ABSTRACT
Veteran and solitary trees are key structures supporting biodiversity in many wooded ecosystems. Their global decline threatens numerous organisms associated with them, including several insect species protected by law that serve as umbrella species. The floodplain along the lower Morava and Dyje rivers is considered a hotspot for saproxylic organisms associated with veteran trees. The area is a UNESCO Biosphere Reserve and part of NATURA 2000. Between 2006 and 2015, we mapped 11,596 veteran and habitat trees in the area. The mapping also included the distribution of several insects associated with veteran trees including three beetle species (Cerambyx cerdo, Osmoderma barnabita, and Eurythyrea quercus) and two ant species (Liometopum microcephalum and Lasius fuliginosus). The data on the position, abundance, diameter, forest structure and health of the veteran trees and trees inhabited by the above species are presented in a map created in ArcGIS Online. These data serve as an important source of information for the management of nature conservation of the area.
1. Introduction
Veteran trees are usually large, senescent trees bearing numerous specific microhabitats. They are considered to be key features supporting biodiversity in wooded landscapes of Central Europe as well as temperate and boreal regions in general (CitationFischer, Stott, & Law, 2010; CitationHall & Bunce, 2011; CitationLindenmayer et al., 2014; CitationLindenmayer, Laurance, & Franklin, 2012; CitationManning, Fischer, & Lindenmayer, 2006; CitationRead, 2000; CitationSebek et al., 2016; CitationSiitonen & Ranius, 2015; CitationVodka, Konvička, & Čížek, 2009). These trees mostly grow in open woodlands, including wooded meadows and pastures. They host a wide spectrum of endangered and protected organisms, including mainly insects and lichens, but also bats, birds and other taxa (CitationHartel et al., 2014). Owing to natural disturbances (e.g. windthrows or fires) (CitationAdámek, Bobek, Hadincová, Wild, & Kopecký, 2015; CitationHultberg, Gaillard, Grundmann, & Lindbladh, 2015; CitationNiklasson et al., 2010) and grazing of large herbivores (CitationBengtsson, Nilsson, Franc, & Menozzi, 2000; CitationVera, 2000) European forests have at least partly been open for most of their Holocene history. These natural processes have gradually been replaced by human activities such as coppicing and wood pasture (CitationRackham, 1998; CitationSzabó, 2009; CitationSzabó, Müllerová, Suchánková, & Kotačka, 2015). The open canopy conditions that are required for trees to reach large diameters and veteran state have thus endured until modern times.
The intensification of agriculture and forest management, however, led to the abandonment of traditional silvicultural practices between the eighteenth and twentieth centuries. They were replaced by intensive forestry cultivating mainly even-aged, closed-canopy forests often managed within a clearcut silvicultural system. Open woodlands thus rapidly turned into closed-canopy forests (CitationBürgi, 1999; CitationHédl, Kopecký, & Komárek, 2010; CitationKopecký, Hédl, & Szabó, 2013). Remaining open woodlands containing veteran trees have been preserved mainly in some hunting parks and game reserves, parks and tree alleys (CitationHorak et al., 2014; CitationJonsell, 2012). Open woodlands have mostly disappeared from protected areas where a non-interventionist management regime prevails. Today, they are isolated, fragmented and still in decline (CitationMiklín & Čížek, 2014); the same applies to solitary and veteran trees (CitationČížek & Hauck, 2008). Given the importance of open woodlands and veteran and solitary trees for biodiversity (CitationDolek et al., 2009; CitationHorak et al., 2014; CitationRamírez-Hernández, Micó, de los Ángeles Marcos-García, Brustel, & Galante, 2014; CitationSpitzer, Konvička, Tropek, Tuf, & Tufová, 2008), nature conservation management should focus on their preservation and restoration. Knowledge of landscape history and land use changes together with detailed mapping of the present state is thus crucial for designing effective conservation management measures and goals.
