?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
A growing body of evidence has shown the effects of poor preconception health on adverse pregnancy outcomes and, subsequently, maternal and child morbidity and mortality. However, the cost of poor preconception health remains relatively unexplored. Using the case of Nigeria, this study provides the first estimate of the disease and economic burden of poor preconception health at a country level. Using data from international databases and the scientific literature, the study used a cost-of-illness approach to quantify the foregone productivity and direct healthcare costs resulting from six preconception risk factors (adolescent pregnancy, short birth interval, overweight and obesity, intimate partner violence, female genital mutilation, folate deficiency). The results indicate that 6.7% of maternal deaths, 10.9% of perinatal deaths, and 10.5% of late neonatal deaths were attributable to the selected preconception risk factors in 2020. The economic burden of poor preconception health in Nigeria was estimated at US$ 3.3 billion in 2020, of which over 90% was generated by premature mortality. If prevalence rates remain constant, total economic losses could amount to US$ 46.2 billion by 2035. This analysis paves the way for further studies investigating the economic costs and benefits of preconception interventions and policies in low and middle-income countries.
Introduction
Over the past two decades, numerous studies and initiatives have outlined the potential of preconception care as a means to reduce maternal and neonatal mortality and morbidity (Berghella et al., Citation2010; Berglund & Lindmark, Citation2016; Dorney & Black, Citation2018; Mason et al., Citation2014; Stephenson et al., Citation2018). While pregnancy-related complications are mainly observed during gestation, it is essential to acknowledge that certain conditions develop before conception, which, if not adequately addressed beforehand, may lead to complications during the pregnancy period. By addressing the risk factors responsible for adverse maternal and child health outcomes before pregnancy, preconception care strives to optimise the health status of women, ultimately contributing to healthier pregnancies and improved long-term health for both mothers and their children (Fleming et al., Citation2018).
The World Health Organization defined preconception care as
the provision of biomedical, behavioural and social health interventions to women and couples before conception occurs, aimed at improving their health status and reducing behaviours and individual and environmental factors that could contribute to poor maternal and child health outcomes. (World Health Organization, Citation2013)
Research on preconception care effectiveness has shown promising results, leading researchers and international organisations to call for better integration of preconception care within strategies to prevent maternal and childhood mortality (Atrash et al., Citation2006; Berglund & Lindmark, Citation2016; Dorney & Black, Citation2018; Johnson et al., Citation2008; World Health Organization, Citation2013). However, the overall adoption and implementation of such initiatives have been limited in practice (Dorney & Black, Citation2018). While preconception care has gained increasing attention, the development of comprehensive and integrated strategies is impeded by multiple barriers, notably the lack of comprehensive data on the burden that poor preconception health places on individuals and societies. This absence of understanding encompasses not only the health burden associated with poor preconception care but also the substantial economic burden stemming from the resulting productivity losses, healthcare expenses, and diminished quality of life (Grosse et al., Citation2006). Yet, access to economic data is crucial to garnering public and healthcare professional support and influencing decision-makers to allocate resources to preconception care.
A recent scoping review indicated that the existing economic evaluations, albeit limited in number, tend to support the cost-effectiveness of preconception care (Kotirum et al., Citation2021). However, these evaluations primarily focused on a narrow range of interventions and were predominantly conducted in high-income countries. Investigating the cost-effectiveness of a comprehensive package of preconception care interventions, rather than individual interventions, is essential to determine the economic value of preconception care in the largest possible way. Also, evaluations focusing on low-resource settings are of particular interest since the impact of many preconception interventions is assumed to be more significant in countries where maternal and child mortality is high (Kotirum et al., Citation2021).
This economic impact analysis is the first of a series exploring the costs and benefits of preconception care in low and middle-income countries. Its primary aim is to estimate the disease and economic burden associated with six preconception risk factors, using the case of Nigeria. A secondary aim is to quantify the contribution of the selected preconception risk factors to the overall burden of maternal, perinatal and child mortality or morbidity. Thirdly, the study aims to determine what pathways and preconception risk factors constitute the main driving forces behind the economic burden of poor preconception health.
To our knowledge, this paper is the first that aims to estimate the productivity losses and direct healthcare costs attributable to a set of preconception risk factors at a country level. It constitutes a starting point for researchers and decision-makers to understand better the health and economic costs of inaction towards improving women of reproductive age’s health while paving the way for an investment case on preconception care in low and middle-income countries.
Materials and methods
Approach
This study used a prevalence-based cost-of-illness approach (Jo, Citation2014) to estimate economic losses resulting from six preconception risk factors between 2020 and 2035 in Nigeria. We estimated economic losses through three key pathways: (1) the foregone productivity due to premature mortality, (2) the foregone productivity due to child cognition deficit, and (3) the direct healthcare costs. We used the human capital approach to estimate the productivity losses associated with the selected preconception risk factors. This approach is based on the assertion that health influences individuals’ ability to work, earn income, and contribute to the economy (Robinson, Citation1993). For estimating the direct healthcare costs, we employed a combination of cost estimation methods. For conditions where per-patient average cost data were readily accessible, we used the unit cost method. On the other hand, in cases where per-patient average costs were not directly available, we adopted the cost ingredient method to estimate expenses by itemising and valuing each component of the intervention.
