1. Introduction
With increasing age, frailty is recognized as an emerging new diabetes-related complication [Citation1]. As life expectancy is increasing, the number of older people with comorbid diabetes and frailty is therefore likely to increase [Citation2]. This is recognized to an extent with frailty-specific recommendations in several published clinical guidelines of diabetes management [Citation3,Citation4]. These guidelines categorize frail older people as one homogenous group with a recommendation of relaxed glycemic targets. In addition, it is recommended that the use of the new therapy of glucagon-like peptide-1 receptor agonists (GLP-1RAs) and sodium glucose transporter-2 (SGLT-2) inhibitors are to be avoided due to the high risk of weight loss, dehydration, and hypotension. Insulin therapy is considered a last resort, after diet and oral agents, due to the fear of inducing hypoglycemia in these vulnerable patients [Citation3,Citation4]. However, these fixed assumptions about frailty as a homogeneous concept should be challenged as frailty now appears to have a spectrum of different metabolic phenotypes, which may play a role in deciding what is the optimum glycemic target and what is the most suitable hypoglycemic agent [Citation5].
2. Frailty
Frailty is a state of increased vulnerability to physical or psychological stressors due to decreased physiologic reserve in multiple organ systems, which causes limited capacity to maintain homeostasis [Citation6]. Diabetes increases the risk of frailty likely due to diabetes-related complications and diabetes-associated comorbidities [Citation7]. Several tools are used for screening for frailty, which generally focus on muscle strength and physical function () [Citation8Citation9–12]. Identifying the metabolic phenotype is not currently considered in frailty assessment. Skeletal muscle contains muscle fibers type I, which promotes insulin sensitivity, and type II fibers, which increase insulin resistance and glucose intolerance [Citation13]. Therefore, the predominance of one fiber or another may influence the overall insulin sensitivity of the individual. Aging is associated with increased muscle loss and with the development of frailty, the muscle loss is accelerated and predominantly of type II, which may lead to an overall reduction of insulin resistance [Citation14]. Weight loss is not an absolute necessity for frailty diagnosis, and obesity can be associated with frailty [Citation15]. Therefore, depending on overall body weight, differential loss of muscle fibers and body adipose/muscle tissue ratio, frailty can be seen as a spectrum of metabolic phenotypes with wide variations in insulin resistance. The anorexic malnourished (AM) frail phenotype with significant muscle loss and reduced insulin resistance on one side of the spectrum and the sarcopenic obese (SO) frail phenotype with increased visceral fat and insulin resistance on the other. Between the two ends of the spectrum, there will be wide variations of clinical symptoms among frail older people with type 2 diabetes, which will need future research for precise characterization. However, the two ends of the spectrum may follow different metabolic pathways with a continuous weight loss and reduced insulin resistance in the AM phenotype that may lead to spontaneous resolution of hyperglycemia (a regressive course of diabetes) and a continuous weight gain and increased insulin resistance that lead to persistent hyperglycemia in the SO phenotype (a progressive course of diabetes) [Citation16–18].