In this paper, we present results of an extensive mapping survey of solitary and veteran trees and several saproxylic insect species associated with such trees on 14,600 ha of the floodplain woodlands along the lower reaches of the Dyje and Morava rivers in the south-east of the Czech Republic. High concentrations of solitary and veteran trees in the area are a result of traditional silvicultural practices that have locally been abandoned over the past two centuries. Shifts in the forest management have led to increased canopy closure and the decline of solitary and veteran trees that are crucial to the survival of numerous endangered species (CitationMiklín & Čížek, 2014). Our results should serve as one of the sources for nature conservation and forestry administration of the area.
1.1. Study area and species
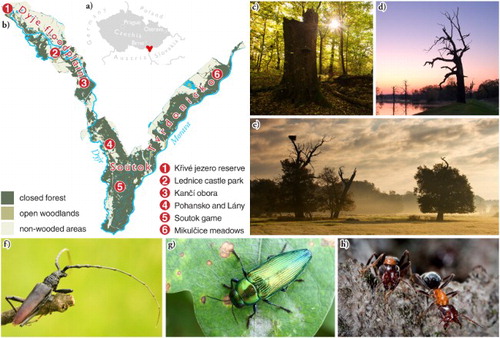
Solitary and veteran trees were mapped in the floodplains along the lower reaches of the Morava (March) and Dyje (Thaya) rivers in the very south-eastern part of the Czech Republic ((a)). With an average yearly temperature of ∼9.6°C and rainfall of ∼500 mm, the study area belongs to one of the warmest and driest localities of the Czech Republic. Out of 146 km2 covered by the study, 60.1% are forests and woodlands, 13.6% are grasslands and 18.0% is arable land (CitationMiklín & Hradecký, 2016a). Pedunculate oak (Quercus robur), narrow-leaved ash (Fraxinus angustifolia), hornbeam (Carpinus betulus) and field maple (Acer campestre) prevail in the forests. The study area can be divided into three parts based on forestry management units, namely (i) Dyje floodplain (north-western part of the area between the Nové Mlýny dam and Břeclav town along the Dyje river), (ii) Tvrdonicko (north-eastern part of the area between Lanžhot and Hodonín towns along the Morava river) and (iii) Soutok (part to the south of Lanžhot town along both the rivers to their confluence) ((b)). Flat terrain of the alluvial landscape (149–184 m a.s.l.) was strongly influenced by regular flooding, enhanced by human impact over the last two millennia (CitationKadlec et al., 2009), and long-lasting human presence (CitationDresler & Macháček, 2013). The contemporary landscape thus has to be understood as a mosaic of natural and semi-natural ecosystems, and its biodiversity has been partially dependent on human management. Due to its importance, the study area is covered by both national and international forms of nature conservation and protected areas. The Lower Morava UNESCO Biosphere Reserve covers the whole area, while its parts are recognised within the NATURA 2000 system (Soutok – Podluží and Niva Dyje Sites of Community Importance; Special Protection Areas of Soutok-Tvrdonicko, Lednice fishponds and Pálava), Trilateral Ramsar Site Floodplains of the Morava-Dyje-Danube Confluence, or several state reserves.
Figure 1. Study area (a) within the Czech Republic and (b) with localities mentioned in the text marked with red circles. Example of (c) veteran tree snag in a closed-canopy forest (Ranšpurk National Nature Reserve), (d) old solitary oak on Černé louky meadows, (e) solitary trees in a meadow near Pohansko, (f) the great capricorn beetle (C. cerdo), (g) the jewel beetle (E. quercus) and (h) the L. microcephalum ant. Photo Jan Miklín (c, d, e, f, g), Nikola Rahmé (g) and Jiří Klváček (h).
