ABSTRACT
Although mammography is the gold standard for breast cancer screening, the World Health Organization recommends clinical breast examination (CBE) as the preferred early detection method in countries with limited resources. However, its effectiveness as a ‘stand-alone’ screening modality compared with other techniques remains unclear. Therefore, we evaluated a risk-based opportunistic breast cancer screening programme using three modalities. Between June and December 2018, we conducted a cross-sectional study in Yogyakarta, Indonesia, of women aged >40 years with at least one risk factor for breast cancer. Subjects underwent CBE, mammography, and ultrasonography. We calculated the proportion of breast lesions detected through each modality and compared their mass size. A total of 503 eligible subjects were screened. Five cases of potential malignant lesions were detected; pathological tests conducted for 4 of them confirmed breast cancer diagnoses. A combined assessment of mammography and ultrasonography examinations revealed 343 breast lesions (68.2%), whereas CBE screening detected only 76 breast lesions (15.1%). The mean lesion sizes detected by mammography or ultrasonography, but not through CBE, were significantly smaller (p-values of 0.037 and 0.007 for mammography and ultrasonography, respectively). In conclusion, mammography and ultrasonography produced higher detection rates for benign and malignant breast lesions compared with CBE.
Introduction
Breast cancer is the most common disease affecting women worldwide, with over 2.26 million women diagnosed with breast cancer in 2020 (International Agency for Research on Cancer, Citation2021). Moreover, this figure is projected to reach 11 million by 2035 (Benson & Jatoi, Citation2012). The incidence of breast cancer is rising everywhere but is particularly alarming in low- and middle-income countries (LMICs) (Torre et al., Citation2017). An estimated 42 cases of breast cancer occur per 100,000 women in Indonesia, with a total of 65,858 new cases diagnosed in 2020 (Sung et al., Citation2021).
Breast cancer mortality is considerably higher in LMICs than in high-income countries, given a larger proportion of later-stage diagnoses (Yip, Citation2016). The high mortality rate is attributed to the lack of early detection, which includes screening and early diagnosis. Early detection is important because treatment is generally less intensive, less toxic, and less expensive for localised cancers, resulting in improved outcomes (Freitas & Weller, Citation2015).
Mammography screening has been successful in detecting early cancers, as well as the detection of previously occult benign breast disease (Gaunt, Citation2004). However, the distinction between benign and malignant lesions is not always simple. However, the distinction between benign and malignant lesions is not always simple. In most cases, the cause of symptoms was a benign breast lesion (Stachs et al., Citation2019). Women with benign breast disease have been reported to have an elevated risk for future breast cancer (Román et al., Citation2021).
The strong argument in favour of early detection for improving patient outcomes has led to the implementation of breast cancer screening programmes worldwide, including in Indonesia. The Indonesian government has implemented a joint programme of breast cancer and cervical cancer screening (Ministry of Health of Indonesia, Citation2015). Under this population-based national cancer screening programme, women aged >40 years are advised to undergo clinical breast examination (CBE) in primary health care centres along with visual acetic acid inspections (Ministry of Health of Indonesia, Citation2015; Wahidin et al., Citation2022). However, the programme’s implementation has been suboptimal, evidenced by the low screening rate (7.34%) in 2018 (Badan Penelitian dan Pengembangan Kesehatan, Citation2019).
Mammography, considered as the gold standard for breast cancer screening, has not been implemented as a national screening programme (World Health Organization, Citation2014). The World Health Organization (WHO) recommends prioritising early diagnosis, such as CBE, of symptomatic women and their prompt treatment in countries with limited resources and weak health systems, where nationwide implementation of mammography screening for breast cancer is not yet feasible. However, CBE as a ‘stand-alone’ screening modality has not been recommended as the effectiveness remains undetermined (Ngan et al., Citation2020).
Recently the government of Indonesia developed a plan to implement a population-based screening programme using the combination of CBE and ultrasonography for women aged 30–65 years old (Ministry of Health of Indonesia, Citation2021). Ultrasonography shows greater sensitivity than mammography in dense breasts, frequent among Asian populations (Fan et al., Citation2015). Furthermore, it is more affordable and widely available in low-resource settings (Sood et al., Citation2019). Ultrasonography, compared to mammography, also lacks ionising radiation and is more easily accepted by patients (Geisel et al., Citation2018).
Within existing population-based screening programmes in Indonesia, age is the only condition used to define the target population. However, the future programme will implement risk-based screening. It is aimed at increasing benefits and decreasing harm, has been proposed as an alternative to age-based screening (Shieh et al., Citation2016). A risk-based screening programme for breast cancer could have the greatest potential for reducing mortality, given that in a population at increased risk the expected disease incidence is higher and therefore the detection rate would also be higher in this population (T. C. Lee et al., Citation2019).
