ABSTRACT
Introduction
The environmental spread of pollutants has led to a persistent exposure of living beings to multiple chemicals, by now become ubiquitous in the surrounding environment. Environmental exposure to these substances has been reported to cause multi- and/or transgenerational health effects. Per- and Polyfluorinated Substances (PFAS) raise great concern, given their known effects both as endocrine disruptors and potential carcinogens. The multi/trans-generational effects of different endocrine disruptors have been investigated by several studies, and harmful effects observed also for PFAS.
Areas covered
This review examines the current data on the multi-trans-generational effects of PFAS, with a focus on their impact on the thyroid axis. The aim is to determine if there is evidence of potential multi-trans-generational effects of PFAS on the thyroid and/or if more research is needed.
Expert opinion
PFAS exposure impacts thyroid homeostasis and can cross the placental barrier. In addition PFAS have shown multi-transgenerational effects in laboratory experiences and animal models, but thyroid disruptive effects of PFAS were also investigated only in a small number of these studies. Efforts are needed to study the adverse effects of PFAS, as not all PFAS are regulated and removal strategies are still being developed.
1. Introduction
The progress of industrialization has led to relevant consequences for the environment surrounding us. One of the implications of industrialization stems from the accumulation in the environment of persistent organic pollutants (POPs) [Citation1]. POPs are organic substances that persist in the environment, accumulate in living organisms and pose a risk to our health and the environment. POPs can be transported by air, water or migratory species across international borders, reaching regions where they were never produced nor used [Citation1]. The life and survival of any organism (animal, human, plant and others) is dependent both on its genetic background and on the influence of the environment to which, it is exposed. However, these factors should not be regarded as ‘independently acting,’ as the interaction of genetics and environment plays a crucial role in determining the phenotype. Indeed, during the last decades, it has been shown that the composition of the environment has a substantial impact on the heredity of an organism without modifying the genetic information. In vitro, in vivo and epidemiological studies have reported that the effects of exposure to several POPs can be transmitted through generations [Citation2–5]. This ‘heredity’ is commonly defined as a multigenerational and/or transgenerational effect of a chemical compound [Citation2–5]. POPs include several classes of compounds, which may be very heterogeneous in their chemical structure. Furthermore, at least some POPs were identified to display an endocrine disruptor (EDC) effect [Citation1].
This review will focus on one class of POPs, specifically identified as EDC, named per-polyfluoroalkyl substances (PFAS). The aim will be to overview currently available data suggesting the possibility that these compounds could exert multi and/or transgenerational effects. More specifically, a critical revision of the literature on the thyroid-disruptive effect of PFAS will be performed with the final aim to evaluate whether currently available data provide evidence for potential multi-trans-generational effects of PFAS on thyroid axis and/or support the need for future research in this field.
2. Methods
A comprehensive narrative review was performed. We searched for relevant literature using Medline, Embase and Cochrane search and including the following terms: (‘Epigenesis, Genetic’[MESH] OR (‘Transgenerational’[All Fields] OR ‘Multigenerational’[All Fields]) AND (‘thyroid’ [MeSH Terms] OR ‘thyroid’[All Fields]) AND (‘PFAS’[All Fields] OR ‘PFOA’[All Fields] OR ‘PFBS’[All Fields] OR ‘PFOS’[All Fields] OR ‘Endocrine Disruptors’[All Fields]). Publications from 1974 up to 2024 were included.
3. Multigenerational and/or transgenerational effect: what is the difference?
Direct exposure during adulthood to a contaminated environment generates alterations that may be passed to children and in turn to grandchildren through epigenetic changes that occur in the parents’ germline. The concept of multi-generational and/or transgenerational effect might require further clarification, as it is not a mere transmission of a character from mother to child (please refer to the scheme reported in ).
Figure 1. Multigenerational and transgenerational effects of prenatal exposure to environmental contaminants during adulthood, direct exposure leads to changes in epigenetics, which can be passed to children and grandchildren. Exposure of adult male F0 or non-pregnant female germ cells results in an F1 that can be affected, a phenomenon called ‘multigenerational’ transmission. The F2 generation, although not directly exposed, may exhibit EDC-induced effects, this phenomenon is called”transgenerational” transmission (a). The fetuses F1 of directly exposed F0 pregnant females are themselves considered directly exposed, as is the F2 generation (b). The F3 generation is the first not to be directly exposed to the compounds, but any induced inheritance effects are due to ‘transgenerational’ transmission.
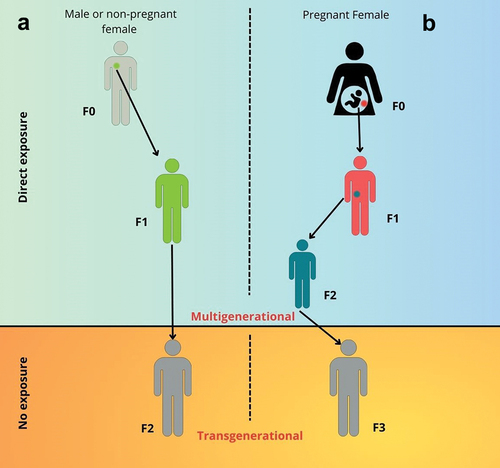
Briefly, we will start with the example of an individual persistently exposed to a surrounding environment containing potentially harmful contaminants. The subject could be either a male or a female (), or a pregnant female (). In the case of adult males or non-pregnant females (F0 ancestral), their preconception exposure germ cells give birth to an F1 progeny, which would be exposed (multigenerational transmission) (). Thus, the term multigenerational is used to describe effects that are transmitted to one or more subsequent generations [Citation6]. Potential mechanisms accounting for these types of effects include maternal transfer of contaminants (i. e. from the mother to egg in the case of oviparous species) or direct exposure of germ cells (i.e. during gestation in mammals). On the other hand, to be considered ‘transgenerational,’ the effect must appear in individuals who were never directly exposed to the environmental contaminant. Such effects are often thought to occur through epigenetic marks, although other mechanisms such as genetics, altered metabolism, and parental behavior are also possible. Transgenerational inheritance, first appears in the F2 or F3 generation, depending on the exposure scenario, the gender, and the species-specific reproductive modality [Citation7]. The scenario is quite different when the F0 is a pregnant female exposed to a contaminated environment (). In this case, the exposure to environmental contaminants of her offspring (the F1 generation), occurs directly in utero [Citation3,Citation4,Citation6]. Consequently, being germ cells derived from the F1 in pregnancy, also the F2 generation will be exposed [Citation3,Citation4,Citation6]. The effects in the F1 or F2 generations that are both exposed to environmental contaminants are referred to as multigenerational [Citation3,Citation4,Citation6]. The first generation not directly exposed to environmental contaminants is the F3, which is referred to as ancestral exposure. The effects observed in F3 are known as ‘transgenerational’ [Citation3,Citation4,Citation6].