2. Methods
The mapping of solitary and veteran trees took place from 2006 to 2015. All solitary trees of a diameter in breast height (DBH) > 40 cm were recorded outside closed-canopy stands. All trees apparently older than the surrounding stand and trees with veteran characteristics (e.g. trees with hollows or a partly dead trunk, see CitationRead, 2000) were recorded in the forests. The following data were recorded for each tree: (i) coordinates (measured with a global positioning system receiver in WGS84 coordinate system, afterwards converted into the national S-JTSK system); (ii) species; (iii) DBH of the trunk; (iv) health condition of the tree (1 – healthy, 2 – ca. one-third of the tree crown dried, 3 – ca. one-half of the tree crown dried, 4 – ca. two-thirds of the tree crown dried, 5 – dead tree, log or stump).
Moreover, the presence of the following mostly endangered and protected species was registered: (i) the hermit beetle (Osmoderma barnabita), (ii) the great capricorn beetle (Cerambyx cerdo), (iii) jewel beetle (Eurythyrea quercus), (v) jet ant (Lasius fuliginosus) and (vi) velvety tree ant (Liometopum microcephalum). The hermit beetle (i) inhabits hollows of various broadleaved trees in Europe (CitationRanius et al., 2005). The great capricorn beetle (ii) inhabits mainly large, old oaks growing outside closed-canopy conditions (CitationBuse, Ranius, & Assmann, 2008). The jewel beetle (iii) inhabits bare and dry, but hard wood of old oaks (CitationBílý, 2002). All the three species prefer sun-exposed trees. The jet ant (v) is a common species nesting in hollowed tree trunks. Among the mapped species, it is the only one that is neither endangered nor protected. The velvety tree ant (vi) also nests in tree trunks, it prefers large trees and is considered endangered (CitationSchlaghamersky & Omelkova, 2007) The species (i–iii) are ‘target species’ of the Sites of Community Importance. The presence of adult individuals or their remains, larvae and larval frass indicates the presence of (i). Characteristic exit holes indicated the presence of (ii) and (iii). Individuals walking on a tree served as a proof of presence of (v) and (vi). Among these data – visualised on the maps – several other characteristics usable for further analyses were recorded, for example, tree habitats or estimation of percentage without bark. Some other species including the Rosalia longicorn (Rosalia alpina) or Stag beetle (Lucanus cervus) were mapped in a related project in selected localities. All the data were processed and maps created using Esri ArcGIS (Desktop and Online) software.
3. Results
In total, 11,596 trees were mapped within the study area, with oaks (mainly pedunculate oak) being the most frequent species (63.2%), followed by narrow-leaved ash (F. angustifolia; 9.7% share) and willows (Salix spp.; 8.8%) (). The great capricorn beetle was recorded on 2988 oaks, the hermit beetle on 254 trees (53.1% willows and 39.0% oaks), E. quercus jewel beetle on 332 oaks, L. microcephalum ant on 1397 trees (90.0% were oaks), and L. fuliginosus ant on 627 trees (50.1% on oaks and 18.2% on willows). Hollows were recorded on 2610 trees (51.5% were oaks and 25.7% willows).
Table 1. Mapped veteran and solitary trees and insect species.
Average DBH of all recorded trees was 97 cm. The highest average trunk diameters were those of poplars (Populus spp. 105 cm), willows and oaks (both 101 cm). In total, 756 trees with DBH > 150 cm were recorded, 582 of them were oaks. The largest recorded tree was a pedunculate oak with a 260-cm DBH ().
Table 2. Trunk diameters of mapped veteran and solitary trees.
In total, 54.1% of the trees were classified as healthy, while 18.8% were dead (). Nearly half of the oaks were healthy, while 25.3% were dead and at least half of the crown was dead in 8.5% of the oaks. Unsurprisingly, tree health on average worsened with increasing DBH (). In total, 68.6% of trees with DBH < 75 cm were classified as healthy, whereas only 20.4% of trees with DBH > 150 cm. As many as 39.0% of the trees with DBH > 150 cm and only 11.3% of those with DBH < 75 cm were dead. Generally, there are some differences in health among tree species. The health state of oaks, for example, was worse than that of ash, most likely owing to the lower average DBH of the latter.
