ABSTRACT
The emergent threat of antimicrobial resistance (AMR) has resulted in debates around the use and preservation of effective antimicrobials. Concerns around AMR reflect a history of increasing dependence on antibiotics to address disease epidemics rooted in profound structural and systemic challenges. In the context of global health, this process, often referred to as pharmaceuticalisation, has commonly occurred within disease programmes, of which lessons are vital for adding nuance to conversations around antimicrobial stewardship. Tuberculosis (TB) is a notable example. A disease which accounts for one-third of AMR globally and remains the leading cause of death from a single infectious agent in many low – and middle-income countries, including South Africa. In this scoping review, we chart TB science in South Africa over 70 years of programming. We reviewed published manuscripts about the programme and critically reflected on the implications of our findings for stewardship. We identified cycles of programmatic responses to new drug availability and the emergence of drug resistance, which intersected with cycles of pharmaceuticalisation. These cycles reflect the political, economic, and social factors influencing programmatic decision-making. Our analysis offers a starting point for research exploring these cycles and drawing out implications for stewardship across the TB and AMR communities.
Introduction
Antimicrobial Resistance (AMR) is a rising concern globally (IACG, Citation2019; O’Neill, Citation2016). AMR is caused by mutations in microbial organisms that render them less susceptible to available antimicrobial medicines. AMR is driven by selective pressure, including the overuse of antimicrobial drugs and, in particular, non – or partial adherence. Human and animal health has come to rely heavily on antimicrobial medicines, especially antibiotics, to cure and prevent disease, creating the ideal conditions for accelerating AMR (WHO, Citation2015). AMR makes infections more challenging to treat, increases their likelihood of spreading, and can result in severe illness or death. 700,000 people die of drug-resistant infections yearly – a number expected to increase to 10 million by 2050 if no intervention is made (World Bank Group, Citation2021).
In 2015, the WHO declared AMR a threat to global health (WHO, Citation2015). Recognition of AMR has resulted in a renewed push to rationalise and reduce the use of antimicrobial medicines. ‘Stewardship’, the most recent framework through which such efforts have been discussed, intends to optimise clinical outcomes whilst limiting the use of antibiotics, to minimise the occurrence of AMR (Poots, Citation2012). Interventions that have been proposed and implemented under the rubric of stewardship are numerous but include hospital therapeutics committees, formulary restrictions, audit and feedback, clinical guideline development and education and awareness campaigns (Cox et al., Citation2017; A. Wilkinson et al., Citation2018).
Much of the emphasis of stewardship has tended to be placed on restricting and correcting individual behaviour to protect drug efficacy (Broom et al., Citation2021; Cox et al., Citation2017; A. Wilkinson et al., Citation2018). Although evidence suggests interventions of this nature can be effective, evidence of their long-term sustainability remains limited and is especially limited in low-resource settings, as was found in a systematic review (A. Wilkinson et al., Citation2018). A growing number of qualitative studies in resource-limited settings, both in high-income countries (Broom et al., Citation2017, Citation2021) and low – and middle-income countries (Dixon et al., Citation2021; MacPherson et al., Citation2022; Pearson & Chandler, Citation2019), have drawn attention to the numerous contextual factors that shape and limit the choices of prescribers and patients – especially poverty and inequality, inadequate health and hygiene infrastructure, high-pressure prescribing environments, and the lack of effective alternatives to drug-based care.
Further, Biehl (Citation2007) has described how the over-use of antimicrobials is itself reflective of a historical and deepening reliance on antibiotics to do the work of caring – a process sometimes referred to as ‘pharmaceuticalisation’ (Biehl, Citation2007). Chandler (Citation2019), following the work of Bowker and Star (Citation1970), has characterised AMR as a moment of ‘infrastructural inversion’ – where antibiotics, having become part of the infrastructure of healthcare systems globally, have become hyper-visible by virtue of their breakdown, prompting us to consider how we ended up in the current predicament. With this breakdown increasingly visible, several historical analyses have sought to understand the processes through which antibiotics have been designed into health systems globally and why attempts to ration or ‘steward’ their use have tended to have limited traction in practice (Chandler, Citation2019; Dixon et al., Citation2021; María Jesús Santesmases, Citation2017; Monnais & Tousignant, Citation2006; Podolsky, Citation2015). Global tensions between expanding and limiting antibiotic use have played out within disease-specific programmes. Contained within siloes of programmatic activity, the dynamics of antibiotic use and resistance and the lessons that we may learn in the current moment of crisis, remain a rich and underexplored area of social and historical analysis. Notable among these is tuberculosis (TB), a disease that makes up a third of the total AMR burden (WHO, Citation2014).
TB is caused by Mycobacterium tuberculosis and is estimated to be responsible for 1.2 million deaths per year (WHO, Citation2020). An estimated 10.6 million people developed TB in 2021, of whom 450 000 were reported to have rifampicin resistant (RR-TB) TB or multi-drug resistant (MDR-TB) TB (WHO, Citation2022a). Drug susceptible (DS)-TB is treated with a combination of 4 drugs. RR-TB and MDR-TB are treated with a variety of second-line drugs with individually tailored regimens. The treatment duration for DS-TB is 4–6 months, and for RR – and MDR-TB is 9–18 months (WHO, Citation2022b). Long treatment duration increases the risk of non – or partial adherence. This is particularly pertinent in LMIC settings, where enduring patterns of structural violence (Farmer, Citation1997) create barriers to meeting desired stewardship protocols. These include the impact of poverty, inequality, urbanisation, migration, and conflict. Similarly, Lönnroth et al. (Citation2009) discussed how control strategies remain constricted when implemented without addressing the circumstances or risk factors that render one vulnerable to disease.
South Africa is a middle-income country in sub-Saharan Africa and has one of the world’s highest TB burdens (Ayles et al., Citation2022; WHO, Citation2020) and the third-largest population of people treated for DR-TB (Cox et al., Citation2017; WHO, Citation2020; Wood et al., Citation2011). TB is the country's leading cause of death from a single infectious agent (StatsSA, Citation2018). South Africa also has one of the world’s most long-standing and sophisticated TB programmes, with a wealth of academic and applied experience through the programme’s rich history. Many resources and much expertise have been dedicated to expanding and controlling the use of antibiotics within South Africa’s TB programme. Yet, rates of TB and DR-TB remain among the highest in the world, making South Africa a rich case study and source of insights for AMR and stewardship.
In this scoping review, we aim to explore what has been written about tuberculosis management and drug resistance within TB programming in South Africa. The scoping review methodology is calibrated towards the inclusion of perspectives and discourses from the biomedical sciences and scholarship concentrated in journal databases, which will enable us to provisionally identity patterns and trends in the programme and its attendant discourses over time that can be subjected to deeper social and historical analysis through future research. In this regard, the scope of review does not extend to other historical accounts and humanities scholarship, much of which would not be captured by a scoping review, but which has nonetheless informed the framing of our questions, interpretations, and conclusions. Also beyond the scope due to the volume of published scientific literature are the multifaced influences that developments in other country contexts have had on TB in South Africa, which can similarly be pursued in future work.
We aim to chart TB science in South Africa over 70 years of programming. Specifically, (a) we will chart how drug resistance has figured in manuscripts published about the South African TB programme, (b) analyse patterns in new drug availability and emergent resistance, and (c) critically reflect on implications for AMR stewardship.
Methods
Study setting
This review spans the period from the availability of the first TB drugs in South Africa (1950–2021). During these 70 years, South Africa’s TB control strategy intersected with shifting social and political contexts – most notably, apartheid and liberation – that fragmented its health system. Apartheid was a political system that violently subjugated South Africa’s ‘non-white’[1] population, through institutionalised racial segregation, between 1948 and 1994. The Group Areas Act of 1950 consigned one’s ability to live, access services, and operate businesses to specific areas designated by race. Areas assigned to ‘non-white’ populations were historically underfunded by the government (Mabin, Citation1992). This included the health system, which today continues to carry the legacy of apartheid through its disparity in quality of service. The apartheid system was built on earlier colonial systems of labour migration management, which included the ‘barracking’ of black men in labour camps associated with mines and seasonal farm labour systems (Packard, Citation1989; Van Rensburg et al., Citation2005). Consequently, there were multiple intersecting cyclical patterns of migration in the country with poor integration of health services across the homeland and other internal geographic borders and systems (Ponthieu & Incerti, Citation2016).