The economic burden was projected from the baseline year of 2020 across three timeframes: 6 years (2020–2025), 11 years (2020–2030), and 16 years (2020–2035). We used population and fertility projections from UN World Population Prospects to reflect Nigeria’s future demographic and birth pattern changes (United Nations, Citation2022). The level of exposure to the selected preconception risk factors, as well as morbidity and mortality indicators, were maintained at the baseline level. This approach simulates a hypothetical ‘status quo’ scenario, assuming no new interventions or improvements throughout the selected timeframe. A discount rate of 3% was applied to calculate the net present value of the economic losses at baseline, thereby reflecting the time value of money. Further details on the model’s parameters and data sources are provided in the supplemental materials.
Conceptual framework
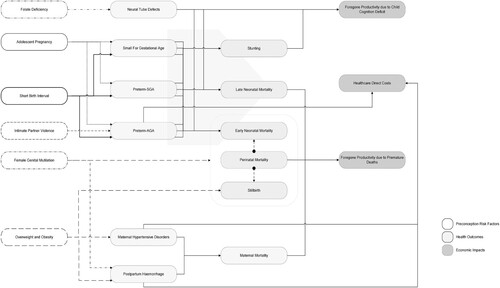
We developed a conceptual framework that delineates the intricate pathways through which a selected set of six preconception risk factors may influence various pregnancy, maternal and child outcomes (). This framework, informed by the results of a scoping review and causal loop diagram study conducted by the authors (Poix & Elmusharaf, Citation2023), served as the foundation for this analysis.
We defined a preconception risk factor as any risk factor associated with adverse maternal, perinatal and neonatal outcomes whose effects could be eliminated or mitigated by acting before pregnancy starts. Based on this definition, we included the following preconception risk factors: (1) adolescent pregnancy, (2) short birth interval, (3) folate deficiency, (4) overweight and obesity, (5) intimate partner violence, and (6) female genital mutilation (). The criteria for inclusion were the availability of (i) prevalence rates from the literature or relevant datasets and (ii) access to risk estimates linking each preconception risk factor to at least one adverse health outcome.
Table 1. Description of the preconception risk factors included in the analysis.
The health outcomes included in the model encompassed a broad spectrum of pregnancy, maternal and child health issues, including (1) preterm birth with appropriate for gestational age, (2) preterm birth with small-for-gestational-age, (3) small-for-gestational-age, (4) neural tube defects, (5) stunting, (6) perinatal mortality, (7) neonatal mortality, (8) maternal hypertensive disorders, (9) postpartum haemorrhage, and (10) maternal mortality. The health outcomes were selected based on (i) their measurability and (ii) the access to risk estimates that establish direct or indirect associations with the identified preconception risk factors.
Population-attributable fraction (PAF) estimation method
The population-attributable fraction (PAF) is a common epidemiological measure that quantifies the proportion by which a specific outcome within a population could be reduced if certain risk factors were either eliminated or shifted to a lower-risk category. In this study, we employed the PAF to assess the contribution of the selected preconception risk factors to the health outcomes mentioned above.
Following the methodology proposed by Bryce et al. (Citation2022), we calculated PAFs using a multi-step process that accounts for the influence of multiple risk factors simultaneously. First, we derived relative risks from high-quality meta-analyses linking the selected preconception risk factors and health outcomes. In two meta-analyses, risk relationships were reported in the form of odds ratios (Nesari et al., Citation2018; Vats et al., Citation2021). Since relative risks are required for PAF estimation, we converted the odds ratios from these meta-studies into relative risks using the formula developed by Zhang and Yu (Citation1998):
Where RR is the relative risk, OR is the odds ratio, and P0 is the prevalence of the outcome of interest in the non-exposed group.
In the conversion process from odds ratios to relative risks, we encountered the challenge of not having direct access to P0, as data was derived from a meta-analysis. To approximate its value, we employed a multi-step approach. This involved retrieving P0 and the sample size from each original study included in the meta-analysis. We then calculated the sample size-weighted average of P0 across all these studies when available. Additionally, we ensured the consistency of P0 estimates across studies by verifying that they consistently fell within a comparable range. This validation step was crucial to address the challenge of missing or unreported P0 values in some studies while maintaining the internal consistency of our estimates.
In the subsequent step, we utilised Levin’s formula to estimate the individual PAFs for each preconception risk factor (Levin, Citation1953). These individual PAFs represent the fraction of adverse outcomes attributable to a specific risk factor when considered in isolation.