Figure 2. Tree health dependence on the species trunk diameter; (a) all species, (b) oaks (Quercus spp.), (c) narrow-leaved ash (F. angustifolia), (d) willows (Salix spp.), (e) Elm (Ulnus spp.), (f) Poplars (Populus spp.) and (g) other species.
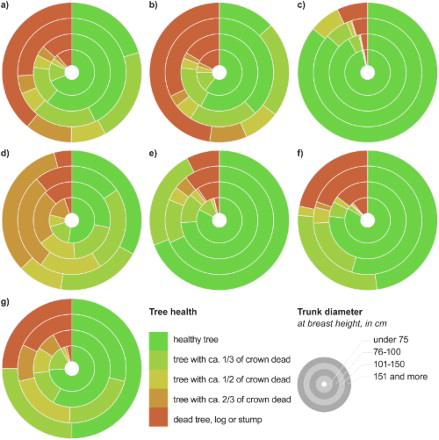
Table 3. Health conditions of mapped veteran and solitary trees.
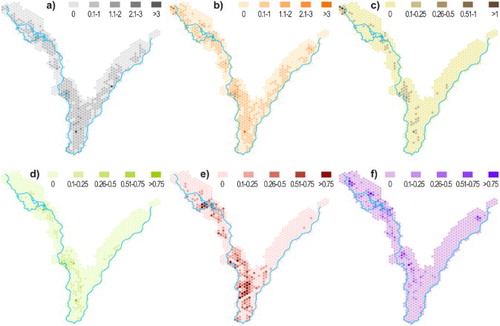
Analysis of the spatial distribution of the trees revealed several clearly pronounced hotspots (). In general, the western part of the study area (along the Dyje river) is densely covered. Tree hotspots include (see (b) for location) the Křivé jezero National Nature Reserve (with the highest share of willows), the area of Lednický Chateau park (park around Lednice Chateau), Kančí obora near the town of Břeclav, open woodlands and meadows with solitary trees around Pohansko and Lány Chateaus, and the east-central part of the Soutok game reserve. In the floodplain of the Morava river (Tvrdonicko), the only site rich in veteran trees concerns meadows near Mikulčice in the north-eastern part of the study area. The hotspots of mapped insect species () are mostly identical with those of the trees. Individual hotspots also differed for tree health. The oaks in Lednice Chateau park, for example, were generally in better health conditions than the oaks on the meadows in Pohansko and Lány despite similar land cover condition (both places were classified by CitationMiklín and Hradecký (2016a) as open woodlands).
Figure 3. Density of mapped trees within a hexagon net (in trees per ha; regular hexagons with side length 300 m and area 23.4 ha), in trees per ha; (a) all species, (b) oaks (Quercus spp.), (c) narrow-leaved ash (F. angustifolia, (d) willows (Salix spp.), (e) Elm (Ulnus spp.) and (f) Poplars (Populus spp.).
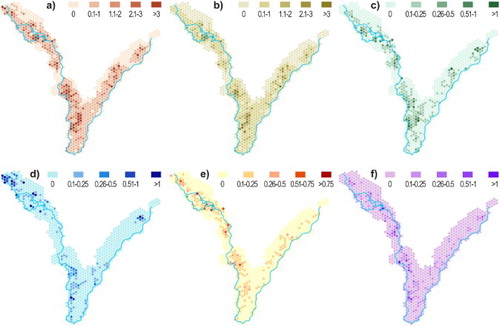
Figure 4. Density of trees inhabited by mapped insect species within a hexagon net (in trees per hectare, regular hexagons with side length 300 m, area 23.4 ha), in trees per ha; (a) great capricorn beetle (C. cerdo), (b) hollows, (c) hermit beetle (O. barnabita), (d) jewel beetle (E. quercus), (e) L. microcephalum ant, and (f) L. fuliginosus ant.
