ABSTRACT
Introduction
Guidelines for type 2 diabetes (T2D) recommend individualized HbA1c targets to take into account patient age or frailty. We synthesized evidence from randomized controlled trials and observational studies for intensive glycemic control (HbA1c target ≤58 mmol/mol) versus standard care, in elderly (age ≥60 years) or frail adults with T2D.
Methods
Searches were performed utilizing recognized terms for T2D, frailty, older age, and HbA1c control and outcomes of interest. Meta-analysis was performed where possible. Primary outcomes included all-cause mortality, severe hypoglycemia, and hospital admission rates. Vascular complications, cognitive decline, and falls/fractures were secondary outcomes.
Results
7,528 studies were identified of which 15 different clinical studies were selected. No difference was noted in all-cause mortality with intensive control (pooled hazard ratio 0.96, 95% confidence interval 0.90–1.03), but risk of severe hypoglycemia increased (2.45, 2.22–2.72). Intensive control was associated reductions in microvascular (0.73, 0.68–0.79) and macrovascular complications (0.84, 0.79–0.89). Outcome data for risk of hospitalization, cognition, and falls/fractures were limited.
Conclusion
Intensive glycemic control was associated with reduced rates of complications but increased severe hypoglycemia. Significant heterogeneity exists and the impact of different drug regimens is unclear. Caution is needed when setting glycemic targets in elderly or frail individuals.
1. Introduction
Diabetes and frailty, generally considered to be a state of multi-morbidity and poor physiological reserve and limited resistance to physiological insults or illnesses [Citation1], are increasingly coinciding in our aging population. The impact of diabetes on elevating the risk of frailty is therefore recognized and represented in frailty scoring systems such as the electronic frailty index [Citation2–4].
Current management of type 2 diabetes (T2D), irrespective of age or co-morbidity, is centered on the management of glucose levels, assessed by HbA1c. The National Institute for Health and Care Excellence in the UK and the American Diabetes Association in the USA have broadly similar recommendations for HbA1c targets in T2D of <48 mmol/mol (6.5%) if this can be accomplished without introducing significant hypoglycemia or HbA1c <53 mmol/mol (7%) in the majority of people at high risks of hypoglycemia [Citation5,Citation6]. However, these glycemic targets are derived from decades old randomized controlled trial (RCT) evidence where those with frailty are particularly under-represented. As such, the potential benefits and risks of intensive glycemic control identified in these works may not translate to frail or elderly populations [Citation7,Citation8]. Nonetheless, the existing evidence demonstrated a significant reduction in the risk of microvascular complications such as retinopathy and nephropathy, but less clear evidence supporting tight glycemic targets to reduce the risk of overall mortality or macrovascular complications. Furthermore, there is also evidence for the potential harms of tight glycemic control. The RCT Action to Control Cardiovascular Risk in Diabetes (ACCORD), aiming for a HbA1c <42 mmol/mol in its intervention group, was stopped early due to findings that intensive glucose lowering was associated with an increase in mortality [Citation9,Citation10]. Crucially, this trial, when compared to similar trials [Citation7,Citation8], included more elderly and co-morbid population. This finding is supported by more contemporaneous observational studies which have demonstrated increased mortality with lower HbA1c levels in people with T2D and a “U-shaped” curve in which those with very high or very low HbA1c levels experienced the worst outcomes [Citation11,Citation12]. These observations are apparent in similar analyses by our group as well as another, using an elderly cohort [Citation13,Citation14].
In view of this, treatment guidelines on target HbA1c levels have emphasized the need for patient centered care and collaborative goal setting, which takes into account factors like advanced age or limited life-expectancy, multiple co-morbidity, and disease duration [Citation6]. Although HbA1c targets are recommended to be adapted in view of patient-specific factors, no specific numerical values are given – this leaves final target setting to clinician discretion following discussion with the person with T2D, with any goal being mutually agreed, and with a clear underlying rationale. Other guidelines, such as the 2014 International Diabetes Federation (IDF) meanwhile, adopt less glucose centric approaches and advocate strongly for holistic care, consideration of non-pharmacological management where appropriate, and the setting of individualized goals to reduce the risks of hospitalization and minimize hypoglycemia [Citation15,Citation16]. Additionally, separate recommendations are also made on how treatment should be adjusted to account for differing levels of physical dependence, dementia, and end of life care. Glucose control, however, remains to be an important consideration in the consensus statement by the European Diabetes Working Party for Older People (EDWPOP) which advocated for a HbA1c target of 53–59 mmol/mol for those with mild functional impairment at one end of the frailty spectrum to >70 mmol/mol in those with advanced dementia, with an emphasis on avoiding hypoglycemia and more holistic care [Citation17].
Although consensus and experience tell us that lower HbA1c targets are likely to introduce additional risks, evidence to support this is lacking. Where present, it is heterogenous and uses variable definitions of frailty and differing treatment regimens to achieve glycemic targets.
Although age is independent to frailty, and not all people of age>60 are frail, we can examine this as a proxy to try and unify current studies and their varied outcomes. It has been established that advancing age with T2D is associated with increased mortality and need for social care [Citation18]. In addition, complications of T2D due to suboptimal glycemic management can cause significant functional limitations and frailty, often more so than in younger or less frail counterparts. For example, significant eye disease [Citation19] in an elderly or frail person is associated with more profound decreases in function or loss of independence, which further increased frailty compared with younger individuals [Citation20]. Despite this, attempts to achieve this tight glycemic control in an elderly cohort, and prevent such complications, were associated with increased rates of both severe hypoglycemia [Citation21,Citation22] and falls [Citation23] – both of which can have similar derogatory impacts on quality of life. Thus, although glucose centric approaches may not be completely appropriate in frail or elderly individuals, the understanding of the role of glycemia in the prevention of complications and possible worsening frailty, while minimizing the risk of hypoglycemia, is an increasingly important area for study [Citation17]. Given the conflicting evidence for tight glycemic control, especially in an elderly or frail cohort, this systematic review was undertaken to explore and synthesize the current available RCT and observational evidence assessing the risk versus benefits of tight glycemic control on multiple relevant outcomes in a frail and/or elderly population.