Table 1. Frailty assessment tools
3. Glucose-lowering therapy: a phenotype-dependent choice
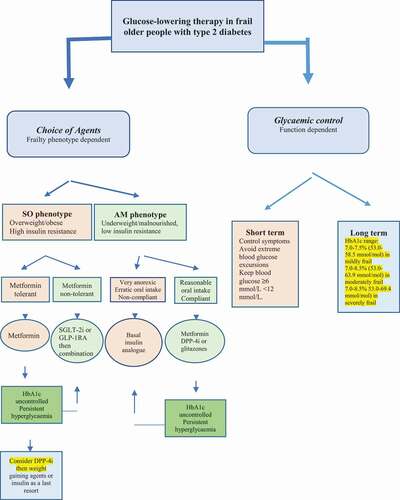
In frail older people with diabetes, the potential effect on body weight and frailty phenotype should be considered when prescribing glucose-lowering therapy. The AM frail phenotype is likely to be of advanced age, have multiple comorbidities, and less tolerance to drug therapy likely due to associated organ dysfunction. For this phenotype, agents that induce significant weight loss such as acarbose, GLP-1RAs and SGLT-2 inhibitors should be avoided due to the increased risk of further weight loss, dehydration, hypotension, and falls. Insulin secretagogues such as sulfonylureas or glinides, although they have the advantage of desirable weight gain in this phenotype, they should not be considered due to their high risk of hypoglycemia. In the milder form of this phenotype such as people who are still compliant with oral therapy and nutrition, metformin, dipeptidyl peptidase-4 (DPP-4) inhibitors or glitazones can be first-line therapy, mainly due to their lower risk of hypoglycemia. However, in patients with severe malnutrition and less compliant with oral therapy, insulin could be the first-line therapy. Insulin therapy has been shown to produce a sustained improvement in the older people’s well-being [Citation19]. Weight gain associated with insulin will be an advantage in this frail phenotype', but other insulin-associated side effects such as the inconvenience of frequent injections, blood glucose monitoring, and the increased risk of hypoglycemia should be considered as potential disadvantages. The most convenient and simple regimen is the use of one of the long-acting basal insulin analogues that can be given once daily at bedtime because of its simplicity and less risk of hypoglycemia. For example, the use of long-acting insulins (glargine or detemir) has been shown to reduce emergency department visits or hospitalization due to hypoglycemia compared with NPH insulin in older people with type 2 diabetes [Citation20]. In the SO phenotype metformin remains the preferred first-line agent due to its cardiovascular benefits, weight neutral effects and a potential positive effect on frailty [Citation21,Citation22]. The new therapies of GLP-1RAs and SGLT-2 inhibitors should be considered as second line or first line in patients not tolerant to metformin due to their advantage of inducing significant weight loss in addition to their cardiovascular and renal protection [Citation23]. DPP-4 inhibitors are a well-tolerated option with little side effects. Acarbose can be considered as an add on therapy if well tolerated. Although, it can cause diarrhea, it may have some cardiovascular benefits, low risk of hypoglycemia and it promotes weight loss [Citation24]. Insulin secretagogues and glitazones should be avoided in this frailty phenotype, and insulin should be considered as last resort due to the increased risk of further weight gain (). In both frailty phenotypes, the whole spectrum of therapy should be taken into account to reduce polypharmacy and the unnecessary burden of medications’ side effects and interactions.
Figure 1. Glucose-lowering therapy in frail older people with type 2 diabetes mellitus. The choice of an agent is dependent on the metabolic phenotype of frailty while glycemic control is dependent on functional level. SO, s arcopenic obese; AM, anorexic malnourished; SGLT-2i, sodium glucose transporter-2 inhibitor; GLP-1 RA, glucagon-like peptide-1 receptor agonists; DPP-4i, dipeptidyl peptidase-4 inhibitor.
4. Glycemic control-function dependent
The general aims of glycemic control are to achieve symptomatic benefits with avoidance of extreme dysglycaemia (hyperglycemia and hypoglycemia), in the short term, and prognostic outcomes, in the long term. Hyperglycemia increases the risk of frailty probably through inducing mitochondrial dysfunction, microvascular damage, increased inflammation and oxidative stress [Citation25]. Hypoglycemia may increase the risk of frailty by inducing repeated minor subclinical cerebral injuries or recurrent falls and fractures that may, over time, lead to functional impairment [Citation26]. Therefore, the ideal short-term glycemic control is to avoid the wide excursions in blood glucose levels to reduce the time patients spent in dysglycaemia. Zaslavsky et al. have found a U-shaped relationship between blood glucose levels and the risk of incident frailty with blood glucose levels <160 mg/dL (8.9 mmol/L) and >180 mg/dL (10 mmol/L) to be associated with increased risk of frailty (p = 0.001). [Citation27] The ideal long-term glycemic control or HbA1c is less clear. Previous studies have found that HbA1c ≥8.0 to be associated with low walking speed [Citation28] and an HbA1c >7.0% with functional disability [Citation29]. Tighter control (HbA1c <7.0%) did not show a beneficial effect on physical function and was associated with an increased risk of hypoglycemia, ffalls,and fractures [Citation30]. The U-shaped relationship demonstrated by Zaslavsky et al. has also found that an HbA1c of 7.6% (59.6 mmol/mol) to be associated with the lowest risk of frailty [Citation27] .Therefore, an HbA1c around a target of 7.5% (58.5 mmol/mol) may be a reasonable target to reduce the risk of frailty in most older people with diabetes. However, overall glycemic control should depend on overall physical function of the individual and life expectancy. A target range of 7.0–7.5% (53.0–58.5 mmol/mol) in mildly frail, 7.0–8.0% (53.0–63.9 mmol/mol) in moderately frail and 7.0–8.5% (53.0–69.4 mmol/mol) in severely frail and blood glucose levels ≥6 and <12 mmol/L have been suggested [Citation3]. Other goals of therapy are to avoid further reduction of muscle mass and function. In the SO phenotype, the use of GLP-1RAs and SGLT-2 inhibitors should be considered regardless of their function as these agents, with their novel mode of action, have very little risk of hypoglycemia and offer cardio-renal benefits independent of tight glycemic control. Therefore, the focus in this phenotype is to improve their metabolic profile, reduce weight but maintain muscle mass, through exercise as feasible and reduce cardiovascular risk. While, in the AM phenotype, the focus is to avoid further weight loss, maintain muscle mass, reduce risk of hypoglycemia and improve their quality of life ().