In 1994, the African National Congress (ANC) was voted into power, bringing the apartheid state to a close. The newly reconstituted Republic of South Africa began consolidating the apartheid fragmented health services by developing a universal healthcare system inspired by the Gluckman Commission of the mid-1940s (Harrison, Citation1993). South Africa's new health service offered comprehensive primary health services to all its citizens and was government funded. The health service currently comprises the National Department of Health (NDoH) and nine provincial health departments, which, in the case of Cape Town, includes a second health district – the Cape Metropolitan Health District (Alliance for Health Policy and Systems Research, Citation2017). In addition, a primary health care (PHC) system was also established. PHC provides affordable clinics in rural areas and addresses the growing disease burden within impoverished communities (Phillips, Citation2014). However, the legacy of apartheid remains in contemporary South Africa, which has yet to reconcile its gaps in inequality (Gordon et al., Citation2020). These, in combination with limited health expenditure enforced by macroeconomic policy decisions in the 1990s (Foster, Citation2005), have meant that poverty, issues with access to health services, and vulnerability to poor health outcomes remain a persistent struggle of the health system (Coovadia et al., Citation2009).
Design
We followed Arksey and O’Malley’s five-phased framework (Arksey & O’Malley, Citation2005) for scoping reviews and the recommendations of the Joanna Briggs Institute (The Joanna Briggs Institute, Citation2015). Namely, (1) specifying the research question, (2) identifying relevant studies, (3) study selection, (4) charting the data, and (5) collating, summarizing, and reporting results. We also followed the PRISMA-ScR checklist to report our findings.
Specifying the research question
We asked: ‘How did emergent resistance figure relative to the availability of new TB drugs and their use within the SA TB programme?’
Identifying relevant studies
With the assistance of a medical librarian, we collected scientific, published literature from electronic databases (SABINET, PubMed, Scopus, Web of Science, and Africa-Wide Information). Articles were identified and selected using MESH terms and a Boolean search. A keyword search was used on the predominantly South African database (SABINET) that did not offer Boolean search functionality. The MESH/Keyword terms used were:
‘The review covers the era from 1950 to 2021. This period spans the introduction of the first TB drug (streptomycin) to the programmatic use of the most recent novel drugs (bedaquiline and delamanid).’ Our search was broad to accommodate the fragmented way AMR and TB research has historically evolved in disease-specific programmes in global health (Packard, Citation2016a).Study selection
Titles using the search terms were extracted to Microsoft Excel, and duplicates were removed. Initially, manuscript titles were screened by the first (RR) and last authors (GH). Titles were excluded if they discussed countries outside of South Africa, had a research subject that was not human, or were not specific to TB, AMR, antibiotics (antitubercular and other). This resulted in a long list of 1,681 abstracts to progress to screening. We also realised that many of these included titles which, whilst technically meeting the inclusion criteria, were unlikely to be relevant to answering our stated research question. Therefore, we agreed first to sort the included titles (and abstracts if available) into 26 broad categories (multi-coding possible). Once sorted, we further excluded those manuscripts that did not contribute directly to our understanding of how resistance figured in the South African TB programme and were instead about (a) coinfection with other diseases, (b) clinical descriptions of TB microbiology, pathology or radiology but not resistance, (c) screening or diagnosis of disease (not resistance), (d) TB care for paediatric patients and mothers, (e) site of disease, (f) disease epidemiology/modelling, (g) knowledge, attitudes, and perceptions about TB, (h) TB transmission, (i) Health workers/infection prevention control, (j) epidemic or health service costing, (k) models of TB service delivery, (l) risks for infection or disease progression, (m) surgery, (n) antibiotics not used in TB care, (o) symptoms, adverse effects, or mortality, and (p) pharmacokinetics/pharmacology. We then proceeded to the abstract and full-text review of the remaining manuscripts as planned by RR and GH, with conflicts resolved by JD.
Manuscripts were excluded if the content did not speak to (a) policies, guidelines, control efforts, strategies, and messaging, (b) prescribing practices, (c) clinician/patient perceptions or experiences of TB, (d) context – such as opinion pieces, responses, editorials, and historiographies.
Findings
We identified 461 unique manuscripts included in the scoping review – .
A brief history of TB drugs becoming available relative to South African political context
We have developed below, based on the literature reviewed, to illustrate the cyclic patterns we observed in the South African TB programme over the 70-year period. The timeline presents key events and transitions through a colour-coded system. It additionally shows how these events took place through antibiotic eras indicated at the top of the figure, and political eras indicated in the lower part of the figure. The colours can be interpreted as follows: The green segments denote significant milestones in the discovery of new antibiotics. Brown segments represent moments when care was decentralised. The red portions indicate the emergence of drug resistance. Yellow reflects the use of antibiotics prophylactically. The blue segments illustrate where medicines were combined to create more effective regimens, and finally, purple represents moments where the health system had to rely on centralised care once more.
Figure 2. Timeline depicting cyclic patterns observed in the South African TB program over the 70-year period. References for the image can be found in a dedicated section of the reference list.
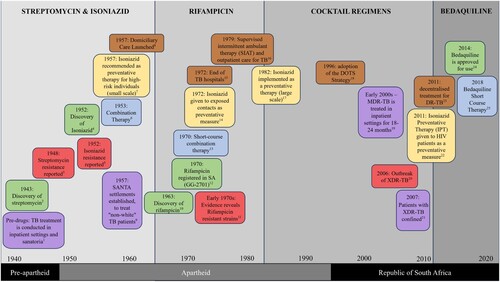
In South Africa, there have been several key developments in antitubercular drug availability and usage over the past 70 years. Most notable is the introduction of Streptomycin (SM), Isoniazid (INH) and Para-aminosalicylic acid (PAS) in the late 1940s and early 1950s; Rifampicin (RIF), which was introduced to South Africa in the early 1970s (Cox et al., Citation2017); and Bedaquiline (BDQ), which became more widely available between 2012 and 2016. The introduction of pharmaceuticals in each of these ‘eras’ significantly shifted the TB programme's approach to control and containment.
Novel medicines initially showed high success in curing TB and, in later iterations, allowed treatment to be shortened and staggered. Their introduction cast doubt on the necessity of hospitalisation to treat TB (H. Grusin, Citation1966) and introduced the possibility of treating TB on an outpatient basis. In the early twentieth century, health officials recognised the benefits of outpatient treatment. Not only did it improve access to treatment, but it also reduced the burden on hospitals during a severe bed shortage. Health officials recognised that a growing TB burden and a weak, resource-limited health infrastructure could not provide adequate inpatient treatment and rolled out accordingly. Since then, several examples of such services have been implemented in TB control strategies over the last 70 years.
However, outpatient treatment was not without risk. With each iteration of outpatient care adopted, the end user was, in one way or another, made responsible for ensuring treatment was not interrupted. Historical accounts (Packard, Citation1989; Van Rensburg et al., Citation2005) of TB in South Africa demonstrate the challenges faced by TB patients seeking and continuing care – an issue that persists today (Human et al., Citation2010; Kandel et al., Citation2008; Snyman et al., Citation2018). To remedy this, a focus on behavioural change interventions was embraced in the late 1950s. Under the framing of ‘Health education’ (Ferguson, Citation1960), public health programmes set out to teach patients, amongst other things, how to adhere to treatment. However, these interventions have become increasingly narrower in focus over time, leaning more heavily on observation. Conversely, a return to hospitalised, inpatient care also occurred several times during this history – driven by the increasing prevalence of emergent resistance and the challenges associated with its specialised management in resource-limited settings.
Four patterns of programmatic responses around new drug availability and resistance
We found four recurring, interwoven patterns of programmatic responses to new drugs becoming available and the development of resistance: first, optimism and emboldening following the discovery of a novel drug. Second, the freedom to decentralise care on account of the confidence in the drug. Third, an emphasis on the individual end-user behaviour to preserve the drug, and finally, a recentralisation of care once resistance emerges.
