in light of study limitations. There was the potential for selection bias in the recruitment of patients as members of patient groups may have had different treatment circumstances than those who were not members of patient groups, resulting in dissimilar experiences. However, the inclusion of HCPs may have mitigated such effects and improved the validity of the findings. Additionally, patients self-reported that their HCPs attributed their disease to HPV; however, HPV was not definitively identified as the cause of their disease. HPV can be the causative agent of a high proportion of cancers included in this study (National Cancer Institute, Citation2021), thus, findings are still highly salient for understanding the burdens faced by people with HPV-attributed cancers. Due to the sensitive nature of the topic, there is a possibility of social desirability bias, whereby participants may have answered untruthfully to present themselves in a positive way. However, interview guide questions were designed and standardised to be administered in an open-ended and non-judgmental way to reduce bias. In addition, contextual information was provided for patients to minimise reluctance and there was flexibility of interview approach to promote a comfortable interview environment.
Despite these limitations, this study provides key contributions in the field by elucidating the substantial physical, psychological, social, and financial HPV-related burdens experienced by men and women in Indonesia. Findings highlight the need for programmes and policy that reduce stigma, which was identified as a key driver of psychological and social challenges. Discrepancies between patient and HCP narratives may be ameliorated by improved HCP education efforts, which will likely benefit patients by improving HCPs understanding and subsequent ability to support patients. Despite the invaluable findings from this study, there is a need for additional research investigating these concepts to further validate findings and more precisely quantify the burden of HPV-related disease.
Acknowledgements
The authors would like to thank the researchers at Adept Field Solutions and their local associates who facilitated the fieldwork for the study.
Disclosure statement
This research is sponsored by CORE, Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA, of which MF, SK, IS, and SV are employees. Merck Sharp & Dohme LLC are the manufacturer of the HPV vaccines GARDASIL® and GARDASIL® 9 (Recombinant, Adsorbed), of which MF, SK, IS, and SV may own stocks and/or stock options. These interests have been fully disclosed to Taylor & Francis, and measures to control for potential conflict arising from sponsor involvement were in place for the entirety of the research project. For example, RP, DR, RN, AB, and CO are employees of Adelphi Values PROVE™. Adelphi Values PROVE™ was compensated by CORE, Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA, for the analysis of data and development of the manuscript. I addition, Adelphi Values PROVE™ consulted a third-party fieldwork agency, Adept Field Solutions, to conduct the in-depth individual interviews and focus groups with patients in Indonesia. MP received grants and honoraria from various pharmaceutical companies, all unrelated to this work except a targeted grant by Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA to support this specific research and subsequent manuscript. This study was funded by Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA. The funder contributed to the design of the study, collection, analysis, and interpretation of data as well as the writing and revision of the manuscript.
Additional information
Funding
References
- Alonzo, A. A., & Reynolds, N. R. (1995). Stigma, HIV and AIDS: An exploration and elaboration of a stigma trajectory. Social Science & Medicine, 41(3), 303–315. https://doi.org/10.1016/0277-9536(94)00384-6
- Arrossi, S., Ramos, S., Straw, C., Thouyaret, L., & Orellana, L. (2016). HPV testing: A mixed-method approach to understand why women prefer self-collection in a middle-income country. BMC Public Health, 16(1), 832. https://doi.org/10.1186/s12889-016-3474-2
- Bhatia, N., Lynde, C., Vender, R., & Bourcier, M. (2013). Understanding genital warts: Epidemiology, pathogenesis, and burden of disease of human papillomavirus. Journal of Cutaneous Medicine and Surgery, 17(2), S47–S54. https://doi.org/10.2310/7750.2013.13072
- Braun, V., & Clarke, V. (2012). Thematic analysis. In APA handbook of research methods in psychology. Research designs: Quantitative, qualitative, neuropsychological, and biological (Vol. 2., pp. 57–71). American Psychological Association. https://doi.org/10.1037/13620-004
- Casas, C. P. R., Albuquerque, R. C. R., Loureiro, R. B., Gollner, A. M., Freitas, M. G., Duque, G., & Viscondi, J. Y. K. (2022). Cervical cancer screening in low- and middle-income countries: A systematic review of economic evaluation studies. Clinics, https://doi.org/10.1016/j.clinsp.2022.100080
- Castle, P. E. (2008). How does tobacco smoke contribute to cervical carcinogenesis? Journal of Virology, 82(12), 6084–6086. https://doi.org/10.1128/JVI.00103-08
- Centers for Disease Control and Prevention. (2022). Human Papillomavirus (HPV): Genital HPV Infection. Retrieved 19th July from https://www.cdc.gov/std/hpv/stdfact-hpv.htm.