4. Multi-trans generational effects following PFAS exposure
Per-polyfluoroalkyl substances (PFASs) are a class of chemicals constituted by a polar head group attached to a chain of C-F bonds [Citation8]. The unique chemistry of these compounds renders them effectively indestructible, thus a prime candidate for high-heat industrial processes and long-lasting consumer goods such as nonstick cookware and waterproofed outerwear. PFAS are also referred to as ‘forever chemicals’ due to their persistence and ubiquitous environmental distribution, being therefore included in the group of POPs [Citation9]. Humans and animals are constantly exposed to PFAS, during the entire course of their life. Drinking water is a significant source of exposure to PFAS in humans [Citation4], and drinking water treatment plants are not designed to remove these contaminants from source water. Likewise, wastewater treatment plants do not intentionally filter out PFASs [Citation10]. PFAS are detected virtually everywhere in wildlife, multiple environmental matrices, and in humans [Citation11,Citation12]. Moreover, the general population displays serum PFAS levels in the μg/L range (>999 ng/L) [Citation13]. Perfluoro-octanoic acid (PFOA) and perfluoro-octane sulfonic acid (PFOS) represent the two most studied and widespread compounds [Citation14,Citation15].
Although PFOA and PFOS were phased out of production due to their well-characterized health adverse effects, industries have introduced new congeners (not always free from adverse effects) to replace them. Serum levels of PFAS have been associated with multiple health endpoints, including elevated cholesterol and other serum lipid parameters, as well as liver enzymes, changes in thyroid hormones serum levels and increased incidence of thyroid disease, increased risk of preeclampsia, reduced antibody response, and reduced birth weight [Citation16]. Thus, the utility of PFASs is offset by their bioaccumulation and toxic health effects. Of particular concern, when evaluating potential hereditary effects of PFAS, is the evidence that these compounds readily cross the placental barrier, as demonstrated by studies reporting a strong positive correlation between PFAS levels in maternal and cord samples. PFAS concentrations in maternal plasma are therefore a predictor for the level of cord plasma [Citation17–22]. This would potentially expose the fetus during sensitive periods throughout intra-uterine development [Citation20,Citation23]. Moreover, some PFAS can be excreted through breastmilk, and given the importance of the health benefits of breastfeeding for both mother and infant, this kind of ‘transmission’ is also of particular concern [Citation24,Citation25].
It is known that chemical assault during critical windows in development can have effects later in life [Citation26]. Thus, since PFAS do cross the placenta, studying early-life exposure and the heritable and potential multi-transgenerational effects could provide some information/should be encouraged.
4.1. Laboratory experiences
Epigenetic modifications of DNA or chromatin constitute a ‘biological memory’ of the environmental exposure history which modulates gene regulatory networks in current and future generations [Citation27]. There is growing evidence that PFAS exposure confers heritable effects on subsequent generations via epigenetic mechanisms rather than direct genotoxicity [Citation28].
A study performed in rodents found that nonspecific methylation therapy administered together with PFOS to F0 females produced an amelioration of birth outcomes in F1 pups as compared to F0 which were exposed to PFOS but did not receive methylation therapy [Citation29].
Several studies aimed at assessing the effects of PFAS exposure in two animal models (i.e. zebrafish and c.elegants), are very suitable for the investigation of multi-trans generational effects of chemicals.
Zebrafish represents one of the best models to assess multi-trans generational effects in exposure studies due to its high fecundity, rapid embryogenesis, short life cycle, and small size, it is also less expensive to establish/maintain compared to mammalian models. In addition, several studies have reported homologies in lipid metabolism between zebrafish and humans [Citation30].
In the study by Cui et al. zebrafish exposure to PFOS caused liver fatty degeneration which was more severe in F0 males than in females. Consistent with morphological changes in the liver, similar alterations in the intestine were observed. Notably, the expression of genes related to lipid metabolism was markedly altered in F1 larvae, suggesting potential trans-generational effects [Citation31]. The study by Shi et al. was the first to investigate the potential adverse effects of long-term F-53B exposure on lipid metabolism in both F0 and F1 generations in zebrafish. They showed that the transcriptional levels of several genes associated with lipid metabolism in F1 larvae were changed after exposure to F-53B (a PFOS alternative), though the transcriptional profiles showed the opposite trend in F0 adult fish [Citation32].
Bouwmeester et al. showed that moderate-range of PFOA exposure increased methylation associated with vtg1, a gene involved in fertility in zebrafish, suggesting that epigenetic mechanisms mediate each generation’s response to exposure in terms of behavior and gene expression [Citation33]. Other studies demonstrated that changes in transgenerational toxicity might be critical toxic effects due to exposure to PFOS [Citation34–36]. Du et al. recently showed that chronic exposure to PFOS adversely impacts development, reproduction in the F0 generation of exposed zebrafishes, and offspring embryonic growth. The Authors suggested that the down-regulation of genes and hormones might be responsible for transgenerational toxicity [Citation37]. Finally, Chen et al. evaluated PFBS life-cycle exposure at environmentally realistic concentrations in fish. They observed a skewed sex ratio toward male dominance, and impairment of reproductive functions in female fish, as assessed by extremely small ovaries, blocked oocyte development, and reduced egg production. PFBS was found to gradually accumulate in F0 adults during continuous exposure but it was rapidly eliminated when depurated in clean water. Parental exposure also transferred PFBS pollutants to F1 offspring eggs. Although no trace of PFBS was detected in F1 adults and F2 eggs, adverse effects from parental exposure persisted in F1 and F2 offspring. These transgenerational effects implicate PFBS as an ongoing threat to the fitness and sustainability of fish population [Citation38].
