ABSTRACT
Background
Type 1 diabetes mellitus (T1DM) is associated with adverse maternal and fetal outcomes. Continuous glucose monitoring (CGM) during pregnancy is associated with better glycemic control in women with T1DM. However, no clear benefits have been demonstrated in reducing adverse feto-maternal outcomes in pregnant women with T1DM.
Design and Methods
This is a retrospective, single-center study of pregnant women with T1DM to evaluate the impact of CGM use on glycemic control and feto-maternal outcomes in pregnant women with T1DM.
Results
Of 265 women with T1DM, 92 (34.7%) used CGM, and 173 (65.3%) were managed with capillary blood glucose (CBG) monitoring. The mean (SD) age and BMI at the first visit were 29.4 (4.7) years and 27.2 (5.2) kg/m2, respectively. The mean (SD) HbA1c at the first-trimester visit was 63 (1) mmol/mol, and in the last trimester was 51 (1%). There was no difference in the mean changes in HbA1c between the two groups. Women using CGM had lower insulin requirements (1.02 + 0.37 vs. 0.87 + 0.04 units/kg, p = 0.01). The two groups had no significant differences in maternal or fetal outcomes.
Conclusion
CGM use in pregnant T1DM women is not associated with improved fetomaternal outcomes.
1. Introduction
Type 1 diabetes mellitus (T1DM) is associated with adverse maternal and fetal outcomes. Pregnancy-related complications in uncontrolled T1DM include an increased risk of miscarriage, preterm birth, preeclampsia, induction of labor, and cesarean section (C-section). Neonates born to women with T1D have an increased risk of congenital malformations, large for gestational age (LGA), macrosomia, birth injuries, neonatal hypoglycemia, and admission to intensive care units [Citation1,Citation2]. Furthermore, intrauterine hyperglycemia predisposes offspring to obesity, type 2 diabetes, and cardiovascular disease in early adulthood [Citation3].
Fetal hyperinsulinemia is stimulated by maternal hyperglycemia during the second and third trimesters and is associated with excessive fetal weight gain. In addition, increased HbA1c (>6%) in late pregnancy and a rise in blood glucose during specific times of the day (late morning and afternoon in the second trimester and evening in the third trimester) are associated with adverse fetal outcomes [Citation2]. Optimum glycemic control during pregnancy is associated with decreased risk of preeclampsia, preterm birth, and LGA [Citation4]. As a result, the American diabetes association (ADA) recommends that the ideal HbA1c during pregnancy should be < 6% (42 mmol/L) if it can be achieved without the risk of hypoglycemia [Citation5]. The NICE guideline recommends an HbA1c ≤ 6.5% (48 mmol/L), fasting blood glucose ≤5.3 mmol/L, 1-hour post-meal blood glucose ≤7.8 mmol/L, and 2-hour post-meal blood glucose ≤6.4 mmol/L in pregnant women with diabetes [Citation6]. Achieving these glycemic targets during pregnancy is a complex process due to changes in insulin sensitivity and variations in insulin absorption during the different stages of pregnancy [Citation7]. In pregnant women with T1D, only up to 40% of women can achieve these glycemic targets. Furthermore, there is a 5-fold increase in the rate of hypoglycemia to achieve tighter glycemic control [Citation8]. Hypoglycaemia is also related to increased weight gain in T1D women, likely due to increased carbohydrate intake in response to frequent hypoglycemic episodes [Citation9].
Continuous glucose monitoring (CGM) can help in the appropriate adjustment of insulin regimens, thus achieving the target glycemic levels. The Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group reported a significant improvement in HbA1c and time spent in the target glucose range at 26 weeks of CGM use in women with T1DM [Citation10]. A meta-analysis reported significantly reduced HbA1c levels and hypoglycemia in women with T1D with CGM use [Citation11]. Yu et al. compared CGM plus routine capillary blood glucose (CBG) monitoring versus routine CBG alone in pregnant women with gestational diabetes (GDM) and showed lesser duration of hyperglycemia and hypoglycemia, lower incidence of preeclampsia, preterm birth, primary C-section, LGA and macrosomia as compared to the routine CBG group alone [Citation12]. In an open-labeled randomized controlled trial (RCT) on women with GDM, Paramasivan et al. reported a lower mean HbA1c at 37 weeks in the CGM group compared to the routine care, with 92% of women achieving an HbA1c < 5.8% in the CGM group compared to 66% in the control group [Citation13]. However, studies on CGM in pregnant women with T1D have reported variable results. Some reported no difference in feto-maternal outcomes, while others reported improved outcomes with CGM use compared to routine CBG monitoring [Citation14–16]. In this study, we aimed to assess the impact of CGM use on feto-maternal outcomes in pregnant women with T1D.