2. Methods
2.1. Protocol and registration
A version protocol for this systematic review and meta-analysis was prospectively registered with PROSPERO (registration number: CRD42020175114). The final version deviates, as speculated in the initial protocol [Citation24], by the inclusion of observational studies, due to a paucity of RCT data for some of the outcomes. The reporting of this review has been undertaken in the format recommended by the PRISMA guidelines [Citation25]. A summary of the PICO (population, intervention, comparison, and outcome) model for this systematic review is shown in .
Table 1. A summary of the PICO (population, intervention, comparison, and outcome) model used for this systematic review and meta-analysis.
2.2. Eligibility criteria
RCT and observational data (cohort, case-control, and cross-sectional designs) comparing intensive versus conventional glycemic control, as defined by treatment to a pre-specified HbA1c level, in adults aged ≥60 years or with frailty (as defined by study design) were eligible for inclusion. Subgroup analyses focusing on frail or elderly cohorts from studies including a broader range of patients were eligible for inclusion. Studies published prior to 1990 or published in a non-English language were excluded. Other forms of publication or study design, such as case-series, reviews, or opinion pieces, were excluded.
Studies included had a population with age ≥60 years or specifically included frail individuals irrespective of age, using a recognized definition or assessment tool, in their design. Although those aged ≥60 may not be frail, the lack of studies identified during an informal scoping review and preparatory work meant that criteria had to be widened from the initial age of ≥65. Only studies involving individuals with T2D were included. Studies including people with type 1 diabetes mellitus, or other forms of diabetes (maturity onset diabetes of the young, type 3c diabetes, gestational diabetes) were excluded, unless outcomes for those with T2D were reported separately.
Studies were selected if their primary intervention was treatment, using any therapy, to a pre-specified HbA1c level irrespective of the eventual target attained. The pre-specified HbA1c target must have been less than 59 mmol/mol (7.5%). The comparison group of usual care should have “standard care” with or without a pre-specified level of HbA1c.
The primary outcomes of interest were all-cause mortality, severe hypoglycemia (i.e. that requires third party assistance, paramedic callout, or admission) or hospital admission for any reason. Secondary outcomes of interest included the following:
Macrovascular complications: myocardial infarctions, stroke, or heart failure
Microvascular complications: nephropathy, retinopathy, foot disease (including amputations), or neuropathy
Cognitive decline: assessed by mini-mental state examination or similar, dementia
Falls and fractures
2.3. Data sources and search strategy
The following electronic databases were searched for English language papers, within the date limits of 01/01/1990 and 25/01/2021: Cumulative Index to Nursing & Allied Health Literature (CINAHL); The Cochrane Central Register of Controlled Trials (CENTRAL); EMBASE; MedLine and PubMed.
The search terms used in OVID:Medline are displayed in Appendix 1 as an example of the terms used. These were adapted if needed for each database. Language and date limits were applied during the search, but no others were used.
2.4. Study screening and selection
Studies were imported into EndNote v9.3 for reference management purposes. Reference lists of any secondary literature found were hand-searched and cross-referenced to ensure no relevant studies were excluded. Titles were screened for irrelevance, following which abstracts were reviewed prior to full-text screening. Full-text studies were selected for inclusion providing they met the pre-specified criteria. Where full-text versions were not available for access, the authors were contacted to obtain results. If this was not possible then the study was excluded from the analysis. All studies were reviewed independently by two reviewers (TC and JJO). Any disputes regarding inclusion/exclusion were adjudicated by a third reviewer (II).
2.5. Data extraction
An adapted version of Cochrane’s data collection form for RCTs and non-RCTs was used, tailored specifically to the outcomes of this review [Citation26]. The adapted form is available as Appendix 2. Key data to be extracted included HbA1c targets, outcomes of interest, and participant characteristics including age, frailty, baseline HbA1c, duration of diabetes, weight, BMI, ethnicity, relevant co-morbidities, and medications (specifically insulin use). Outcomes were captured as hazard ratios (HR) with 95% confidence intervals to facilitate meta-analysis if possible. If this data was not available, then available outcomes were summarized in a narrative format for subsequent synthesis.
2.6. Bias assessment
Bias assessments were conducted using Cochrane’s Collaboration bias assessment tool for RCTs [Citation27]. For observational studies, the relevant version (e.g. cross-sectional, cohort) of The Joanna Briggs Institute (JBI) Critical Appraisal Checklist was used [Citation28]. Bias was assessed both within studies and across each individual outcome. Bias studies were not excluded from narrative synthesis or narrative review, however, to reduce the risk of introducing further bias into this review. The bias of included studies is one potential limitation of the study design.