A further good model for studying the effects of exposure to pollutants throughout generations is represented by C. elegans and again, studies have demonstrated that PFBS exerts trans-generational effects. Indeed, a severe impairment of body bending and head thrashing was observed in the P0 worms exposed to PFBS. Similarly, F1 progeny of exposed nematodes also showed a negative effect on locomotion as P0. Nevertheless, from the F2 generation, nematodes recovered from the negative effect on locomotion and restored healthy movement. Hence, it is clear that PFBS harms the body’s movement mechanism, and this can be transferred to the progeny. Continuous exposure of C. elegans to PFBS, even at the lower concentrations that are commonly found in AFFF-affected areas (e.g. 150 μg L−1) is likely to reduce life span in progenies, although no acute effects could be observed 10.1002/etc.5055. Another study assessed the short-time exposure, continuous exposure, and long-term effects of PFOS over several generations of C. elegans. The results confirmed that PFOS affected locomotion, reproduction, lifespan & and chemotaxis behavior in C. elegans and also in subsequent generations [Citation39]. Li et al. evaluated the effects of PFBS and PFHxS on C. elegans by multi- and trans-generational experiments. The multi-generational effects were assessed in continuous exposure throughout four generations (i.e. F1 to F4). Results showed that PFBS did not affect the lipid profile in F1 but disturbances of the lipid metabolism and the insulin and insulin-like (IIS) pathway were found in F4. Similar results were observed also for PFHxS exposure in F1 and F4. The results of trans-generational experiments showed that the effects of PFBS and PFHxS on lipid metabolism and IIS pathway were not recovered in the offspring of F1 (i.e. T1-T3) and F4 (i.e. T1’−T3’) which were not continuously exposed. PFHxS showed a common pattern to up-regulate daf-7 in both multi- and trans-generational effects [Citation40].
5. PFAS as thyroid disruptors
The studies reported in the previous paragraph evaluated the potential multi-transgenerational effects of PFAS. However, these studies did not take into account the thyroid disruptive effects of these compounds which, are known to be not only POPs but also Endocrine Disrupting chemicals (EDCs). EDCs are defined as exogenous compounds or mixtures that impair the homeostasis of the endocrine system, interfering with endogenous hormones at several levels (i.e. hormonal synthesis, release, transport and metabolism). These interactions consequently cause adverse health effects in an intact organism, or its progeny, or in sub-populations [Citation41,Citation42]. To date, the number of studies evaluating the effects of EDCs has grown exponentially, and thousands of EDCs have been identified [Citation43,Citation44]. EDCs have recently been shown to promote a transgenerational phenotype involving several diseases and transgenerational (epigenetic) effects as observed in germ cells [Citation45–47].
Through binding to nuclear receptors, EDCs are capable of altering the expression of enzymes involved in both steroid and thyroid hormone metabolism [Citation47]. In particular, PFAS were recognized to be EDCs but more specifically, they were demonstrated to interfere with thyroid functions at several levels by altering deiodinase activity, slowing down thyroid hormone metabolism, reducing iodine absorption by thyroid cells, competitively inhibiting thyroid transport protein TTR, and antagonizing complexes arising from the thyroid hormone-responsive elements (TREs) [Citation47–49]. Thus, PFAS are currently considered also as ‘Thyroid disruptors.’
6. Could PFAS exert multi-transgenerational thyroid-disruptive effects?
6.1. Human studies
Thyroid hormones play crucial roles in the physiological development of the neurological system both in the fetus and early childhood. In the first 16 weeks of gestation, the fetal thyroid gland is not fully functioning, being the fetus’s requirements dependent upon placental thyroid hormone transfer from the mother [Citation50–52]. Thyroid hormones (TH) are crucial for brain development as they regulate critical processes such as precursor cell differentiation, maturation, migration, neurotransmitter synthesis and synaptogenesis [Citation53–55]. Maternal TH are particularly critical in the early stages of fetal development, since their deficiency is associated with potentially severe irreversible damage to the offspring’s brain development [Citation53,Citation56–59].
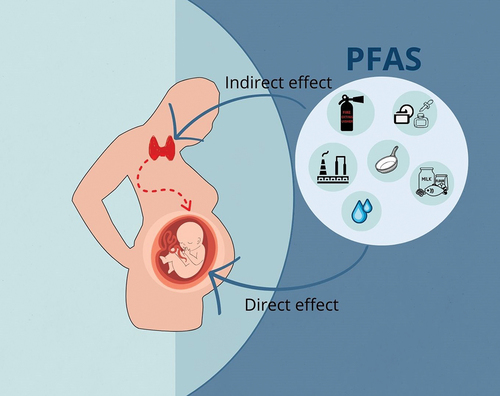
In recent years, an increasing number of population-based epidemiological studies have investigated the effects of PFAS exposure on maternal and neonatal thyroid function during pregnancy. Some studies demonstrated that prenatal exposure to PFAS compounds can cause alterations in the homeostasis of TH in both pregnant women (indirect effect) and fetal TH levels (direct effects) () [Citation60–63]. However, other studies failed to demonstrate any association between PFAS exposure and changes in maternal TSH or FT4 during pregnancy as well as in TT4 levels in newborns [Citation64,Citation65].
Figure 2. Graphical representation of exposure of pregnant women to PFAS according to sources and routes of exposure. Typical routes of exposure that people come in contact with are food, water, air, and dust. These exposures can occur directly or indirectly, causing alterations in the fetus.
A recent meta-analysis of 13 eligible studies attempted to elucidate the effects of PFAS on thyroid hormones during pregnancy [Citation66] indicating the existence of a consistent association between PFOS, PFOA and PFDA exposure and maternal TSH. There are currently few longitudinal studies specifically aimed at evaluating whether changes in maternal TH and TSH, possibly dependent upon PFAS exposure, may produce clinically relevant consequences, such as adverse pregnancy outcomes or developmental implications for the fetus [Citation67,Citation68]. In difference with other POPs, classified as EDCs, no epidemiological study investigated the transgenerational effect of PFAS on thyroid function through epigenetic modifications.
Kim et al. investigated the association between prenatal exposure to certain POPs, including OCPs (organochlorine pesticides), PBDEs (polybrominated diphenyl ethers), and PCBs (polychlorinated biphenyls) and DNA methylation in three genes that are essential for placental thyroid hormone (TH) supply. These genes include iodothyronine deiodinases such as deiodinase type 3 (DIO3), plasma membrane transporters like monocarboxylate transporter 8 (MCT8), and binding proteins within trophoblasts such as transthyretin (TTR) [Citation59]. The results showed that exposure to prenatal POPs was associated with epigenetic changes in placental key thyroid-regulating genes, such as DIO3 and MCT8. The correlation between chemical exposure and DNA methylation differed by gender. Female infants showed a correlation between p, p-dichlorodiphenyldichloroethylene and BDE-47 serum concentrations, and placental DIO3 methylation. In males, p,p’-dichlorodiphenyltrichloroethane serum concentration was positively correlated with MCT8 methylation. Overall, the study found a positive and significant relationship between the serum concentrations of certain POPs and placental methylation in infants. These results would suggest that similarly to other POPs, an effect of the DNA methylation mechanism could be exerted also by PFAS [Citation48,Citation60].
