Cancer is a leading cause of premature mortality in patients with type 2 diabetes mellitus (T2DM) [Citation1] and T2DM is strongly associated with site-specific cancers including hepatocellular carcinoma (HCC) [Citation2]. In 2020, 830,200 people died from HCC and the incidence of HCC is expected to increase by 55% in the next 20 years [Citation3]. HCC is now the fastest growing indication for liver transplantation [Citation4] and is predicted to become the third most common cause of cancer death worldwide by 2030 [Citation5]. HCC has a very poor prognosis with a 5-year survival of just ~ 20%; however, if cases are identified at an early-stage, curative treatments are available which include surgical resection, liver transplant, or tumor ablation [Citation6].
A major risk factor for the increasing numbers of HCC is the increasing global prevalence of T2DM [Citation3,Citation5,Citation7]. T2DM is strongly associated with central obesity, insulin resistance (IR), and other features of the metabolic syndrome; and of these linked risk factors, IR in particular is strongly linked with the development of liver steatosis, inflammation, fibrosis, and liver cirrhosis. When insulin resistance is present, in the absence of excess alcohol consumption, it is most likely that NAFLD is responsible for the development of chronic liver disease. Importantly, patients with NAFLD-related cirrhosis have a risk of developing HCC at least similar to [Citation8] that reported for patients with cirrhosis occurring from other etiologies.
There is a high prevalence of all chronic liver diseases in people living with T2DM compared to the general population [Citation9]. All stages of NAFLD occur with T2DM [Citation10–13], and we now know that there is a bi-directional causality between NAFLD and T2DM [Citation14,Citation15]. A recent study of 561 patients from the United States showed a high prevalence of liver fibrosis and cirrhosis in patients with T2DM, leading to the authors advocating the need for screening [Citation11]. This study showed that, in patients with T2DM, significant fibrosis was present in 6% and severe fibrosis or cirrhosis in 9% of patients [Citation11].
NAFLD represents a spectrum of liver conditions that begins with hepatic steatosis and progresses to nonalcoholic steatohepatitis (NASH), liver fibrosis, and cirrhosis. Occasionally, hepatic steatosis occurs without changes in easily measured concentrations of liver enzymes such as alanine aminotransferase (ALT) that is commonly measured in primary care. However, it is important to recognize that increases in ALT concentration do not parallel the stages of liver disease and serum concentrations of ALT occurring within the laboratory normal range may occur in NAFLD. Sometimes patients with NAFLD may have ALT concentrations below laboratory upper limits of normal and consequently, people living with T2DM may have undiagnosed NAFLD. With that in mind, the American College of Gastroenterology (ACG) suggests that current upper limits of normal are too high and that there should be sex-specific thresholds for the upper limits of normal. The ACG recommend ALT upper limits of normal of 33 U/L for men and 25 U/L for women, respectively, and that individuals with enzyme catalytic activity concentrations above these upper limits should be further investigated [Citation16]. Despite uncertainty as to what level of ALT should trigger further investigations, concern has also been expressed about the assays used for measurement of ALT concentration [Citation17]. Nevertheless, it seems likely that many laboratories’ current upper limit of normal of 40 U/L is probably too high, and a lower threshold for defining a normal ALT result should be used.
Understanding how sex influences NAFLD is important for risk stratification and management of the disease, particularly as there is a disparity between men and women in the prevalence and severity of NAFLD [Citation18]. We know that women with prior gestational diabetes mellitus are more prone to metabolic syndrome [Citation19,Citation20] and have a higher risk of NAFLD compared with women without a history of gestational diabetes mellitus [Citation21,Citation22]. Moreover, the presence of gestational diabetes and NAFLD synergistically increases the risk of developing diabetes [Citation23]. Indeed, NAFLD was initially considered a female disease [Citation24]; however, studies have shown that NAFLD is as common in men as in women [Citation25]. A recent systematic review concluded that women have a lower risk of NAFLD than men [Citation18], but once NAFLD is established the risk of progressing to advanced fibrosis is higher in women [Citation18].