2.7. Synthesis of results
Data presented as hazard ratios were synthesized by meta-analysis using generic inverse variance with log[HR] and standard errors in Stata 16 SE. A random-effects model was utilized to reflect the broad base from which the search was conducted and the desire to broaden the generalizability of the findings, as has been suggested as good practice by Borenstein et al. [Citation29]. Inter-study heterogeneity and variation were assessed and reported using Q and I2 statistics [Citation30,Citation31]. Where data were available from a combination of RCT and observational data for the impact of HbA1c levels on outcome of interest, an overall estimate of effect using hazard ratios from both randomized control trials and cohort studies complemented by subgroup analysis by study design. This was undertaken due to a lack of RCT studies and the relative observational nature of many of the RCT extensions. Data of interest from other types of study, such as cross-sectional or case-control, has been synthesized in narrative. For outcomes with an extreme paucity of study data or inter-study heterogeneity or where data was not in an appropriate format to include in meta-analysis, a narrative synthesis of the results has been presented or included alongside meta-analysis.
3. Results
3.1. Literature search and study selection
A total of 7,528 records were identified. Of which 567 records were identified through CINAHL, 98 records from Cochrane CENTRAL, 2,605 records from OVID:EMBASE, 1,747 records from OVID:Medline and 2,511 records identified through PubMed. 2,537 were immediately excluded as duplicates. Initial screening of the remaining 4,996 records titles and abstracts resulted in the further exclusion of 4,936 records which did not meet the inclusion criteria. Hand searching of the bibliographies of secondary literature or relevant subanalyses not identified by the search but included on clinicaltrials.gov yielded an additional 7 records of potential relevance.
67 records were assessed for eligibility by examining full-text content, of which 21 papers reporting the results of 15 different clinical studies with outcomes of interest were identified for final inclusion. While 6 of the studies had outcomes relevant to the review, the data presented was not in a format suitable for meta-analysis and therefore the results of these have been discussed in a narrative format. For the remaining 9 studies, at least some outcome data was available for synthesis by meta-analysis. However, for some outcomes within these studies, data may have been explored or synthesized narratively either due to a general paucity of data for that outcome across the included studies or within the study itself. Two of the studies identified for inclusion – the ACCORD trial [Citation9,Citation10] and ADVANCE trial [Citation8,Citation32] – required the assessment of multiple manuscripts (6 and 3, respectively) to fully ascertain and assess the relevant results, trial design, and risk of bias. Reasons for exclusion are demonstrated in the flowchart (. Further detail on exclusions can be found in Appendix 3.
Figure 1. Flow chart showing the systematic review process and the numbers excluded at each stage, with reasons where appropriate. Further detail can be found in Appendix 3.
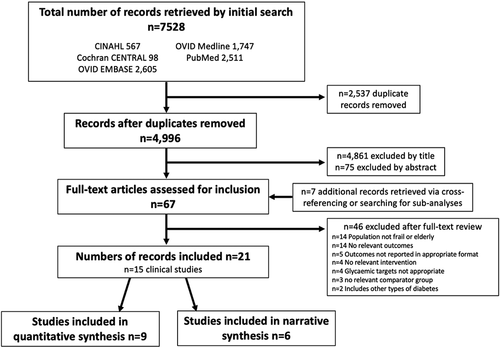
Twelve of the identified studies were observational studies – 7 cohort, 3 cross-sectional, or 2 case-control by design, and only three were RCTs. The study characteristics for the included observational studies are shown in and for the RCTs in .
Table 2. Study characteristics of the observational studies included in this review.
Table 3. Study characteristics of the randomized controlled trials included in this review.
3.2. Risk of bias assessment
Bias assessment concluded potential sources of bias in all but one study. However, much of this bias was due to lack of clarity in the reviewed manuscripts. Furthermore, of the 5 studies with the highest risk of potential bias, 3 (Puar et al. [Citation40], van Hateren et al. [Citation42], Davis et al. [Citation33]) are included in narrative synthesis only. The RCT with the highest risk of bias, Chen et al. [Citation46], has significantly smaller numbers than the ADVANCE [Citation8,Citation32] and ACCORD [Citation9,Citation10] trials, so is unlikely to influence the outcome of meta-analysis significantly. The full results of the bias assessment are included in the tables in Appendices 4 and 5a-c.
3.3. All-cause mortality
Of the identified studies, eight had outcome data for mortality (six observational studies and two RCTs). The meta-analysis of the data from these studies demonstrated in suggests that tight HbA1c targets were not associated with any significant change in all-cause mortality rates (HR 0.96; 95% CI 0.90–1.03, random effects). Findings were similar in the CPRD-based study by Ling et al., which could not be included in meta-analysis as it subdivided cardiovascular and non-cardiovascular mortality without reporting a hazard ratio for all-cause mortality and without elaborating on which causes were included [Citation38]. In this study, no clear increased risk of mortality was noted for a HbA1c of ≤53 mmol/mol for both cardiovascular mortality (HR 0.98; 95% CI, 0.91–1.06) and non-cardiovascular mortality (HR 1.05; 95% CI 0.99–1.11).
Figure 2. Forest plot showing individual study and pooled hazard ratios (subgroup and overall) for the effect of intensive glycemic control versus standard control on the risk of mortality in frail and/or elderly patients with type 2 diabetes.
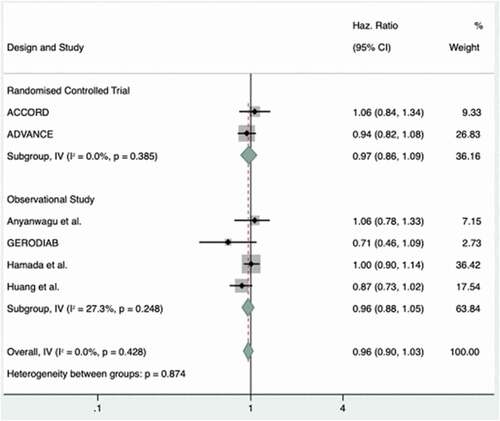
In contrast, the 2020 study by Ying et al. reported an odd’s ratio for all-cause mortality of 1.94 (95% CI 1.41–2.68) for tight glycemic control (HbA1c<48 mmol/mol). This is an outlier compared to the other outcomes of the other included studies which are otherwise homogenous in their outcomes with a Q statistic of 4.91 (5 degrees of freedom [d.f.]) and I2 0.00%.