To compare the performance of alternative screening methods, we implemented a risk-based opportunistic breast cancer screening programme in 2018 using three modalities: CBE, mammography, and ultrasonography. In addition, we also evaluated the detection of benign breast disease using those modalities. We invited individuals with risk factors for breast cancer to participate in our study to evaluate a risk-based opportunistic breast cancer screening programme in Yogyakarta Province, Indonesia.
Materials and methods
A cross-sectional study was conducted between June and December 2018 at Dr Sardjito Hospital, a type-A government hospital. The study was approved by the local ethics committee of the Faculty of Medicine, Public Health, and Nursing, Universitas Gadjah Mada, Yogyakarta, Indonesia (KE/FK/0133/EC) on February 22, 2018.
Subjects
Women aged >40 years living in Yogyakarta Province, Indonesia, with at least one breast cancer risk factor, were eligible to participate in this study. The risk factors considered were: (1) age >50 years; (2) a family history of breast cancer, ovary cancer, thyroid cancer, or colon cancer; (3) a history of benign breast lesions; (4) age at first menstruation (menarche) < 12 years; (5) menopause at the age of >55 years; (6) absence of pregnancy until aged 30 years old; (7) history of chest radiotherapy; (8) absence of breastfeeding history; (9) history of hormonal contraception usage; (10) history of oestrogen hormonal therapy; and (11) abortion history. We excluded women who had been diagnosed with breast cancer, who had radiotherapy within six months prior to the study, or who had undergone mammography screening in the last year.
Invitations to enrol in the study were broadcast via social media, email, and posters displayed in hospital. Women interested in participating were asked to provide consent electronically before completing an online form to self-identify any breast cancer risk factors. If they had at least one risk factor, they were invited to come to Dr Sardjito Hospital to undergo further investigative procedures. The subjects were asked to provide informed consent prior to participating and were told that they could withdraw from the study at any time. Their eligibility criteria were assessed by the research team during interviews. Eligible women were enrolled in the study and underwent examinations of their vital signs, CBE, mammography, and ultrasonography on the same day. Subjects who underwent these screening examinations on different days were excluded due to the possibility of different results.
Data collection
CBE was conducted by a male or female general practitioner or surgeon, and the results were recorded in the case report form. Breast lesions were defined as any lumps detected during inspection or palpation of the breast. After completing the CBE, the subjects underwent digital mammography examinations.
A female radiographer guided the subjects to perform the mammography. A Breast Imaging Reporting and Data System (BI-RADS®) score of 2 indicated benign lesions, whereas a BI-RADS® score of 4 or 5 indicated malignant lesions. Following the mammography, female radiologists performed ultrasonography. Benign breast lesions were defined by a BI-RADS® score of 2 or 3, whereas malignant lesions were defined by a BI-RADS® score of 4 or 5.
After assessing the results of mammography and ultrasonography separately, which means that the results of one modality were not influenced by another modality. In the end, the radiologists made a final assessment using both results of mammography and ultrasonography to define breast lesions according to a combined BI-RADS® score for mammography and ultrasonography. Benign breast lesions were defined by a BI-RADS® score of 2 or 3, whereas malignant lesions were defined by a BI-RADS® score of 4 or 5.
Results of all examinations were recorded in the case report form. Subjects with suspected malignancies according to mammography or ultrasonography results were advised to undergo pathology examinations entailing fine needle aspiration biopsy (FNAB), and the results were recorded. Prior to and after each screening examination, the subjects were interviewed by the research staff using a questionnaire with items to elicit their knowledge and experience of the screening examinations.
Data analysis
Categorical data on the subjects’ baseline characteristics were presented as frequencies and percentages, and means with 95% confidence interval were used for numerical data. We also calculated the number of breast lesions detected using each screening modality. The number of breast cancer cases confirmed through pathology examinations was also displayed. An independent t-test was performed to compare the sizes of breast lesions detected using each screening modality. P < 0.05 was considered statistically significant.
We then calculated the accuracy of breast lesion detection through CBE compared with that obtained with the gold standard (mammography), noting their sensitivity, specificity, positive predictive value, and negative predictive value. Data on the subjects’ knowledge and experience were recorded as frequencies. We further calculated costs associated with each screening modality as (total cost * number of cases detected)/total subjects. The analysis was performed using SPSS version 21 (SPSS, Chicago, IL, USA).