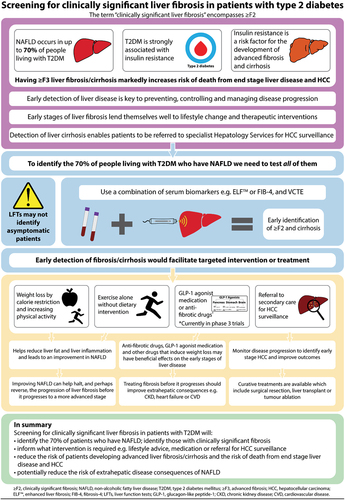
When patients have cirrhosis, they are considered at high risk of developing HCC and surveillance (for detecting HCC) is relatively simple with liver ultrasound. International guidance recommends biannual surveillance in these patients; yet despite that, less than one-third of incident cases of HCC are identified via surveillance in patients with T2DM [Citation26]. This is important as cancers that are identified via surveillance have better outcomes [Citation27]. Accordingly, the NHS England Cancer Alliance have recently incorporated the early detection of HCC into its success metrics as it strives to achieve the objectives of the NHS long-term plan.
To engage patients with T2DM into HCC surveillance, it is necessary to first identify patients with cirrhosis. In the past, liver disease was hard to identify because it usually progresses without signs or symptoms. However, several approaches have now been validated in patients with T2DM to identify asymptomatic disease. These include panels of blood tests, including FIB-4 [Citation28] and the Enhanced Liver Fibrosis (ELF™) test [Citation29], and a simple scan of the liver which uses vibration controlled-transient elastography (VCTE) to assess liver stiffness [Citation28,Citation30,Citation31] as a marker of liver fibrosis. In recent years, thresholds of liver stiffness measurements (expressed in kPa) have been established to correspond to the likelihood of a given fibrosis stage. These thresholds have been validated against liver histology assessment of liver fibrosis [Citation32].
The 2021 Korean Clinical Practice Guidelines for Diabetes Mellitus recommend evaluating adults with T2DM for NAFLD [Citation33]. Additionally, the American Diabetes Association updated their guidelines to recommend that patients with T2DM and pre-diabetes are tested for NAFLD [Citation34]. However, routine screening of liver disease in patients with T2DM is not currently recommended by the National Institute for Health and Care Excellence (NICE) [Citation35,Citation36] or the European Association for the Study of Diabetes [Citation37]. This is despite the high background prevalence of liver disease in patients with T2DM [Citation11,Citation13], the availability of validated diagnostic tests for detecting high probability of advanced liver fibrosis, and recent calls for screening for liver fibrosis in patients with T2DM [Citation11]. In 2021, NICE highlighted a lack of evidence in this area and called for further research. Existing NICE guidance [Citation38] recommends that targeted testing for liver disease should be restricted to patients with T2DM and a fatty liver on ultrasound (detecting liver steatosis) or harmful alcohol consumption or abnormal liver function tests [Citation38]. However, routine measurement of liver function tests are not recommended in the NICE guidelines for people with diabetes [Citation39], which means people with diabetes will not, as a matter of routine care, access diagnostic pathways for liver disease from their annual diabetes reviews (unless measurements of liver enzymes are undertaken for another clinical reason).
Community liver pathways are being developed in the UK to help guide primary care physicians through standardized and evidence-based processes that deal with the risk factors associated with liver disease. These pathways could help screen people to identify patients with T2DM who have evidence of potentially progressive liver disease, and who are at high risk of HCC [Citation40,Citation41]. However, currently, it is important to recognize that these primary care-focused pathways reflect NICE guidance by only testing patients with abnormal liver function tests or who have abnormal ultrasound imaging [Citation38]. This is a sub-optimal pathway for detecting liver disease in patients with T2DM. Moreover, liver function tests are not part of the routine annual diabetes review and half of patients with liver disease who are at risk of HCC have normal liver function tests [Citation42]. Consequently, relying on abnormal liver enzyme concentrations to trigger further investigation of potential liver disease is fraught with problems for people living with T2DM. By virtue of having T2DM, these people are already in a high risk group for liver fibrosis, cirrhosis, and HCC. Rather, it is important to consider whether screening all patients with T2DM for evidence of liver fibrosis should be implemented. It is now evident that NAFLD is not only associated with increased risk of adverse liver disease outcomes such as cirrhosis and end stage liver disease, but NAFLD is also associated with increased risk of CVD [Citation43], heart failure [Citation44], and CKD [Citation45].
