ABSTRACT
Background
We investigated how a personalized care-planning software and linked mobile-app may aid people to self-manage their type 2 diabetes (T2D) more effectively.
Research Design and Methods
People with T2D and glycated hemoglobin (HbA1c) greater than 58 mmol/mol (7.5%) were randomized to either an intervention group receiving a personalized care plan, or the control group receiving usual care. Quality of life (QoL) was measured for both groups using validated questionnaires and one-on-one interviews with a subset of 12 participants from each group.
Results
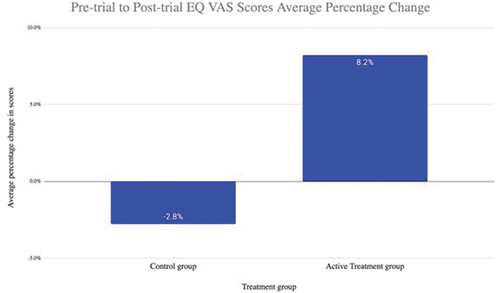
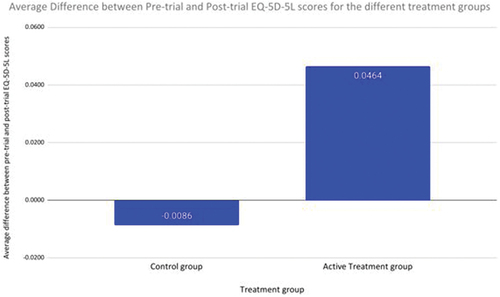
QoL for the active treatment group increased, by their EQ −5D-5 L score increasing on average by 0.046, whereas it decreased for the control group on average by 0.009. The EQ Visual Analogue Score (VAS) of the intervention group also increased by 8.2%, whereas the control group had a reduction in EQ VAS score of 2.8% (p = 0.008 for difference).
Conclusion
In this prospective RCT, the findings point to how the provision of personalized care plans can result in an improvement in individuals’ self-rated QoL. This may lead to longer term health benefits.
1. Introduction
Over the last 20 years, the importance of incorporating a person’s perspective into care and policy decisions has become increasingly apparent to both clinicians and policymakers [Citation1,Citation2]. As a consequence, many trials now collect information on individuals’ perceived health states or their perceived health-related quality of life (HRQoL). These patient reported outcome measures (PROMs) include ‘any report coming directly from individuals, without interpretation by physicians or others, about how they function or feel in relation to a health condition and its therapy’ [Citation3]. PROMs are an important addition to previous methods of measuring clinical outcomes, as these may not capture the full experience or benefits of a specific intervention. Therefore, PROMs are increasingly used as primary and secondary endpoints in RCTs such as this one [Citation1,Citation2].
Quality of life (QoL) is an essential goal for which all individuals strive toward, regardless of their personal circumstances [Citation4]. QoL is influenced by a combination of various factors, such as material living conditions, social interactions as well as health. HRQoL is an established concept in health care that takes different health-related aspects of QoL into account [Citation5]. HRQoL can be influenced by different factors, one of which is suffering from a chronic disorder, such as T2D. People with T2D are at risk of severe complications due to having high blood glucose levels. Most people with T2D have at least one complication, and cardiovascular complications are the leading cause of morbidity and mortality in these individuals [Citation6]. Maintaining independence, enabling self-management, and adapting one’s lifestyle are crucial to counteract the negative effects of a chronic disorder [Citation5]. For example, individuals with T2D have to carefully monitor their blood glucose levels and must avoid certain foods whilst following a strict diet.
For individuals with long-term conditions (LTC), those who can participate in social activities despite their chronic disorder report an improvement in their overall health status [Citation7]. By offering digital interventions, such as mobile apps, individuals can be supported in living with and improving their LTC, facilitating social interaction and thus improving their HRQoL [Citation8]. Examples of such interventions include apps to help people change their behavior toward a healthier lifestyle [Citation5].
In this study, we investigated how a personalized care-planning software and linked mobile app may aid people to manage their diabetes more effectively [Citation9]. The purpose of this evaluation was to determine the way that the intervention might influence an individual’s experience of having T2D in relation to their QoL and self-management. Our evaluation included responses to interviews conducted with five participants in the active treatment group.