Results
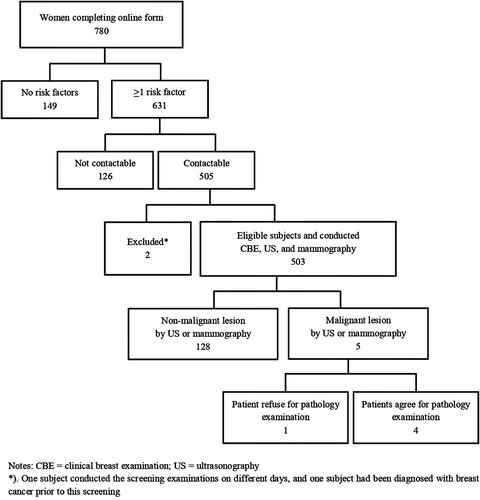
Initially, we only targeted women employed in the academic hospital and/or the school of medicine. However, as only one-third of the recruitment target was met, we broadcast an open call to all women in Yogyakarta Province. shows that 780 women responded to the invitation, of whom 631 (81%) had at least one breast cancer risk factor. However, 128 of these women could not be contacted for a screening appointment, resulting in 505 potential subjects.
Two of these women were excluded from the study: one had previously been diagnosed with breast cancer and another conducted her screening examinations on different days because of inflammation due to mastitis, which would make mammography examination painful. Therefore, she only underwent CBE and ultrasonography on the examination day; the mammography was conducted after she recovered from mastitis.
Ultimately, a total of 503 eligible subjects were enrolled in this screening programme, all of whom underwent CBE, mammography, and ultrasonography consecutively. The subjects’ characteristics are presented in , which also lists their risk factors for breast cancer development. Mean age (±SD) of the 503 subjects was 50.95 ± 7.63. Of all the women, 103 (38.4%) had a positive family history of any cancer, with 29 having a family history of breast cancer. A total of 71 subjects (14.1%) had early menarche, 73 (14.5%) had late menopause, 113 (22.5%) had a positive history of hormonal contraceptive use, and 44 (8.8%) were nulliparous.
Breast lesion detection using three modalities of breast cancer screening
Table 1. Baseline characteristics of subjects (N = 503).
presents a summary of the results of lesion detection using each modality. Suspected malignant lesions were detected in 5 of the 503 subjects who completed the screening programme, of whom 4 agreed to undergo FNAB, which confirmed breast cancer.
shows the number of breast lesions (benign and malignant) detected using each modality. Breast lesions were detected through CBE in 76 subjects (15.1%). Mammography and ultrasonography produced a higher number of detections, compared to CBE. When ultrasonography was used alone, breast lesions were found in 225 subjects (44.7%), whereas mammography resulted in the detection of lesions in 280 subjects (55.7%). Furthermore, 343 breast lesions (68.2%) were found through combined assessments using mammography and ultrasonography.
and show that two suspected malignant lesions detected by mammography and ultrasonography were not detected as lesions through CBE. If we used the US result, the size of palpable mass was more than 2 cm. However, one of those suspected malignant lesions was not detected when being examined by mammography alone, but was detected by ultrasonography alone.
Table 2. Breast lesion detection through clinical breast examination, mammography, and ultrasonography.
Table 3. Description of subjects suspected with malignancy through the examination of mammography and ultrasound.
shows a comparison of mass size detected by CBE, ultrasonography, and mammography. Mean mass sizes detected by CBE as well as mammography and ultrasonography were approximately 1.54 cm, whereas mean mass size detected by mammography or ultrasonography but not with CBE was significantly smaller (p = 0.037 and 0.007 for mammography and ultrasonography, respectively). The mean mass size detected by mammography but not through CBE was 0.99 cm, while the mean mass size detected by ultrasonography but not through CBE was 0.77 cm. Moreover, the mean mass size detected by mammography, which could or could not be detected by ultrasonography, did not differ significantly (p = 0.128). Masses detected by ultrasonography but not by mammography (0.73 cm) were smaller than masses detected by mammography but not by ultrasonography (0.99 cm).
| b. | The accuracy of CBE compared to mammography to detect breast lesion | ||||
Table 4. Comparison of mass size detected through clinical breast examination, mammography, and ultrasonography*.
shows a cross-tabulation of breast lesions detected through CBE versus those detected by mammography. The sensitivity and specificity of CBE for detecting breast lesions compared with those of mammography were 15.0% and 84.8%, respectively, and the positive and negative predictive values were 55.3% and 44.3%, respectively.
Table 5. The accuracy of clinical breast examination versus mammography for breast lesion detection.