- Charlie Parker, S. S. A. G. (2020). Sage research methods foundations https://doi.org/10.4135/9781526421036831710
- Corrigan, P. W. (2014). The stigma of disease and disability: Understanding causes and overcoming injustices. American Psychological Association. http://www.jstor.org/stable/j.ctv1chrz90
- de Martel, C., Georges, D., Bray, F., Ferlay, J., & Clifford, G. M. (2020). Global burden of cancer attributable to infections in 2018: A worldwide incidence analysis. The Lancet Global Health, 8(2), e180–e190. https://doi.org/10.1016/S2214-109X(19)30488-7
- Elhalawani, H., Mohamed, A. S. R., Elgohari, B., Lin, T. A., Sikora, A. G., Lai, S. Y., Abusaif, A., Phan, J., Morrison, W. H., Gunn, G. B., Rosenthal, D. I., Garden, A. S., Fuller, C. D., & Sandulache, V. C. (2020). Tobacco exposure as a major modifier of oncologic outcomes in human papillomavirus (HPV) associated oropharyngeal squamous cell carcinoma. BMC Cancer, 20(1), 912. https://doi.org/10.1186/s12885-020-07427-7
- Endarti, D., Kristina, S. A., Farida, M. A., & Andriani, T. (2018). Knowledge, perception, and acceptance of HPV vaccination and screening for cervical cancer among women in Yogyakarta province, Indonesia. Asian Pacific Journal of Cancer Prevention. https://doi.org/10.22034/APJCP.2018.19.4.1105
- Endarti, D., Riewpaiboon, A., Thavorncharoensap, M., Praditsitthikorn, N., Hutubessy, R., & Kristina, S. A. (2015). Evaluation of health-related quality of life among patients with cervical cancer in Indonesia. Asian Pacific Journal of Cancer Prevention, 16(8), 3345–3350. https://doi.org/10.7314/APJCP.2015.16.8.3345
- Expatistan. (2022). Cost of living in Indonesia. Retrieved 6/9/22 from https://www.expatistan.com/cost-of-living/country/indonesia.
- Ginjupalli, R., Mundaden, R., Choi, Y., Herfel, E., Oketch, S. Y., Watt, M. H., Makhulo, B., Bukusi, E. A., & Huchko, M. (2022). Developing a framework to describe stigma related to cervical cancer and HPV in western Kenya. BMC Women's Health, 22(1), 39. https://doi.org/10.1186/s12905-022-01619-y
- GLOBOCAN. (2020). Indonesia fact sheet. Retrieved 10 May from https://gco.iarc.fr/today/data/factsheets/populations/360-indonesia-fact-sheets.pdf.
- Hatzenbuehler, M. L., Phelan, J. C., & Link, B. G. (2013). Stigma as a fundamental cause of population health inequalities. American Journal of Public Health, 103(5), 813–821. https://doi.org/10.2105/AJPH.2012.301069
- Hennink, M. M., Kaiser, B. N., & Weber, M. B. (2019). What influences saturation? Estimating sample sizes in focus group research. Qualitative Health Research, 29(10), 1483–1496. https://doi.org/10.1177/1049732318821692
- Himawan, K. K. (2020). Singleness, sex, and spirituality: How religion affects the experience of being single in Indonesia. Mental Health, Religion & Culture, 23(2), 204–215. https://doi.org/10.1080/13674676.2020.1767555
- Holipah, H., Sulistomo, H. W., & Maharani, A. (2020). Tobacco smoking and risk of all-cause mortality in Indonesia. PloS one, 15(12), e0242558. https://doi.org/10.1371/journal.pone.0242558
- Islami, F., Ferlay, J., Lortet-Tieulent, J., Bray, F., & Jemal, A. (2016). International trends in anal cancer incidence rates. International Journal of Epidemiology, 46(3), dyw276–dyw938. https://doi.org/10.1093/ije/dyw276
- Joo, E.-J., Chang, Y., Kwon, M.-J., Cho, A., Cheong, H. S., & Ryu, S. (2019). High-Risk human papillomavirus infection and the risk of cardiovascular disease in Korean women. Circulation Research, 124(5), 747–756. https://doi.org/10.1161/CIRCRESAHA.118.313779
- Kompas.com. (2019). Program Vaksin HPV Terhambat, Apa Kabar Anak Perempuan di Indonesia? [HPV vaccination program has been delayed. What is going to happen to Indonesian girls?]. Retrieved 27 July 2022 from https://sains.kompas.com/read/2019/12/20/190200923/program-vaksin-hpv-terhambat-apa-kabar-anak-perempuan-di-indonesia?page = all.