Where PAFu is the individual PAF, RR is the relative risk, and Pe is the prevalence of the risk factor.
We then computed a combined PAF that accounts for the presence of multiple preconception risk factors simultaneously.
Where PAFt is the combined PAF, and PAFu is the individual PAF.
Finally, we used the following formula to normalise these individual PAFs. The normalisation process adjusts the individual PAFs proportionally, ensuring that their combined effect accurately reflects the joint contribution of all risk factors to the health outcome:
Where PAFa is the normalised PAF, PAFu is the individual PAF, and PAFt is the combined PAF.
Due to the lack of risk estimates in the form of relative risks or odds ratios, we could not employ this method to assess the fraction of maternal deaths attributable to pre-pregnancy overweight and obesity and female genital mutilation. To address this issue, we used an alternative approach. First, we extracted the percentage of maternal deaths attributed to postpartum haemorrhage and maternal hypertensive disorders from the literature (Say et al., Citation2014). Then, we multiplied this percentage by the PAF of postpartum haemorrhage and maternal hypertensive disorders associated with the two related preconception risk factors. For instance, considering that 15.0% of maternal deaths were attributable to postpartum haemorrhage and 2.9% of postpartum haemorrhage episodes were attributable to pre-pregnancy overweight, we assumed that 0.4% of maternal deaths were attributable to pre-pregnancy overweight. This alternative approach allowed us to approximate the contribution of these two preconception risk factors to maternal mortality.
Finally, the PAF of neural tube defects associated with folate deficiency was not calculated but was directly obtained from Moench-Pfanner et al. (Citation2016). Due to the many challenges in accessing accurate and relevant data on folate deficiency during the preconception period, we made the decision to use an estimate of 72.0%, which had already been tested in a previous model.
Foregone productivity due to maternal mortality attributable to the preconception risk factors
The foregone productivity due to maternal mortality is the economic valuation of the potential years of working life lost due to maternal deaths attributable to preconception risk factors. These productivity losses represent the potential contribution deceased women would have made to the economy if they had not died. The net present value of these economic losses was calculated as follows:
Where Di is the maternal deaths attributable to the selected preconception risk factors in a 5-year age group i, LFPR is the labour force participation rate, A is the midpoint age of the 5-year age i, R is the retirement age, GDPW is the GDP per worker, r is the discount rate.
Foregone productivity due to perinatal and neonatal mortality attributable to the preconception risk factors
The foregone productivity due to perinatal and neonatal mortality represents the cumulative economic contribution that individuals would have made to the economy if they had survived into adulthood and participated in the labour force. The net present value of these productivity losses was calculated as follows:
Where D is the perinatal and neonatal deaths attributable to the selected preconception risk factors, LFPR is the labour force participation rate, S is the age when individuals would have started working, R is the retirement age, GDPW is the GDP per worker, r is the discount rate.
Foregone productivity due to child cognition deficit attributable to the preconception risk factors
This pathway is grounded in the premise that exposure to some of the selected preconception risk factors contributes to the development of neural tube defects and stunting in children. These two health conditions, in turn, can lead to cognitive deficits, subsequently resulting in reduced schooling years, lower earnings in adulthood and, ultimately, diminished national productivity (Hoddinott et al., Citation2013). To calculate the impact of stunting on future lifetime earnings, we relied on data and assumptions derived from the Lives Saved Tool (Avenir Health, Citation2023). These two assumptions are that (1) one standard deviation decrease in height-for-age z-score in two-year-old stunted children corresponds to a 0.47-year decrease in future schooling and that (2) each additional year of schooling lost results in a 3.8% decrease in future earnings (Avenir Health, Citation2023). By combining these assumptions with data on the prevalence of stunted children across different severity levels (moderate and severe), also sourced from the Lives Saved Tool, we estimated reduction coefficients reflecting the reduction in future lifetime earnings resulting from stunting.
To estimate the impact of neural tube defects on future lifetime earnings, we relied on data and assumptions from a previous model developed by Moench-Pfanner et al. (Citation2016). In this model, it was assumed that children with moderate impairment due to neural tube defects would experience a 50% reduction in future earnings, while children with severe impairment would face a 100% reduction in future earnings.
We employed the concept of reduction in future earnings as a proxy to estimate the potential decrease in productivity attributable to the selected preconception risk factors. Additionally, to avoid double counting, we specifically focused on calculating the future loss of productivity in a birth cohort of children aged 2. Calculations were then conducted for each year between 2020 and 2035. The following formula was used to calculate the net present value of these productivity losses:
Where LFPR is the labour force participation rate, c refers to the conditions ms for moderate stunting, ss for severe stunting, mn for moderate neural tube defects, and sn for severe neural tube defects, N is the number of children affected by a condition c attributable to the selected preconception risk factors, RF is the reduction coefficient for a condition c, S is the age when individuals would have started working, R is the retirement age, GDPW is the GDP per worker, r is the discount rate.