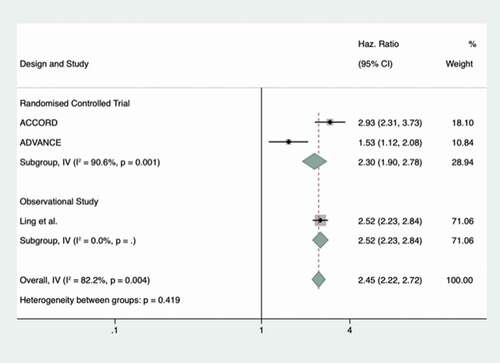
3.4. Severe hypoglycemia
Five studies had outcome data for severe hypoglycemia, including three RCTs. The three studies included in meta-analysis are shown in the forest plot (. There was a significant increase in the rate of hypoglycemia with intensive glycemic control – strongly favoring standard over tight control (HR 2.30; 95% CI 1.71–3.09, random-effects model). Inter-study heterogeneity was high with a Q statistic of 11.24 (2 d.f.) and I2 statistic = 82.2%. Two studies were not included in meta-analysis, but did contain some data of interest. The first study by Davis et al. [Citation33] investigated nursing home residents with a HbA1c≤53 mmol/mol. Severe hypoglycemia occurred at a rate of 36.8 events per 100 person/months for those aged >65 years. The rate of hypoglycemia further increased when stratified by age, with event rates per 100 person/months of 46.2 and 65 in those aged >75 years and >85 years respectively. This study also showed that hypoglycemia in those with more intensive glycemic control increases further with advancing age. Secondly, the study by Chen et al. [Citation46] was a controlled trial performed on a small group of people with T2D and Alzheimer’s disease. Participants were randomized to three different types of glycemic management – with “strength” corresponding to a target HbA1c of <53 mmol/L and “mitigate” responding to much less stringent control. The 30 people in each of these groups were compared, and an odds ratio between these groups was calculated. This showed a significant and large increase in hypoglycemia in intensive versus standard control in this cohort (odds ratio 10.55, 95% CI 1.23–90.66).
3.5. Hospital admission
Three observational studies had outcome data available for hospitalization rates, although none of these studies were suitable for meta-analysis and one only contained data for hospitalization rates secondary to hyperglycemia (or related complications). No RCTs reported hospitalization rates as an outcome.
The 2011 Diabetes and Aging study [Citation36] reported significantly lower rates of hospitalization for hyperglycemic complications in those with a HbA1c ≤53 mmol/mol (7%) compared to those with levels >53 mmol/mol (HR 0.27, 95% CI 0.08–0.46). Rates were lowest in the <42 mmol/mol group and highest, as might be expected, in those with the highest HbA1c levels (>97 mol/mol in this study). Similarly, van Hateren et al.’s 2010 analysis of their Dutch cohort showed reductions in hospitalization rates within the same HbA1c group of ≤53 mmol, for intensive versus standard glycemic control (HR 0.56, 95% CI 0.33–0.95) [Citation42]. However, this is with the caveat that this analysis only included those with a diabetes duration <5 years. In contrast to the above, Oliver et al. reported higher rates of admission in their cohort among those described as being “over-treated” – defined as those with a HbA1c ≤53 mmol/mol – and suggested a significant increase in risk of hospitalization in those who were “over-treated” (the calculated odds ratio 1.8, 95% 1.23–2.64) [Citation39].
The designs of these studies are heterogenous, and the evidence available gives no uniform signal on whether intensives or standard control reduces hospitalization rates in an elderly population.
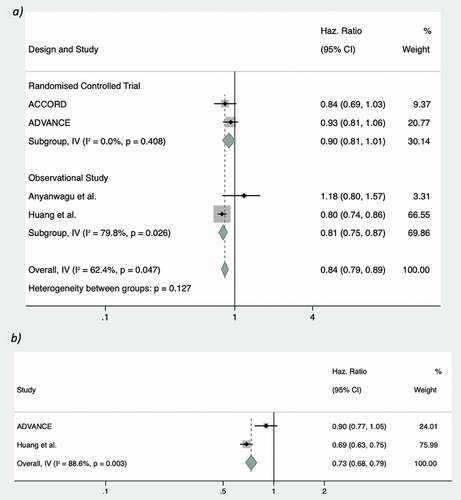
3.6. Macrovascular disease
Of the included studies, four contained outcome data for macrovascular disease, two being RCTs. All of the studies with data available for this outcome were suitable for inclusion in meta-analysis, summarized in the forest plot (. The overall result slightly favored intensive glycemic control (HR 0.88, 95% CI 0.77–1.00, random effects) versus standard glycemic control (P = 0.047). This was similar in both observational and RCT subgroups. All studies, except Anyanwagu et al. [Citation13], favored intensive glycemic control in reducing the rates of macrovascular disease. The calculated Q and I2 statistic for the RCT subgroup suggested little heterogeneity (I2 0.00%; Q 0.68, 1 d.f.) but both the Q and I2 values for the observational were consistent with high levels of heterogeneity (I2 79.8%; Q 4.96, 1 d.f.). The net effect of these translated into moderate levels of inter-study heterogeneity when the data was pooled, with an overall I2 of 62.4% and Q statistic of 7.98 (3 d.f.).