- Lebel, S., & Devins, G. M. (2008). Stigma in cancer patients whose behavior may have contributed to their disease. Future Oncology. https://doi.org/10.2217/14796694.4.5.717
- Linda Rae Bennett, S. M. D. (2022). INDONESIA TAKES AN IMPRESSIVE STEP TOWARD CERVICAL CANCER CONTROL. Retrieved 02 August 2022 from https://pursuit.unimelb.edu.au/articles/indonesia-takes-an-impressive-step-toward-cervical-cancer-control#:~:text = In%20April%2C%20Indonesian%20Health%20Minister,routine%20free%20immunisations%20administered%20nationwide.
- Lu, Y., Xie, Z., Luo, G., Yan, H., Qian, H. Z., Fu, L., Wang, B., Huang, R., Cao, F., Lin, H., You, R., Tan, L., Yu, T., Chen, M., Li, C., Liu, X., Lei, W., & Zou, H. (2022). Global burden of oropharyngeal cancer attributable to human papillomavirus by anatomical subsite and geographic region. Cancer Epidemiology, 78, 102140. https://doi.org/10.1016/j.canep.2022.102140
- Ma'som, M., Bhoo-Pathy, N., Nasir, N. H., Bellinson, J., Subramaniam, S., Ma, Y., Yap, S. H., Goh, P. P., Gravitt, P., & Woo, Y. L. (2016). Attitudes and factors affecting acceptability of self-administered cervicovaginal sampling for human papillomavirus (HPV) genotyping as an alternative to Pap testing among multiethnic Malaysian women. BMJ Open, 6(8), e011022. https://doi.org/10.1136/bmjopen-2015-011022
- Mayadev, J., Lim, J., Durbin-Johnson, B., Valicenti, R., & Alvarez, E. (2018). Smoking decreases survival in locally advanced cervical cancer treated with radiation. American Journal of Clinical Oncology, 41(3), 295–301. https://doi.org/10.1097/COC.0000000000000268
- Menezes, F. D. S., Fernandes, G. A., Antunes, J. L. F., Villa, L. L., & Toporcov, T. N. (2021). Global incidence trends in head and neck cancer for HPV-related and -unrelated subsites: A systematic review of population-based studies. Oral Oncology, 115, 105177. https://doi.org/10.1016/j.oraloncology.2020.105177
- Murdiyarso, L. S., Kartawinata, M., Jenie, I., Widjajahakim, G., Hidajat, H., Sembiring, R., Nasar, I. M., Cornain, S., Sastranagara, F., & Utomo, A. R. H. (2016). Single and multiple high-risk and low-risk human papillomavirus association with cervical lesions of 11,224 women in Jakarta. Cancer Causes & Control, 27(11), 1371–1379. https://doi.org/10.1007/s10552-016-0816-4
- National Cancer Institute. (2021). HPV and cancer. Retrieved 14 January from https://www.cancer.gov/about-cancer/causes-prevention/risk/infectious-agents/hpv-and-cancer.