Direct healthcare costs
Direct healthcare costs refer to the money spent on medical care (hospitalisations, physicians, medications, and other medical expenditures) to treat and manage disease cases or adverse events attributable to the selected preconception risk factors. Due to limited data availability, we did not assess the direct healthcare costs to manage small-for-gestational-age births and neural tube defects. Therefore, we limited our analysis to the costs of managing preterm births, maternal hypertensive disorders, and postpartum haemorrhage episodes. The following formula was used:
Where N is the number of individuals affected by a specific condition attributable to the selected preconception risk factors, C is the average cost of treating a specific condition, CR is the percentage of affected individuals who received treatment for a specific condition, pb refers to preterm birth, pph refers to postpartum haemorrhage, and mhd refers to maternal hypertensive disorders.
The average cost of managing one preterm birth case and one postpartum haemorrhage episode was derived from previous studies conducted in Rwanda and Nigeria (Ngabonzima et al., Citation2022; Theunissen et al., Citation2021). The per-patient cost of managing maternal hypertensive disorders was estimated using costing and treatment regimen assumptions from the OneHealth Tool (Avenir Health, Citation2023) and the average outpatient visit cost in Nigeria, sourced from the WHO-CHOICE database. To account for inflation, the average outpatient visit costs were adjusted using annual inflation rates from 2010 to 2020, as reported by the International Monetary Fund (International Monetary Fund, Citation2023). The UHC Service Coverage sub-index on reproductive, maternal, newborn and child health was used as a proxy indicator to estimate the coverage rate of the three interventions (World Health Organization, Citation2023).
Discounting
We adjusted the discounting period for each year between 2020 and 2035 to reflect the time elapsed between the year they were calculated and the baseline year (2020). This adjustment ensured that all economic losses presented in this analysis are expressed as their net present value in 2020.
Data sources
A detailed account of the parameters, data sources, and assumptions used to estimate the population attributable fractions, the productivity losses and direct healthcare costs are provided in the supplementary materials.
Sensitivity analysis
We conducted a one-way sensitivity analysis to assess how changes in some model parameters impact the total economic burden. The analysis involved varying the discount rates of future costs and the assumed number of working years in a lifetime. To examine the impact of demographic projections on the results, we replicated the analysis using both low and high-fertility scenarios from UN World Population Prospects (United Nations, Citation2022).
Results
Disease burden attributable to the selected preconception risk factors
and show the PAF of the six preconception risk factors for the selected health outcomes in Nigeria in 2020. 6.6% of all preterm births with appropriate for gestational age were attributable to adolescent pregnancy, short birth interval, and intimate partner violence before pregnancy. Adolescent pregnancy and short birth spacing were also responsible for 14.4% of preterm births with small-for-gestational-age and 3.7% of small-for-gestational-age births. 72.0% of neural tube defects in neonates were attributable to folate deficiency. Moreover, we estimated that 10.9% of perinatal deaths and 10.5% of late neonatal deaths were attributable to the selected preconception risk factors, perinatal death being the only health outcome associated with all of them. For stunting in two-year-old children, 1.6% of cases were attributable to adolescent pregnancy, short birth interval, and intimate partner violence before pregnancy. In terms of maternal health outcomes, pre-pregnancy overweight and obesity emerged as the only preconception risk factor associated with maternal hypertensive disorders, with a PAF estimated at 34.3%. Additionally, 7.4% of postpartum haemorrhage cases and 6.7% of maternal deaths were attributable to the effects of pre-pregnancy overweight and obesity and female genital mutilation.
Table 2. Population-attributable fractions of preconception risk factors for selected perinatal and neonatal health outcomes in Nigeria in 2020.
Table 3. Population-attributable fractions of preconception risk factors for selected maternal health outcomes in Nigeria in 2020.
Economic burden attributable to the selected preconception risk factors
In 2020, the net present value of the foregone productivity and direct healthcare costs generated by the six selected preconception risk factors was estimated at US$ 3.3 billion. From 2020 to 2022, the annual economic burden was equivalent to 0.72% of Nigeria’s GDP on average. If the prevalence of the preconception risk factors remains at the same level, and accounting for future population and fertility changes, the cumulated economic burden is expected to reach US$ 19.0 billion by 2025, US$ 33.3 billion by 2030, and US$ 46.2 billion by 2035.