3.7. Microvascular disease
Two of the identified studies reported the required a composite outcome for rates of microvascular complications but when included in meta-analysis suggested no overall benefit of intensive glycemic control on microvascular outcomes (HR 0.78, 95% CI 0.69–1.02, random effects), as shown in . In addition to this overall outcome, the more recent analysis of those aged≥65 in the ADVANCE study by Ohkuma et al. failed to reach significance for this outcome [Citation47]. No subgroup analysis was performed as only one study of each type was identified. The I2 value of 88.6% and Q statistic of 8.76 (1 d.f.) represent potentially high levels of heterogeneity between the two studies.
One further study, a subanalysis of the ACCORD study by Tang et al. [Citation45] reported odds ratios for cardiac autonomic neuropathy only and was not included in meta-analysis. In this study, intensive versus standard glycemic control was not associated with any increased or decreased odds of developing cardiac autonomic neuropathy in those aged ≥65 years (odds ratio 0.98; 95% CI 0.8–1.21) and was therefore found to be of neutral risk or benefit.
3.8. Memory and cognition
Two RCTs contained outcome data on the effect of intensive glycemic control on memory or cognitive function. The results were not presented in a format suitable for meta-analysis. The first RCT by Chen et al. did not favor intensive glycemic control on reducing diagnoses of dementia in their cohort (odds ratio 0.63, 95% CI 0.21–1.88) [Citation46]. Conversely, Ohkuma et al.’s ADVANCE analysis revealed a near neutral effect of intensive glycemic control (HR 1.01, 95% CI 0.9–1.12) [Citation47]. These results give no clear overall signal of effect and should be interpreted with caution due to the small numbers in the study by Chen et al. and significant differences between the two studies.
3.9. Falls and fractures
Fall or fracture outcomes were reported by three studies but were not in a format suitable for meta-analysis. Two of these were observational studies. The one RCT, an analysis from ACCORD, showed no overall effect of intensive glycemic control versus standard control on risk of falls (HR 1.1, 95% CI 084–1.43); however, this analysis included some participants aged less than 65 years [Citation44]. Favoring less stringent control, the observational study by Schwartz et al. showed significantly increased falls in those with intensive glycemic control in insulin users with HbA1c <42 mmol/mol (odds ratio 4.36, 95% CI 1.32–14.46) compared to those with a HbA1c >64 mmol/mol (8%) [Citation41]. A further study found that 45% of those admitted to hospital with a fracture had a low HbA1c (<48 mmol/mol) [Citation40].
Overall, these results are suggestive of an increased risk of falling with lower HbA1c levels, but there is such marked variation in outcomes, design, and reporting that any clear conclusion is impossible to draw. A summary of all systematic review and meta-analysis is described in .
Table 4. Table summarizing the results of the systematic review for each outcome including numbers of observational or randomized controlled trials (RCT) included, pooled hazard ratios (HRs), assessments of heterogeneity and bias, and overall conclusions.
4. Discussion
The estimated overall effect for the impact of intensive glycemic control on all-cause mortality was neutral. The outcomes of the included studies were heterogenous. While several favored tight glycemic management in reducing mortality, others reported significantly increased mortality. Ultimately, the neutrality of the pooled HR along with the significant differences in study design and divergence in individual study results means it is difficult for us to conclude one way or another whether intensive glycemic control reduces mortality rates.
Despite differences in study design and significant heterogeneity, it is reasonable to conclude from this systematic review that intensive glucose control is associated with increased rates of severe hypoglycemia in frail or elderly individuals. The identified studies were universally in favor of less intensive control with the pooled HR for intensive control among meta-analyzed studies further supported by the results of the studies included in narrative. The impact of hypoglycemia on quality of life is well established, and increased rates of hypoglycemia will likely have a deleterious effect on frailty as well as potentially increased rates of cognitive decline or vascular complications [Citation48–50]. However, it is also important to recognize that most of the selected studies included large numbers (or even exclusively) of those treated with insulin and/or sulphonylureas where the hypoglycemia risks are highest.
The effect of HbA1c target on hospital admissions is similarly unclear, very little data was available; the data identified was not suitable for meta-analysis, and the results of the studies on an individual level were conflicting with two favoring intensive control and one significantly favoring less stringent control.
Our meta-analysis favors intensive control for reducing the risk of macrovascular complications to a degree of statistical significance. Additionally, we have demonstrated a trend, although failing to meet statistical significance, toward reductions in microvascular complication rates. These results are not unique to frail and elderly people with diabetes as similar findings in younger cohorts have been demonstrated in earlier meta-analyses [Citation51].
Evidence to assess the role of intensive glycemic control in reducing cognitive decline or the risk of falls and fractures is, however, lacking. The identified studies were small and contained limited quantitative data. It has been suggested that as intensive control increases the rate of cognitive decline and falls due to increased rates of hypoglycemia, but this is not fully borne out by current data and further dedicated studies are needed beyond the current evidence derived from imaging or animal studies [Citation52–54].
4.1. Limitations and strengths
While there are numerous RCTs examining intensive glycemic control in people with T2D, there is less data on those who are elderly or frail. The identified studies are heterogenous in design including the range of medications used and the level of HbA1c targeted. It can be presumed that differing levels of HbA1c less than 53 mmol/mol likely confer differing levels risk although the exact effect of this is not clear, especially to the individual. Recent works have highlighted the positive impacts of sodium-glucose linked transported 2 inhibitor (SGLT2s) and glucagon-like-peptide 1 agonists (GLP1RAs) on adverse cardiovascular outcomes but did not stratify this reporting by HbA1c levels attained therefore could not be included [Citation55]. Unfortunately, the included RCTs are from more than a decade ago, in an era where many of these drugs with cardiovascular or mortality benefits were not available [Citation56] – it may be that lowering HbA1c to a lower target level with these drugs would result in a different outcome to the drug regimens commonly used in the past. In contrast, there are studies underway to assess whether SGLT2s may increase sarcopenia and therefore increase frailty [Citation57] – an interaction that may need to be considered moving forwards. Given this, it is difficult to predict if the risks and benefits of intensive control would shift if newer medications were used to achieve these targets. Finally, some of the included studies lacked data for some outcomes of interest making some conclusions difficult to draw.