5. Conclusion and future perspectives
Frailty is a metabolically heterogeneous condition, with at least two different phenotypes, and the choice of hypoglycemic agents should be tailored to suit each type. The new therapies of GLP-1RAs and SGLT-2 inhibitors should be considered early in the SO but avoided in the AM phenotypes, while insulin should be considered early in the AM but a last resort in the SO phenotypes. Glycemic control remains based on function with tighter targets in less frail and relaxed goals in severely frail individuals. However, the use of GLP-1RAs and SGLT-2 inhibitors should be considered in the SO phenotype regardless of function due to their prognostic benefit that is independent of glycemic control. Future research is required to further characterize the metabolic properties of frail older people with diabetes to help precisely tailor hypoglycemic treatment. A recent study has identified five subtypes of patients with type 2 diabetes with different characteristics, insulin resistance, disease prprogression,nd risk of diabetes-related complications, which is a step toward practising a precision medicine [Citation31]. In addition, novel glucose-lowering agents that have a positive effect on frailty are still needed.
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Additional information
Funding
References
- Sinclair AJ, Abdelhafiz AH, and Rodríguez-Mañas L. Frailty and sarcopenia-newly emerging and high impact complications of diabetes. J Diabetes Complications. 2017;31:1465–1473.
- Hanlon P, Fauré I, Corcoran N, et al. Frailty measurement, prevalence, incidence, and clinical implications in people with diabetes: a systematic review and study-level meta-analysis. Lancet Healthy Longev. 2020;1:e106–e116 .
- Sinclair AJ, Abdelhafiz A, Dunning T, et al. An international position statement on the management of frailty in diabetes mellitus: summary of recommendations 2017. J Frailty Aging. 2018;7:10–20.
- LeRoith D, Biessels GJ, Braithwaite SS, et al. Treatment of diabetes in older adults: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2019;104:1520–1574.
- Abdelhafiz AH, Emmerton D, and Sinclair AJ. Impact of frailty metabolic phenotypes on the management of older people with type 2 diabetes mellitus. Geriatr Gerontol Int. 2021;21:614–622.
- Bergman H, Ferrucci L, Guralnik J, et al. Frailty: an emerging research and clinical paradigm-issues and controversies. J Gerontol A Biol Sci Med Sci. 2009;62A:731–737.
- Castrejon-Perez RC, Aguilar-Salinas CA, Gutierrez-Robledo LM, et al. Frailty, diabetes, and the convergence of chronic disease in an age-related condition: a population-based nationwide cross-sectional analysis of the Mexican nutrition and health survey. Aging Clin Exp Res. 2018;30:935–941.
- Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–56.
- Clegg A, Bates C, Young J, et al. Development and validation of an electronic frailty index using routine primary care electronic health record data. Age Ageing. 2016;45:353‐60.
- Kojima G. Frailty defined by FRAIL scale as a predictor of mortality: a systematic review and meta-analysis. J Am Med Dir Assoc. 2018;19:480–483.
- Rockwood K, Theou O. Using the clinical frailty scale in allocating scarce health care resources. Can Geriatr J. 2020;23:254–259.