Optimism and opportunity with novel drug discovery
Streptomycin, Isoniazid and Para-aminosalicylic acid (1950-1970): In the early 1950s, SM, INH and PAS provided an effective solution to treating TB. Before their discovery, the only treatments available to physicians were invasive surgeries, absolute rest, and long retention in hospitals (Cooper, Citation1954; de Villiers, Citation1957; Fatti Crawshaw et al., Citation1950). Health officials were emboldened by the ‘new weapons’ they had available. Pharmaceuticals enabled not only curative interventions but an opportunity for South Africa to rethink its entire approach to TB control. De Villiers, the acting Medical Officer of Health at the time, described the pharmaceutical advance as one that shifted South Africa’s position from ‘defence’ to ‘attack’ (de Villiers, Citation1957).
The discovery of INH presented an opportunity to trial it as an effective chemoprophylaxis. Preventative measures had been an interest of the South African TB control efforts prior to the advent of the chemotherapeutic era. The improvement of living conditions had been at the forefront of their preventative strategy (Cluver, Citation1965; de Villiers, Citation1957; Dormer, Citation1960; Marais, Citation1960), alongside a limited use of the BCG vaccines.Footnote1 Although used in a limited capacity, INH was made available to breastfeeding mothers (Collins, Citation1982), hospital staff and other vulnerable contacts that would have extensive exposure to the disease (Dormer, Citation1960).
Rifampicin (1970–2014): Rifampicin was discovered in the early 1960s, but it was not until trials were conducted (Dormer & Salinger, Citation1971; Holland, Citation1972) that it was registered and used among South African TB patients in 1973 (Cox et al., Citation2017). Rifampicin (RIF) was considered to be a TB wonder drug. It arrived when large proportions of hospital-admitted TB patients were infected with strains resistant to one of the first-line treatments (INH, SM or PAS) (Salinger & Dormer, Citation1972). The benefits of combining RIF with already established treatment regimens were evident. New recommendations were made for its use in treating ‘poly-resistant’ cases when drug sensitivity testing was impossible (Salinger & Dormer, Citation1972).
The introduction of RIF, like SM and INH, revolutionised how TB was treated. The ‘rifampicin effect’ (Collins, Citation1972), when used in combination with INH, PAS and streptomycin, rifampicin allowed the overall treatment length to be reduced. It ushered in the era of intermittent short-course chemotherapy (Bell & Yach, Citation1988) and transformed the standard treatment regimen of 18–24 months to 6 months.
Reportedly, INH prevention also ramped up in the rifampicin era (Collins, Citation1982), and more patients gained access than were able to in decades prior. INH prophylaxis was given to healthy but infected patients at risk of developing the disease (Dormer, Citation1960). Treatment lengths were not standardised, but treatment length was estimated to be ‘at least 6 months and preferably 1 year’ (Benatar, Citation1982).
Bedaquiline (2014–2021): Following decades of no new TB drugs, in 2012, Bedaquiline (BDQ) was approved by the US Food and Drug Administration (FDA) for compassionate use in South Africa. This followed a growing MDR-TB epidemic and an XDR-TB outbreak in 2006 (Conradie et al., Citation2014; Geffen, Citation2016). BDQ’s discovery raised optimism as it was a new class of drug with no existing resistance – a stark contrast to the cocktail regimens, that included drugs such as ethambutol and pyrazinamide, that had come to be relied upon over the previous decades. Additionally, BDQ was a more cost-effective option (Lu et al., Citation2017) and offered the opportunity to shift away from painful injectables and drugs with severe adverse effects (Bouton et al., Citation2019). BDQ’s approval increased the survival rate of MDR-TB patients significantly. The improved treatment outcomes (Ndjeka et al., Citation2015; Olayanju et al., Citation2018) led to South Africa making BDQ a first-line treatment in 2018 (Bouton et al., Citation2019), prior to WHO guidelines which followed in 2019 (Chiang et al., Citation2020).
Decentralised care as a solution to overcrowded hospitals.
Domiciliary Care: Domiciliary care was an early outpatient TB treatment model implemented in South Africa in the mid-1950s (Cooper, Citation1954). This approach involved providing TB patients with medications, instructions for self-administration, and regular follow-up visits from a health worker. It was reported to be effective in treating TB and significantly reduced the length of hospitalisation required (Cooper, Citation1954; Saville-Lewis, Citation1953). The model was strongly advocated for as a solution to a growing crisis of hospital bed shortages that left many (particularly ‘non-European’ patients) untreated (Cooper, Citation1954; Saville-Lewis, Citation1953).
Supervised Intermittent Ambulatory Treatment (SIAT): In the 1970s, SIAT was launched in South Africa shortly after the RIF short-course regimen demonstrated positive treatment outcomes. This treatment approach, which allowed TB patients to return home between supervised sessions, was reported to be effective in improving treatment outcomes and reducing the burden on hospitals. The advantages were said to be: the shortening of treatment through the use of RIF and reducing the likelihood of patients ‘defaulting’ (Escreet & Cowie, Citation1981; Whitehouse, Citation1980). They additionally included lowering the incidence of relapse (Dormer & Salinger, Citation1971; Escreet & Cowie, Citation1981), fewer side effects (Whitehouse, Citation1980), and easing the strain on health system resources (financial and other) (Atwell & Pearson, Citation1980; Dormer & Salinger, Citation1971; Escreet & Cowie, Citation1981; Kent, Citation1982; Pearson, Citation1977; Whitehouse, Citation1980; Zabow & Pearson J, Citation1982). SIAT was advantageous for the health system, as it relieved pressure on hospitals by limiting patient interaction. It also significantly reduced health system expenditure by removing the substantial expense of hospitalisation (Atwell & Pearson, Citation1980).
Decentralised DR-TB Units: In 2011, following the standardisation of DR-TB treatment regimens, decentralised TB units were introduced in South Africa as a management strategy for MDR-TB. These units, which employed directly observed treatment short-course (DOTS) principles, were promoted as a strategy to bring TB care closer to patients. The units were based in primary healthcare facilities and community settings. This improved drug access and enabled the programme to reach more people. Prior to this, MDR-TB had been treated in an inpatient setting, which resulted in waitlists and bed shortages – similar to that of the past. Decentralised DR-TB units, much like domiciliary care and SIAT before it, allowed for a reduced burden on hospitals (Berhanu et al., Citation2016; Evans et al., Citation2018).
End-user behaviour change interventions
SANTA settlements and education programs: Concern for patient adherence to TB drugs is seen as early as the 1950s. Patients were described as ‘difficult to control’ for ‘defaulting’ on treatment protocol (de Villiers, Citation1957). Research cited: little concern, impulsive behaviour (Crowhurst Archer, Citation1960), ‘ignorance, lack of money for travelling […] and distance’ (Cluver, Citation1965) as contributing factors to interrupted treatment. An effort to discourage ‘defaulting’ through health education arose (Cluver, Citation1965). ‘Settlements’ provided by the South African National Tuberculosis Association (SANTA) were deemed the appropriate vectors to transmit this information (Marais, Citation1960). SANTA settlements were informal inpatient treatment clinics set up by local welfare and charity organisation. The locations provided inpatient care to South Africa’s black and ‘coloured’Footnote2 population in rural parts of the country (Saville-Lewis, Citation1953). In addition to expanding access to treatment, settlements allowed for close monitoring of patients while taking their treatment outside of a dedicated TB hospital.
Supervised Intermediate Ambulatory Treatment (SIAT): SIAT continued the intervention of patient observation during treatment in a clinical setting but allowed patients to return home after receiving their medications. Research efforts into ensuring a patient’s ‘compliance’ in the context of SIAT interventions continued (Bell & Yach, Citation1988; Escreet & Cowie, Citation1981). Patient anxieties about families and the future of employment were also mentioned, along with the lack of adequately trained medical personnel and high staff turnover (Griffiths & Makgothi, Citation1981; Yeats, Citation1986).
DOTS: In 1996, Directly Observed Therapy, Short course (DOTS) became a widely adopted approach following the WHO’s declaring of TB a threat to global health. Through DOTS, the NTCP endeavoured to ‘cure 85% of all smear-positive TB cases detected; to reduce the interruption rate to less than 10%; to detect 70% of estimated TB cases; to attain a smear conversion rate of 85% in new smear-positive cases and 80% in re-treatment cases’ (Van Rensburg et al., Citation2005, p. 28). DOTS was reported to improve treatment adherence and patient outcomes, by providing TB treatment under the direct observation of a health worker or other designated observer. It was implemented in hospitals, clinics, and community-based settings – reducing the burden on hospitals and creating greater patient access (Weyer & Stander, Citation1996; D. Wilkinson et al., Citation1996; D. Wilkinson & de Cock, Citation1996).