Although there are significant limitations this review highlights several important points. The key strength of this study is that it aimed to assess a wide range of important outcomes including those of particular interest to the care of frail and elderly individuals with diabetes. In doing this, we have highlighted the overall lack of robust evidence to support current practice. In addition, this study reaffirms that older patients with T2D are at significant risk of hypoglycemia irrespective impact of the heterogeneity in the studies used. The impact of glycemic control on other outcomes of interest is unclear in view of the limitations of the study, and the evidence around glycemic targets and important frailty-specific outcomes such as fractures, falls, or cognitive impairment are near non-existent.
4.2. Implication for further research
At present consensus and experience appear to be in agreement for how glycemic control in frail individuals should be managed. Nevertheless we should continue to seek to support this with robust observational or RCT evidence which, as we have demonstrated, is currently severely lacking. Further studies must be undertaken using recognized frailty scoring systems and recognize the potential differing impacts of newer glucose lowering therapies on outcomes of interest. Particular focus should be paid to important frailty-specific outcomes of interest. Furthermore, there needs to be consideration in all diabetes research of the interplay of frailty on outcomes, and the possible phenotypic differences that may affect outcomes in those with and without frailty.
5. Conclusion
This systematic review and meta-analysis have confirmed the risks of severe hypoglycemia resulting from intensive glycemic control in frail and elderly people with type 2 diabetes. While intensive glycemic control has no overall effect on mortality, it may reduce the risk of macrovascular complications and, although less significant, microvascular complications too. However, the heterogeneity of studies included suggests that these results should be interpreted with caution. When it comes to glucose control in the elderly or frail individual, a balance of risk and benefits need to be made, tailored to the individual and although a more holistic and less glucose centric approach is needed, an understanding of the role of glucose levels in this balance will continue to be key. Finally, our review highlights the significant gaps in current evidence and emphasizes the need for further works using recognized markers of frailty to establish as well as important frailty-specific outcome measures such as falls, fractures of cognitive impairment.
6. Expert opinion
Our systematic review and meta-analysis highlight the paucity of evidence from which current guidelines regarding glycemic control in the elderly or frail are derived. These highlight the need for holistic and individualized care. While there is a shift away from a glucose centric approach toward a more holistic approach of hyperglycemia management, taking into account patient co-morbid conditions and functional status, it must be acknowledged that the management of glucose levels remains a key component to prevent complications and maintain quality of life.
Although clinical experience, guidelines, and consensus would support an individualized HbA1c target in the elderly, there is little data currently available to support the objective assessment of an optimal HbA1c target, especially for frailty-specific outcomes such as cognitive decline or the risk of falls or fractures. Our systematic review and meta-analysis presented here confirmed that reduced HbA1c targets were associated with reductions in rates of micro- and macrovascular complications but with associated increased rates of hypoglycemia, with no significant positive effects on all-cause mortality.
Further studies are therefore needed. The era of the large scale randomized controlled trial looking at glycemic targets is likely to have passed, but epidemiological data is available and observational studies must be performed to assess the association between HbA1c levels and key markers of frailty as well as rates of diabetes complications so that clinicians can make informed decisions about glucose management. This should incorporate valid scoring systems that recognize frailty as its own entity rather than relying on subjective or age-derived definitions – for example this could utilize the electronic frailty score. This would allow for easy “in consultation” recognition of frail individuals with diabetes and appropriate and evidenced-based HbA1c targets to be suggested.
In addition to this quantitative work, the burden of diabetes management in elderly or frail individuals with diabetes must be assessed through qualitative work. This could be done using structured interviews or surveys of individuals and their carers. We need to gain insight not only into the risks of polypharmacy and the side-effects of medication on one the hand and the burden of diabetes symptoms and complications on quality of life on the other. The opinions of people with diabetes and their carers are likely to be the most important factor in delineating how our guidelines should evolve in the future.
Combining qualitative work with observational work should allow us to draw robust conclusions around the importance, or lack thereof, of achieving HbA1c targets in a frail and elderly population. In the future, we anticipate that work will have been completed to address the limited data available, especially for frailty-specific end-points, utilizing validated methods for recognition of frailty (e.g. the electronic frailty index). This work can inform the evolution of already existing guidance produced from consensus and clinical experience, which should also evolve in view of any qualitative work done around the experiences of people with diabetes and frailty and their carers. It may be that the conclusions of the current guidelines will not change, but the evidence from which they are derived will be significantly greater and clinicians will have the confidence to set higher or lower HbA1c targets in frail individuals.
Declaration of interest
TSJ Crabtree has received speaker fees from NovoNordisk, Abbott Diabetes Care, and Sanofi. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Additional information
Funding
References
- Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A. 2001;56(3):M146–M157.
- Yoon S-J, Kim K-I. Frailty and disability in diabetes. Ann Geriatr Med Res. 2019;23(4):165–169.
- García-Esquinas E, Graciani A, Guallar-Castillón P, et al. Diabetes and risk of frailty and its potential mechanisms: a prospective cohort study of older adults. J Am Med Dir Assoc. 2015 2015/09/01/;16(9):748–754.