Distribution of the economic burden
In 2020, the foregone productivity due to premature deaths (maternal, late neonatal, and perinatal mortality) emerged as the primary driving force behind the economic burden, with a net present value estimated at US$ 3.1 billion (). The economic losses generated by perinatal mortality, which includes stillbirths and early neonatal deaths, contributed to 73.6% of the economic burden. The contribution of maternal deaths to the total economic losses was estimated at 9.7%. The cumulative cost of premature deaths attributable to the six preconception risk factors is projected to reach US$ 17.6 billion by 2025, US$ 31.0 billion by 2030, and US$ 42.9 billion by 2035. The long-term economic impact of child cognition deficit resulting from stunting and neural tube defects accounted for 6.5% of the economic burden in 2020, with a net present value of US$ 213 million. Over a 15-year horizon, the cumulated economic losses are estimated to exceed US$ 3.0 billion. Direct healthcare costs had a minor contribution to the total economic burden (0.5%). Cumulatively, these costs are expected to reach US$ 101 million by 2025, US$ 176 million by 2030, and US$ 245 million by 2035.
Table 4. Economic burden by pathway (US$ million).
shows that pre-pregnancy overweight and obesity emerged as the first contributor to the total economic burden (33.8%), with a net present value of US$ 1.1 billion in 2020 and cumulated economic losses projected to reach US$ 15.6 billion between 2020 and 2035. Short birth intervals represented 23.8% of the economic burden, with associated economic losses estimated at US$ 785 million in 2020. The economic impact of folate deficiency was US$ 630 million in 2020 and an expected cumulative total of US$ 8.8 billion by 2035. Intimate partner violence before pregnancy and adolescent pregnancy contributed to 9.5% and 7.9% of the total economic burden, respectively. Lastly, the economic losses generated by female genital mutilation accounted for 5.8% of the total economic losses, representing a net present value of US$ 192 million in 2020. These economic losses are projected to reach a cumulative total of US$ 2.7 billion between 2020 and 2035.
Table 5. Economic burden by preconception risk factor (US$ million).
Sensitivity analysis
shows how varying some model parameters affects the total economic burden from 2020 to 2035. Using 5% and 7% discount rates, the total economic burden decreased by 50.3% and 73.1%, respectively. A more conservative estimate (−4.0%) is obtained when the total number of working years in a lifetime used in the model is reduced from 49 to 45. Regarding the fertility scenarios, the total economic burden was US$ 43.1 billion (−6.6%) under the low variant scenario and US$ 49.2 billion (+6.5%) under the high variant scenario.
Table 6. Sensitivity analysis.
Discussion
Poor preconception health increases the risks of pregnancy complications and adverse health outcomes for future mothers and children, resulting in an additional morbidity and mortality burden that has long-term and irreversible effects on the healthcare system and the country’s economy. In this study, we used the case of Nigeria to model the productivity losses and direct healthcare costs associated with a selection of six preconception risk factors, including adolescent pregnancy, short birth interval, folate deficiency, pre-pregnancy overweight and obesity, intimate partner violence before pregnancy, and female genital mutilation. To our knowledge, this research is the first that aims to quantify the current and future economic burden stemming from poor preconception health at a country level.
We estimated that the effects of the six preconception risk factors on maternal, child and pregnancy outcomes cost Nigeria about US$ 3.3 billion in 2020, which is equivalent to 0.76% of the country’s GDP in the same year. If these risk factors are not tackled, the cumulative economic burden will amount to US$ 19.0 billion by 2025, US$ 33.3 billion by 2030, and US$ 46.2 billion by 2035. Our findings revealed that over 90% of this burden is due to the impact of premature deaths, including perinatal, late neonatal and maternal deaths, on productivity. From 2020 to 2035, premature deaths attributable to poor preconception health could result in the potential loss of more than 900,000 individuals in the future workforce, posing profound implications for the national economy. The second major contributor is the foregone productivity stemming from neural tube defects and stunting in childhood. The effects of childhood stunting on future earnings and productivity at adult age have been established within the literature on malnutrition (Hoddinott et al., Citation2013). In our study, we estimated that approximately 1.6% of all stunting cases could be attributable to the selected preconception risk factors, with prematurity and small-for-gestational-age births playing an intermediary role. Our cost estimates for this pathway are conservative since we solely focused on two-year-old children to avoid double-counting the stunting cases over time. The direct healthcare costs contributed to less than 1% of the total economic burden. Such a distribution is common in cost-of-illness studies because direct costs are generally less substantial and enduring than indirect productivity losses. However, in our analysis, this distribution pattern is amplified by the fact that our model included only the costs for managing preterm births, postpartum haemorrhage, and episodes of maternal hypertensive disorders. The inclusion of direct costs due to other pregnancy complications, such as small-for-gestational-age births or neural tube defects, would have increased the contribution of this pathway to the total economic burden, but probably to a limited extent only.
