1. Introduction
Malnutrition may be classified as acute or chronic and by severity into mild, moderate, and severe forms. Features of chronic malnutrition include diminished weight gain, stunted growth, mental apathy, and developmental delay.
Acute protein-energy malnutrition (PEM) manifests itself most commonly as marasmus (adapted form) but also as kwashiorkor; however, some children may have signs of both (marasmic kwashiorkor). The occurrence of nutritional edema in the two latter forms heralds a state of decompensation (mal-adapted form). One diagnoses marasmus when weight-for-height (WFH) is more than 3 SDs below the mean for age and sex (weight-for-height Z score [WHZ] of less than −3), whereas kwashiorkor is characterized by pitting pedal edema, independent of height or weight. Children may also present with marasmic kwashiorkor, with edema superimposed upon on severe wasting. Similarly, severe stunting is a height (or length) of more than 3 SDs below that expected for age (height-for-age Z-score [HAZ] of <−3). In addition, children with kwashiorkor exhibit behavioral symptoms such as lethargy and apathy when left alone, with increased irritability with physical contact [Citation1].
Moderate malnutrition is defined by anthropometric values between −3 and −2 SDs from the expected. Mild or ‘at-risk’ malnutrition is considered if any of the above-described indexes fall below 1 standard deviation less than the median value for the reference population (Z score <−1 SD). The mid-upper-arm circumference (MUAC) is considered a measure of lean body mass and correlates strongly with WHZ. It is a strong predictor of mortality [Citation1,Citation2].
Nutritional stunting may occur at several epochs of an individual’s life history: in utero, caused by insufficient maternal nutrition and/or intrauterine undernutrition; during infancy by a lack of breastfeeding until 6 months of age, or during later infancy due to the delayed introduction of complementary feeding, inadequate (quantity and quality) complementary feeding, or impaired absorption of nutrients, especially due to multiple episodes of infectious diseases [Citation3].
The consequences of child stunting may be both immediate and longer term. They include increased morbidity and mortality, poor child development and learning capacity, increased risk of infections and non-communicable diseases, increased susceptibility to accumulate central fat, lower fat oxidation, lower energy expenditure, insulin resistance and a higher risk to develop diabetes, hypertension, dyslipidemia, lowered working capacity, and unfavorable maternal reproductive outcomes in adulthood. Furthermore, stunted children, who experienced rapid weight gain after 2 years, have an increased risk of becoming overweight or obese later in life (components of the metabolic syndrome) [Citation2–4].
2. Infection and malnutrition
Infection and malnutrition are closely linked. Malnutrition is the primary cause of immunodeficiency worldwide. Protein-energy malnutrition (PEM) greatly increases susceptibility to major human infectious diseases in low- and middle-income countries, particularly in children. Despite strong evidence of associations among undernutrition, infection, and an increased risk of death for infants and young children, the mechanisms driving this vulnerability are not fully understood. Efforts to determine causal pathways are hampered by the complex interplay between nutritional status and infection that results in this vicious cycle. Infection results in undernutrition due to nutrient loss, reduced uptake, and increased energy requirements. Undernutrition drives an increased risk of infection by reducing gut barrier function, modifying the intestinal microbiota, altering regulation of inflammatory adipocytokines, and limiting the uptake of key micro- and macronutrients [Citation5].
Infection itself contributes to malnutrition. The relationship of malnutrition to immune suppression and infection is further complicated by the profound effects of a number of infections on the nutritional status; for example, gastrointestinal infection can lead to diarrhea and malabsorption; HIV/AIDS, tuberculosis, and other chronic infections can cause cachexia and anemia, and intestinal parasites can cause anemia and nutrient deprivation.
PEM is a common cause of secondary immune deficiency and susceptibility to infection in humans. Severe malnutrition during early childhood leads to immunodeficiency that represents a key factor in susceptibility to infections and has therefore been termed nutritionally acquired immunodeficiency syndrome [Citation6].
In addition to protein–calorie deficiency, micronutrient deficiencies (e.g. iron, zinc, and vitamins A, D, and B12) have negative effects such as growth faltering, impaired intellect, higher susceptibility to infection, and increased mortality [Citation7].
Severe protein malnutrition in the new born and in infants causes atrophy of the thymus with reduced cell numbers and subsequently ill-developed peripheral lymphoid organs. Moreover, acute malnutrition inhibits activation-induced T cell glucose metabolism. This step is required to shift the T cells from oxidative to glycolytic metabolism and is critical to maintain T cell function. Decreased glucose availability inhibits T cell cytokine production and proliferation [Citation8,Citation9].
Plasma concentrations of several mediators of the inflammatory cascade have been studied in children with PEM. The data support the notion that children with edematous malnutrition show increased inflammatory reactivity that may contribute to edema formation [Citation10]. In children with anorexia nervosa (another form of PEM), research is suggestive of increased levels of various pro-inflammatory cytokines as well as the spontaneous production of tumor necrosis factor [Citation11].