Given that VCTE scanning is now increasingly becoming available in many communities within primary care settings, it should be possible for all patients to undergo liver fibrosis biomarker blood testing together with VCTE to determine whether they have evidence of liver fibrosis. Since the early stages of NASH are potentially amenable to the benefits of lifestyle changes, and there are now incretin receptor agonist drugs, that are licensed for both the treatment of T2DM and weight loss (that can also benefit liver disease in NAFLD [Citation46]), there is a powerful argument in 2023 supporting the detection of NASH. If the early stages of NAFLD are diagnosed, agents such as GLP-1 receptor agonists could be prescribed in patients with T2DM.
Since thresholds for each of the liver fibrosis stages have now been validated in a large series of patients who have undergone liver biopsy as the gold standard for staging liver fibrosis [Citation32], there is a case to suggest that all people with T2DM should undergo liver fibrosis biomarker testing and VCTE. That said, whether such an approach in routine care is cost effective and how often patients should be tested for liver fibrosis is uncertain. Additionally, whether VCTE and liver biomarkers can be used to monitor progression or amelioration of liver fibrosis over time is uncertain [Citation47–49]. Nevertheless, considerable progress is being made in this area for people living with diabetes and it is likely that these issues can be resolved in the near future.
In conclusion, the balance of evidence is now shifting in favor of screening all patients with T2DM for liver fibrosis with a combination of liver fibrosis biomarkers and VCTE. Detecting low-stage fibrosis would facilitate targeting patients toward lifestyle measures and certain treatments and detecting cirrhosis would enable patients to be referred for HCC surveillance (). Generating good evidence of cost effectiveness from randomized-controlled trials comparing such an approach with a control arm that uses contemporaneous usual care as a comparator arm is crucial, and hopefully it will soon be difficult for policy makers not to recommend screening of all people living with T2DM for liver fibrosis.
Figure 1. Screening for clinically significant liver fibrosis in patients with type 2 diabetes.
Abbreviations
| ACG | = | American College of Gastroenterology |
| ALT | = | Alanine aminotransferase |
| CKD | = | Chronic kidney disease |
| CVD | = | Cardiovascular disease |
| ELF™ | = | Enhanced liver fibrosis test |
| FIB-4 | = | Fibrosis 4 index |
| GLP-1 | = | Glucagon-like peptide-1 |
| HCC | = | Hepatocellular carcinoma |
| IR | = | Insulin resistance |
| kPa | = | Kilopascal |
| NAFLD | = | Nonalcoholic fatty liver disease |
| NASH | = | Nonalcoholic steohepatitis |
| NICE | = | National Institute for Health and Care Excellence |
| NHS | = | National Health Service |
| T2DM | = | Type 2 diabetes mellitus |
| U/L | = | Units per liter |
| VCTE | = | Vibration controlled transient elastography |
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Acknowledgments
The authors would like to thank the NIHR Southampton Biomedical Research Centre and the University of Southampton for their support.