Improving preconception health presents a compelling opportunity to advance towards reducing maternal and child mortality in Nigeria. To obtain significant results, it is essential to develop comprehensive strategies encompassing multiple interventions such as family planning (Government of Malawi, Citation2014; Kennedy et al., Citation2013; United Nations Population Fund, Citation2021a, Citation2021b), child marriage prevention, folic acid fortification or supplementation (UNICEF Madagascar, Citation2017), and physical activity promotion. Concurrently, efforts to address all forms of violence against women are crucial, as they also contribute to the maternal and child mortality and morbidity burden. Furthermore, it is important to note that investing in the health of women of reproductive age may have benefits that extend far beyond the pregnancy pathway. For example, reducing overweight and obesity in women of reproductive age will not only reduce the risk of pregnancy-related complications but also contribute to a broader public health goal by alleviating the burden of associated non-communicable diseases. These additional benefits underscore the far-reaching impact of creating supportive environments for women of reproductive age.
Our study has several limitations that must be mentioned. Firstly, the analysis does not provide a comprehensive assessment of the economic burden associated with poor preconception health since it covers only six preconception risk factors and a limited number of health outcomes. Secondly, our projections assume a constant prevalence rate for the preconception risk factors throughout the whole study period. Therefore, we did not account for a potential change due to changing lifestyles and environmental influences or the impact of health interventions or policies. For example, the prevalence of overweight and obesity among Nigerian women aged 15–49 increased by almost 8% between 2003 and 2018 (ICF, Citation2023), and this trend is expected to continue in the future. Thirdly, measuring the economic implications of premature mortality poses several challenges. Our study used a human capital approach centred around the GDP per worker. This approach offers a broader economic perspective than wage-based methods but does not capture impacts on the informal economy, which plays a significant role in Nigeria (Onwe, Citation2013). If using a full-income approach may address this limitation, this methodology also carries a risk of overestimation. Specifically, it could disproportionately inflate the economic impact of premature mortality, especially when compared with direct healthcare costs and productivity losses associated with morbidity. Finally, the model does not factor in future productivity growth due to the considerable uncertainty surrounding this variable. Introducing assumptions with such a level of uncertainty could have compromised the results. Thus, we chose a more cautious and conservative approach by not adjusting the GDP per worker over time.
This study provides a first step towards better understanding the economic burden of poor preconception health. While comparing Nigeria to other countries could offer insightful context, the lack of comparable data poses challenges in making direct comparisons. To advance our understanding, we aim to repeat this economic burden assessment by including several countries and assessing how the economic impact varies depending on countries’ level of economic development and epidemiological profile. Future analyses will also benefit from developing the healthcare direct costs pathway and valuing productivity losses beyond paid labour. Moreover, this research lays the foundation for a global investment case on preconception care that could guide decision-makers in low and middle-income countries towards effective interventions, policy responses, and resource allocation strategies.
Poor preconception health contributes to maternal and child mortality and morbidity, causing substantial economic losses in Nigeria. Addressing early and rapidly repeated pregnancies, overweight and obesity, folate deficiency, and gender-based violence presents an opportunity to significantly improve pregnancy outcomes, save lives and generate potential economic savings. It is essential to investigate the economics of preconception health further to inform policy-makers and public health authorities on the best ways to allocate resources. An important step in that direction is quantifying the economic returns of investing in preconception interventions and policies, notably in low and middle-income countries.
Ethical approval
Ethics approval was not required for this economic impact analysis. We used publicly accessible documents and data to conduct the analysis.
Supplemental Material
Download MS Word (103 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Atrash, H. K., Johnson, K., Adams, M., Cordero, J. F., & Howse, J. (2006). Preconception care for improving perinatal outcomes: The time to act. Maternal and Child Health Journal, 10(5 Suppl), S3–S11. https://doi.org/10.1007/s10995-006-0100-4
- Avenir Health. (2023). OneHealth Tool 6.29, Lives Saved Tool (LiST).
- Barker, M., Dombrowski, S. U., Colbourn, T., Fall, C. H. D., Kriznik, N. M., Lawrence, W. T., Norris, S. A., Ngaiza, G., Patel, D., Skordis-Worrall, J., Sniehotta, F. F., Steegers-Theunissen, R., Vogel, C., Woods-Townsend, K., & Stephenson, J. (2018). Intervention strategies to improve nutrition and health behaviours before conception. The Lancet, 391(10132), 1853–1864. https://doi.org/10.1016/S0140-6736(18)30313-1
- Berghella, V., Buchanan, E., Pereira, L., & Baxter, J. K. (2010). Preconception care. Obstetrical & Gynecological Survey, 65(2), 119–131. https://doi.org/10.1097/OGX.0b013e3181d0c358
- Berglund, A., & Lindmark, G. (2016). Preconception health and care (PHC)—A strategy for improved maternal and child health. Upsala Journal of Medical Sciences, 121(4), 216–221. https://doi.org/10.1080/03009734.2016.1191564
- Bryce, E., Gurung, S., Tong, H., Katz, J., Lee, A. C., Black, R. E., & Walker, N. (2022). Population attributable fractions for risk factors for spontaneous preterm births in 81 low- and middle-income countries: A systematic analysis. Journal of Global Health, 12, 04013. https://doi.org/10.7189/jogh.12.04013
- Dorney, E., & Black, K. I. (2018). Preconception care. Australian Journal of General Practice, 47(7), 424–429. https://doi.org/10.31128/AJGP-02-18-4485
- Fleming, T. P., Watkins, A. J., Velazquez, M. A., Mathers, J. C., Prentice, A. M., Stephenson, J., Barker, M., Saffery, R., Yajnik, C. S., Eckert, J. J., Hanson, M. A., Forrester, T., Gluckman, P. D., & Godfrey, K. M. (2018). Origins of lifetime health around the time of conception: Causes and consequences. The Lancet, 391(10132), 1842–1852. https://doi.org/10.1016/S0140-6736(18)30312-X
- Government of Malawi. (2014). The Government of Malawi’s investment case for reproductive, maternal, newborn, child and adolescent health and nutrition (https://www.globalfinancingfacility.org/sites/gff_new/files/documents/Malawi-GFF-Investment-Case.pdf.