The results of the mapping are presented in an online map (http://goo.gl/oeBgtn) created with ArcGIS Online (CitationESRI, 2016) allowing users to combine several layers and zooming over the map (see the instructional video in Supplementary material). Among data from the mapping, an orthophotomap (used as a basemap) and land cover data (from the years 1938 and 2009 adopted from CitationMiklín and Hradecký 2016a with simplified categories) are available. Tree data are separated into layers by individual species (except for elms, which are popular with amateur entomologists as they host a large number of rare species), using visual variables of colour (five categories according to tree health) and size (matching trunk diameter). A pop-up window for each tree contains information on the date of mapping, presence of the great capricorn beetle, tree health (categorised as per colour) and trunk diameter (in cm). It is also possible to visualise the density of all the mapped trees on a heat map (CitationDeBoer, 2015). The same visualisation is used for species data (hollows, great capricorn beetle, hermit beetle, jewel beetle, L. microcephalum and L. fuliginosus ants). For conservation reasons, the data on the website map are not available as point symbols to prevent precisely identify their location. Nevertheless, they provide a good overview of priority areas for veteran trees and their associated biota.
Several series of detailed maps were printed on the basis of the online map presented in the paper for the national nature conservation administration and majority forest owner company, for example, maps of the distribution of selected species or maps of the distribution of solitary and veteran trees. These maps were prepared as atlases with several dozens of map sheets covering the whole area at a detailed scale (1:5000) (see the Main map for examples).
4. Discussion and conclusions
The herein presented information demonstrates that the target area is rich in veteran trees, pointing to their hotspots and the hotspots of associated organisms that were the subject of mapping.
Solitary and veteran trees are keystone features supporting the biodiversity of wooded landscapes. The larger a tree is, the greater its importance (CitationLindhe, Lindelöw, & Åsenblad, 2005). In the mapped area, tree veterans are crucial not only for the mapped endangered and protected beetles, but also for a wide range of other organisms including lichens, birds and insects (CitationSebek et al., 2016; CitationVondrák et al., 2016). The number of the trees is, however, in long-term decline, which increases the risk of extinction for their associated biota (CitationČížek & Hauck, 2008).
It is difficult to estimate the age of a tree from its DBH as the increment growth depends on numerous regional and local factors such as climate, water and nutrient availability, and current and past canopy closure (CitationAltman et al., 2013). In a recent study based on extensive coring, however, the largest oaks in the study area were estimated to be up to 400 years old (CitationAltman, Doležal, & Čížek, 2016).
CitationMiklín and Hradecký (2016b) found an effect of current and past land cover on the distribution of veteran trees and their associated insect species. The areas classified as open woodlands today showed higher numbers of tree veterans. Yet in a closed forest, there were more veteran trees if a particular stand was open in 1938 than if it was closed. This points to the importance of open canopy conditions for the existence of veteran trees. Only in open or semi-open conditions do oaks reach large dimensions preferred by, for example, saproxylic beetles (CitationAltman et al., 2013). Open grown-up tree veterans are preferred even after canopy closure due to changes in forest management (CitationMiklín & Čížek, 2014). A substantial increase in canopy closure in woodlands of the area during the past century has thus threatened the existence of veteran trees and their associated biota in two ways. First, it decreases survival of existing tree veterans; second, it prevents younger trees from gaining veteran tree characteristics. An exceptionally high rate of logging over the past three decades has posed another threat to the biodiversity of the area (CitationMiklín & Čížek, 2014).
Since all except one of the mapped insect species are protected by law, the maps presented in the paper offer evidence needed by conservation authorities and already serve as a base for management decisions.
Software
All the processing and analyses of mapped data as well as paper maps were carried out using Esri ArcGIS (9.3 to 10.2). The online map was created in ArcGIS Online. For design of printed maps, Adobe Creative Suite (InDesign, Photoshop and Illustrator) was used. The instructional video was recorded using with Active Presenter 6 (Free edition).