Recentralisation of care
Hospitalisation for MDR-TB: By 1996, the South African Department of Health standardised the treatment of MDR-TB in inpatient settings due to the longer and more complex treatment regimens required, as well as the higher mortality rates and worse treatment outcomes associated with MDR-TB compared to drug-sensitive TB. At this stage, there was no standardised policy for treating MDR-TB, and it was treated in two phases, including both outpatient and inpatient settings. Patients were hospitalised for six months and treated with injectables, which required monitoring for adverse effects and strict adherence. Patients would follow up monthly, on an outpatient basis, for 18 months after the injectable period (Loveday & Cox, Citation2017). The recentralisation of care for MDR-TB patients led to long waitlists and reduced access to treatment for these patients.
Hospitalisation for XDR-TB: In 2006, South Africa experienced an outbreak of extensively drug-resistant TB (XDR-TB) in the Tugela ferry area (Bateman, Citation2007; Coetzee & Koornhof, Citation2006; Wise, Citation2006). XDR-TB is resistant to all first-line drugs and three (or more) of the six second-line anti-tuberculosis drugs (de Lange, Citation2006). At the time, XDR-TB was associated with a mortality rate of approximately 80% (London, Citation2009). During the outbreak, the median survival time for those infected was 16 days after diagnosis (Jones et al., Citation2008). Treatment for XDR-TB relied on older medications that had painful adverse effects on the user. The treatment was high risk and required prolonged hospitalisation to monitor the patient. Sufferers were treated in an inpatient setting for up to 2 years (London, Citation2009). This renewed the bed crisis of the early twentieth century (Andrews et al., Citation2007; Loveday & Cox, Citation2017), forcing doctors to triage patients (Njaramba & Naidoo, Citation2007).
Discussion
We found 461 manuscripts relevant to understanding how resistance has figured in the South African TB programme. Over the 70 years included in the review, we found four patterns around the emergence of new drugs and resistance: (1) optimism following the discovery of a novel drug, (2) decentralisation of care, (3) an emphasis on the individual, end-user behaviour to preserve drug efficacy, and (4) recentralisation of care. These cycles are not neat, complete, or mutually exclusive, with legacies of previous cycles overlapping with and inflecting subsequent ones.
Such scholarship, applied to a variety of diseases or health programmes, has noted how pharmaceuticals have become central to the way in which health systems are organised to deliver care. In diverse contexts and across various diseases and health programmes, research by Comaroff (Citation2006), Biehl (Citation2007), Nguyen (Citation2010), and Prince and Marsland (Citation2013) emphasise the central role of pharmaceuticals in healthcare delivery. For instance, as Biehl (Citation2007) has documented in Brazil and Nguyen (Citation2010) in West Africa, the advent of antiretrovirals, particularly in the context of HIV, narrowed public health interventions towards expanding access and ensuring adherence to drugs, effectively creating what Nguyen (Citation2010) characterised as ‘therapeutic citizenship.’ These insights are congruent with our own, revealing yet another instance of pharmaceuticalisation in the realm of TB control over an extended period in a high-burden country.
As concerns surrounding AMR mount, particular attention has been drawn to the overreliance on antibiotics, particularly in the treatment of TB. Scholars such as Bowker and Star (Citation1970) and Chandler (Citation2019) have posited that antibiotics have been utilised to bridge gaps in healthcare system infrastructure, and in turn, have ultimately assumed the role of infrastructure themselves. The over-reliance this has encouraged conflicts with organised AMR efforts to steward antibiotics and limit their loss of efficacy. More recently, the attention of AMR stewardship has turned to considering the historical processes that have made antibiotics a vital component of care. For example, Denyer Willis and Chandler (Citation2019) have argued, based on work in Uganda, that antibiotics have been used to fill gaps in the health system, and water and sanitation infrastructure. In turn, they have become infrastructure themselves.
In recognising that pharmaceutical dependency has been written into health systems and economies over decades, even centuries (Packard, Citation2016b), another fruitful avenue of research has been tracing the historical processes that have made antibiotics such a vital component of our lives. Dixon et al. (Citation2021) in Zimbabwe, for example, traced current discourses around the ‘irrational’ use of antibiotics back to the push for essential medicines and rational drug use in the 1970s and 1980s. Looking back further, historical accounts have shown how debates that surrounded antibiotics and their use unfolded around the time of their discovery in the earlier twentieth century (Bud, Citation2007; María Jesús Santesmases, Citation2017; Podolsky, Citation2015), as well as how the advent of these ‘wonder drugs’ took shape following their ‘arrival’ in Africa (Palanco Lopez et al., Citation2022). Several accounts, including that by Monnais (Citation2019) in Vietnam, have further shown that the antecedents of pharmaceutical dependence can be found in the ‘social lives’ of medicines in the colonial era. Our findings echo these recurrent patterns, offering further substantiation for their existence.
Our findings offer an example of the historical processes through which pharmaceuticalisation – specifically a systemic dependence on antibiotics – occurs. In this instance, it was applied over the long term to a TB program in a high-burden country. Our analysis is especially significant given the extent to which current AMR deaths are attributed to TB (approximately 30%) (Johnson & Johnson, Citation2023) and the rich lessons from this programme that have, to the present day, become bracketed off from the broader AMR conversation.
The recurrent cycle reveals a narrowing to dependence on pharmaceuticals occurring in tandem with the increasing neglect of ‘social medicine’ (Adams et al., Citation2019) and environmental upliftment. Beginning with hope and optimism when new TB antibiotics were discovered, they were quickly used to shore up the TB control programme and broader health system, specifically by enabling decentralised treatment out of a necessity to treat patients outside of burdened hospitals. With antibiotics an integral component of the infrastructure of outpatient care, we next see a recasting of responsibility for prevention and management away from social and structural factors and onto patients and communities. This was enacted through socio-behavioural interventions such as educational campaigns run by SANTA, SIAT and DOTS to ensure adherence would be followed in the new modes of care. Finally, as the efficacy of drugs inevitably waned, the recentralisation of care occurred, and the transmission of DR-TB strains forced the health system to bring patients back into inpatient care for closer monitoring. By showing how this cycle has repeated throughout history in the context of an ever-dwindling supply of antibiotics, and increasingly extensive drug resistance, our analysis points towards the intractability of these patterns, despite the evident limitations and perhaps futile nature of attempting to control TB in this manner.
Strengths of our analysis include the long time period covering the history of chemotherapies in the South African TB programme, the inclusion of manuscripts from multiple databases, an inclusive set of search criteria, and the application of rigorous scoping review steps (Arksey & O’Malley, Citation2005) for screening and review. Limitations on extrapolation from our findings arise because we did not include grey or otherwise (un)published literature, such as relevant insights from critical social science scholarship in books, which may bias the results toward over-representing novel, positive readings. A further limitation is that, while we are confident in the patterns presented, some contextual nuance is lost without other historical and qualitative data, such as archival analysis of the TB programme and qualitative interviews with people involved in the TB programme at the time. Such primary research, as well as ongoing collaborative engagement across the evolving TB and AMR conversations, is needed to further detail and draw out the implications of the findings from this scoping review.
Additionally, our focus on AMR within South Africa may have overlooked intersecting scholarship and policy developments that have occurred concurrently in other parts of Africa and beyond. For example, international collaboration and research (McMillen, Citation2015), particularly through the work of the Medical Research Council (Fox et al., Citation1999), have been suggested to have played a pivotal role in shaping TB treatment strategies. Notable contributions have come from trials and research in other African and Asian countries, such as Tanzania (Gradmann, Citation2019) and India (Amrith, Citation2004), which have informed and been part of the narrative of TB control in South Africa. This global interplay underscores the fact that, although our focus was on South Africa, TB treatment did not evolve in isolation. Over the historical period reviewed, wars were fought, national and internal borders changed, and countries were renamed – all affecting TB transmission networks, control efforts, and treatment strategies. Such literature could provide additional insights into the regional dynamics of AMR that could be used in future research.