- Pulok MH, Theou O, van der Valk AM, et al. The role of illness acuity on the association between frailty and mortality in emergency department patients referred to internal medicine. Age Ageing. 2020;49:1071–1079.
- Pette D, Peuker H, Staron RS. The impact of biochemical methods for single fibre analysis. Acta Physiol Scand. 1999;166:261–277.
- Sonjak V, Jacob K, Morais JA, et al. Fidelity of muscle fibre reinnervation modulates ageing muscle impact in elderly women. J Physiol. 2019;597:5009–5023.
- Watanabe D, Yoshida T, Watanabe Y, Kyoto-Kameoka Study Group. et al. A U-Shaped relationship between the prevalence of frailty and body mass index in community-dwelling Japanese older adults: the Kyoto-Kameoka study. J Clin Med. 2020;9:1367.
- Kovesdy CP, Park JC, Kalantar-Zadeh K. Glycemic control and burnt-out diabetes in ESRD. Semin Dial. 2010;23:148–156.
- Kalyani RR, Varadhan R, Weiss CO, et al. Frailty status and altered glucose-insulin dynamics. J Gerontol A Biol Sci Med Sci. 2012;67:1300–1306.
- Goulet ED, Hassaine A, Dionne IJ, et al. Frailty in the elderly is associated with insulin resistance of glucose metabolism in the postabsorptive state only in the presence of increased abdominal fat. Exp Gerontol. 2009;44:740–744.
- Reza M, Taylor C, Towse K, et al. Insulin improves well-being for selected elderly type 2 diabetic subjects. Diabetes Res Clin Pract. 2002;55:201–207.
- Bradley MC, Chillarige Y, and Lee H, et al. Severe hypoglycemia risk with long-acting insulin analogs vs neutral protamine hagedorn insulin. JAMA Intern Med. 2021;181:598–607.
- Maruthur NM, Tseng E, Hutfless S, et al. Diabetes medications as monotherapy or metformin-based combination therapy for type 2 diabetes. a systematic review and meta-analysis. Ann Intern Med. 2016;164:740–751.
- Crowley MJ, Diamantidis CJ, McDuffie JR, et al. Clinical outcomes of metformin use in populations with chronic kidney disease, congestive heart failure, or chronic liver disease: a systematic review. Ann Intern Med. 2017;166:191–200.
- Abdelhafiz AH, Sinclair AJ. Cardio-renal protection in older people with diabetes with frailty and medical comorbidities - A focus on the new hypoglycaemic therapy. J Diabetes Complications. 2020;34:107639.
- Chang YC, Chuang LM, Lin JW, et al. Cardiovascular risks associated with second-line oral antidiabetic agents added to metformin in patients with Type 2 diabetes: a nationwide cohort study. Diabet Med. 2015;32:1460–1469.
- Stout MB, Justice JN, Nicklas BJ, et al. Physiological aging: links among adipose tissue dysfunction, diabetes, and frailty. Physiology (Bethesda). 2017;32:9–19.
- Abdelhafiz AH, Rodríguez-Ma˜nas L, and Morley JE, et al. Hypoglycemia in older people-a less well recognized risk factor for frailty. Aging Dis. 2015;10:156–167.
- Zaslavsky O, Walker RL, Crane PK, et al. Glucose levels and risk of frailty. J Gerontol A Biol Sci Med Sci. 2016;71:1223–1229.
- Yoon JW, Ha YC, Kim KM, et al. Hyperglycemia is associated with impaired muscle quality in older men with diabetes: the Korean longitudinal study on health and aging. Diabetes Metab J. 2016;40:140–146.
- Godino JG, Appel LJ, Gross AL, et al. Diabetes, hyperglycemia, and the burden of functional disability among older adults in a community-based study. J Diabetes. 2017;9:76–84.
- Yau CK, Eng C, Cenzer IS, et al. Glycosylated hemoglobin and functional decline in community dwelling nursing home-eligible elderly adults with diabetes mellitus. J Am Geriatr Soc. 2012;60:1215–1221.
- Ahlqvist E, Storm P, Karajamaki A, et al. Novel subgroups of adult-onset diabetes and their association with outcomes: a data-driven cluster analysis of six variables. Lancet Diabetes Endocrinol. 2018;6:361–369.