In sum, the link between infection and malnutrition appears to be complex. It is not only an imbalance between Th1 and Th2 cytokines, but many other processes are actively contributing. These include impaired intestinal cell turnover, disruption of gut barrier function, effect of microbial toxins, and other host responses [Citation12]. These could offer a potential explanation how malnutrition predisposes to infection and/or inflammation.
3. Inflammation and nutrition
Emerging evidence also suggests that immune system function is heavily influenced by signals of cellular stress, including those of nutrient availability. The integrated stress response (ISR) is a coordinated cellular program that allows cells to respond to such micro-environmental stressors, including endoplasmic reticulum (ER) stress and nutrient deprivation [Citation13]. It has been shown that nutritional status can influence the host response to pathogen and influence the genetic make-up of the viral genome. Nutritionally deficient mice infected with viral quasispecies developed severe pathology. Isolation and sequencing of virus recovered from the nutritionally deficient host demonstrate selection of a variant genotype [Citation14].
Pro-inflammatory cytokines balance anabolism and catabolism and maintain normal myogenesis. During muscle wasting (as in PEM), their enhanced expression can lead to marked destructive metabolism in skeletal muscle [Citation15]. In the human, IL-6 profoundly alters amino acid turnover and causes a substantial decrease in plasma amino acid concentrations with a concomitant decrease in muscle protein turnover and a modest increase in net muscle degradation. TNF-α can act directly on mature muscle to accelerate protein degradation. These data can explain the marked disturbance of circulating amino acids (both quantity and quality) as well as muscle wasting and edema in infants with kwashiorkor [Citation10,Citation15,Citation16].
Malnutrition-related changes in intestinal microbiota contribute to growth faltering and dysregulated inflammation and immune function because the integrity of the gastrointestinal mucosa is commonly impaired and, together with reduced gastric acid secretion, leads to an increased susceptibility to some pathogens [Citation8]. Malnutrition also causes immunosuppression through a variety of mechanisms, including the involvement of leptin and the hypothalamic–pituitary–adrenal axis [Citation6].
4. Inflammation and the endocrine system
Cortisol increases markedly in children with PEM, especially those with infection. High levels of glucocorticoids induce muscle atrophy both in vivo and in vitro by stimulating protein breakdown and inhibiting protein synthesis. Glucocorticoids suppress the GH/IGF1 axis through inhibiting GH secretion and downregulating GH receptors in the liver, thereby inhibiting IGF1 production and activity [Citation17].
GH levels are high in children with PEM and represent an important means of mobilizing fat stores to maintain euglycemia in states of undernutrition [Citation18].
Growth hormone (GH) and IGF-1 also have immunoregulatory effects. A study on 147 infants with malnutrition showed that infection and inflammation were linked to evidence of GH resistance, and the low level of IGF-1 was associated with growth impairment [Citation19].
GH and IGF-1 may act to protect the host from lethal bacterial infection by promoting the maturation of myeloid cells, stimulating phagocyte migration, priming phagocytes for the production of superoxide anions and cytokines, and enhancing opsonic activity. Therefore, GH resistance and low IGF1 may compromise these immune-regulatory functions during malnutrition and infection [Citation20]. In addition, high TNFα during infection/sepsis mediates hepatic GH resistance by inhibiting the duration of signaling via the Janus kinase 2/STAT5 pathway [Citation21]. This form of GH resistance contributes to the catabolism of muscle protein.
Cytokines, normally upregulated under conditions of chronic inflammation, can act individually or in combination suppress longitudinal bone growth by direct local effects at the growth plate level, in addition to systemically suppressing IGF1 [Citation22]. The increase in local muscle cytokines produced during inflammation makes the muscle GH-resistant and reduces its own IGF-I production. Not only decreased IGF-I production by muscle but also decreased muscle sensitivity to the anabolic effects of IGF-I may contribute to muscle wasting observed in children with severe PEM [Citation23].
Pro-inflammatory cytokines increase glucose, alanine, and triacylglycerol utilization but inhibit insulin secretion in a clonal pancreatic β-cell line. This can explain in part the low insulin/glucose ratio and decreased insulin secretion in children with PEM. Insulin is a potent anabolic stimulus for muscle proteins. Insulin deficiency (in the presence of high cortisol) leads to a protein catabolic state with loss of muscle mass and explains, in part, muscle wasting during PEM [Citation24].
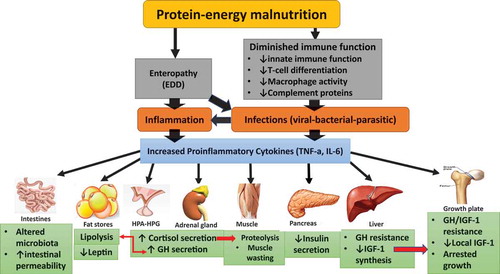
It appears that pro-inflammatory cytokines dominate the anti-inflammatory cytokines in PEM. These systemic inflammatory mediators can directly and indirectly contribute to muscle wasting and growth delay in these children. They are mediated through their potent effects on many tissues and organs (liver, muscle, pancreas, and adipose tissue), the hypothalamic–pituitary–adrenal (HPA) axis, GH–IGF1 axis, growth plate, pancreatic beta-cells, and the digestive system. Collectively, these alterations lead to a catabolic state (muscle protein wasting, subcutaneous fat loss, and anorexia–cachexia). Finally, myokines secreted by skeletal muscle itself in response to inflammation have been implicated as autocrine and endocrine mediators of cachexia, as well as potential modulators of this debilitating condition ().