6.2. Animal models
Most of the available data report on the effects of PFAS exposure on pregnant animals and subsequent evaluation of the effects on the progeny. Although these types of experiments would not strictly meet the criteria for establishing multi/transgenerational effects, they will be briefly overviewed before discussing the limited available multi/transgenerational evidence.
The following studies were designed to understand whether the exposure to PFAS (including long-chain as PFOA, PFOS, PFBS, PFNA, F53-B, 8:8 PFPIA, short-chain as PFBS and new PFAS molecules as GenX) could exert adverse effects on the progeny through interference with the maternal thyroid status or by directly acting on the fetal development.
Conley et al. characterized maternal and postnatal toxicities of oral GenX in Sprague-Dawley rats during sexual differentiation by assaying maternal thyroid hormones. Pregnant mice exposed to different concentrations of GenX had lower thyroid hormone levels. In detail, total T4 levels were significantly lower in exposed versus non-exposed mice while total T3 levels were undetectable for higher exposure levels [Citation69]. These finding are consistent with the previous data, reporting that PFOS and PFOA decreased the levels of both T3 and T4 in the mother’s serum [Citation70]. Another study compared the toxicity of PFOA and GenX in pregnant mice. Interestingly, high levels of PFOA or GenX were found in the placenta of the exposed mice. Additionally, the study observed an increase in placental weight, a higher number of placental lesions, and lower embryo-placenta weight ratios in exposed mice. Of note, GenX seemed to specifically disrupt placental thyroid hormones [Citation71]. Further, in vivo reproductive studies have linked the concentrations of PFBS exposure with adverse effects on thyroid and sex hormones [Citation72,Citation73]. The recent in vivo study by Crute et al. was the first to assess the potential effects of Perfluorobutanesulfonic acid (PFBS) on placental thyroid hormone gene expression in the New Zealand White rabbit model [Citation74]. In particular, in utero PFBS exposure leads to adverse effects on maternal, fetal, and placental outcomes. Briefly, nulliparous female rabbits were supplied drinking water containing 0, low or high concentrations of PFBS. The results showed no statistically significant changes in maternal hormones, however, trends for lower T4 levels and higher T3:T4 ratios were observed in PFBS low-exposed dams. Moreover, lower T3 and T4 levels were observed in PFBS highly exposed dams. Finally, the analysis in fetal placentas showed changes in the expression of the mRNA levels of several genes involved in thyroid hormone metabolism [Citation74]. Moving to another animal model, Zhang et al. recently reported an increase in T4 and T3 levels in zebrafish larvae that were exposed to 8:8 perfluoroalkyl phosphinic acid (8:8 PFPiA). Furthermore, changes in several genes involved in thyroid physiology (CRH, TSHβ, UGT1AB) were observed [Citation75]. Another recent study on zebrafish exposed to 8:8 PFPiA found an increase in the expression of genes involved in thyroid physiology (in this case the genes were CRHB, TSHR, and NKX2.1).
Fini et al. evaluated the effects of exposing the human amniotic fluid to chemical contaminants during embryonic development and how thyroid hormone signaling could be affected in Xenopus laevis [Citation76]. The results showed that exposure to a mixture of chemicals, including PFOA and PFOS, at the concentrations found in human amniotic fluid, could impact thyroid hormone-dependent transcription, gene expression, and brain development during early embryogenesis. Short-term exposure to PFOA and PFOS showed a dose-dependent effect on fetal development within 72 hours, confirming the adverse effect of PFAS exposure.
Taken together the above evidence demonstrated that the effect of PFAS exposure on the progeny could be both indirect (as a consequence of the exposure on maternal thyroid homeostasis) and direct (effect on fetal development).
Rather surprisingly, it should be acknowledged that few studies so far have evaluated the potential multi-transgenerational effects of PFAS in terms of thyroid disruption. The study by Liu et al. was the first to demonstrate a thyroidal multi-transgenerational effect following exposure to perfluorooctanoate (PFNA) in zebrafish. Indeed, PFNA exposure leads to increased T3 plasma levels in both F(0) and F(1) adults, as well as histological changes in the thyroid follicles of F(0) male zebrafish. Moreover, an upregulation of TTR and a down-regulation of UDP-glucuronosyltransferases in F(0) adult males were also observed. The Authors also provide evidence for a trans-generational effect as demonstrated by altered expression of genes related to the synthesis of thyroid hormones and metabolism in F(1) larvae [Citation77].
Shi et al. evaluated whether the exposure to different concentrations of F-53B (0, 5, 50, or 500 μg/L) for 180 days in fishes, could exert transgenerational thyroid-disrupting effects. Thyroid disturbances were investigated in the three (F0, F1, and F2) generations. The acquired F1 embryos were raised in uncontaminated water for an additional 180 days to obtain the F2 generation.
For F0 adult fish, thyroxine (T4) increased whereas 3,5,3’-triiodothyronine (T3) decreased in all groups. For F1 embryos, parental exposure resulted in F-53B transfer as well as an increase in T4 levels. At 5 days post-fertilization, the increase in T4 and decrease in T3 were paralleled by a decrease in body length, increase in mortality, and increase in uninflated posterior swim bladder occurrence in F1 larvae. Of note, thyroid hormone levels were not changed significantly in F1 adult fish or F2 offspring compared with the control, while significant changes in the transcription levels of several genes involved in the hypothalamus-pituitary-thyroid axis were observed [Citation78].
7. Conclusions
The present review was principally focused on one class of POPs, specifically identified as EDC, which are named per-polyfluoroalkyl substances (PFAS). The aim was to overview currently available data suggesting the possibility that, similarly to what was observed for another health-related issue, these compounds could exert multi and/or transgenerational effects also in terms of thyroid-disruptive effects.