2. Materials and methods
This is an observational, single-center, retrospective study to evaluate the effect of CGM use on glycemic control and maternal and fetal outcomes in pregnant women with T1D who established care in the National Diabetes Center (NDC) at the Women’s Wellness and Research Centre (WWRC), Anonymized (HMC), Qatar. HMC is the largest secondary and tertiary care provider in Qatar. The Women’s Wellness and Research Center is the largest maternity care provider in Qatar, with 16–18 thousand deliveries per year. Almost all the women in the CGM arm used flash glucose monitoring (Freestyle libre 1).
2.1. Inclusion criteria
All T1D women with pregnancy lasting 24 weeks or more, who started attending the NDC-WWRC at or before 15-week gestation between NaN Invalid Date to 30 October 2021 were included in the study.
2.2. Exclusion criteria
We excluded women who delivered outside the HMC facilities.
2.3. Data collection & definitions of outcomes
Data were collected through electronic medical records (Cerner) for the included women by the members of the research study. We defined the pre-pregnancy HbA1c target as ≤53 mmol/mol and the third-trimester target as ≤48 mmol/mol. We classified the ambulatory glucose profile ranges into Time above range-TAR (>140 mg/dl or 7.8 mmol/l)), Time in range-TIR (63–140 mg/dl or 3.5–7.8 mmol/l), and Time below range-TBR (<63 mg/dl or 3.5 mmol/l) [Citation17]. Per the international consensus, women with T1D during pregnancy should achieve < 25% TAR, >70% TIR, and < 5% TBR. Macrosomia was defined as a birth weight greater than 4000 grams [Citation18]. LGA was defined as fetal weight more than the 90th percentile for the gestational age, and SGA was defined as fetal weight less than the 10th percentile for the gestational age [Citation19]. Gestational weight gain (GWG) was defined as excessive if it exceeded the Institute of Medicine (IOM) recommendations [Citation20]. Preterm labor was defined as delivery before the 37th week of gestation. Pregnancy-induced hypertension (PIH) and pregnancy-induced toxemia (PET) were diagnosed based on the American College of Obstetrics and Gynecology classification [Citation21].
2.4. Statistical analysis
We used descriptive statistics to present the demographic characteristics of the study cohort. We categorized the cohort into three groups: Qatari, Arab, and others. We used ethnic-specific cutoff points to categorize BMI into normal, overweight, and obese [Citation22]. We summarized continuous variables as means ± SD or medians (IQR) as appropriate. We summarized categorical variables as percentages. The Shapiro-Wilk test was used to analyze the normality of the data. We used an unpaired t-test and Man-Whitney U test to compare continuous variables as appropriate. Chi-square and Fisher’s tests were used to compare categorical variables. A p-value of < 0.05 was considered significant. We used STATA 15 for the analysis.
2.5. Ethical approval
The study was approved by the Medical Research Centre (MRC) Anonymized, Qatar (Protocol ID: Anonymized).
2.6. Informed consent
Due to the study’s retrospective design, informed consent was waived by the Medical Research Centre (MRC) at Anonymized, Qatar.
2.7. Practice at NDC-WWRC
The referred patients to the NDC were seen by the dietitian to provide an individualized dietary plan based on their pre-gestation weight. The clinic offers a combined diabetes and obstetric service where patients are scheduled for appointments every 2–4 weeks, depending on their medical status. The patients received treatment involving basal insulin analogs and short-acting insulin that were adjusted to the amounts of carbohydrates intake. The insulin doses were adjusted based on the data obtained from home blood glucose values. The recommended glycemic targets for patients utilizing CBG as ADA guidelines were as follows: pre-prandial levels <5.3 mmol/L (95 mg/dL) and 2 hour postprandial levels <6.7 mmol/L (120 mg/dL).
CGM devices are available free of charge to all Qatari citizens. CGM is offered to non-Qatari nationals for half the regular cost. The NDC-WWRC has arrangements with charitable organizations to cover the cost of the CGM for those who are unable to pay. Hence, since the time CGM was available in Qatar, all pregnant women with type 1 DM are offered to use it. Nevertheless, some women might still opt out. All women using CGM are also asked to measure capillary glucose seven times per day, before and 2 hours after each meal and at bedtime.