2. Methods
2.1. Randomised control trial (RCT) participants
This prospective RCT compared baseline and 6-month clinical data from 197 people with T2D across 3ee surgeries in the South of the UK plus 10 surgeries in Eastern Cheshire (UK). In these data set, the participant age range was 22–85 years and the average age was 63.2 years. Out of a total of 197 participants, 116 (58.9%) were male, 65 (33.0%) were female and 16 (8.1%) did not report their gender. The QoL analysis was conducted for a subset of this sample, 12 participants from each group, using validated questionnaires and one-on-one interviews. This subset was representative of the sample as a whole.
2.2. Recruitment and randomization
Inclusion Criteria
Participants who:
are capable of reading the Patient information Sheet (PIS) and giving informed consent themselves
are over the age of 18
are living with Type 2 Diabetes Mellitus
have a smartphone and are able to use three apps that are not categorized as Utilities (i.e. clock, calculator, phone, etc.)
have an HbA1c of over 58 mmol/mol
Exclusion Criteria
Participants who:
are pregnant
are unable to read or speak English owing to the fact that the content of the app is only available in English at this time
are in another research study that clashes with the burdens of this study or where the tasks of any other study combined with this study are deemed too burdensome for the individual, as determined by the lead investigator at each GP practice and the Chief Investigator of this study.
Consecutive patients identified as potentially suitable for the study were recruited either in a face-to-face consultation or a telephone consultation in primary care. If interested, they were given or sent by post or e-mail the details of the study, with informed consent taken if they were happy to participate.
Once the participant consented to be part of the study, they were randomized using Sealed Envelope software (London, UK), to either the control or active treatment group.
2.3. Intervention
People with T2D with glycated hemoglobin (HbA1c) greater than 58 mmol/mol (7.5%) were randomized to either the active intervention group (usual care + app) or control group (usual care). The intervention group received a co-created personalized care plan which addressed individuals’ objectives and concerns, and involved daily lifestyle prompts, self-management tools and a range of educational content and local services. Randomization did not influence other decisions about diabetes management. QoL and individuals’ activation were determined quantitatively and qualitatively.
2.4. Quality of life analysis
QoL was self-rated by participants using the online survey “Healum Diabetes Study Survey’’ which included the NICE-validated EQ-5D-5 L questionnaire [Citation10]. Participants were sent a link by text message from their GP to complete this survey at two time points, once at the beginning of their time on the trial (before their care plan appointment for the intervention group), and again after 6 months on the trial. The responses were extracted and matched together for each individual to allow a comparison of their QoL before and after their 6 months in the trial.
The survey involved:
1. EQ-5D-5 L questionnaire [Citation10] which is a widely used generic measure of health status consisting of two parts, the EQ-5D-5 L descriptive system and accompanying EQ visual analogue scale (EQ VAS).
The descriptive system comprises five dimensions: mobility, self-care, usual activities, pain/discomfort and anxiety/depression, with each dimension having five levels: no problems, light problems, moderate problems, severe problems, and extreme problems. A summary index with a maximum score of 1 can be derived from these five dimensions by conversion with a table of scores. The maximum score of 1 indicates the best health state. The EQ VAS records the individual’s self-rated health on a vertical visual analogue scale, where the top endpoint is labeled ‘The best health you can imagine’ and the bottom endpoint is ‘The worst health you can imagine.’
2. Questions relating to individuals’ engagement with their health. These open-ended questions were designed to assess the knowledge, skills and confidence of individuals to manage their health.
The responses to the engagement questions were grouped into responses: ‘Yes;’ ‘Somewhat Yes;’ ‘Neutral;’ ‘Somewhat No’ and ‘No,’ whereby an answer of ‘Yes’ indicated higher engagement in their health.
2.5. Statistical methods
The outcome measure in this RCT was the changes in EQ-5D-5 L scores and EQ VAS scores from the beginning of the trial to 6 months later for participants. Analysis was restricted to those participants with two responses to the survey and those we could identify as either part of the active treatment group or the control group. We calculated the mean change in EQ-5D-5 L score and EQ VAS score during the trial relative to the baseline score for each individual. As the responses to the engagement questions were grouped into ‘yes,’ ‘no,’ etc. there was no average analysis on these responses.
2.6. Ethics approval
This study gained approval from the West Midlands REC18 June 2020 (IRAS ID 272,569). The study was performed in accordance with the Declaration of Helsinki 1964 and its later amendments. The delivery of the research was carried out exclusively by … … All participants gave informed consent in relation to participation in the study and were aware that their anonymized data would be analyzed and included in peer review publications.