Additional information
Funding
References
- Pearson-Stuttard J, Bennett J, Cheng YJ, et al. Trends in predominant causes of death in individuals with and without diabetes in England from 2001 to 2018: an epidemiological analysis of linked primary care records. Lancet Diabetes Endocrinol. 2021;9(3):165–173. doi: 10.1016/S2213-8587(20)30431-9
- Wang P, Kang D, Cao W, et al. Diabetes mellitus and risk of hepatocellular carcinoma: a systematic review and meta-analysis. Diabetes Metab Res Rev. 2012;28(2):109–122. doi: 10.1002/dmrr.1291
- Rumgay H, Arnold M, Ferlay J, et al. Global burden of primary liver cancer in 2020 and predictions to 2040. J Hepatol. 2022;77(6):1598–1606. doi: 10.1016/j.jhep.2022.08.021
- Wong RJ, Cheung R, Ahmed A. Nonalcoholic steatohepatitis is the most rapidly growing indication for liver transplantation in patients with hepatocellular carcinoma in the U.S. Hepatology. 2014;59(6):2188–2195. doi: 10.1002/hep.26986
- Akinyemiju T, Abera S, Ahmed M, et al. The burden of primary liver cancer and underlying etiologies from 1990 to 2015 at the global, regional, and national level: results from the global burden of disease Study 2015. JAMA Oncol. 2017;3(12):1683–1691. doi: 10.1001/jamaoncol.2017.3055
- Galle PR, Forner A, Llovet JM. EASL Clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69(1):182–236. doi: 10.1016/j.jhep.2018.03.019
- Petrick JL, Florio AA, Znaor A, et al. International trends in hepatocellular carcinoma incidence, 1978-2012. Int J Cancer. 2020;147:317–330. doi: 10.1002/ijc.32723
- Orci LA, Sanduzzi-Zamparelli M, Caballol B, et al. Incidence of hepatocellular carcinoma in patients with nonalcoholic fatty liver disease: a systematic review, meta-analysis, and meta-regression. Clin Gastroenterol Hepatol. 2022;20:283–92 e10. doi: 10.1016/j.cgh.2021.05.002
- Wild SH, Morling JR, McAllister DA, et al. Type 2 diabetes and risk of hospital admission or death for chronic liver diseases. J Hepatol. 2016;64(6):1358–1364. doi: 10.1016/j.jhep.2016.01.014
- Ciardullo S, Muraca E, Perra S, et al. Screening for non-alcoholic fatty liver disease in type 2 diabetes using non-invasive scores and association with diabetic complications. BMJ Open Diabetes Res Care. 2020;8(1):e000904. doi: 10.1136/bmjdrc-2019-000904
- Lomonaco R, Godinez Leiva E, Bril F, et al. Advanced liver fibrosis is common in patients with type 2 diabetes followed in the outpatient setting: the need for systematic screening. Diabetes Care. 2021;44(2):399–406. doi: 10.2337/dc20-1997
- Wild SH, Walker JJ, Morling JR, et al. Cardiovascular disease, cancer, and mortality among people with type 2 diabetes and alcoholic or nonalcoholic fatty liver disease hospital admission. Diabetes Care. 2018;41(2):341–347. doi: 10.2337/dc17-1590
- Younossi ZM, Golabi P, de Avila L, et al. The global epidemiology of NAFLD and NASH in patients with type 2 diabetes: a systematic review and meta-analysis. J Hepatol. 2019;71(4):793–801. doi: 10.1016/j.jhep.2019.06.021
- Liu Z, Zhang Y, Graham S, et al. Causal relationships between NAFLD, T2D and obesity have implications for disease subphenotyping. J Hepatol. 2020;73(2):263–276. doi: 10.1016/j.jhep.2020.03.006
- Byrne CD. Banting memorial lecture 2022: ‘type 2 diabetes and nonalcoholic fatty liver disease: partners in crime’. Diabet Med. 2022;39(10):e14912. doi: 10.1111/dme.14912
- Kwo PY, Cohen SM, Lim JK. ACG Clinical guideline: evaluation of abnormal liver chemistries. Am J Gastroenterol. 2017;112(1):18–35. doi: 10.1038/ajg.2016.517
- Panteghini M, Adeli K, Ceriotti F, et al. American liver guidelines and cutoffs for “normal” ALT: a potential for overdiagnosis. Clin Chem. 2017;63(7):1196–1198. doi: 10.1373/clinchem.2017.274977
- Balakrishnan M, Patel P, Dunn-Valadez S, et al. Women have a lower risk of nonalcoholic fatty liver disease but a higher risk of progression vs men: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2021;19:61–71 e15. doi: 10.1016/j.cgh.2020.04.067
- Buckley BS, Harreiter J, Damm P, et al. Gestational diabetes mellitus in Europe: prevalence, current screening practice and barriers to screening. A review. Diabet Med. 2012;29(7):844–854. doi: 10.1111/j.1464-5491.2011.03541.x
- Lauenborg J, Mathiesen E, Hansen T, et al. The prevalence of the metabolic syndrome in a Danish population of women with previous gestational diabetes mellitus is three-fold higher than in the general population. J Clin Endocrinol Metab. 2005;90:4004–4010. doi: 10.1210/jc.2004-1713
- Forbes S, Taylor-Robinson SD, Patel N, et al. Increased prevalence of non-alcoholic fatty liver disease in European women with a history of gestational diabetes. Diabetologia. 2011;54(3):641–647. doi: 10.1007/s00125-010-2009-0
- Cho Y, Chang Y, Ryu S, et al. History of gestational diabetes and incident nonalcoholic fatty liver disease: The Kangbuk Samsung Health Study. Am J Gastroenterol. 2023; Publish Ahead of Print. doi: 10.14309/ajg.0000000000002250.