3. Results
3.1. Baseline characteristics of all patients
A total of 265 women were included in the analysis. The mean age was 29.4 ± 4.7 years and BMI was 27.2 ± 5.2 kg/m2. The majority were either overweight (N = 85, 32.8%) or obese (N = 77, 29.7%). Most women were Qataris (N = 133, 50.2%), followed by non-Qatari Arabs (N = 115, (43.4%). The mean duration of DM was 14.1 ± 7.2 years. The baseline HbA1c at the first-trimester visit was 63 ± 1 mmol/mol; only 76 (28.7%) women had HbA1c within the target (≤53 mmol/mol). A total of 92 (34.7%) women used CGM, while 173 (65.3%) used CBG throughout pregnancy ().
Table 1. Comparison of baseline characteristics in pregnant women with type 1 diabetes mellitus using CGM versus those not using CGM.
3.2. Comparison of women on CGM versus standard CBG monitoring
3.2.1. Comparison of baseline characteristics
More Qatari women were managed with CGM during pregnancy compared to non-Qatari Arabs or other ethnicities (67.4% vs. 30.4% vs. 2.2%, p < 0.001). More women in the CBG routine care group were overweight (38.7% vs. 22%, p 0.009). There were no statistically significant differences in age, DM duration, baseline HbA1c, and comorbid conditions between the two groups ().
3.2.2. Comparison of maternal outcomes
Compared to women managed with CBG, women using CGM had lower insulin requirements (1.02 ± 0.37 vs. 0.87 ± 0.04 units/kg, p = 0.01). Women on CGM had a higher proportion of C-section delivery than those without CGM (76.1% vs. 63%, p = 0.03). However, there was no statistically significant difference in the primary C-section rate (45.1% vs. 37.6%, p = 0.3). Both the CGM and CBG groups had a relatively good decline in HbA1c between initial and last values (12 ± 2 mmol/mol, p < 0.001 and 13 ± 1 mmol/mol, p < 0.001; respectively). There was no significant difference in the mean changes in HbA1c between the 1st trimester and 3rd trimester in women on CGM vs. CBG (). Last trimester HbA1c, proportion achieving the HbA1c target (≤6.5%), weekly and total GWG, PIH, PIT, preterm labor, and proportions of patients with excessive GWG did not differ between the two groups ().
Table 2. Comparison of maternal outcomes in pregnant women with type 1 diabetes mellitus using CGM versus those not using CGM.
3.2.3. Comparison of fetal outcomes
More women with CGM had macrosomia than those with CBG monitoring (13% vs. 4.2%, p 0.009). Neonatal weight, fetal weight percentiles, birth injury, stillbirth, and neonatal ICU admissions were similar between the two groups ().
Table 3. Comparison of fetal outcomes in pregnant women with type 1 diabetes mellitus using CGM versus those not using CGM.
3.3. Glycemic control in the CGM group
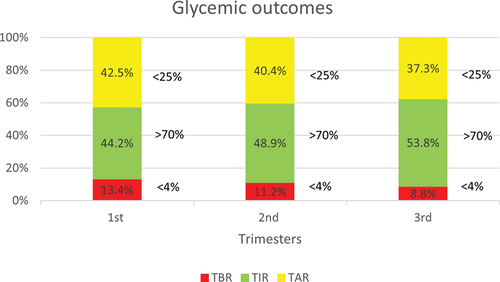
The mean (SD) TAR, TIR and TBR were 42.5 ± 18.3%, 44.2 ± 15.3%, and 13.4 ± 8.5%, respectively, in the first trimester; 40.4 ± 16.8%, 48.9 ± 15.8%, and 11.2 ± 6.45%, respectively, in the second trimester; and 37.4 ± 18.3%, 53.8 ± 18.2%, and 8.8 ± 7.1%, respectively, in the third trimester ().
4. Discussion
In this retrospective study on 265 pregnant women with T1D, almost two-thirds were overweight or obese based on ethnic-specific BMI and only one-third had an HbA1c within target. All women in this cohort from both groups achieved a significant change in HbA1c between first and third trimester. Despite a high baseline and last trimester HbA1c, only 7.3% of infants developed macrosomia, and one-third developed LGA. In women using CGM, TIR improved from 44.2 ± 15.3% in the first trimester to 53.8 ± 18.2% in the third trimester. There were no significant differences in last trimester HbA1c, GWG, neonatal weight, PIH, PIT, preterm labor, primary C-section, fetal weight percentiles and birth injury between the two groups.