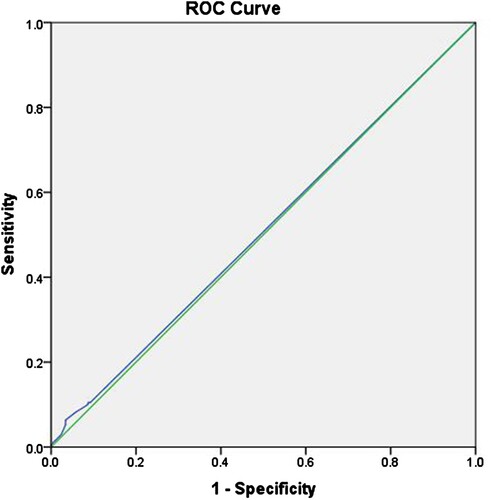
visualises the receiver operating characteristic (ROC) curve. The accuracy of CBE in detecting breast lesions according to mass size was defined as the area under the ROC curve. shows that the area under the curve (AUC) was 0.507. Although this value indicates purely random performance, this ROC analysis demonstrated a cutoff lesion size, which can increase the sensitivity and specificity of CBE performance. shows that the optimal accuracy of mass size detected through CBE, compared with mammography, was associated with a size of ≥0.15 cm, with a sensitivity of 10.5% and a specificity of 91.3%.
| c. | Subjects' knowledges and experiences of breast cancer screening | ||||
Table 6. ROC curves characteristics forclinical breast examination to detect breast lesion (using mammography as gold standard).
Table 7. Summary of test properties of clinical breast examination (CBE) to detect breast lesion (using mammography as gold standard) at selected cutoffs for CBE mass size.
shows responses to statements about the subjects’ knowledge of breast cancer screening prior to the screening and their experience after the screening. A total of 167 of the 503 subjects (33.2%) felt more anxious before undergoing mammography than they did before undergoing CBE (20.5%) or ultrasonography (22.5%) because they feared that cancer would be detected. Moreover, they felt embarrassed, and were worried that the procedure would be painful. Compared to CBE or ultrasonography, more subjects felt uncomfortable undergoing a mammography examination because of embarrassment, pain, a tickling sensation, and a feeling that the examination was dirty. Interestingly, virtually all the subjects (>98%) were willing to repeat this screening examination.
Table 8. Women’s knowledge and experiences of breast cancer screening.
Discussion
We implemented a risk-based opportunistic breast cancer screening programme and examined 503 women at risk for breast cancer in Yogyakarta Province, Indonesia. A total of 5 suspected cases of breast cancer were subsequently detected. The women in four of these cases agreed to undergo a pathology examination, which confirmed the cancer diagnosis. The detection rate of breast cancer in our study was relatively similar to that obtained in a previous opportunistic breast cancer screening initiative in Jakarta, Indonesia in which 13 out of 1,179 women were found to have breast cancer (Kardinah et al., Citation2014). However, this screening programme was done among women living in Jakarta regardless their risk status for breast cancer.
The approach to breast cancer screening has changed over time from being a general approach to becoming a more personalised, risk-based approach (C. I. Lee et al., Citation2017). Risk-based screening could optimise benefits while minimising harm (Harkness et al., Citation2020). There are several calculators that can measure the risk of breast cancer, such as the Tyrer-Cuzick Risk Calculator, the BCSC Risk Calculator, and the BCRAT Calculator (Himes et al., Citation2016; Matsuno et al., Citation2011; Vachon et al., Citation2015). The criteria for risk factors that they use vary, but in general they produce a calculation which combines more than one risk. Commonly used criteria include age, a history of breast cancer in first-degree relatives, and a history of breast biopsy. However, in our study, having at least one risk factor was sufficient for the subjects’ eligibility. We applied this criterion because of the time constraint for recruiting subjects. Thus, 81% of the women who completed the online form had at least one risk factor. This is a relatively large group of women of whom not all are at increased risk. Therefore, a risk calculator should be used to obtain an optimal selection for screening, as it will represent women at risk better than when using just the ‘one risk factor’ cutoff.
A screening test that is both highly sensitive and highly specific is desirable, but in practice, this is often infeasible. Mammography is considered the gold standard for breast cancer screening with estimated sensitivity and specificity values of 86.9% and 88.9%, respectively (Breast Cancer Research Consortium, Citation2017). The WHO and various international cancer networks also suggest that mammography screening is effective for reducing mortality (Oeffinger et al., Citation2015; Senkus et al., Citation2015; World Health Organization, Citation2014). Currently, there is no nationwide, population-based breast cancer screening programme in Indonesia that uses mammography.