- Nyblade, L., Stockton, M., Travasso, S., & Krishnan, S. (2017). A qualitative exploration of cervical and breast cancer stigma in karnataka, India. BMC Women's Health, 17(1), 58. https://doi.org/10.1186/s12905-017-0407-x
- Okunade, K. S. (2020). Human papillomavirus and cervical cancer. Journal of Obstetrics and Gynaecology, 40(5), 602–608. https://doi.org/10.1080/01443615.2019.1634030
- Östensson, E., Hellström, A.-C., Hellman, K., Gustavsson, I., Gyllensten, U., Wilander, E., Zethraeus, N., & Andersson, S. (2013). Projected cost-effectiveness of repeat high-risk human papillomavirus testing using self-collected vaginal samples in the Swedish cervical cancer screening. Acta Obstetricia et Gynecologica Scandinavica, 92(7), 830–840. https://doi.org/10.1111/aogs.12143
- Petersen, P. E. (2009). Oral cancer prevention and control – The approach of the World Health Organization. Oral Oncology, 45(4-5), 454–460. https://doi.org/10.1016/j.oraloncology.2008.05.023
- Prayudi, P. K. A., Permatasari, A. A. I. Y., Winata, I. G. S., & Suwiyoga, K. (2016). Impact of human papilloma virus vaccination on adolescent knowledge, perception of sexual risk and need for safer sexual behaviors in Bali, Indonesia. Journal of Obstetrics and Gynaecology Research, 42(12), 1829–1838. https://doi.org/10.1111/jog.13123
- Racey, C. S., & Gesink, D. C. (2016). Barriers and facilitators to cervical cancer screening Among women in rural Ontario, Canada: The role of self-collected HPV testing. The Journal of Rural Health. https://doi.org/10.1111/jrh.12136
- Scott, N., Crane, M., Lafontaine, M., Seale, H., & Currow, D. (2015). Stigma as a barrier to diagnosis of lung cancer: Patient and general practitioner perspectives. Primary Health Care Research & Development, 16(6), 618–622. https://doi.org/10.1017/S1463423615000043
- Setiawan, D., Andrijono, Hadinegoro, S. R., Meyta, H., Sitohang, R. V., Tandy, G., Perwitasari, D. A., & Postma, M. J. (2020). Cervical cancer prevention in Indonesia: An updated clinical impact, cost-effectiveness and budget impact analysis. PloS one, 15(3), e0230359. https://doi.org/10.1371/journal.pone.0230359
- Suk, R., Hong, Y.-R., Rajan, S. S., Xie, Z., Zhu, Y., & Spencer, J. C. (2022). Assessment of US preventive services task force guideline–concordant cervical cancer screening rates and reasons for underscreening by age, race and ethnicity, sexual orientation, rurality, and insurance, 2005 to 2019. JAMA Network Open, 5(1), e2143582–e2143582. https://doi.org/10.1001/jamanetworkopen.2021.43582
- Suls, J., & Rothman, A. (2004). Evolution of the biopsychosocial model: Prospects and challenges for health psychology. Health Psychology, 23(2), 119–125. https://doi.org/10.1037/0278-6133.23.2.119
- Sung, H., Ferlay, J., Siegel, R. L., Laversanne, M., Soerjomataram, I., Jemal, A., & Bray, F. (2021). Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer Journal for Clinicians, 71(3), 209–249. https://doi.org/10.3322/caac.21660
- UK Health Security Agency. (2018). Ten years on since the start of the HPV vaccine programme – what impact is it having? Retrieved 21/10/2022 from https://ukhsa.blog.gov.uk/2018/06/18/ten-years-on-since-the-start-of-the-hpv-vaccine-programme-what-impact-is-it-having/.
- Vahabi, M., & Lofters, A. (2016). Muslim immigrant women’s views on cervical cancer screening and HPV self-sampling in Ontario, Canada. BMC Public Health, 16(1), 868. https://doi.org/10.1186/s12889-016-3564-1
- Wahidin, M. (2018). Cervical cancer screening financing in Indonesia. Journal of Global Oncology, 4(Supplement 2), 93s–93s. https://doi.org/10.1200/jgo.18.22700
- Wijayanti, K. E., Schütze, H., & MacPhail, C. (2021). Parents’ attitudes, beliefs and uptake of the school-based human papillomavirus (HPV) vaccination program in Jakarta, Indonesia - A quantitative study. Preventive Medicine Reports, 101651–103355. (Print) https://doi.org/10.1016/j.pmedr.2021.101651
- World Health Organization. (2021). Human Papillomavirus (HPV) vaccination coverage. Retrieved 21/10/2022 from https://immunizationdata.who.int/pages/coverage/hpv.html?CODE = IDN&ANTIGEN = &YEAR = .
- World Health Organization. (2022a). Cancer. Retrieved 2022 from https://www.who.int/news-room/fact-sheets/detail/cancer.
- World Health Organization. (2022b). Cervical cancer. Retrieved 26/10/2022 from https://www.who.int/news-room/fact-sheets/detail/cervical-cancer.
- Yabroff, K. R., Reeder-Hayes, K., Zhao, J., Halpern, M. T., Lopez, A. M., Bernal-Mizrachi, L., Collier, A. B., Neuner, J., Phillips, J., Blackstock, W., & Patel, M. (2020). Health insurance coverage disruptions and cancer care and outcomes: Systematic review of published research. JNCI: Journal of the National Cancer Institute, https://doi.org/10.1093/jnci/djaa048
- Zhao, G., Okoro, C. A., Li, J., & Town, M. (2018). Health insurance status and clinical cancer screenings Among U.S. Adults. American Journal of Preventive Medicine, 54(1), e11–e19. https://doi.org/10.1016/j.amepre.2017.08.024