PFAS are well-known thyroid disruptors and consistent evidence in literature both in vivo and in vitro, showed interference with thyroid homeostasis by these compounds. Given the importance of the thyroid gland in the development of an individual in all the stages of life (from the embryo to the elderly) it is also important to consider this aspect in multi-trans generational studies. At present, the possibility of multi-trans generational effects following PFAS exposure was proven in several animal models but in none of these studies, a thyroid disruptive effect was taken into account. On the other hand, while several studies regarding the exposure of pregnant mothers to a given PFAS with subsequent evaluation of the thyroidal effects in the developing embryo are available, there are only two studies specifically designed to evaluate the possibility of thyroidal multi-trans-generational effects following PFAS exposure.
8. Expert opinion
There is increasing evidence of multi-transgenerational effects due to exposure to several Persistent Organic Pollutants (POPs). POPs include a group of chemical compounds known as Endocrine Disruptors, due to their ability to interfere with the endocrine system causing multiple detrimental effects that, at least in some cases, could be hereditable. Among EDCs, Per and poly-fluoroalkyl substances (PFAS) are of particular concern given their persistence in the environment and their adverse health effects on human health. The multi-trans generational effects of these compounds were evidenced by laboratory experiences on mice, zebrafish and c.elegans, however, more studies are needed to better understand their hereditable effects. Of note, PFAS are not only EDCs but also thyroid disruptors. Of note, PFAS are EDCs and more specifically they may act as thyroid disruptors, as demonstrated by a large body of evidence, (both epidemiological and in vitro), showing their ability to interfere with thyroid function. The thyroid gland and its hormones are crucial in embryogenesis and fetal development but also in childhood and during the life of an individual. For this reason, taking into account the effects of thyroid disruptors in multi-transgenerational studies appears mandatory. On the other hand, multi-transgenerational studies that consider the thyroid disruptive effects of PFAS are still scanty. Furthermore, it should be highlighted that in vitro data and studies on animal models have evaluated the adverse effects of PFAS exposure at rather high and acute concentrations. Because humans display prolonged exposure to fairly low concentrations of many types of PFASs, it could be suggested to study the effects of low-level chronic exposure and/or PFAS mixtures, on developing organisms, by using a multi-transgenerational approach.
It should be acknowledged that evaluating transgenerational epigenetic effects by epidemiological studies can be challenging. Indeed in humans, the rather long gestational period and lifespan together with the high prevalence of thyroid diseases represent a further challenge for understanding whether thyroid diseases are caused by multi/transgenerational inheritance or rather are acquired post-conception. The issue is further complicated by the fact that humans are constantly exposed not only to multiple PFAS congeners but also to other environmental contaminants that could differently affect germ cells. In this view, the use of animal models, if on one hand represents the best approach for the investigation of the potential multi or transgenerational effect of a given substance, on the other hand may be not fully representative of the real environmental exposure (‘mixture’ of compounds). However, in animal models, the effect of the exposure to a single compound and/or to a mixture could be directly evaluated. Although further studies on this issue should be strongly encouraged, and despite some limitations of the currently available literature, it should be acknowledged that PFAS exposure can be potentially hazardous for living beings. The fact that these effects may still be observed even several years after exposure strongly points toward the notion that multi/transgenerational mechanisms could be involved. For this reason, efforts to monitor the long-term effects of PFAS exposure on mother-child cohorts are needed. Moreover, regulatory agencies should be aware of the potential long-term toxicity of these compounds, strengthening the indication for tighter regulation of PFAS production and environmental spread. Currently, several long-chain PFAS compounds (PFOS and PFOA included) are strictly regulated. Although first-generation PFAS have been phased out, the problem has probably not been solved. Considering that these chemical compounds are persistent and rather ubiquitous (being detected worldwide), especially in water sources, the solution for preventing exposure appears challenging. Indeed, in addition to abolishing their production by all countries, even more important is to identify rapid and ecologic methods to eliminate PFAS already present in the environment. In conclusion, we will be dealing with exposure to these substances for a long time, especially since removal strategies are still being developed and far from proven to be effective, in addition not all PFAS are regulated at present by restriction laws. More efforts in finding methods for the removal of PFAS from the environment, as well as studies aimed at further characterize adverse effects of PFAS in human, animal and environmental health are strongly encouraged.
From this overview the available literature, the following considerations can be made: ì) a large body of evidence indicates that PFAS exposure impacts thyroid homeostasis at several levels; ìì) owing to their chemical structure PFAS readily cross the placental barrier, as assessed by PFAS detection in the cord serum and/or in milk of lactating mothers; ììì) multi-trans generational effects have been documented for PFAS in several animal models; iv) although the effects of PFAS exposure have been extensively studied so far, very limited data evaluating their multi-trans generational thyroidal effects are available.
Taking together the above considerations, it would seem reasonable to propose the need for future studies specifically designed with this aim.
Article highlights
PFAS are synthetic compounds persistent in the environment, causing potential health adverse effects
The exposure to PFAS exert multi-transgenerational effects
PFAS are known to be EDC and in particular thyroid disruptors
Available data show evidence for potential multi-trans-generational effects of PFAS on the thyroid axis even if few studies were performed
The need for future research in this field is encouraged
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Additional information
Funding
References
- ECHA. Understanding POPs. Available from: https://echa.europa.eu/understanding-pops
- Robaire B, Delbes G, Head JA, et al. A cross-species comparative approach to assessing multi- and transgenerational effects of endocrine disrupting chemicals. Environ Res. 2022 Mar;204(Pt B):112063. doi: 10.1016/j.envres.2021.112063
- Skinner MK. What is an epigenetic transgenerational phenotype? F3 or F2. Reprod Toxicol. 2008 Jan;25(1):2–6. doi: 10.1016/j.reprotox.2007.09.001
- Xin F, Susiarjo M, Bartolomei MS. Multigenerational and transgenerational effects of endocrine disrupting chemicals: a role for altered epigenetic regulation? Semin Cell Dev Biol. 2015 Jul;43:66–75. doi: 10.1016/j.semcdb.2015.05.008
- Rattan S, Brehm E, Gao L, et al. Di(2-ethylhexyl) phthalate exposure during prenatal development causes adverse transgenerational effects on female fertility in mice. Toxicol Sci. 2018 Jun 1;163(2):420–429. doi: 10.1093/toxsci/kfy042
- Rebuzzini P, Fabozzi G, Cimadomo D, et al. Multi- and transgenerational effects of environmental toxicants on mammalian reproduction. Cells. 2022 Oct 09;11(19):3163. doi: 10.3390/cells11193163
- Brehm E, Flaws JA. Transgenerational effects of endocrine-disrupting chemicals on male and female reproduction. Endocrinology. 2019 Jun 01;160(6):1421–1435. doi: 10.1210/en.2019-00034
- (NIH) Nioehs. Perfluoroalkyl and polyfluoroalkyl substances (PFASs). 2023. Available from: https://www.pops.int/TheConvention/ThePOPs/TheNewPOPs/tabid/2511/Default.aspx
- program UE. The new POPs under the Stockholm convention. 2023. Available from: https://www.pops.int/TheConvention/ThePOPs/TheNewPOPs/tabid/2511//Default.aspx
- ECHA. XV restriction report. 2023.