Conclusion
Our scoping review analysed 70 years of TB science in South Africa. We identified cycles in programmatic responses to the availability of new drugs and emergent resistance, culminating in the current debates around the introduction of BDQ. We critically reflected on the implications of these cycles for AMR stewardship. There are many shared concerns between the fields of TB and AMR, and collaborative research will be required to better understand and respond to the intractable predicament of emergent resistance and dwindling drug supply. Our analysis suggests that despite the programmatic and scientific awareness of the risks of emergent AMR, novel antimicrobials will be used with limited stewardship, driven by socio-political dynamics inherent to public health systems. We suggest this analysis adds further weight to the cause of re-interrogating the logics of AMR stewardship – away from unhelpful binary positions of prescription versus withholding, and toward management of use aligned with cycles of drug development and deployment.
In addition, we note that the observed reliance on pharmaceuticals within these cycles can be problematic when viewed through the lens of global health. Programmes and policies shaped predominantly around drug availability and efficacy may falter as resistance emerges, often reverting progress made in treatment settings. Hence, we advocate that global public health initiatives designed for TB, expand their approach by bringing together the often siloed and discrete critical analysis of AMR and TB.
This approach should integrate a stronger emphasis on social care and support, and the strengthening of health systems, aiming to mitigate the impact of antibiotic resistance. By doing so, we can better safeguard the strides made in combating diseases and ensure a more resilient response to the inevitable challenges posed by AMR, ultimately contributing to the global effort against infectious diseases.
Acknowledgements
Acknowledgement and thanks must be given to Professor Clare Chandler and Professor Alison Grant for their valuable insights and feedback throughout the course of this work. We would also like to express our appreciation to Yusuf Ras, the librarian at Stellenbosch University, who played a crucial role in facilitating our scoping review. This combined expertise has been instrumental in shaping this research and ensuring its relevance and impact.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
Notes
1 Although first used in South Africa in 1941 (Collins, Citation1982), the BCG vaccine was not embraced until de Villiers incorporated it into his short-term strategy. Prior to 1957, BCG had been used sparingly for nurses who had high exposure to the disease. After seeing BCGs success, the short-term strategy extended its use to children, with the aim of vaccinating them all before leaving school (de Villiers, Citation1957).
2 ‘Coloured’ is a racial classification in southern Africa and is composed primarily of people who were formally understood to be ‘mixed race’, but have since developed their own independent culture/ethnicity group. 'Coloured' is contentious. It was largely a lie invented to suit / support apartheid's hierarchy of races that coloured people are descendant from white farmers and their black labourers. Genetically, most coloured people are descendant from Khoi and San peoples who were resident in the Cape (and had held back the spread of black / bantu-speaking peoples from the north and east. And then there are also the descendants of south-east Asian (Malaysian and Indonesian mainly) slaves.
References
- Adams, V., Behague, D., Caduff, C., Löwy, I., & Ortega, F. (2019). Re-imagining global health through social medicine. Global Public Health, 14(10), 1383–1400. https://doi.org/10.1080/17441692.2019.1587639
- Alliance for Health Policy and Systems Research. (2017). Primary Health Care Systems Case Study from South Africa. World Health Organization, 1–12.
- Amrith, S. (2004). In search of a ‘Magic Bullet’ for tuberculosis: South India and Beyond, 1955–1965. Social History of Medicine, 17(1), 113–130. https://doi.org/10.1093/shm/17.1.113
- Andrews, J., Basu, S., Scales, D., Maru, D. S.-R., & Subbaraman, R. (2007). XDR-TB in South Africa: No time for denial or complacency. PLoS Medicine, 4(1), 0019–0025. https://doi.org/10.1371/journal.pmed.0040050
- Arksey, H., & O’Malley, L. (2005). Scoping studies: Towards a methodological framework. International Journal of Social Research Methodology: Theory and Practice, 8(1), 19–32. https://doi.org/10.1080/1364557032000119616
- Atwell, A. G., & Pearson, P. J. O. (1980). Routine outpatient short-course chemotherapy for pulmonary tuberculosis. A radiographic presentation. South African Medical Journal, 58(26), 1041–1046. https://doi.org/10.10520/AJA20785135_12088
- Ayles, H., Mureithi, L., & Simwinga, M. (2022). The state of tuberculosis in South Africa: What does the first national tuberculosis prevalence survey teach us? The Lancet Infectious Diseases, 22(8), 1094–1096. https://doi.org/10.1016/S1473-3099(22)00286-9
- Bateman, C. (2007). XDR-TB or not XDR-TB? That is the question. South African Medical Journal, 97(5), 318–322,318,320,320,322. https://doi.org/10.1016/j.meegid.2011.07.019
- Bell, J., & Yach, D. (1988). Tuberculosis patient compliance in the western Cape, 1984. South African Medical Journal, 73(1), 31–33. https://doi.org/10.10520/AJA20785135_8879
- Benatar, S. R. (1982). Tuberculosis in the 1980s, with particular reference to South Africa. South African Medical Journal, 62(11), 359–364. https://doi.org/10.10520/AJA20785135_7455
- Berhanu, R., Schnippel, K., Mohr, E., Hirasen, K., Evans, D., Rosen, S., & Sanne, I. (2016). Early outcomes of decentralized care for rifampicin-resistant tuberculosis in Johannesburg, South Africa: An observational cohort study. PLoS ONE, 11(11), https://doi.org/10.1371/journal.pone.0164974
- Biehl, J. (2007). Pharmaceuticalization: AIDS treatment and global health politics. Anthropological Quarterly, 80(4), 1083–1126. https://doi.org/10.1353/anq.2007.0056
- Bouton, T. C., de Vos, M., Ragan, E. J., White, L. F., van Zyl, L., Theron, D., Robert Horsburgh, C., Warren, R. M., & Jacobson, K. R. (2019). Switching to bedaquiline for treatment of rifampicin-resistant tuberculosis in South Africa: A retrospective cohort analysis. PLoS ONE, 14(10), 1–6. https://doi.org/10.1371/journal.pone.0223308
- Bowker, G. C., & Star, S. L. (1970). Sorting things out: Classification and its consequences. The American journal of nursing, 70(8), https://doi.org/10.2307/3421475
- Broom, A., Broom, J., Kirby, E., Gibson, A., & Davis, M. (2017). Health & place antibiotic optimisation in ‘ the bush ‘ : Local know-how and core–periphery relations. Health & Place, 48, 56–62. https://doi.org/10.1016/j.healthplace.2017.09.003
- Broom, A., Kenny, K., Prainsack, B., & Broom, J. (2021). Antimicrobial resistance as a problem of values? Views from three continents. Critical Public Health, 31(4), 451–463. https://doi.org/10.1080/09581596.2020.1725444
- Bud, R. (2007). Penicillin: triumph and tragedy. Oxford University Press.
- Chandler, C. I. R. (2019). Current accounts of antimicrobial resistance: Stabilisation, individualisation and antibiotics as infrastructure. Palgrave Communications, 5(53), 15–17. https://doi.org/10.1057/s41599-019-0263-4
- Chiang, C. Y., Chiang, C. Y., Chiang, C. Y., Trébucq, A., Piubello, A., Rieder, H. L., Rieder, H. L., Schwoebel, V., van Deun, A., & van Deun, A. (2020). The looming threat of bedaquiline resistance in tuberculosis. European Respiratory Journal, 55(6), 3–5. https://doi.org/10.1183/13993003.00718-2020
- Cluver, F. W. P. (1965). The scourge of tuberculosis. South African Medical Journal, 39(9), 181–182. https://journals.co.za/doi/epdf/10.10520AJA20785135_37871
- Coetzee, G., & Koornhof, H. (2006). MDR / XDR tuberculosis in South Africa. Southern African Journal of Epidemiology and Infection, 21(3), 150–151. https://doi.org/10.10520/EJC80747
- Collins, T. F. (1972). The new approach to tuberculosis. South African Medical Journal, 46(10), 260–261. https://doi.org/10.10520/AJA20785135_31877
- Collins, T. F. (1982). The history of southern Africa’s first tuberculosis epidemic. South African Medical Journal, 62(21), 780–788. https://doi.org/10.10520/AJA20785135_7579
- Comaroff, J. (2007). Beyond Bare Life: AIDS, (Bio)Politics, and the Neoliberal Order. Public Culture, 19(1), 197–219. doi:10.1215/08992363-2006-030
- Conradie, F., Meintjes, G., Hughes, J., Maartens, G., Ferreira, H., Siwendu, S., Master, I., & Ndjeka, N. (2014). Clinical access to Bedaquiline Programme for the treatment of drug-resistant tuberculosis. South African Medical Journal, 104(3), 164–166. https://doi.org/10.7196/samj.7263
- Cooper, E. D. (1954). The domiciliary treatment of pulmonary tuberculosis as a public service. South African Medical Journal, 28(10), 1954. https://journals.co.za/doi/10.10520AJA20785135_30257
- Coovadia, H., Jewkes, R., Barron, P., Sanders, D., & Mcintyre, D. (2009). The health and health system of South Africa: historical roots of current public health challenges. The Lancet, 374(9692), 817–834. https://doi.org/10.1016/S0140-6736(09)60951-X
- Cox, H., Dickson-Hall, L., Jassat, W., Moshabela, M., Kielman, K., Grant, A., Nicol, M., Black, J., Mlisana, K., Vanleeuw, L., & Loveday, M. (2017a). Drug-resistant tuberculosis in South Africa: History, progress and opportunities for achieving universal access to diagnosis and effective treatment. South African Health Review, 20, 157–168.