- Clegg A, Bates C, Young J, et al. Development and validation of an electronic frailty index using routine primary care electronic health record data. Age Ageing. 2016;45(3):353–360.
- National Institute for Health and Care Excellence UK. Type 2 diabetes in adults: management [NG28]: National Institute for Health and Care Excellence UK; 2015 [cited 2019 Aug 15]. Available from: www.nice.org.uk/guidance/ng28/
- American Diabetes Association. 6. Glycemic targets: standards of medical care in diabetes—2019. Diabetes Care. 2019;42(Supplement 1):S61.
- RC T, RR H, CA C, et al. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. 1998;352(9131):837–853.
- Patel A, MacMahon S, Chalmers J, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008 Jun 12;358(24):2560–2572.
- Ismail-Beigi F, Craven T, Banerji MA, et al. Effect of intensive treatment of hyperglycaemia on microvascular outcomes in type 2 diabetes: an analysis of the ACCORD randomised trial. Lancet. 2010;376(9739):419–430.
- Action to Control Cardiovascular Risk in Diabetes Study GroupGerstein HC, Miller ME, Genuth S, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008; 358(24):2545–2559.
- Li W, Katzmarzyk PT, Horswell R, et al. HbA1c and all-cause mortality risk among patients with type 2 diabetes. Int J Cardiol. 2016;202:490–496.
- Arnold LW, Wang Z. The HbA1c and all-cause mortality relationship in patients with type 2 diabetes is J-shaped: a meta-analysis of observational studies. Rev Diabet Stud. 2014 Summer;11(2):138–152.
- Anyanwagu U, Mamza J, Donnelly R, et al., Relationship between HbA1c and all-cause mortality in older patients with insulin-treated type 2 diabetes: results of a large UK cohort study. Age Ageing. 2019;48(2): 235–240.
- Anyanwagu UC, Mamza JB, Mehta R, et al. Association between hba1c with cardiovascular (CV) events and all-cause mortality in elderly patients with insulin-treated type 2 diabetes (T2D). Diabetes. 2016;65(Supplement 1):A368.
- Dunning T, Sinclair A. The IDF global guideline for managing older people with type 2 diabetes: implications for nurses. J diabetes nurs. 2014;18(4):145–150.
- Dunning T, Sinclair A, Colagiuri S. New IDF guideline for managing type 2 diabetes in older people. Diabetes Res Clin Pract. 2014;103(3):538–540.
- Sinclair A, Morley JE, Rodriguez-Mañas L, et al. Diabetes mellitus in older people: position statement on behalf of the International Association of Gerontology and Geriatrics (IAGG), the European Diabetes Working Party for Older People (EDWPOP), and the international task force of experts in diabetes. J Am Med Dir Assoc. 2012 Jul;13(6):497–502.
- Brown AF, Mangione CM, Saliba D, et al. Guidelines for improving the care of the older person with diabetes mellitus. J Am Geriatr Soc. 2003 May;51(5 Suppl Guidelines):S265–80.
- Morisaki N, Watanabe S, Kobayashi J, et al. Diabetic control and progression of retinopathy in elderly patients: five-year follow-up study. J Am Geriatr Soc. 1994 Feb;42(2):142–145.
- Sloan FA, Ostermann J, Brown DS, et al. Effects of changes in self-reported vision on cognitive, affective, and functional status and living arrangements among the elderly. Am J Ophthalmol. 2005;140(4):618.e1–618.e12.
- Heller SR, Pratley RE, Sinclair A, et al. Glycaemic outcomes of an individualized treatMent aPproach for oldER vulnerable patIents: a randomized, controlled stUdy in type 2 diabetes mellitus (IMPERIUM). Diabetes Obes Metab. 2018;20(1):148–156.
- Miller ME, Bonds DE, Gerstein HC, et al. The effects of baseline characteristics, glycaemia treatment approach, and glycated haemoglobin concentration on the risk of severe hypoglycaemia: post hoc epidemiological analysis of the ACCORD study. BMJ. 2010;340:b5444.
- Nelson JM, Dufraux K, Cook PF. The relationship between glycemic control and falls in older adults. J Am Geriatr Soc. 2007 Dec;55(12):2041–2044.
- Ogendo -J-J. Systematic review and meta-analysis of randomised control trials addressing the outcomes of intensive versus conventional HbA1c control in older, frail adults with type 2 diabetes mellitus. CRD42020175114 PROSPERO PROSPERO 2020: PROSPERO; 2020 [22/01/2021]. Available from: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42020175114
- Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535.
- Higgins J, Thomas J, Chandler J, et al. Cochrane handbook for systematic reviews of interventions version 6.1. 2020.
- Higgins JPT, Altman DG, Gøtzsche PC, et al. The Cochrane collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928.
- Moola SM, Tufanaru Z, Aromataris C, et al. chapter 7: systematic reviews of etiology and risk, JBI Man Evid Synth. 2020.
- Borenstein M, Hedges LV, Higgins JP, et al. A basic introduction to fixed-effect and random-effects models for meta-analysis. Res Synth Methods. 2010 Apr;1(2):97–111.
- Higgins JPT, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557.
- Cochran WG. The combination of estimates from different experiments. Biometrics. 1954;10(1):101–129.
- Patel A, Chalmers J, Poulter N. ADVANCE: action in diabetes and vascular disease. J Hum Hypertens. 2005;19 Suppl 1:S27–32.
- Davis KL, Wei W, Meyers JL, et al. Association between different hemoglobin A1c levels and clinical outcomes among elderly nursing home residents with type 2 diabetes mellitus. J Am Med Dir Assoc. 2014 Oct;15(10):757–762.