3. Results
3.1. Descriptives
We were able to match the pre-trial and post-trial surveys for 12 individuals in the control group, and another 12 in the active treatment group. This subset group of 24 individuals was representative of the larger study group in terms of age, gender, and baseline HbA1c. The mean average number of days in between completion of the first and second survey for the control group was 233 days (±se 22.5 days), whereas for the active treatment group was 191 days (±se 31.2 days). The range in number of days from completion of the first survey to the second for the control group was between 126 days and 250 days, whereas for the active treatment group was 152 days to 230 days.
The mean starting HbA1c for members of the control group who fitted the inclusion criteria was 68.9 mmol/mol (±se 1.7 mmol/mol) and that of the active treatment group was 70.6 mmol/mol (±se 1.6 mmol/mol). For the treatment group, the end HbA1c result decreased to a mean average of 64.1 mmol/mol (±se 1.4 mmol/mol), with the control group end HbA1c increasing to an average of 69.1 mmol/mol (±se 1.8 mmol/mol) (p for difference in HbA1c change = 0.009). There was no statistically significant difference in BMI change between the groups.
3.2. Changes in total EQ-5D-5 L score
Individuals in the active treatment group who completed the questionnaire twice, at month 0 and month 6 of the trial, demonstrated an improvement in their self-measured QoL score by their EQ-5D-5 L rating increasing on average by 0.046 (). For those in the control group, the QoL decreased, shown by their EQ-5D-5 L rating decreasing on average by 0.009 ().
Figure 1. (a) Differences in EQ-5D-5 L scores for control and treatment group between the pre-trial and post-trial surveys completed. An increase in EQ-5D-5 L score represents a higher self-rated quality of life for the individual. (b) Average percentage change in EQ5D5L VAS scores for control and treatment groups from the pre-trial and post-trial surveys completed. An increase in EQ5D5L VAS score represents a higher self-rated quality of life for the individual.
3.3. Changes in EQ VAS score
Patients in the active treatment group who completed the questionnaire twice, at month 0 and month 6 of the trial, demonstrated an improvement in their self-measured standard of their health on that day shown by their VAS score increasing on average by 8.2% (). For those patients in the control group, their VAS score changed on average by −2.8% indicating a reduction in patients’ self-measured standard of their health ().
3.4. Changes in engagement question responses
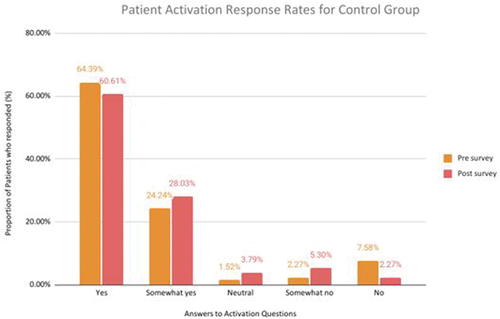
These questions (listed in ) were answered by 12 participants in the active treatment group and 12 in the control group. A comparison between pre- and post-trial responses to questions related to individual’s engagement with their health, indicated that members of the active treatment group reported higher engagement. For the part of the survey regarding an individual’s engagement in their health, an answer of ‘yes’ represents good engagement. For the control group’s pre-trial survey responses, 64.4% of responses were ‘yes’ and in the post-trial survey, 60.6% were ‘yes,’ whereas for the active treatment group, 60.6% of pre-trial responses were ‘yes,’ which increased to 76.5% post-trial ().
Figure 2. (a) Activation responses to the activation questionnaires from the active treatment group in the pre-trial survey and post-trial surveys. An increase in the number of ‘yes’ responses indicates better health engagement for the individual. (b) Activation responses to the activation questionnaires from the control group in the pre-trial survey and post-trial surveys. An increase in the number of ‘yes’ responses indicates better health engagement for the individual.
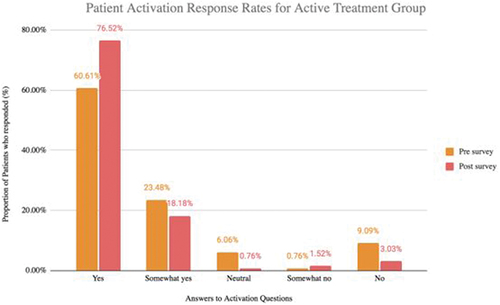
Table 1. The questions relating to engagement in the survey completed by participants in the control and active treatment groups.