- Grosse, S. D., Sotnikov, S. V., Leatherman, S., & Curtis, M. (2006). The business case for preconception care: Methods and issues. Maternal and Child Health Journal, 10(5 Suppl), S93–S99. https://doi.org/10.1007/s10995-006-0101-3
- Harika, R., Faber, M., Samuel, F., Kimiywe, J., Mulugeta, A., & Eilander, A. (2017). Micronutrient status and dietary intake of iron, vitamin A, iodine, folate and zinc in women of reproductive age and pregnant women in Ethiopia, Kenya, Nigeria and South Africa: A systematic review of data from 2005 to 2015. Nutrients, 9(10), 1096. https://doi.org/10.3390/nu9101096
- Hemsing, N., Greaves, L., & Poole, N. (2017). Preconception health care interventions: A scoping review. Sexual & Reproductive Healthcare, 14, 24–32. https://doi.org/10.1016/j.srhc.2017.08.004
- Hoddinott, J., Alderman, H., Behrman, J. R., Haddad, L., & Horton, S. (2013). The economic rationale for investing in stunting reduction. Maternal & Child Nutrition, 9 (Suppl 2), 69-82. https://doi.org/10.1111/mcn.12080
- ICF. (2023). The DHS Program STATcompiler. Funded by USAID.. http://www.statcompiler.com.
- International Monetary Fund. (2023). Inflation rate, average consumer prices. Retrieved June 06, 2023, from https://www.imf.org/external/datamapper/PCPIPCH@WEO/OEMDC/ADVEC/WEOWORLD/NGA.
- Jack, B. W., Atrash, H., Coonrod, D. V., Moos, M. K., O'Donnell, J., & Johnson, K. (2008). The clinical content of preconception care: An overview and preparation of this supplement. American Journal of Obstetrics and Gynecology, 199(6 Suppl 2), S266–S279. https://doi.org/10.1016/j.ajog.2008.07.067
- Jo, C. (2014). Cost-of-illness studies: Concepts, scopes, and methods. Clinical and Molecular Hepatology, 20(4), 327–337. https://doi.org/10.3350/cmh.2014.20.4.327
- Johnson, K., Atrash, H., & Johnson, A. (2008). Policy and finance for preconception care. Women’s Health Issues, 18(6 Suppl), S2–S9. https://doi.org/10.1016/j.whi.2008.09.006
- Kennedy, E. C., Mackesy-Buckley, S., Subramaniam, S., Demmke, A., Latu, R., Robertson, A. S., Tiban, K., Tokon, A., & Luchters, S. (2013). The case for investing in family planning in the pacific: Costs and benefits of reducing unmet need for contraception in Vanuatu and the Solomon Islands. Reproductive Health, 10(1), 30. https://doi.org/10.1186/1742-4755-10-30
- Kotirum, S., Kiatpongsan, S., & Kapol, N. (2021). Systematic review of economic evaluation studies on preconception care interventions. Health Care for Women International, 42(4-6), 503–517. https://doi.org/10.1080/07399332.2020.1817025
- Levin, M. (1953). Symposium on endemiology of cancer of lung: Occurrence of lung cancer in man. Acta - Unio internationalis Contra Cancrum, 9, 531–541.