The Indonesian government is currently implementing a CBE-based screening programme, using age (≥40 years) as the only condition for defining the target population (Ministry of Health of Indonesia, Citation2015). Our study showed that the sensitivity and specificity of CBE for detecting breast lesions (both benign and malignant lesions) were 15.0% and 84.8%, respectively. A systematic review reported a higher accuracy of CBE in screening malignant lesions, but the reported sensitivity (40%–69%) was much lower than the specificity (93%–97%) as in our findings (Ngan et al., Citation2020). In our study, two of the five suspected malignant lesions were not detected through CBE. Therefore, stand-alone CBE should not replace mammography for screening. Instead, CBE should be used as intended, an early diagnosis modality for palpable masses. Clinical breast assessment which includes history taking of breast symptoms, a general medical examination, and CBE performed by a trained health care provider should be applied as a comprehensive examination for early diagnosis of breast cancer (Duggan et al., Citation2020).
One previous study reported that the sensitivity of mammography could be compromised by higher breast density in Asian women (Wang et al., Citation2020) A complementary secondary image-based test, such as ultrasonography, which is potentially better suited for dense breast tissue examination (Fan et al., Citation2015), could be useful as an adjunct modality in Asian populations. Recently the government of Indonesia developed plan to implement a new strategy of breast cancer screening by using the combination of CBE and ultrasonography (Ministry of Health of Indonesia, Citation2021). Our study found that ultrasonography detected one more case of suspected malignant lesions than mammography. Unfortunately, this subject refused to undergo pathology examination, so we are unsure about the definite diagnosis.
Furthermore, our study found that mammography and ultrasonography could detect more breast lesions of a significantly smaller size than CBE could. Our study also revealed that masses detected by ultrasonography (but not by mammography) were smaller than those detected by mammography (but not by ultrasonography). Benign masses (e.g. cysts) can be better visualised through ultrasonography than mammography. Since cyst lesions would show up as low- or iso-density in a mammography examination, it is very difficult to differentiate them from normal fibro glandular tissue.
However, for the detection of breast cancer it is the high-density lesions or microcalcifications that are of relevance, and for these, ultrasonography was less accurate than mammography in our study (Gharekhanloo et al., Citation2018). As the type of lesion, whether it is malignant or benign, is a more crucial characteristic for the prognosis of breast cancer than the size of lesion, mammography would still be the gold standard for breast cancer screening. In addition, ultrasonography and CBE are more operator dependent than mammography which makes this modality more challenging for a screening test (Madjar, Citation2008; Madjar et al., Citation1999).
The idea of analysing not only malignancy, but also benign lesions (all breast lesions) is because we did not do pathology examination in all subjects with lesions. The combination of mammography and ultrasonography is sufficient as the gold standard for detecting breast lesions, as it is not necessary to differentiate between malignancy and benign. Furthermore, we believe that this approach provides novel and broader evidence of breast diseases, not only breast cancer, as it is rarely evaluated among researchers.
We also explored the subjects’ experiences of undergoing the screening procedures. More women reported anxiety before undergoing mammography than before undergoing CBE and ultrasonography. This anxiety was associated with fear of a cancer diagnosis, embarrassment, and apprehension regarding pain during the procedure. Subjects also found mammography to be more uncomfortable compared with CBE and ultrasonography. This finding is in line with those of our previous study and of Mohan et al., indicating that fear of a cancer diagnosis, embarrassment, and discomfort because of the presence of male staff were the most reported barriers (Aidalina & Syed Mohamed, Citation2018; Choridah et al., Citation2021; Mohan et al., Citation2021).
Despite these commonly reported emotional barriers, virtually all the subjects in our study (>98%) were willing to repeat this screening examination, particularly if the examination was free of charge, as in our screening programme. This finding is in line with that of Bourdeanu et al. (Citation2020), who found that fear of cancer and the benefits of and barriers to mammograms are not statistically significant predictors of having had a mammogram. One of the most important predictors was recommendations by physicians and health care providers, who are the primary information sources (Bourdeanu et al., Citation2020). This finding was also confirmed by that of our previous study, namely that Indonesian women would prioritise this examination if it was recommended by their doctors or the national health service (Choridah et al., Citation2021).