- Calafat AM, Kato K, Hubbard K, et al. Legacy and alternative per- and polyfluoroalkyl substances in the U.S. general population: paired serum-urine data from the 2013-2014 national health and nutrition examination survey. Environ Int. 2019 Oct;131:105048. doi: 10.1016/j.envint.2019.105048
- Giesy JP, Kannan K. Global distribution of perfluorooctane sulfonate in wildlife. Environ Sci Technol. 2001 Apr;35(7):1339–1342. doi: 10.1021/es001834k
- Prevention CCfDCa. Fourth report on human exposure to environmental chemicals, updated tables. 2019.
- Supporting documents for drinking water health advisories for PFOA and PFOS. Available from: https://www.epa.gov/ground-water-and-drinking-water/supporting-documents-drinking-water-health-advisories-pfoa-and-pfos
- Commission E. Health and environmental impacts prompt a call for strict ruling on ubiquitous ‘forever chemicals’ 2023. Available from: https://environment.ec.europa.eu/news/health-and-environmental-impacts-prompt-call-strict-ruling-ubiquitous-forever-chemicals-2023-10-19_en
- Post GB, Cohn PD, Cooper KR. Perfluorooctanoic acid (PFOA), an emerging drinking water contaminant: a critical review of recent literature. Environ Res. 2012 Jul;116:93–117. doi: 10.1016/j.envres.2012.03.007
- Spratlen MJ, Perera FP, Lederman SA, et al. Cord blood perfluoroalkyl substances in mothers exposed to the world trade center disaster during pregnancy. Environ Pollut. 2019 Mar;246:482–490. doi: 10.1016/j.envpol.2018.12.018
- Cai D, Li QQ, Chu C, et al. High trans-placental transfer of perfluoroalkyl substances alternatives in the matched maternal-cord blood serum: evidence from a birth cohort study. Sci Total Environ. 2020 Feb 25;705:135885. doi: 10.1016/j.scitotenv.2019.135885
- Pascali JP, Piva E, Bonasoni MP, et al. Analysis and distribution of per- and polyfluoroalkyl substances in decidua and villi placenta explants. Environ Res. 2023 Jul 15;229:115955. doi: 10.1016/j.envres.2023.115955
- Workman CE, Becker AB, Azad MB, et al. Associations between concentrations of perfluoroalkyl substances in human plasma and maternal, infant, and home characteristics in Winnipeg, Canada. Environ Pollut. 2019 Jun;249:758–766. doi: 10.1016/j.envpol.2019.03.054
- Mamsen LS, Jönsson BAG, Lindh CH, et al. Concentration of perfluorinated compounds and cotinine in human foetal organs, placenta, and maternal plasma. Sci Total Environ. 2017 Oct 15;596-597:97–105. doi: 10.1016/j.scitotenv.2017.04.058
- Yu G, Luo F, Nian M, et al. Exposure to perfluoroalkyl substances during pregnancy and fetal BDNF level: a prospective cohort study. Front Endocrinol. 2021;12:653095. doi: 10.3389/fendo.2021.653095
- Gützkow KB, Haug LS, Thomsen C, et al. Placental transfer of perfluorinated compounds is selective–a Norwegian mother and child sub-cohort study. Int J Hyg Environ Health. 2012 Feb;215(2):216–219. doi: 10.1016/j.ijheh.2011.08.011
- Goeden HM, Greene CW, Jacobus JA. A transgenerational toxicokinetic model and its use in derivation of Minnesota PFOA water guidance. J Expo Sci Environ Epidemiol. 2019 Mar;29(2):183–195. doi: 10.1038/s41370-018-0110-5
- Nyberg E, Awad R, Bignert A, et al. Inter-individual, inter-city, and temporal trends of per- and polyfluoroalkyl substances in human milk from Swedish mothers between 1972 and 2016. Environ Sci Process Impacts. 2018 Aug 16;20(8):1136–1147. doi: 10.1039/C8EM00174J
- Ingber SZ, Pohl HR. Windows of sensitivity to toxic chemicals in the motor effects development. Regul Toxicol Pharmacol. 2016 Feb;74:93–104. doi: 10.1016/j.yrtph.2015.11.018
- Bowers EC, McCullough SD. Linking the epigenome with exposure effects and susceptibility: the epigenetic seed and soil model. Toxicol Sci. 2017 Feb;155(2):302–314. doi: 10.1093/toxsci/kfw215
- Kim S, Thapar I, Brooks BW. Epigenetic changes by per- and polyfluoroalkyl substances (PFAS). Environ Pollut. 2021 Jun 15;279:116929. doi: 10.1016/j.envpol.2021.116929
- Tian J, Xu H, Zhang Y, et al. SAM targeting methylation by the methyl donor, a novel therapeutic strategy for antagonize PFOS transgenerational fertilitty toxicity. Ecotoxicol Environ Saf. 2019 Nov 30;184:109579. doi: 10.1016/j.ecoenv.2019.109579
- Anderson JL, Carten JD, Farber SA. Zebrafish lipid metabolism: from mediating early patterning to the metabolism of dietary fat and cholesterol. Methods Cell Biol. 2011;101:111–141.