- Cox, J. A., Vlieghe, E., Mendelson, M., Wertheim, H., Ndegwa, L., Villegas, M. V., Gould, I., & Levy Hara, G. (2017b). Antibiotic stewardship in low- and middle-income countries: The same but different? Clinical Microbiology and Infection, 23(11), 812–818. https://doi.org/10.1016/j.cmi.2017.07.010
- Crowhurst Archer, B. (1960). The psychiatric approach to rehabilitation. South African Medical Journal, 34(3), 242–244. https://doi.org/10.10520/AJA20785135_41438
- de Lange, M. (2006). Tuberculosis and the emergence of drug resistance. Professional Nursing Today, 10(5), 3–5.
- Denyer Willis, L., & Chandler, C. (2019). Quick fix for care, productivity, hygiene and inequality: Reframing the entrenched problem of antibiotic overuse. BMJ Case Reports, 4(4), 1–6. https://doi.org/10.1136/bmjgh-2019-001590
- de Villiers, J. P. (1957). The Tuberculosis problem in South Africa in the light of recent advances. South African Medical Journal, 31(50), 1274–1277. https://journals.co.za/doi/epdf/10.10520AJA20785135_44814
- Dixon, J., Manyau, S., Kandiye, F., Kranzer, K., Clare, I., & Chandler, R. (2021). Antibiotics, rational drug use and the architecture of global health in Zimbabwe. Social Science & Medicine, 272, 113594. https://doi.org/10.1016/j.socscimed.2020.113594
- Dormer, B. A. (1960). Tuberculosis. South African Medical Journal, 34(15), 291–294. https://doi.org/10.10520/AJA20785135_41586
- Dormer, B. A., & Salinger, P. L. (1971). A comparative trial with combinations of rifampicin, ethambutol and isoniazid (hydronsan) in previously untreated cases of pulmonary tuberculosis. South African Medical Journal, 45(25), 697–699. https://doi.org/10.10520/AJA20785135_31334
- Escreet, B. C., & Cowie, R. L. (1981). Short-course chemotherapy for pulmonary tuberculosis - a 100-day interrupted regimen. South African Medical Journal, 60(25), 951–955. https://doi.org/10.10520/AJA20785135_14165
- Evans, D., Sineke, T., Schnippel, K., Berhanu, R., Govathson, C., Black, A., Long, L., Rosen, S., EvansDenise, S. T., Schnippel, K., Berhanu, R., Govathson, C., Black, A., Long, L., Rosen, S., Evans, D., Sineke, T., Schnippel, K., … Rosen, S. (2018). Impact of Xpert MTB/RIF and decentralized care on linkage to care and drug-resistant tuberculosis treatment outcomes in Johannesburg, South Africa. BMC Health Services Research, 18(1), 973. https://doi.org/10.1186/s12913-018-3762-x
- Farmer, P. (1997). Social scientists and the new tuberculosis. Social Science & Medicine (1982), 44(3), 347–358. doi:10.1016/S0277-9536(96)00143-8
- Fatti Crawshaw, V. H., Reginald, G. R., & Wilson, H. V. (1950). Pulmonary tuberculosis : The results of treatment at Baragwanath Non-European Hospital. South African Medical Journal, 24(51), 1065–1069. https://doi.org/10.10520/AJA20785135_25810
- Ferguson, D. L. (1960). Health Education in South Africa. South African Medical Journal, 34(3), 263–264.
- Foster, K. E. (2005). Clinics, communities, and cost recovery: Primary health care and neoliberalism in postapartheid South Africa. Cultural Dynamics, 17(3), 239–266. https://doi.org/10.1177/0921374005061990
- Fox, W., Ellard, G. A., & Mitchison, D. A. (1999). Studies on the treatment of tuberculosis undertaken by the British Medical Research Council Tuberculosis Units, 1946-1986, with relevant subsequent publications. The International Journal of Tuberculosis and Lung Disease, 3(2), S231–S279(49).
- Geffen, N. (2016). Anything to stay alive: The challenges of a campaign for an experimental drug. Developing World Bioethics, 16(1), 45–54. https://doi.org/10.1111/dewb.12084
- Gordon, T., Booysen, F., & Mbonigaba, J. (2020). Socio-economic inequalities in the multiple dimensions of access to healthcare: The case of South Africa. BMC Public Health, 20(1), 1–13. https://doi.org/10.1186/s12889-020-8368-7
- Gradmann, C. (2019). Treatment on trial: Tanzania’s national tuberculosis program, the international union against Tuberculosis and Lung Disease, and the Road to DOTS, 1977-1991. Journal of the History of Medicine and Allied Sciences, 74(3), 316–343. https://doi.org/10.1093/JHMAS/JRZ029
- Griffiths, M. L., & Makgothi, M. M. (1981). Tuberculosis management in a rural community - factors in failure. South African Medical Journal, 59(1), 14–16. https://doi.org/10.10520/AJA20785135_13526
- Grusin, H. (1966). The results of treatment of pulmonary tuberculosis among Africans. South African Medical Journal, 40(26), 617–620. https://doi.org/10.10520/AJA20785135_37386
- Harrison, D. (1993). The National Health Services Conunission, 1942-1944-its origins and outcome. South African Medical Journal, 83, 679–684.
- Holland, M. D. (1972). Rifampicin - what dose for pulmonary tuberculosis? South African Medical Journal, 46(20), 604–608.
- Human, S. P., Smith, J. E., & Tshabalala, D. L. (2010). Factors influencing tuberculosis treatment interruptions. Africa Journal of Nursing and Midwifery, 12(2), 48–57. https://doi.org/10.10520/EJC19353
- IACG (Interagency Coordination Group on Antimicrobial Resistance). (2019). No Time to Wait: Securing the Future from Drug-Resistant Infections: Report to the Secretary- General of the United Nations.
- Johnson & Johnson. (2023). Safeguarding medicines for multidrug-resistant tuberculosis (MDR-TB) treatment.
- Jones, K. D. J., Hesketh, T., & Yudkin, J. (2008). Extensively drug-resistant tuberculosis in sub-Saharan Africa: an emerging public-health concern. Transactions of the Royal Society of Tropical Medicine and Hygiene, 102(3), 219–224. https://doi.org/10.1016/j.trstmh.2007.11.014
- Kandel, T. R., Mfenyana, K., Chandia, J., & Yogeswaran, P. (2008). The prevalence of and reasons for interruption of anti-tuberculosis treatment by patients at Mbekweni Health Centre in the King Sabata Dalindyebo (KSD) District in the Eastern Cape province : original research. South African Family Practice, 50(6), 47. doi:10.1080/20786204.2008.10873785
- Kent, P. (1982). The relevance of short-course tuberculosis chemotherapy to the African situation. South African Medical Journal, 62, 8–12. https://doi.org/10.10520/AJA20785135_7410
- London, L. (2009). Confinement for extensively drug-resistant tuberculosis: Balancing protection of health systems, individual rights and the public’s health. International Journal of Tuberculosis and Lung Disease, 13(10), 1200–1209.