The question with the largest difference in comparing the pre-trial and pre-trial survey responses for the active treatment group was ‘I have been able to manage lifestyle changes, like exercise and diet.’ For this question, 16.7% of the active treatment group’s pre-trial responses were ‘yes,’ compared to 58.3% of the post-trial responses, demonstrating an extra 41.6% of responses being ‘yes’ after individuals received the intervention. For the control group, 50% of pre-trial responses were ‘yes,’ compared to 66.7% post-trial, showing a smaller increase than the active treatment group. Another question with a large difference in responses from pre-trial to post-trial was ‘I know what support and interventions are available for my health concerns.’ For the active treatment group 50% of pre-trial responses were ‘yes,’ and post-trial 83.3% were ‘yes.’ For the control group, 66.67% of responses were ‘yes’ for this question pre-trial, compared to 58.3% post-trial.
3.5. Interview feedback
As of 1 January 2023, 5 individuals who had completed 6 months in the study, had been interviewed to find out how they use the app, what benefits they have derived from use, what they find useful and how the app may be improved. A survey of responses made on consistent responses among the group of five individuals are as follows: 5/5 responders said that the app was simple to set up; 3/5 said the app was easy to use; 4/5 said that the tracking function was useful; 4/5 said the app was motivational and 4/5 said they would continue to use the app if given the opportunity.
Through the feedback interviews, it became apparent that the app helps to increase individuals’ motivation to change their habits and self-manage their condition. This is supported by the following statements from app users, ‘The main problem for me before was that I wasn’t taking active steps to manage my diabetes. I think the app is a very useful tool – it has the right things on there to help and motivate you. Quite often all you need is a reminder – for example I forget that I shouldn’t be eating cake. The app reminds me to do certain things and keep on top of my management.’ Another app user stated ‘Having the app has made me feel more motivated. Before, whenever I went to the GP it was all about the drugs I must take and that was it. I was never really told about the things I could do myself to help my diabetes management..’ A third app user said ‘I did find it quite useful as a sort of nag, a little bit of conscience sitting on your shoulder saying you really need to get your weight down – so in that sense that constant reminder was quite useful.’
4. Discussion
4.1. Findings
In this prospective primary care based study in the UK, we investigated how a personalized care-planning software and linked mobile app may aid people to manage their diabetes more effectively [Citation9]. Here we address the secondary outcome of the RCT: does the Healum app improve the individuals’ engagement levels measured by their capability, opportunity, and/or motivation to change their behavior. We report on the experience of people with T2D over a 3 to 6-month period of using the app, as well as the experience for a comparison group not using the app but receiving usual care.
We previously reported an analysis of app access, where 30% of users used the app at least 10 times in the first month of app access, dropping to 20% in the second month [Citation11]. We also reported how regular and continued user access to the app over time was associated with a greater reduction in HbA1c than occasional access [Citation9]. For example, those individuals who used the app just once during the trial had an average proportionate HbA1c change of −1.5%, compared to −8.5% for those who used it at least two times during the trial [Citation9]. There is a myriad of different diabetes management support apps available for download free or for a small cost, but hardly any of them have been validated in an RCT as we have done here.
These results indicate an improvement in PROMs (EQ-5D-5 L and EQ VAS) over the course of the trial, when comparing the active treatment group to the control group. Specifically, there was an improvement within the intervention group in the areas of Mobility and Anxiety/Depression. The active treatment group also showed an improvement specifically in the areas of ‘managing lifestyle changes, like exercise and diet,’ as well as ‘knowing what support and interventions are available.’ These survey responses suggest that having a synchronized care plan and app lead to individuals understanding their health problems better, as well as how to prevent them, while knowing what interventions are available to support them, and most importantly, feel more confident in reducing problems with their diabetes. The qualitative feedback from the app user interviews conducted supports the notion that individuals were more actively involved in managing their health through having a care plan and having access to the app. Therefore, indicating a self-management app could be a reliable addition to usual care for individuals with T2D.
Increasing digitalization of health care brings opportunities to enable much greater access to evidence-based interventions for individuals [Citation12,Citation13]. With the evolution of diabetes technology, those living with T2D now have access to a wide range of tools with which to reduce fluctuations in their blood glucose level and achieve good glycemic control. The use of personalized care planning has been previously effective at improving the experience of care amongst people with T2D and other LTCs [Citation12,Citation13]. Diabetes technology can also have a beneficial impact on psychosocial health by reducing the burden of diabetes, with the longer term potential to reduce rates of chronic complications, decrease the cost of diabetes care, and improve the QoL for individuals [Citation14].