- Doucet J, Verny C, Balkau B, et al. Haemoglobin A1c and 5-year all-cause mortality in French type 2 diabetic patients aged 70 years and older: the GERODIAB observational cohort. Diabetes Metab. 2018 Dec;44(6):465–472.
- Hamada S, Gulliford MC. Mortality in individuals aged 80 and older with Type 2 diabetes mellitus in relation to glycosylated hemoglobin, blood pressure, and total cholesterol. J Am Geriatr Soc. 2016 Jul;64(7):1425–1431.
- Huang ES, Liu JY, Moffet HH, et al. Glycemic control, complications, and death in older diabetic patients: the diabetes and aging study. Diabetes Care. 2011;34(6):1329–1336.
- Lee R, Sloane R, Pieper C, et al. Effect of hemoglobin A1c and treatment regimen on fracture risk among older men with diabetes mellitus. J Bone Miner Res. 2018;33(Supplement 1):272.
- Ling S, Zaccardi F, Lawson C, et al. Glucose control, sulfonylureas, and insulin treatment in elderly people with Type 2 diabetes and risk of severe hypoglycemia and death: an observational study. Diabetes Care. 2021;44(4):dc200876.
- Oliver A, Chodosh J, Ferris R, et al. Over-treatment of older adults with diabetes and dementia. J Am Geriatr Soc. 2019;67(Supplement 1):S120.
- Puar THK, Ng JM, Chen RY, et al. The correlation between HbA1c and risk of femoral fractures in elderly patients. Diabetologia. 2011;54(SUPPL. 1):S518.
- Schwartz AV, Vittinghoff E, Sellmeyer DE, et al. Diabetes-related complications, glycemic control, and falls in older adults. Diabetes Care. 2008 Mar;31(3):391–396.
- Van Hateren KJJ, Landman GWD, Kleefstra N, et al. Diabetes duration: a crucial factor when determining individual target levels of glycaemic control in old age? (ZODIAC-20). Diabetologia. 2010;53(SUPPL. 1):S166.
- Ying DG, Ko SH, Li YC, et al. Association between intensive glycemic control and mortality in elderly diabetic patients in the primary care: a retrospective cohort study. Prim Care Diabetes. 2020;14(5):476–481.
- Schwartz AV, Margolis KL, Sellmeyer DE, et al. Intensive glycemic control is not associated with fractures or falls in the ACCORD randomized trial. Diabetes Care. 2012;35(7):1525–1531.
- Tang Y, Shah H, Bueno Junior CR, et al. Intensive risk factor management and cardiovascular autonomic neuropathy in Type 2 Diabetes: the ACCORD trial. Diabetes Care. 2021;44(1):164.
- Chen Y, Wang J, Wang LJ, et al. Effect of different blood glucose intervention plans on elderly people with type 2 diabetes mellitus combined with dementia. Eur Rev Med Pharmacol Sci. 2017 Jun;21(11):2702–2707.
- Ohkuma T, Chalmers J, Cooper M, et al. The comparative effects of intensive glucose lowering in diabetes patients aged below or above 65 years: results from the ADVANCE trial. Diabetes Obesity Metab. 2021;23:1292–1300.
- Desouza CV, Bolli GB, Fonseca VH. Diabetes, and cardiovascular events. Diabetes Care. 2010;33(6):1389.
- Williams SA, Shi L, Brenneman SK, et al. The burden of hypoglycemia on healthcare utilization, costs, and quality of life among type 2 diabetes mellitus patients. J Diabetes Complications. 2012;26(5):399–406.
- Abdelhafiz AH, McNicholas E, Sinclair AJ. Hypoglycemia, frailty and dementia in older people with diabetes: reciprocal relations and clinical implications. J Diabetes Complications. 2016;30(8):1548–1554.
- Boussageon R, Bejan-Angoulvant T, Saadatian-Elahi M, et al. Effect of intensive glucose lowering treatment on all cause mortality, cardiovascular death, and microvascular events in type 2 diabetes: meta-analysis of randomised controlled trials. BMJ. 2011;343:d4169.
- Suh SW, Gum ET, Hamby AM, et al. Hypoglycemic neuronal death is triggered by glucose reperfusion and activation of neuronal NADPH oxidase. J Clin Invest. 2007 April/02/;117(4):910–918.
- Languren G, Montiel T, Julio-Amilpas A, et al. Neuronal damage and cognitive impairment associated with hypoglycemia: an integrated view. Neurochem Int. 2013;63(4):331–343.
- Kirchhoff B, Lugar H, Smith S, et al. Hypoglycaemia‐induced changes in regional brain volume and memory function. Diabetic Med. 2013;30(4):e151–e156.
- Karagiannis T, Tsapas A, Athanasiadou E, et al. GLP-1 receptor agonists and SGLT2 inhibitors for older people with type 2 diabetes: a systematic review and meta-analysis. Diabetes Res Clin Pract. 2021 Apr;174:108737.
- Røder ME. Major adverse cardiovascular event reduction with GLP-1 and SGLT2 agents: evidence and clinical potential. Ther Adv Chronic Dis. 2017;9(1):33–50.
- Yabe D, Shiki K, Suzaki K, et al. Rationale and design of the EMPA-ELDERLY trial: a randomised, double-blind, placebo-controlled, 52-week clinical trial of the efficacy and safety of the sodium-glucose cotransporter-2 inhibitor empagliflozin in elderly Japanese patients with type 2 diabetes. BMJ Open. 2021 Apr 7;11(4):e045844.