- Mason, E., Chandra-Mouli, V., Baltag, V., Christiansen, C., Lassi, Z. S., & Bhutta, Z. A. (2014). Preconception care: Advancing from ‘important to do and can be done’ to ‘is being done and is making a difference’. Reproductive Health, 11(3), S8. https://doi.org/10.1186/1742-4755-11-S3-S8
- Moench-Pfanner, R., Silo, S., Laillou, A., Wieringa, F., Hong, R., Hong, R., Poirot, E., & Bagriansky, J. (2016). The economic burden of malnutrition in pregnant women and children under 5 years of age in Cambodia. Nutrients, 8(5), https://doi.org/10.3390/nu8050292
- Nesari, M., Olson, J. K., Vandermeer, B., Slater, L., & Olson, D. M. (2018). Does a maternal history of abuse before pregnancy affect pregnancy outcomes? A systematic review with meta-analysis. BMC Pregnancy and Childbirth, 18(1), 404. https://doi.org/10.1186/s12884-018-2030-8
- Ngabonzima, A, Asingizwe, D, Cechetto, D, Mukunde, G, Nyalihama, A, Gakwerere, M, & Epstein, D M. (2022). Evaluating the medical direct costs associated with prematurity during the initial hospitalization in Rwanda: a prevalence based cost of illness study. BMC Health Services Research, 22(1), 953.
- Ngabonzima, A, Asingizwe, D, Cechetto, D, Mukunde, G, Nyalihama, A, Gakwerere, M, & Epstein, D M. (2022). Evaluating the medical direct costs associated with prematurity during the initial hospitalization in Rwanda: a prevalence based cost of illness study. BMC Health Services Research, 22(1), 953.
- Onwe, O. J. (2013). Role of the Informal Sector in Development of the Nigerian Economy: Output and Employment Approach. (Ed.),^(Eds.).
- Poix, S., & Elmusharaf, K. (2023). Investigating the pathways from preconception care to preventing maternal, perinatal and child mortality: A scoping review and causal loop diagram. Preventive Medicine Reports, 34, 102274. https://doi.org/10.1016/j.pmedr.2023.102274
- Robinson, R. (1993). Cost-benefit analysis. BMJ, 307(6909), 924–926. https://doi.org/10.1136/bmj.307.6909.924
- Say, L., Chou, D., Gemmill, A., Tunçalp, Ö, Moller, A. B., Daniels, J., Gülmezoglu, A. M., Temmerman, M., & Alkema, L. (2014). Global causes of maternal death: A WHO systematic analysis. The Lancet Global Health, 2(6), e323–e333. https://doi.org/10.1016/S2214-109X(14)70227-X
- Stephenson, J., Heslehurst, N., Hall, J., Schoenaker, D., Hutchinson, J., Cade, J. E., Poston, L., Barrett, G., Crozier, S. R., Barker, M., Kumaran, K., Yajnik, C. S., Baird, J., & Mishra, G. D. (2018). Before the beginning: Nutrition and lifestyle in the preconception period and its importance for future health. The Lancet, 391(10132), 1830–1841. https://doi.org/10.1016/S0140-6736(18)30311-8
- Theunissen, F., Cleps, I., Goudar, S., Qureshi, Z., Owa, O. O., Mugerwa, K., Piaggio, G., Gülmezoglu, A. M., Nakalembe, M., Byamugisha, J., Osoti, A., Mandeep, S., Poriot, T., Gwako, G., Vernekar, S., & Widmer, M. (2021). Cost of hospital care of women with postpartum haemorrhage in India, Kenya, Nigeria and Uganda: a financial case for improved prevention. Reproductive Health, 18(1), 18. https://doi.org/10.1186/s12978-020-01063-x
- UNICEF Madagascar. (2017). Madagascar nutrition investment case 2017 (https://www.unicef.org/madagascar/media/676/file/Plan%20d'Investissement%20sur%20la%20Nutrition%202017%20(full%20report)%20(EN).pdf.
- United Nations. (2022). World population prospects 2022, Online Edition.
- United Nations Population Fund. (2021a). Investment cases towards ending unmet need for family planning and gender-based violence in Namibia. https://esaro.unfpa.org/sites/default/files/pub-pdf/namibia_investment_report.pdf.
- United Nations Population Fund. (2021b). Investment cases towards ending unmet need for family planning in Bostwana. https://esaro.unfpa.org/sites/default/files/pub-pdf/botswana_investment_report_1.pdf.
- Vats, H., Saxena, R., Sachdeva, M. P., Walia, G. K., & Gupta, V. (2021). Impact of maternal pre-pregnancy body mass index on maternal, fetal and neonatal adverse outcomes in the worldwide populations: A systematic review and meta-analysis. Obesity Research & Clinical Practice, 15(6), 536–545. https://doi.org/10.1016/j.orcp.2021.10.005
- World Health Organization. (2013). Meeting to develop a global consensus on preconception care to reduce maternal and childhood mortality and morbidity. https://apps.who.int/iris/bitstream/handle/10665/78067/9789241505000_eng.pdf?sequence=1&isAllowed=y.
- World Health Organization. (2023). Global Health Observatory Data Repository. https://www.who.int/data/gho.
- Zhang, J., & Yu, K. F. (1998). What’s the relative risk?A method of correcting the odds ratio in cohort studies of common outcomes. JAMA, 280(19), 1690–1691. https://doi.org/10.1001/jama.280.19.1690