Our results demonstrated that a risk-based approach for screening women with breast cancer successfully implemented in the Yogyakarta Province, Indonesia. However, as we recruited subjects via social media, women who considered themselves at risk because of their family histories would have been more likely to respond. Furthermore, with the study self-selection of recruitment process, our study might have included women with symptoms and explained the high prevalence of breast cancer. During the eligibility assessment, we did not ask whether the women currently had a palpable lump. This could be the reason why some women signed up to the study, which could essentially change ‘screening’ to ‘early diagnosis’. However, this information was not captured in the data.
The eligibility criterion we selected in our study was having at least one risk factor, which was very wide and resulted in a high proportion of women enrolled. In the future, this criterion for participation in a screening programme should be refined using a risk factor calculator. Accordingly, the screening group size would be smaller, but yield to detect for breast cancer may be similar, thereby ensuring that the programme is more cost-effective.
In our study, subjects with suspected benign breast lesions from mammography or ultrasonography examination were not being followed up with FNAB. Therefore, we could have missed a case of breast cancer, which might influence the accuracy result. Furthermore, a longer follow-up study is also required to evaluate whether the initial increase in early-stage disease promotes long-term improvement in cancer morbidity and mortality using similar or fewer healthcare resources. Nonetheless, in the absence of population-based screening programmes, a well-designed risk-based opportunistic programme embedded within a functional healthcare system may represent an attractive method for early detection as the first step toward improving breast cancer mortality in Indonesia.
Conclusion
A considerable number of breast cancer cases were identified in this study. Furthermore, detection rates for benign and malignant breast lesions were higher for mammography and ultrasonography compared with CBE. The analysis of accuracy of these modalities revealed that CBE is not a reliable screening modality for either benign or malignant breast lesions. Despite some emotional barriers, virtually all subjects in our study (>98%) were willing to repeat this screening examination.
Acknowledgements
The authors thank to the following groups and individuals for their contributions to the study: study participants; the research teams in Radiology Research and Training Office (Faculty of Medicine, Public Health, and Nursing – Universitas Gadjah Mada); the research teams in Radiology Installation (Dr. Sardjito Hospital); Dhite Bayu Nugroho, MD, PhD; Afdina Melya Ganes Febiyanti, B.Med.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Aidalina, M., & Syed Mohamed, A. S. J. (2018). The uptake of mammogram screening in Malaysia and its associated factors: A systematic review. The Medical Journal of Malaysia, 73(4), 202–211.
- Badan Penelitian dan Pengembangan Kesehatan. (2019). Laporan nasional riset kesehatan dasar (2018th ed.). Lembaga Penerbit Balitbangkes, Kementerian Kesehatan, Republik Indonesia, Badan Penelitian dan Pengembangan Kesehatan.
- Benson, J. R., & Jatoi, I. (2012). The global breast cancer burden. Future Oncology, 8(6), 697–702. https://doi.org/10.2217/fon.12.61
- Bourdeanu, L., Alatrash, M., Ketchedjian, N., & Pate, B. (2020). Perceived fears, barriers, and benefits regarding breast cancer screening: A comparison of Lebanese and Lebanese-American women. JCO Global Oncology, 6(6), 1200–1210. https://doi.org/10.1200/GO.20.00019
- Breast Cancer Research Consortium. (2017). Sensitivity, specificity, false negative rate for screening mammogram examinations from 2007-2013. https://www.bcsc-research.org/statistics/screening-performance-benchmarks/screening-sens-spec-false-negative.