- Cho Y, Chang Y, Ryu S, et al. Synergistic effect of non-alcoholic fatty liver disease and history of gestational diabetes to increase risk of type 2 diabetes. Eur J Epidemiol. 2023;38(8):901–911. doi: 10.1007/s10654-023-01016-1
- Sanyal AJ, American Gastroenterological A. AGA technical review on nonalcoholic fatty liver disease. Gastroenterology. 2002;123:1705–1725. doi: 10.1053/gast.2002.36572
- Weston SR, Leyden W, Murphy R, et al. Racial and ethnic distribution of nonalcoholic fatty liver in persons with newly diagnosed chronic liver disease. Hepatology. 2005;41(2):372–379. doi: 10.1002/hep.20554
- Tan DJH, Ng CH, Lin SY, et al. Clinical characteristics, surveillance, treatment allocation, and outcomes of non-alcoholic fatty liver disease-related hepatocellular carcinoma: a systematic review and meta-analysis. Lancet Oncol. 2022;23(4):521–530. doi: 10.1016/S1470-2045(22)00078-X
- Singal AG, Zhang E, Narasimman M, et al. HCC surveillance improves early detection, curative treatment receipt, and survival in patients with cirrhosis: a meta-analysis. J Hepatol. 2022;77(1):128–139. doi: 10.1016/j.jhep.2022.01.023
- Xiao G, Zhu S, Xiao X, et al. Comparison of laboratory tests, ultrasound, or magnetic resonance elastography to detect fibrosis in patients with nonalcoholic fatty liver disease: a meta-analysis. Hepatology. 2017;66(5):1486–1501. doi: 10.1002/hep.29302
- Younossi ZM, Felix S, Jeffers T, et al. Performance of the Enhanced liver fibrosis test to estimate advanced fibrosis among patients with nonalcoholic fatty liver disease. JAMA Netw Open. 2021;4(9):e2123923. doi: 10.1001/jamanetworkopen.2021.23923
- Siddiqui MS, Vuppalanchi R, Van Natta ML, et al. Vibration-controlled transient elastography to assess fibrosis and steatosis in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2019;17:156–63 e2. doi: 10.1016/j.cgh.2018.04.043
- Vuppalanchi R, Siddiqui MS, Van Natta ML, et al. Performance characteristics of vibration-controlled transient elastography for evaluation of nonalcoholic fatty liver disease. Hepatology. 2018;67(1):134–144. doi: 10.1002/hep.29489
- Eddowes PJ, Sasso M, Allison M, et al. Accuracy of FibroScan controlled attenuation parameter and liver stiffness measurement in assessing steatosis and fibrosis in patients with nonalcoholic fatty liver disease. Gastroenterology. 2019;156(6):1717–1730. doi: 10.1053/j.gastro.2019.01.042
- Hur KY, Moon MK, Park JS, et al. 2021 Clinical practice guidelines for diabetes mellitus of the Korean Diabetes Association. Diabetes Metab J. 2021;45:461–481. doi: 10.4093/dmj.2021.0156
- ElSayed NA, Aleppo G, Aroda VR, et al. 4. Comprehensive medical evaluation and assessment of comorbidities: standards of care in diabetes-2023. Diabetes Care. 2023;46:S49–S67. doi: 10.2337/dc23-S004
- National Institute for Health and Care Excellence (NICE). Type 2 diabetes in adults: management. London: NICE; 2015. ( NICE guideline [NG28]). https://www.nice.org.uk/guidance/ng28