- Cui Y, Lv S, Liu J, et al. Chronic perfluorooctanesulfonic acid exposure disrupts lipid metabolism in zebrafish. Hum Exp Toxicol. 2017 Mar;36(3):207–217. doi: 10.1177/0960327116646615
- Shi G, Cui Q, Wang J, et al. Chronic exposure to 6: 2 chlorinated polyfluorinated ether sulfonate acid (F-53B) induced hepatotoxic effects in adult zebrafish and disrupted the PPAR signaling pathway in their offspring. Environ Pollut. 2019 Jun;249:550–559. doi: 10.1016/j.envpol.2019.03.032
- Bouwmeester MC, Ruiter S, Lommelaars T, et al. Zebrafish embryos as a screen for DNA methylation modifications after compound exposure. Toxicol Appl Pharmacol. 2016 Jan 15;291:84–96. doi: 10.1016/j.taap.2015.12.012
- Ankley GT, Kuehl DW, Kahl MD, et al. Reproductive and developmental toxicity and bioconcentration of perfluorooctanesulfonate in a partial life-cycle test with the fathead minnow (Pimephales promelas). Environ Toxicol Chem. 2005 Sep;24(9):2316–2324. doi: 10.1897/04-634R.1
- Chen T, Zhang L, Yue JQ, et al. Prenatal PFOS exposure induces oxidative stress and apoptosis in the lung of rat off-spring. Reprod Toxicol. 2012 Jul;33(4):538–545. doi: 10.1016/j.reprotox.2011.03.003
- Keiter S, Baumann L, Färber H, et al. Long-term effects of a binary mixture of perfluorooctane sulfonate (PFOS) and bisphenol a (BPA) in zebrafish (danio rerio). Aquat Toxicol. 2012 Aug 15;118-119:116–129. doi: 10.1016/j.aquatox.2012.04.003
- Du J, Tang J, Xu S, et al. Parental transfer of perfluorooctane sulfonate and ZnO nanoparticles chronic co-exposure and inhibition of growth in F1 offspring. Regul Toxicol Pharmacol. 2018 Oct;98:41–49. doi: 10.1016/j.yrtph.2018.07.005
- Chen L, Lam JCW, Hu C, et al. Perfluorobutanesulfonate exposure skews sex ratio in fish and transgenerationally impairs reproduction. Environ Sci Technol. 2019 Jul 16;53(14):8389–8397. doi: 10.1021/acs.est.9b01711
- Chowdhury MI, Sana T, Panneerselvan L, et al. Perfluorooctane sulfonate (PFOS) induces several behavioural defects in Caenorhabditis elegans that can also be transferred to the next generations. Chemosphere. 2022 Mar;291(Pt 2):132896. doi: 10.1016/j.chemosphere.2021.132896
- Li Z, Yu Z, Yin D. Multi- and trans-generational disturbances of perfluorobutane sulfonate and perfluorohexane sulfonate on lipid metabolism in Caenorhabditis elegans. Chemosphere. 2021 Oct;280:130666. doi: 10.1016/j.chemosphere.2021.130666
- Macedo S, Teixeira E, Gaspar TB, et al. Endocrine-disrupting chemicals and endocrine neoplasia: a forty-year systematic review. Environ Res. 2023 Feb 1;218:114869. doi: 10.1016/j.envres.2022.114869
- Santangeli S, Consales C, Pacchierotti F, et al. Transgenerational effects of BPA on female reproduction. Sci Total Environ. 2019 Oct 1;685:1294–1305. doi: 10.1016/j.scitotenv.2019.06.029
- Attina TM, Hauser R, Sathyanarayana S, et al. Exposure to endocrine-disrupting chemicals in the USA: a population-based disease burden and cost analysis. Lancet Diabetes Endocrinol. 2016 Dec;4(12):996–1003. doi: 10.1016/S2213-8587(16)30275-3
- Hassan S, Thacharodi A, Priya A, et al. Endocrine disruptors: unravelling the link between chemical exposure and women’s reproductive health. Environ Res. 2023 Oct 12;241:117385. doi: 10.1016/j.envres.2023.117385
- Skinner MK, Manikkam M, Guerrero-Bosagna C. Epigenetic transgenerational actions of endocrine disruptors. Reprod Toxicol. 2011 Apr;31(3):337–343. doi: 10.1016/j.reprotox.2010.10.012
- Anway MD, Skinner MK. Epigenetic transgenerational actions of endocrine disruptors. Endocrinology. 2006 Jun;147(6 Suppl):S43–9. doi: 10.1210/en.2005-1058
- Dutta S, Sengupta P, Bagchi S, et al. Reproductive toxicity of combined effects of endocrine disruptors on human reproduction. Front Cell Dev Biol. 2023;11:1162015. doi: 10.3389/fcell.2023.1162015
- Butt CM, Stapleton HM. Inhibition of thyroid hormone sulfotransferase activity by brominated flame retardants and halogenated phenolics. Chem Res Toxicol. 2013 Nov 18;26(11):1692–1702. doi: 10.1021/tx400342k
- Noyes PD, Lema SC, Macaulay LJ, et al. Low level exposure to the flame retardant BDE-209 reduces thyroid hormone levels and disrupts thyroid signaling in fathead minnows. Environ Sci Technol. 2013 Sep 3;47(17):10012–10021. doi: 10.1021/es402650x
- Patel J, Landers K, Li H, et al. Delivery of maternal thyroid hormones to the fetus. Trends Endocrinol Metab. 2011 May;22(5):164–170. doi: 10.1016/j.tem.2011.02.002
- Vissenberg R, Manders VD, Mastenbroek S, et al. Pathophysiological aspects of thyroid hormone disorders/thyroid peroxidase autoantibodies and reproduction. Hum Reprod Update. 2015 May;21(3):378–387. doi: 10.1093/humupd/dmv004
- Chen Z, Meima ME, Peeters RP, et al. Thyroid hormone transporters in pregnancy and fetal development. Int J Mol Sci. 2022 Dec 1;23(23):15113. doi: 10.3390/ijms232315113
- Bernal J. Thyroid hormone receptors in brain development and function. Nat Clin Pract Endocrinol Metab. 2007 Mar;3(3):249–259. doi: 10.1038/ncpendmet0424
- Morreale de Escobar G, Obregon MJ, Escobar Del Rey F. Role of thyroid hormone during early brain development. Eur J Endocrinol. 2004 Nov;151 Suppl 3:U25–37. doi: 10.1530/eje.0.151u025
- de Escobar GM, Obregón MJ, Del Rey FE. Maternal thyroid hormones early in pregnancy and fetal brain development. Best Pract Res Clin Endocrinol Metab. 2004 Jun;18(2):225–248. doi: 10.1016/j.beem.2004.03.012
- Calzà L, Fernández M, Giardino L. Role of the thyroid system in myelination and neural connectivity. Compr Physiol. 2015 Jul 1;5(3):1405–1421.