Veteran trees and saproxylic insects in the floodplains of lower Morava and Dyje rivers, Czech Republic.avi
Download Microsoft Video (AVI) (48.9 MB)Veteran trees and saproxylic insects in the floodplains of lower Morava and Dyje rivers, Czech Republic.pdf
Download PDF (5.6 MB)Acknowledgements
The authors would like to thank Monika Hradecká for language editing and Veronika Kapustová for recording the demonstration video.
Disclosure statement
No potential conflict of interest was reported by the authors.
ORCID
Jan Miklín http://orcid.org/0000-0002-0125-2539
Additional information
Funding
References
- Adámek, M., Bobek, P., Hadincová, V., Wild, J., & Kopecký, M. (2015). Forest fires within a temperate landscape: A decadal and millennial perspective from a sandstone region in Central Europe. Forest Ecology and Management, 336, 81–90. doi:10.1016/j.foreco.2014.10.014
- Altman, J., Doležal, J., & Čížek, L. (2016). Age estimation of large trees: New method based on partial increment core tested on an example of veteran oaks. Forest Ecology and Management, 380, 82–89. doi:10.1016/j.foreco.2016.08.033
- Altman, J., Hédl, R., Szabó, P., Mazůrek, P., Riedl, V., Müllerová, J., … Doležal, J. (2013). Tree-rings mirror management legacy: Dramatic response of standard oaks to past coppicing in Central Europe. PLoS One, 8, e55770. doi:10.1371/journal.pone.0055770
- Bengtsson, J., Nilsson, S. G., Franc, A., & Menozzi, P. (2000). Biodiversity, disturbances, ecosystem function and management of European forests. Forest Ecology and Management, 132, 39–50. doi:10.1016/S0378-1127(00)00378-9
- Bílý, S. (2002). Summary of the bionomy of the Buprestid beetles of Central Europe (Coleoptera: Buprestidae). Acta Entomologica Musei Nationalis Pragae, 10, 1–104.
- Bürgi, M. (1999). A case study of forest change in the Swiss lowlands. Landscape Ecology, 14, 567–576. doi:10.1023/A:1008168209725
- Buse, J., Ranius, T., & Assmann, T. (2008). An endangered longhorn beetle associated with old oaks and its possible role as an ecosystem engineer. Conservation Biology, 22, 329–337. doi:10.1111/j.1523-1739.2007.00880.x
- Čížek, L., & Hauck, D. (2008). Extinční dluh v našich lesích: Fauna starých stromů na Břeclavsku [Extintion debt in our forests: Fauna of old trees in Břeclavsko]. Lesnická práce, 87, 19–21.
- DeBoer, M. (2015). Understanding the heat Map. Cartographic Perspectives, 80, 39–43. doi:10.14714/CP80.1314
- Dolek, M., Freese-Hager, A., Bussler, H., Floren, A., Liegl, A., & Schmidl, J. (2009). Ants on oaks: Effects of forest structure on species composition. Journal of Insect Conservation, 13, 367–375. doi:10.1007/s10841-008-9181-2
- Dresler, P., & Macháček, J. (2013). Vývoj osídlení a kulturní krajiny dolního Podyjí v raném středověku [The history of settlement and the cultural landscape in the lower Dyje (Thaya) River region in the Early Middle Ages]. Archeologické Rozhledy, 65, 663–675. Retrieved March 23, 2016, from http://is.muni.cz/repo/1136255/Dresler-Machacek_663-705.pdf.