References
- Grover Z, Ee LC. Protein energy malnutrition. Pediatr Clin North Am. 2009;56(5):1055–1068.
- Canani RB, Costanzo MD, Leone L, et al. Epigenetic mechanisms elicited by nutrition in early life. Nutr Res Rev. 2011;24(2):198–205.
- Briend A, Khara T, Dolan C. Wasting and stunting – similarities and differences: policy and programmatic implications. Food Nutr Bull. 2015;36(1Suppl):S15–23.
- De Sanctis V, Soliman A, Alaaraj N, et al. Early and long-term consequences of nutritional stunting: from childhood to adulthood. Acta Biomed. 2021;92(1):e2021168.
- Gwela A, Mupere E, Berkley JA, et al. Undernutrition, host immunity and vulnerability to infection among young children. Pediatr Infect Dis J. 2019;38(8):e175–e177.
- Schaible UE, Kaufmann SH. Malnutrition and infection: complex mechanisms and global impacts. PLoS Med. 2007;4(5):e115.
- Katona P, Katona-Apte J. The interaction between nutrition and infection. Clin Infect Dis. 2008;46(10):1582–1588.
- Ibrahim MK, Zambruni M, Melby CL, et al. Impact of childhood malnutrition on host defense and infection. Clin Microbiol Rev. 2017;30(4):919–971.
- Gerriets VA, MacIver NJ. Role of T cells in malnutrition and obesity. Front Immunol. 2014;5:379.
- Sauerwein RW, Mulder JA, Mulder L, et al. Inflammatory mediators in children with protein-energy malnutrition. Am J Clin Nutr. 1997;65(5):1534–1539.
- Gibson D, Mehler PS. Anorexia nervosa and the immune system – a narrative review. J Clin Med. 2019;8(11):1915.
- Liu J, Bolick DT, Kolling GL, et al. Protein malnutrition impairs intestinal epithelial cell turnover, a potential mechanism of increased cryptosporidiosis in a murine model. Infect Immun. 2016 Nov 18;84(12):3542–3549.
- Ravindran R, Loebbermann J, Nakaya HI, et al. The amino acid sensor GCN2 controls gut inflammation by inhibiting inflammasome activation. Nature. 2016;531(7595):523–527.
- Beck MA, Handy J, Levander OA. Host nutritional status: the neglected virulence factor. Trends Microbiol. 2004 Sep;12(9):417–423.
- Sharma B, Dabur R. Role of pro-inflammatory cytokines in regulation of skeletal muscle metabolism: a systematic review. Curr Med Chem. 2020;27(13):2161–2188.
- Van Hall G, Steensberg A, Fischer C, et al. Interleukin-6 markedly decreases skeletal muscle protein turnover and increases nonmuscle amino acid utilization in healthy individuals. J Clin Endocrinol Metab. 2008;93(7):2851–2858.
- Manary MJ, Muglia LJ, Vogt SK, et al. Cortisol and its action on the glucocorticoid receptor in malnutrition and acute infection. Metabolism. 2006;55(4):550–554.
- Fazeli PK, Klibanski A. Determinants of GH resistance in malnutrition. J Endocrinol. 2014;220(3):R57–65.
- DeBoer MD, Scharf RJ, Leite AM, et al. Systemic inflammation, growth factors, and linear growth in the setting of infection and malnutrition. Nutrition. 2017 Jan;33:248–253.
- Saito H, Inoue T, Fukatsu K, et al. Growth hormone and the immune response to bacterial infection. Horm Res. 1996;45(1–2):50–54.
- Yumet G, Shumate ML, Bryant P, et al. Tumor necrosis factor mediates hepatic growth hormone resistance during sepsis. Am J Physiol Endocrinol Metab. 2002 Sep;283(3):E472–81.
- Cirillo F, Lazzeroni P, Sartori C, et al. Inflammatory diseases and growth: effects on the GH-IGF axis and on growth plate. Int J Mol Sci. 2017;18(9):1878.
- Webster JM, Kempen LJAP, Hardy RS, et al. Inflammation and skeletal muscle wasting during cachexia. Front Physiol. 2020;11:597675.
- Kiely A, McClenaghan NH, Flatt PR, et al. Pro-inflammatory cytokines increase glucose, alanine and triacylglycerol utilization but inhibit insulin secretion in a clonal pancreatic beta-cell line. J Endocrinol. 2007;195(1):113–123.