In recent years, the use of mobile health applications (apps) has gained significant attention as a promising tool to support individual’s self-management. RCTs evaluating the efficacy of self-management apps often rely on PROMs to assess the impact of these interventions. PROMs provide valuable insights into the individual’s perspective, allowing researchers to capture subjective experiences, symptoms, and QoL measures.
These outcome measures based on individuals’ feedback are indispensable as we increasingly move toward person-centered care. People with diabetes are often under significant psychological distress because of strict adherence to medications, changes in their daily activities, patterns such as diet and exercise, and fear of long-term macrovascular and microvascular complications, which have the potential to undermine their QoL [Citation15]. Therefore, with T2D placing a large burden on QoL, it is important to take into consideration the impact of interventions on not only health outcomes but also on QoL of individuals.
EQ-5D-5 L and EQ VAS are generic measures that can be applied across various health conditions and populations. They provide a standardized approach to evaluate HRQoL, facilitating comparisons between different groups of people and interventions. Both measures are relatively simple and quick to administer, making them feasible for use in clinical research settings, including RCTs. EQ-5D-5 L and EQ VAS have been utilized in numerous disorder-specific studies to assess the impact of interventions on individuals’ HRQoL. EQ-5D-5 L and EQ VAS are frequently used in health technology assessments to inform decision-making related to the allocation of healthcare resources. These measures provide valuable data on the economic and health outcomes associated with different interventions. Quantitative data generally support the use of EQ-5D-5 L surveys but does note some potential limitations in individuals with T2D [Citation16].
Findings similar to ours can be seen in the context of a recently published study in Germany which investigated the desirability of a digital health intervention based on personalized nutritional advice for a group of individuals with T2D, as well as the potential efficacy of such an intervention on glycemic control, self-reported well-being, and motivation to maintain diabetes self-management [Citation17]. Overall, an improvement in PROMs was seen, including a substantial improvement in energy levels, a reduction in worrying about increased blood glucose, improvement in feelings of satiety, and a reduction in difficulty in concentration over the course of the study period [Citation17,Citation18]. Although this study did not use EQ5D5L or EQ VAS as ways to evaluate PROMs, it supports the findings of our study in the potential of a digital health product to deliver effective personalized care.
4.2. Strengths and limitations
A strength of this research conducted is the fact that it was an RCT conducted in a real-world setting, where practice nurses delivered the intervention in an everyday practice setting, which is rare for digital health studies in T2D.
We accept that a major limitation is the small number of people completing the online surveys vs. the total number of participants. This was a consequence of a number of factors, not least the fact that no direct face-to-face contact between the investigation team and participants was possible because the study was performed at the time of the COVID-19 Pandemic. Another limitation is the survey itself, as while EQ-5D-5 L and EQ VAS provide a standardized assessment, they may have limited sensitivity in detecting subtle changes or specific aspects of HRQOL. Supplementing them with condition-specific measures may offer a more comprehensive evaluation.
A potential weakness is the control group. It could be argued that in order realistically to demonstrate the strength of their digital application, the control group would have to be offered an alternative application with some form of engagement, for example, documenting their weekly weight and glucose.
More research with a larger number of people would be beneficial further to understand the impact of this and similar interventions on individuals’ QoL, as well as more studies with longer follow-up in order to assess long-term results.
5. Conclusion
In this prospective RCT, the findings point to how the provision of personalized plans of care co-created in real time by patient and healthcare professionals together, plus support, and education linked to a mobile app, can result in an improvement in an individual’s self-rated QoL and engagement. This has the potential to lead to health benefits in the longer term.
Competing interests
No author has any conflict of interest. The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Authorship
All authors:
1. Made a significant contribution to the work reported, whether that’s in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas.
2. Have drafted or written, or substantially revised or critically reviewed the article.
3. Have agreed on the journal to which the article will be submitted.
4. Reviewed and agreed on all versions of the article before submission, during revision, the final version accepted for publication, and any significant changes introduced at the proofing stage.
5. Agree to take responsibility and be accountable for the contents of the article and to share responsibility to resolve any questions raised about the accuracy or integrity of the published work.