- ESRI. (2016). ArcGIS online. Retrieved July 13, 2016, from http://www.esri.com/software/arcgis/arcgisonline
- Fischer, J., Stott, J., & Law, B. S. (2010). The disproportionate value of scattered trees. Biological Conservation, 143, 1564–1567. doi:10.1016/j.biocon.2006.04.023
- Hall, S. J. G., & Bunce, R. G. H. (2011). Mature trees as keystone structures in Holarctic ecosystems – A quantitative species comparison in a northern English park. Plant Ecology & Diversity, 4, 243–250. doi:10.1080/17550874.2011.586735
- Hartel, T., Hanspach, J., Abson, D. J., Mathe, O., Moga, C. I., & Fischer, J. (2014). Bird communities in traditional wood-pastures with changing management in Eastern Europe. Basic and Applied Ecology, 15, 385–395. doi:10.1016/j.baae.2014.06.007
- Hédl, R., Kopecký, M., & Komárek, J. (2010). Half a century of succession in a temperate oakwood: From species-rich community to mesic forest. Diversity and Distributions, 16, 267–276. doi:10.1111/j.1472-4642.2010.00637.x
- Horak, J., Vodka, S., Kout, J., Halda, J. P., Bogusch, P., & Pech, P. (2014). Biodiversity of most dead wood-dependent organisms in thermophilic temperate oak woodlands thrives on diversity of open landscape structures. Forest Ecology and Management, 315, 80–85. doi:10.1016/j.foreco.2013.12.018
- Hultberg, T., Gaillard, M. J., Grundmann, B., & Lindbladh, M. (2015). Reconstruction of past landscape openness using the landscape reconstruction algorithm (LRA) applied on three local pollen sites in a southern Swedish biodiversity hotspot. Vegetation History and Archaeobotany, 24, 253–266. doi:10.1007/s00334-014-0469-8
- Jonsell, M. (2012). Old park trees as habitat for saproxylic beetle species. Biodiversity and Conservation, 21, 619–642. doi:10.1007/s10531-011-0203-0
- Kadlec, J., Grygar, T., Světlík, I., Ettler, V., Mihaljevič, M., Diehl, J. F., … Svitavská-Svobodová, H. (2009). Morava river floodplain development during the last millennium, Strážnické Pomoraví, Czech Republic. The Holocene, 19, 499–509. doi:10.1177/0959683608101398
- Kopecký, M., Hédl, R., & Szabó, P. (2013). Non-random extinctions dominate plant community changes in abandoned coppices. Journal of Applied Ecology, 50, 79–87. doi:10.1111/1365-2664.12010
- Lindenmayer, D. B., Laurance, W. F., & Franklin, J. F. (2012). Global decline in large old trees. Science, 338, 1305–1306. doi:10.1126/science.1231070
- Lindenmayer, D. B., Laurance, W. F., Franklin, J. F., Likens, G. E., Banks, S. C., Blanchard, W., … Stein, J. A. R. (2014). New policies for old trees: Averting a global crisis in a keystone ecological structure. Conservation Letters, 7, 61–69. doi:10.1111/conl.12013
- Lindhe, A., Lindelöw, Å., & Åsenblad, N. (2005). Saproxylic beetles in standing dead wood density in relation to substrate sun-exposure and diameter. Biodiversity and Conservation, 14, 3033–3053. doi:10.1007/s10531-004-0314-y
- Manning, A. D., Fischer, J., & Lindenmayer, D. B. (2006). Scattered trees are keystone structures – implications for conservation. Biological Conservation, 132, 311–321. doi:10.1016/j.biocon.2006.04.023
- Miklín, J., & Čížek, L. (2014). Erasing a European biodiversity hot-spot: Open woodlands, veteran trees and mature forests succumb to forestry intensification, succession, and logging in a UNESCO biosphere reserve. Journal for Nature Conservation, 22, 35–41. doi:10.1016/j.jnc.2013.08.002
- Miklín, J., & Hradecký, J. (2016a). Confluence of the Morava and Dyje rivers: A century of landscape changes in maps. Journal of Maps, 12, 630–638. doi:10.1080/17445647.2015.1068714
- Miklín, J., & Hradecký, J. (2016b). Změny struktury krajiny v oblasti soutoku Moravy a Dyje [Landscape structure changes at the confluence of the Morava and Dyje rivers]. Geografie, 121, 368–389.