- Choridah, L., Icanervilia, A. V., de Wit, M. J. M., van Asselt, A. D. I., Kurniawan, W. T., Fahmi, Y. I., & Rengganis, A. A. (2021). Knowledge and acceptance towards mammography as breast cancer screening tool among Yogyakarta women and health care providers (mammography screening in Indonesia). Journal of Cancer Education, 36(3), 532–537. https://doi.org/10.1007/s13187-019-01659-3
- Duggan, C., Dvaladze, A., Rositch, A. F., Ginsburg, O., Yip, C. H., Horton, S., Camacho Rodriguez, R., Eniu, A., Mutebi, M., Bourque, J. M., Masood, S., Unger-Saldaña, K., Cabanes, A., Carlson, R. W., Gralow, J. R., & Anderson, B. O. (2020). The breast health global initiative 2018 global summit on improving breast healthcare through resource-stratified phased implementation: Methods and overview. Cancer, 126(Suppl 10), 2339–2352. https://doi.org/10.1002/cncr.32891
- Fan, L., Goss, P. E., & Strasser-Weippl, K. (2015). Current status and future projections of breast cancer in Asia. Breast Care, 10, 372–378. https://doi.org/10.1159/000441818
- Freitas, A. G., & Weller, M. (2015). Patient delays and system delays in breast cancer treatment in developed and developing countries. Ciência & Saúde Coletiva, 20(10), 3177–3189. https://doi.org/10.1590/1413-812320152010.19692014
- Gaunt, L. M. (2004). Radiology of benign breast disease. In Benign breast diseases, 1, 1-2. Springer. https://doi.org/10.1007/978-3-642-18527-4_1
- Geisel, J., Raghu, M., & Hooley, R. (2018). The role of ultrasound in breast cancer screening: The case for and against ultrasound. Seminars in Ultrasound, CT and MRI, 39(1), 25–34. https://doi.org/10.1053/j.sult.2017.09.006
- Gharekhanloo, F., Haseli, M. M., & Torabian, S. (2018). Value of ultrasound in the detection of benign and malignant breast diseases: A diagnostic accuracy study. Oman Medical Journal, 33(5), 380–386. https://doi.org/10.5001/omj.2018.71
- Harkness, E. F., Astley, S. M., & Evans, D. G. (2020). Risk-based breast cancer screening strategies in women. Best Practice & Research Clinical Obstetrics & Gynaecology, 65, 3–17. https://doi.org/10.1016/j.bpobgyn.2019.11.005
- Himes, D. O., Root, A. E., Gammon, A., & Luthy, K. E. (2016). Breast cancer risk assessment: Calculating lifetime risk using the Tyrer-cuzick model. The Journal for Nurse Practitioners, 12(9), 581–592. https://doi.org/10.1016/j.nurpra.2016.07.027
- International Agency for Research on Cancer. (2021, March). World Factsheets of Cancer Today. The Global Cancer Observatory. https://gco.iarc.fr/today/data/factsheets/populations/900-world-fact-sheets.pdf.
- Kardinah, D., Anderson, B. O., Duggan, C., Ali, I. A., & Thomas, D. B. (2014). Short report: Limited effectiveness of screening mammography in addition to clinical breast examination by trained nurse midwives in rural Jakarta, Indonesia. International Journal of Cancer, 134(5), 1250–1255. https://doi.org/10.1002/ijc.28442
- Lee, C. I., Chen, L. E., & Elmore, J. G. (2017). Risk-based breast cancer screening. Medical Clinics of North America, 101(4), 725–741. https://doi.org/10.1016/j.mcna.2017.03.005
- Lee, T. C., Reyna, C., Shaughnessy, E., & Lewis, J. D. (2019). Screening of populations at high risk for breast cancer. Journal of Surgical Oncology, 120(5), 820–830. https://doi.org/10.1002/jso.25611
- Madjar, H. (2008). The practice of breast ultrasound. Thieme.
- Madjar, H., Rickard, M., Jellins, J., & Otto, R. (1999). Ibus guidelines for the ultrasonic examination of the breast. European Journal of Ultrasound, 9(1), 99–102. https://doi.org/10.1016/s0929-8266(99)00016-6
- Matsuno, R. K., Costantino, J. P., Ziegler, R. G., Anderson, G. L., Li, H., Pee, D., & Gail, M. H. (2011). Projecting individualized absolute invasive breast cancer risk in Asian and pacific islander American women. JNCI: Journal of the National Cancer Institute, 103(12), 951–961. https://doi.org/10.1093/jnci/djr154
- Ministry of Health of Indonesia. (2015). Penanggulangan kanker payudara Dan kanker leher rahim (breast cancer and cervical cancer communication), Pub. L. No. 34 (2015). http://hukor.kemkes.go.id/uploads/produk_hukum/PMK_No._29_ttg_Penanggulangan_Kanker_Payudara_dan_Kanker_Leher_Rahim_.pdf.
- Ministry of Health of Indonesia. (2021). Laporan Rapat Kemenkes 30 Desember 2021: Isu Tematik Penanggulangan Kanker (Meeting Report the Ministry of Health 30 December 2021: Thematic Issues for Cancer Management).