Specifically:
AHH
Ethical permission was granted by the West Midlands REC (IRAS ID 272,569). Participatory consent was obtained from all patients.
Additional information
Funding
References
- Guyatt G, Feeny D, Patrick D. Measuring health-related quality of life. Ann internal med. 1993;118(8):622–629. doi: 10.7326/0003-4819-118-8-199304150-00009
- Black N. Patient reported outcome measures could help transform healthcare. BMJ. 2013;346(jan28 1):f167. doi: 10.1136/bmj.f167
- Patrick DL, Burke LB, Powers JH, et al. Patient-reported outcomes to support medical product labeling claims: FDA perspective. Value Health. 2007;10(Suppl 2):S125–S137. doi: 10.1111/j.1524-4733.2007.00275.x
- Bakas T, McLennon SM, Carpenter JS, et al. Systematic review of health-related quality of life models. Health Qual Life Outcomes. 2012;10(1):134. doi: 10.1186/1477-7525-10-134
- Lockl J, Schick D, Stoetzer J, et al. A model to assess the impact of digital technologies on the health-related quality of life. Int J Technol Assess Health Care. 2022;38(1):e81. doi: 10.1017/S0266462322003245
- Zheng Y, Ley SH, Hu FB. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat Rev Endocrinol. 2018;14(2):88–98. doi: 10.1038/nrendo.2017.151
- Marcolino MS, Oliveira JAQ, D’Agostino M, et al. The impact of mHealth interventions: systematic review of systematic reviews. JMIR mHealth uHealth. 2018;6(1):e23. doi: 10.2196/mhealth.8873
- Wildevuur SE, Simonse LWL. Information and communication technology-enabled person-centered care for the “big five” chronic conditions: scoping review. J Med Internet Res. 2015;17(3):e77. doi: 10.2196/jmir.3687
- Heald AH, Roberts S, Albeda Gimeno L, et al. A randomised control trial to explore the impact and efficacy of the healum collaborative care planning software and app on condition management in the type 2 diabetes mellitus population in NHS primary care. Diabetes Therapy. 2023;14(6):977–988. doi: 10.1007/s13300-023-01404-6
- The EuroQol Group. EuroQol-a new facility for the measurement of health-related quality of life. Health Policy. 1990;16(3):199–208. doi: 10.1016/0168-8510(90)90421-9
- Heald AH, Roberts S, Gimeno LA, et al. Enhancing type 2 diabetes treatment through digital plans of care. Patterns of access to a care-planning app over the first 3 months of a digital health intervention. Cardiovasc Endocrinol Metab. 2023;12(2):e0283. doi: 10.1097/XCE.0000000000000283
- Velardo C, Shah SA, Gibson O, et al. EDGE COPD team. Digital health system for personalised COPD long-term management. BMC Med Inform Decis Mak. 2017 20;17(1):19. doi: 10.1186/s12911-017-0414-8
- Foley P, Steinberg D, Levine E, et al. Track: a randomized controlled trial of a digital health obesity treatment intervention for medically vulnerable primary care patients. Contemp Clin Trials. 2016;48:12–20. doi: 10.1016/j.cct.2016.03.006
- Prahalad P, Tanenbaum M, Hood K, et al. Diabetes technology: improving care, improving patient-reported outcomes and preventing complications in young people with type 1 diabetes. Diabet Med. 2018;35(4):419–429. doi: 10.1111/dme.13588
- Polonsky WH. Emotional and quality-of-life aspects of diabetes management. Curr Diab Rep. 2002;2(2):153–159. doi: 10.1007/s11892-002-0075-5
- Matza LS, Boye KS, Stewart KD, et al. A qualitative examination of the content validity of the EQ-5D-5L in patients with type 2 diabetes. Health Qual Life Outcomes. 2015;13(1):192. doi: 10.1186/s12955-015-0373-7
- Ungersboeck M, Tang X, Neeff V, et al. Personalised nutritional recommendations based on individual post-prandial glycaemic responses improve glycaemic metrics and PROMs in patients with type 2 diabetes: a real-world assessment. Nutrients. 2022;14(10):2123. doi: 10.3390/nu14102123
- Stedman M, Rea R, Duff CJ, et al. The experience of blood glucose monitoring in people with type 2 diabetes mellitus (T2DM). Endocrinol Diabetes Metab. 2022;5(2):e00302. doi: 10.1002/edm2.302