- Niklasson, M., Zin, E., Zielonka, T., Feijen, M., Korczyk, A. F., Churski, M., … Brzeziecki, B. (2010). A 350-year tree-ring fire record from Białowieża primeval forest, Poland: Implications for central European lowland fire history. Journal of Ecology, 98, 1319–1329. doi:10.1111/j.1365-2745.2010.01710.x
- Rackham, O. (1998). Savanna in Europe. In K. J. Kirby & C. Watkins (Eds.), The ecological history of European forests (pp. 1–24). Wallingford: CAB International.
- Ramírez-Hernández, A., Micó, E., de los Ángeles Marcos-García, M., Brustel, H., & Galante, E. (2014). The ‘dehesa’, a key ecosystem in maintaining the diversity of Mediterranean saproxylic insects (Coleoptera and Diptera: Syrphidae). Biodiversity and Conservation, 23, 2069–2086. doi:10.1007/s10531-014-0705-7
- Ranius, T., Aguado Martín, L. Ó., Antonsson, K., Carpaneto, G. M., Ballerio, A., Audisio, P., … Ruicanescu, A. (2005). Osmoderma eremita (Coleoptera, Scarabaeidae, Cetoniinae) in Europe. Animal Biodiversity and Conservation, 28, 1–44. Retrieved September 1, 2016, from http://www.bcn.cat/museuciencies_fitxers/imatges/FitxerContingut6653.pdf.
- Read, H. (2000). Veteran trees: A guide to good management. Peterborough: English Nature.
- Schlaghamersky, J., & Omelkova, M. (2007). The present distribution and nest tree characteristics of Liometopum microcephalum (Panzer, 1798) (Hymenoptera: Formicidae) in South Moravia. Myrmecological News, 2007, 1994–4136.
- Sebek, P., Vodka, S., Bogusch, P., Pech, P., Tropek, R., Weiss, M., … Cizek, L. (2016). Open-grown trees as key habitats for arthropods in temperate woodlands: The diversity, composition, and conservation value of associated communities. Forest Ecology and Management, 380, 172–181. doi:10.1016/j.foreco.2016.08.052
- Siitonen, J., & Ranius, T. (2015). The importance of veteran trees for saproxylic insects. In K. J. Kirby & C. Watkins (Eds.), Europe’s changing woods and forests: From wildwood to managed landscapes (pp. 140–153). Wallingford: CAB International.
- Spitzer, L., Konvička, M., Tropek, R., Tuf, I. H., & Tufová, J. (2008). Does closure of traditionally managed open woodlands threaten epigeic invertebrates? Effects of coppicing and high deer densities. Biological Conservation, 141, 827–837. doi:10.1016/j.biocon.2008.01.005
- Szabó, P. (2009). Open woodland in Europe in the Mesolithic and in the Middle Ages: Can there be a connection? Forest Ecology and Management, 257, 2327–2330. doi:10.1016/j.foreco.2009.03.035
- Szabó, P., Müllerová, J., Suchánková, S., & Kotačka, M. (2015). Intensive woodland management in the middle ages: Spatial modelling based on archival data. Journal of Historical Geography, 48, 1–10. doi:10.1016/j.jhg.2015.01.005
- Vera, F. W. M. (2000). Grazing ecology and forest history. Wallingfort: CABI.
- Vodka, Š, Konvička, M., & Čížek, L. (2009). Habitat preferences of oak-feeding xylophagous beetles in a temperate woodland: Implications for forest history and management. Journal of Insect Conservation, 13, 553–562. doi:10.1007/s10841-008-9202-1
- Vondrák, J., Malíček, J., Palice, Z., Coppins, B., Kukwa, M., Czarnota, P., … Acton, A. (2016). Methods for obtaining more complete species lists in surveys of lichen biodiversity. Nordic Journal of Botany. doi:10.1111/njb.01053