- Mohan, D., Su, T. T., Donnelly, M., Hoe, W. M. K., Schliemann, D., Tan, M. M., Reidpath, D., Taib, N. A., & Allotey, P. (2021). Breast cancer screening in semi-rural Malaysia: Utilisation and barriers. International Journal of Environmental Research and Public Health, 18(23), 12293. https://doi.org/10.3390/ijerph182312293
- Ngan, T. T., Nguyen, N. T. Q., Van Minh, H., Donnelly, M., & O'Neill, C. (2020). Effectiveness of clinical breast examination as a ‘stand-alone’ screening modality: An overview of systematic reviews. BMC Cancer, 20(1), 1070. https://doi.org/10.1186/s12885-020-07521-w
- Oeffinger, K. C., Fontham, E. T., Etzioni, R., Herzig, A., Michaelson, J. S., Shih, Y. C., Walter, L. C., Church, T. R., Flowers, C. R., LaMonte, S. J., Wolf, A. M., DeSantis, C., Lortet-Tieulent, J., Andrews, K., Manassaram-Baptiste, D., Saslow, D., Smith, R. A., Brawley, O. W., & Wender, R. (2015). Breast cancer screening for women at average risk: 2015 guideline update from the American cancer society. JAMA, 314(15), 1599–1614. https://doi.org/10.1001/jama.2015.12783
- Román, M., Louro, J., Posso, M., Alcántara, R., Peñalva, L., Sala, M., Del Riego, J., Prieto, M., Vidal, C., Sánchez, M., Bargalló, X., Tusquets, I., & Castells, X. (2021). Breast density, benign breast disease, and risk of breast cancer over time. European Radiology, 31(7), 4839–4847. https://doi.org/10.1007/s00330-020-07490-5
- Senkus, E., Kyriakides, S., Ohno, S., Penault-Llorca, F., Poortmans, P., Rutgers, E., Zackrisson, S., Cardoso, F., & ESMO Guidelines Committee. (2015). Primary breast cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Annals of Oncology, 26(Suppl 5), v8–v30. https://doi.org/10.1093/annonc/mdv298
- Shieh, Y., Hu, D., Ma, L., Huntsman, S., Gard, C. C., Leung, J. W., Tice, J. A., Vachon, C. M., Cummings, S. R., Kerlikowske, K., & Ziv, E. (2016). Breast cancer risk prediction using a clinical risk model and polygenic risk score. Breast Cancer Research and Treatment, 159(3), 513–525. https://doi.org/10.1007/s10549-016-3953-2
- Sood, R., Rositch, A. F., Shakoor, D., Ambinder, E., Pool, K. L., Pollack, E., Mollura, D. J., Mullen, L. A., & Harvey, S. C. (2019). Ultrasound for breast cancer detection globally: A systematic review and meta-analysis. Journal of Global Oncology, 5(5), 1–17. https://doi.org/10.1200/JGO.19.00127
- Stachs, A., Stubert, J., Reimer, T., & Hartmann, S. (2019). Benign breast disease in women. Deutsches Ärzteblatt International, 116(33-34), 565–574. https://doi.org/10.3238/arztebl.2019.0565
- Sung, H., Ferlay, J., Siegel, R. L., Laversanne, M., Soerjomataram, I., Jemal, A., & Bray, F. (2021). Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer Journal for Clinicians, 71(3), 209–249. https://doi.org/10.3322/caac.21660
- Torre, L. A., Islami, F., Siegel, R. L., Ward, E. M., & Jemal, A. (2017). Global cancer in women: Burden and trends. Cancer Epidemiology, Biomarkers & Prevention, 26(4), 444–457. https://doi.org/10.1158/1055-9965.EPI-16-0858
- Vachon, C. M., Pankratz, V. S., Scott, C. G., Haeberle, L., Ziv, E., Jensen, M. R., Brandt, K. R., Whaley, D. H., Olson, J. E., Heusinger, K., Hack, C. C., Jud, S. M., Beckmann, M. W., Schulz-Wendtland, R., Tice, J. A., Norman, A. D., Cunningham, J. M., Purrington, K. S., Easton, D. F., … Couch, F. J. (2015). The contributions of breast density and common genetic variation to breast cancer risk. JNCI: Journal of the National Cancer Institute, 107(5), dju397. https://doi.org/10.1093/jnci/dju397
- Wahidin, M., Febrianti, R., Susanty, F., & Hasanah, S. R. (2022). Twelve years implementation of cervical and breast cancer screening program in Indonesia. Asian Pacific Journal of Cancer Prevention, 23(3), 829–837. https://doi.org/10.31557/APJCP.2022.23.3.829
- Wang, J., Zheng, S., Ding, L., Liang, X., Wang, Y., Greuter, M. J. W., de Bock, G. H., & Lu, W. (2020). Is ultrasound an accurate alternative for mammography in breast cancer screening in an Asian population? A meta-analysis. Diagnostics, 10, 985. https://doi.org/10.3390/diagnostics10110985
- World Health Organization. (2014). WHO position paper on mammography screening. WHO Press.
- Yip, C.-H. (2016). Challenges in the early detection of breast cancer in resource-poor settings. Breast Cancer Management, 5(4), 161–169. https://doi.org/10.2217/bmt-2016-0026