- Loveday, M., & Cox, H. (2013). Carpe diem (‘Seize the day’): Building on the findings of the 2015 World Health Organization evaluation of the multidrug-resistant tuberculosis (MDR-TB) programme to make the most of shortened MDR-TB treatment in South Africa. South African Medical Journal, 107(3), 299–307. https://doi.org/10.5588/ijtld.12.0537
- Lönnroth, K., Jaramillo, E., Williams, B. G., Dye, C., & Raviglione, M. (2009). Drivers of tuberculosis epidemics: The role of risk factors and social determinants. Social Science and Medicine, 68(12), 2240–2246. https://doi.org/10.1016/j.socscimed.2009.03.041
- Lu, X., Smare, C., Kambili, C., el Khoury, A. C., & Wolfson, L. J. (2017). Health outcomes of bedaquiline in the treatment of multidrug-resistant tuberculosis in selected high burden countries. BMC Health Services Research, 17(1), 87. https://doi.org/10.1186/s12913-016-1931-3
- Mabin, A. (1992). Comprehensive segregation: The origins of the group areas act and its planning apparatuses. Journal of Southern African Studies, 18(2), 405–429. https://doi.org/10.1080/03057079208708320
- MacPherson, E. E., Reynolds, J., Sanudi, E., Nkaombe, A., Phiri, C., Mankhomwa, J., Dixon, J., & Chandler, C. I. R. (2022). Understanding antimicrobial resistance through the lens of antibiotic vulnerabilities in primary health care in rural Malawi. Global Public Health, 2630–2646. https://doi.org/10.1080/17441692.2021.2015615
- Marais, D. P. (1960). Whither Tuberculosis. South African Medical Journal, 34(6), 509–511. https://doi.org/10.10520/AJA20785135_41205
- María Jesús Santesmases. (2017). The Circulation of Penicillin in Spain: Health, Wealth and Authority. Palgrave Macmillan. http://www.palgrave.com/gp/series/15183.
- McMillen, C. (2015). Tuberculosis: A global history, 1900 to present. Yale University Press.
- Monnais, L. (2019). The colonial life of pharmaceuticals: Medicines and modernity in Vietnam. Cambridge University Press. https://doi.org/10.1017/9781108567152
- Monnais, L., & Tousignant, N. (2006). The colonial life of pharmaceuticals: Accessibility to healthcare, consumption of medicines, and medical pluralism in French Vietnam, 1905––1945. Journal of Vietnamese Studies, 1(1–2), 131–166. https://doi.org/10.1525/vs.2006.1.1-2.131
- Ndjeka, N., Conradie, F., Schnippel, K., Hughes, J., Bantubani, N., Ferreira, H., Maartens, G., Mametja, D., Meintjes, G., Padanilam, X., Variava, E., Pym, A., & Pillay, Y. (2015). Treatment of drug-resistant tuberculosis with bedaquiline in a high HIV prevalence setting: An interim cohort analysis. The International Journal of Tuberculosis and Lung Disease : The Official Journal of the International Union against Tuberculosis and Lung Disease, 19(8), 979–985. https://doi.org/10.5588/ijtld.14.0944
- Nguyen, V.-K. (2010). The republic of therapy: Triage and Sovereignty in West Africa’s Time of AIDS. Duke University Press.
- Njaramba, P., & Naidoo, S. (2007). Managing multidrug-resistant tuberculosis in hospitalised patients: A review of treatment outcomes. Southern African Journal of Epidemiology and Infection, 22(2), 39–44. https://doi.org/10.1080/10158782.2007.11441283
- Olayanju, O., Limberis, J., Esmail, A., Oelofse, S., Gina, P., Pietersen, E., Fadul, M., Warren, R., & Dheda, K. (2018). Long-term bedaquiline-related treatment outcomes in patients with extensively drug-resistant tuberculosis from South Africa. European Respiratory Journal, 51(5), 1800544. https://doi.org/10.1183/13993003.00544-2018
- O’Neill, J. (2016). Tackling Drug-Resistant Infections Globally: Final Report and Recommendations.
- Packard, R. (1989). White plague, black labor: Tuberculosis and the political economy of health and disease in South Africa. University of California Press.
- Packard, R. (2016a). A history of global health: Interventions into the lives of other peoples. Johns Hopkins Press.
- Packard, R. (2016b). Back to the future: From international to global health. In A History of Global Health (pp. 267–328). Johns Hopkins University Press.
- Palanco Lopez, P., Manyau, S., Dixon, J., Nayiga, S., Manton, J., Kirchhelle, C., & & Chandler, C. I. R. (2022). Antibiotic arrivals in Africa: A case study of yaws and syphilis in Malawi, Zimbabwe and Uganda. Medicine Anthropology Theory, 9(3), 1–31. doi:10.17157/mat.9.3.5633
- Pearson, J. O. (1977). The practical benefits of updated medical control of pulmonary tuberculosis. South African Medical Journal, 52(13), 510. https://journals.co.za/doi/pdf/10.10520AJA20785135_18082
- Pearson, M., & Chandler, C. (2019). Knowing antmicrobial resistance in practice: A multi-country qualitative study with human and animal healthcare professionals. Global Health Action, 12(1), 1599560. https://doi.org/10.1080/16549716.2019.1599560
- Phillips, H. (2014). The return of the pholela experiment: Medical history and primary health care in post-apartheid South Africa. American Journal of Public Health, 104(10), 1872–1876. https://doi.org/10.2105/AJPH.2014.302136
- Podolsky, S. H. (2015). The antibiotic era: Reform, resistance, and the pursuit of a rational therapeutics. Journal of Chemical Information and Modeling, 53(9).
- Ponthieu, A., & Incerti, A. (2016). Continuity of care for migrant populations in Southern Africa. Refugee Survey Quarterly, 35(2), 98–115. https://doi.org/10.1093/rsq/hdw006
- Poots, E. (2012). Strategy for Tackling Antimicrobial Resistance (STAR) 2012-2017.
- Prince, R. J., & Marsland, R. (2013). Making and unmaking public health in Africa: Ethnographic and historical perspectives. Ohio University Press. https://books.google.co.uk/books?id=UvnonQEACAAJ
- Salinger, P. L., & Dormer, B. (1972). Rifampicin, ethambutol, ethionamide and hydronsan in advanced pulmonary tuberculosis. South African Medical Journal, 46(13), 354–358. https://doi.org/10.10520/AJA20785135_31735
- Saville-Lewis, J. (1953). Tuberculosis in East London : With particular reference to the Santa tuberculosis settlement, Fort Grey, and its relation to tuberculosis control. South African Medical Journal, 27(44), 980–983. https://journals.co.za/doi/10.10520AJA20785135_29055
- Snyman, L., Venables, E., Trivino Duran, L., Mohr, E., Azevedo, V. D., Harmans, X., & Isaakidis, P. (2018). “I didn’t know so many people cared about me”: Support for patients who interrupt drug-resistant TB treatment. The International Journal of Tuberculosis and Lung Disease : The Official Journal of the International Union against Tuberculosis and Lung Disease, 22(9), 1023–1030. https://doi.org/10.5588/ijtld.17.0826
- StatsSA. (2018). Mid-year population estimates. In StatsSA.
- The Joanna Briggs Institute. (2015). The Joanna Briggs Institute Reviewers’ Manual 2015: Methodology for JBI scoping reviews. Joanne Briggs Institute, 1–24. www.joannabriggs.org.
- Van Rensburg, D., Janse van Rensburg-Bonthuyzen, E., Heunis, C., & Meulemans, H. (2005). Tuberculosis control in South Africa: Reasons for persistent failure. Acta Academica, 2005(1), 1–55. https://doi.org/10.10520/EJC15071
- Weyer, K., & Stander, M. F. (1996). Multidrug resistant tuberculosis in South Africa. The Lancet, 348(9042), 1658. doi:10.1016/S0140-6736(05)65720-0
- Whitehouse, A. B. (1980). Modern management of tuberculosis in a rural area. South African Medical Journal, 58(17), 695–696. https://journals.co.za/doi/epdf/10.10520AJA20785135_16345
- WHO. (2014). Antimicrobial Resistance: Global Report of Surveillance. https://doi.org/10.1016/j.giec.2020.06.004
- WHO. (2015). Global action plan on antimicrobial resistance. Microbe Magazine, 10(9), 354–355. https://doi.org/10.1128/microbe.10.354.1
- WHO. (2020). WHO consolidated guidelines on tuberculosis. Module 4: treatment - drug-resistant tuberculosis treatment. Online annexes. In Who.
- WHO. (2022a). Global Tuberculosis Report 2022. In Geneva: World Health organization.
- WHO. (2022b). WHO consolidated guidelines on tuberculosis Module 4: Treatment Drug-resistant tuberculosis treatment 2022 update.