- Moog NK, Entringer S, Heim C, et al. Influence of maternal thyroid hormones during gestation on fetal brain development. Neuroscience. 2017 Feb 7;342:68–100. doi: 10.1016/j.neuroscience.2015.09.070
- Hernandez A, Martinez ME, Chaves C, et al. Epigenetic developmental programming and intergenerational effects of thyroid hormones. Vitam Horm. 2023;122:23–49.
- Alcaide Martin A, Mayerl S. Local thyroid hormone action in brain development. Int J Mol Sci. 2023 Aug 2;24(15):12352. doi: 10.3390/ijms241512352
- Wang Y, Rogan WJ, Chen PC, et al. Association between maternal serum perfluoroalkyl substances during pregnancy and maternal and cord thyroid hormones: Taiwan maternal and infant cohort study. Environ Health Perspect. 2014 May;122(5):529–534. doi: 10.1289/ehp.1306925
- Aimuzi R, Luo K, Huang R, et al. Perfluoroalkyl and polyfluroalkyl substances and maternal thyroid hormones in early pregnancy. Environ Pollut. 2020 Sep;264:114557. doi: 10.1016/j.envpol.2020.114557
- Shah-Kulkarni S, Kim BM, Hong YC, et al. Prenatal exposure to perfluorinated compounds affects thyroid hormone levels in newborn girls. Environ Int. 2016 Sep;94:607–613. doi: 10.1016/j.envint.2016.06.024
- Guo J, Zhang J, Wang Z, et al. Umbilical cord serum perfluoroalkyl substance mixtures in relation to thyroid function of newborns: findings from Sheyang mini birth cohort study. Chemosphere. 2021 Jun;273:129664. doi: 10.1016/j.chemosphere.2021.129664
- Preston EV, Webster TF, Oken E, et al. Maternal plasma per- and polyfluoroalkyl substance concentrations in early pregnancy and maternal and neonatal thyroid function in a prospective birth cohort: project viva (USA). Environ Health Perspect. 2018 02;126(2):027013. doi: 10.1289/EHP2534
- Inoue K, Ritz B, Andersen SL, et al. Perfluoroalkyl substances and maternal thyroid hormones in early pregnancy; findings in the Danish national birth cohort. Environ Health Perspect. 2019 11;127(11):117002. doi: 10.1289/EHP5482
- Zhang L, Liang J, Gao A. Contact to perfluoroalkyl substances and thyroid health effects: a meta-analysis directing on pregnancy. Chemosphere. 2023 Feb;315:137748. doi: 10.1016/j.chemosphere.2023.137748
- Shin HM, Oh J, Schmidt JR, et al. Prenatal exposure to per- and polyfluoroalkyl substances, maternal thyroid dysfunction, and child autism spectrum disorder. Endocrinol Metab (Seoul). 2022 Dec;37(6):819–829. doi: 10.3803/EnM.2022.1598
- Ballesteros V, Costa O, Iñiguez C, et al. Exposure to perfluoroalkyl substances and thyroid function in pregnant women and children: a systematic review of epidemiologic studies. Environ Int. 2017 Feb;99:15–28. doi: 10.1016/j.envint.2016.10.015
- Conley JM, Lambright CS, Evans N, et al. Adverse maternal, fetal, and postnatal effects of hexafluoropropylene oxide dimer acid (GenX) from oral gestational exposure in Sprague-Dawley rats. Environ Health Perspect. 2019 03;127(3):37008. doi: 10.1289/EHP4372
- Dong H, Curran I, Williams A, et al. Hepatic miRNA profiles and thyroid hormone homeostasis in rats exposed to dietary potassium perfluorooctanesulfonate (PFOS). Environ Toxicol Pharmacol. 2016 Jan;41:201–210. doi: 10.1016/j.etap.2015.12.009
- Blake BE, Cope HA, Hall SM, et al. Evaluation of maternal, embryo, and placental effects in CD-1 mice following gestational exposure to perfluorooctanoic acid (PFOA) or hexafluoropropylene oxide dimer acid (HFPO-DA or GenX). Environ Health Perspect. 2020 Feb;128(2):27006. doi: 10.1289/EHP6233
- Feng X, Cao X, Zhao S, et al. Exposure of Pregnant Mice to Perfluorobutanesulfonate Causes Hypothyroxinemia and Developmental Abnormalities in Female Offspring. Toxicol Sci. 2017 Feb;155(2):409–419. doi: 10.1093/toxsci/kfw219
- National Toxicology Program. NTP Technical Report on the Toxicity Studies of Perfluoroalkyl Carboxylates (Perfluorohexanoic Acid, Perfluorooctanoic Acid, Perfluorononanoic Acid, and Perfluorodecanoic Acid) Administered by Gavage to Sprague Dawley (Hsd:Sprague Dawley SD) Rats (Revised): Toxicity Report 97 [Internet]. Research Triangle Park (NC): National Toxicology Program; 2022. doi: 10.22427/NTP-TOX-97
- Crute CE, Landon CD, Garner A, et al. Maternal exposure to perfluorobutane sulfonate (PFBS) during pregnancy: evidence of adverse maternal and fetoplacental effects in New Zealand white (NZW) rabbits. Toxicol Sci. 2023 Feb 17;191(2):239–252. doi: 10.1093/toxsci/kfac126
- Zhang S, Guo X, Lu S, et al. Exposure to PFDoA causes disruption of the hypothalamus-pituitary-thyroid axis in zebrafish larvae. Environ Pollut. 2018 Apr;235:974–982. doi: 10.1016/j.envpol.2018.01.015
- Fini JB, Mughal BB, Le Mével S, et al. Human amniotic fluid contaminants alter thyroid hormone signalling and early brain development in xenopus embryos. Sci Rep. 2017 03;7(1):43786. doi: 10.1038/srep43786
- Liu Y, Wang J, Fang X, et al. The thyroid-disrupting effects of long-term perfluorononanoate exposure on zebrafish (danio rerio). Ecotoxicology. 2011 Jan;20(1):47–55. doi: 10.1007/s10646-010-0555-3
- Shi G, Wang J, Guo H, et al. Parental exposure to 6: 2 chlorinated polyfluorinated ether sulfonate (F-53B) induced transgenerational thyroid hormone disruption in zebrafish. Sci Total Environ. 2019 May 15;665:855–863. doi: 10.1016/j.scitotenv.2019.02.198