- National Institute for Health and Care Excellence (NICE). Non-alcoholic fatty liver disease (NAFLD): assessment and management. London: NICE; 2016. ( NICE guideline [NG49]). https://www.nice.org.uk/guidance/ng49
- European Association for the Study of the L, European Association for the Study of D, European Association for the Study of O. EASL-EASD-EASO clinical practice guidelines for the management of non-alcoholic fatty liver disease. Diabetologia. 2016; 59:1121–1140. doi: 10.1007/s00125-016-3902-y
- Harrison P, Hogan BJ, Floros L, et al. Assessment and management of cirrhosis in people older than 16 years: summary of NICE guidance. BMJ. 2016;354:i2850. doi: 10.1136/bmj.i2850
- Glen J, Floros L, Day C, et al. Non-alcoholic fatty liver disease (NAFLD): summary of NICE guidance. BMJ. 2016;354:i4428. doi: 10.1136/bmj.i4428
- Srivastava A, Gailer R, Tanwar S, et al. Prospective evaluation of a primary care referral pathway for patients with non-alcoholic fatty liver disease. J Hepatol. 2019;71(2):371–378. doi: 10.1016/j.jhep.2019.03.033
- Reinson T. Performance of the Enhanced liver fibrosis score, comparison with vibration-controlled transient elastography data, and development of a simple algorithm to predict significant liver fibrosis in a community-based liver service: a retrospective evaluation. J Clin Transl Hepatol. 2023;11(4):800–808. doi: 10.14218/JCTH.2022.00335
- Mansour D, Grapes A, Herscovitz M, et al. Embedding assessment of liver fibrosis into routine diabetic review in primary care. JHEP Rep. 2021;3(4):100293. doi: 10.1016/j.jhepr.2021.100293
- Mantovani A, Csermely A, Petracca G, et al. Non-alcoholic fatty liver disease and risk of fatal and non-fatal cardiovascular events: an updated systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2021;6(11):903–913. doi: 10.1016/S2468-1253(21)00308-3
- Mantovani A, Petracca G, Csermely A, et al. Non-alcoholic fatty liver disease and risk of new-onset heart failure: an updated meta-analysis of about 11 million individuals. Gut. 2022. doi:10.1136/gutjnl-2020-323082.
- Mantovani A, Petracca G, Beatrice G, et al. Non-alcoholic fatty liver disease and risk of incident chronic kidney disease: an updated meta-analysis. Gut. 2022;71(1):156–162. doi: 10.1136/gutjnl-2020-323082
- Targher G, Mantovani A, Byrne CD. Mechanisms and possible hepatoprotective effects of glucagon-like peptide-1 receptor agonists and other incretin receptor agonists in non-alcoholic fatty liver disease. Lancet Gastroenterol Hepatol. 2023;8(2):179–191. doi: 10.1016/S2468-1253(22)00338-7
- Siddiqui MS, Yamada G, Vuppalanchi R, et al. Diagnostic accuracy of noninvasive fibrosis models to detect change in fibrosis stage. Clin Gastroenterol Hepatol. 2019;17(9):1877–85.e5. doi: 10.1016/j.cgh.2018.12.031
- Jennison E, Byrne CD. Recent advances in NAFLD: current areas of contention. Faculty Reviews. 2023;12:10. doi: 10.12703/r/12-10
- Reinson T, Buchanan RM, Byrne CD. Noninvasive serum biomarkers for liver fibrosis in NAFLD: current and future. Clin Mol Hepatol. 2023;29(Suppl):SS157–sS170. doi: 10.3350/cmh.2022.0348