- Wilkinson, A., Ebata, A., & Macgregor, H. (2018). Interventions to reduce antibiotic prescribing in LMICs: A scoping review of evidence from human and animal health systems. Antibiotics, 8(1), https://doi.org/10.3390/antibiotics8010002
- Wilkinson, D., & de Cock, K. M. (1996). Tuberculosis control in South Africa - time for a new paradigm? South African Medical Journal, 86(1), 33–35.
- Wilkinson, D., Pillay, M., Davies, G. R., & Sturm, A. W. (1996). Resistance to antituberculosis drugs in rural South Africa: Rates, patterns, risks, and transmission dynamics. Transactions of the Royal Society of Tropical Medicine and Hygiene, 90(6), 692–695. https://doi.org/10.1016/S0035-9203(96)90440-X
- Wise, J. (2006). Southern Africa is moving swiftly to combat the threat of XDR-TB. Bulletin of the World Health Organization, 84(12), 924–925.
- Wood, R., Lawn, S. D., Johnstone-Robertson, S., & Bekker, L. G. (2011). Tuberculosis control has failed in South Africa - time to reappraise strategy. South African Medical Journal, 101(2), 111–114. https://doi.org/10.7196/SAMJ.4587
- World Bank Group. (2021). Antimicrobial Resistance (AMR). World Bank Brief. https://www.worldbank.org/en/topic/health/brief/antimicrobial-resistance-amr.
- Yeats, J. R. (1986). Attendance compliance for short-course tuberculosis chemotherapy at clinics in Estcourt and surroundings. South African Medical Journal, 70(2), 265–266. https://doi.org/10.10520/AJA20785135_3607
- Zabow, M., & Pearson, J. O. (1982). Short-course chemotherapy for pulmonary tuberculosis - points of interest. South African Medical Journal, 61(23), 867–870. https://doi.org/10.10520/AJA20785135_14684
Image references
- 1. T. F. Collins, “The New Approach to Tuberculosis.,” South African Medical Journal 46, no. 10 (March 1, 1972): 260–61; Wood, R., Bekker, L.G., 2017. An epidemic uncurbed: tuberculosis in Cape Town, South Africa, 1910–2010. Transactions of the Royal Society of South Africa 72, 234–241.
- 2. Van Rensburg, D., Janse van Rensburg-Bonthuyzen, E., Heunis, C., Meulemans, H., 2005. Tuberculosis control in South Africa: reasons for persistent failure. Acta Academica 2005, 1–55.
- 3. Van Rensburg, D., Janse van Rensburg-Bonthuyzen, E., Heunis, C., Meulemans, H., 2005. Tuberculosis control in South Africa: reasons for persistent failure. Acta Academica 2005, 1–55.
- 4. Collins, T.F.B., 1982. The history of southern Africa’s first tuberculosis epidemic. South African Medical Journal 62, 780–788.
- 5. Medical Research Council, 1953. Isoniazid in the Treatment of Pulmonary Tuberculosis. South African Medical Journal 27, 461–462.
- 6. Medical Research Council, 1953. Isoniazid in the Treatment of Pulmonary Tuberculosis. South African Medical Journal 27, 461–462.
- 7. de Villiers, J.P., 1957. The Tuberculosis problem in South Africa in the light of recent advances. South African medical journal 31, 1274–1277.
- 8. de Villiers, J.P., 1957. The Tuberculosis problem in South Africa in the light of recent advances. South African medical journal 31, 1274–1277.
- 9. Van Rensburg, D., Janse van Rensburg-Bonthuyzen, E., Heunis, C., Meulemans, H., 2005. Tuberculosis control in South Africa: reasons for persistent failure. Acta Academica 2005, 1–55.
- 10. Collins, T.F.B., 1982. The history of southern Africa’s first tuberculosis epidemic. South African Medical Journal 62, 780–788.
- 11. Cox, Helen., Dickson-Hall, L., Jassat, W., Moshabela, M., Kielman, K., Grant, A., Nicol, M., Black, J., Mlisana, K., Vanleeuw, L., Loveday, M., 2017. Drug-resistant tuberculosis in South Africa: history, progress and opportunities for achieving universal access to diagnosis and effective treatment. S Afr Health Rev 20, 157–168.
- 12. Cox, Helen., Dickson-Hall, L., Jassat, W., Moshabela, M., Kielman, K., Grant, A., Nicol, M., Black, J., Mlisana, K., Vanleeuw, L., Loveday, M., 2017. Drug-resistant tuberculosis in South Africa: history, progress and opportunities for achieving universal access to diagnosis and effective treatment. S Afr Health Rev 20, 157–168; Dormer, B.A., Salinger P L, 1971. A comparative trial with combinations of rifampicin, ethambutol and isoniazid (hydronsan) in previously untreated cases of pulmonary tuberculosis. South African Medical Journal 45, 697–699; Holland, M.D., 1972. Rifampicin - what dose for pulmonary tuberculosis? South African Medical Journal 46, 604–608.
- 13. Escreet, B.C., Cowie R. L., 1981. Short-course chemotherapy for pulmonary tuberculosis - a 100-day interrupted regimen. South African Medical Journal 60, 951–955; Zabow, M., Pearson J O, 1982. Short-course chemotherapy for pulmonary tuberculosis - points of interest. South African Medical Journal 61, 867–870.
- 14. Collins, T.F., 1972. The new approach to tuberculosis. South African Medical Journal 46, 260–261.
- 15. Collins, T.F., 1972. The new approach to tuberculosis. South African Medical Journal 46, 260–261.
- 16. Glatthaar, E., 1982. Tuberculosis control in South Africa: Where have we gone wrong? and a look into the future. South African Medical Journal 62, 36–41.
- 17. Collins, T.F.B., 1982. The history of southern Africa’s first tuberculosis epidemic. South African Medical Journal 62, 780–788.
- 18. Durrheim, D.N., Belt, E.L., 1996. Tuberculosis control programme guidelines--treatment regimens. South African Medical Journal 86, 1293.
- 19. Schnippel, K., Rosen, S., Shearer, K., Martinson, N., Long, L., Sanne, I., Variava, E., 2013. Costs of inpatient treatment for multi-drug-resistant tuberculosis in South Africa. Trop Med Int Health 18, 109–116.
- 20. Bateman, C., 2015. Tugela Ferry’s extensively drug-resistant tuberculosis--10 years on. S Afr Med J.
- 21. London, L., 2009. Confinement for extensively drug-resistant tuberculosis: Balancing protection of health systems, individual rights and the public’s health. International Journal of Tuberculosis and Lung Disease 13, 1200–1209; Sakoane, R., 2007. XDR-TB in South Africa: Back to TB Sanatoria Perhaps? 4.
- 22. Durrheim, D.N., Belt, E.L., 1996. Tuberculosis control programme guidelines--treatment regimens. South African Medical Journal 86, 1293.
- 23. Berhanu, R., Schnippel, K., Mohr, E., Hirasen, K., Evans, D., Rosen, S., Sanne, I., 2016. Early outcomes of decentralized care for rifampicin-resistant tuberculosis in Johannesburg, South Africa: An observational cohort study. PLoS One 11.
- 24. Conradie, F., Meintjes, G., Hughes, J., Maartens, G., Ferreira, H., Siwendu, S., Master, I., Ndjeka, N., 2014. Clinical access to Bedaquiline Programme for the treatment of drug-resistant tuberculosis. South African Medical Journal 104, 164–166; Ndjeka, N., Hughes, J., Reuter, A., Conradie, F., Enwerem, M., Ferreira, H., Ismail, N., Kock, Y., Master, I., Meintjes, G., Padanilam, X., Romero, R., Schaaf, H.S., Riele, J. Te, Maartens, G., 2020. Implementing novel regimens for drug-resistant TB in South Africa: what can the world learn? Int J Tuberc Lung Dis 24, 1073–1080.
- 25. Ndjeka, N., Hughes, J., Reuter, A., Conradie, F., Enwerem, M., Ferreira, H., Ismail, N., Kock, Y., Master, I., Meintjes, G., Padanilam, X., Romero, R., Schaaf, H.S., Riele, J. Te, Maartens, G., 2020. Implementing novel regimens for drug-resistant TB in South Africa: what can the world learn? Int J Tuberc Lung Dis 24, 1073–1080.