HbA1c during the first and last trimester is associated with adverse maternal and fetal outcomes. Lemaitre et al. reported an association of higher HbA1c levels in the first and last trimester with an increased risk of composite criterion consisting of preterm delivery, preeclampsia, LGA, SGA, and C-section [Citation23]. Bashir et al. also reported a higher risk of macrosomia, LGA, C-section, and neonatal ICU admission in women with higher HbA1c in the last trimester [Citation24]. However, the HbA1c levels at conception is known to play a critical role in achieving glycemic targets throughout pregnancy. For example Secher et al. achieved HbA1c of < 6% (42 mmol/mol) at 36 weeks in 154 pregnant women with pregestational DM, of which 123 had T1DM, however the booking HbA1c was 6.6% (49 mmol/mol) [Citation25]. In our study, the mean HbA1c in the last trimester was 6.9% (52 mol/mol), however, the booking HbA1c was 7.9% (63 mmol/mol). In the CONCEPTT trial on pregnant women with T1D, the mean HbA1c at booking was 6.8% (51 mmol/mol), and 66% of women in the CGM group achieved the target HbA1c of 6.5% (52 mmol/mol)at 34 weeks with a mean HbA1c of 6.35% (46 mmol/mol) [Citation14]. Our findings are similar to recent United Kingdom (UK) data that Murphy et al. reported on 8690 women with T1D [Citation26]. The UK cohort had a lower mean BMI compared to ours (25.9 vs. 27.2%), similar first trimester HbA1c (7.6% [60 mmol/mol] vs. 7.9% 63 [mmol/mol]) and similar last trimester HbA1c (6.7% [50 mmol/mol] vs. 6.9% [51 mmol/mol]). The high hbA1c in 1st trimester warrants the need for effective pre-pregnancy counseling and introduction of strict lifestyle and pharmacological interventions to achieve effective glycemic control and lower hbA1c prior to pregnancy which can help in reducing feto-maternal complications.
The rates of LGA and macrosomia in our cohort are much lower than in other studies. Despite pre-pregnancy optimization and lower third trimester HbA1c, the proportion of LGA (53% vs. 33.3%) and macrosomia (23% vs. 7.3%) was higher in the CONCEPTT trial compared to our cohort [Citation14]. The rates of LGA were also higher in the study by Murphy et al. compared to our cohort (52.2% vs. 33.3% respectively) [Citation26]. Evers et al. reported 48.8% macrosomia despite lower HbA1c in the first and last trimesters (6.5% and 6.2%, respectively) [Citation27]. Similarly, in a retrospective study of 308 women with pregestational DM consisting of 221 T1D women, Ladfors et al. found a 50% rate of LGA infants and a 39% rate of macrosomia despite a lower HbA1c in the first and third trimester 6.7% and 5.9% compared to our cohort [Citation28].
As per the ADA guidelines, in T1D women using CGM, time in range (TIR) (3.5–7.8 mmol/L or 63–140 mg/dl) should be more than 70%, time above range (TAR) (>7.8 mmol/L or >140 mg/dl) should be less than 25% and time below range (TBR) (<3.5 mmol/L or <63 mg/dl) should be less than 4% [Citation17]. Even with CGM use, achievement of these targets during pregnancy is challenging and is associated with a concomitant increased risk of hypoglycemia. In the CONCEPTT trial, the investigators achieved a TIR of 68% at 34 weeks of gestation, which is higher compared to the TIR of 53.8% achieved in the third trimester in our cohort. However, our study population’s baseline TIR was much lower than the CONCEPTT trial (44.2% vs. 52%). Notably, in our study, the improvement in TIR from baseline to the third trimester was instead associated with a decrease in TBR (13.4% vs. 8.8%) and a decrease in TAR (42.5% vs. 37.3). It is critical to note that the improvement in TIR was achieved by the end of the third trimester. Hence, the cumulative exposure to hyperglycemia throughout pregnancy could be the reason behind the high rates of LGA in women with T1DM.
Studies on the impact of CGM use on maternal and fetal outcomes in T1D women have yielded variable results. To date, convincing evidence regarding the utility of CGM in T1D women regarding feto-maternal outcomes is lacking. In our study, besides lower TDD of insulin, CGM use was not associated with improved fetal and maternal outcomes. An RCT comparing CGM vs. routine CBG monitoring in women with pregestational diabetes (123 T1DM and 31 Type 2 diabetes mellitus (T2DM)) reported no difference in average HbA1c, hypoglycemia, and LGA between the two groups. However, CGM was used for six days each time, and some women used CGM only for three days [Citation25]. Similarly, in a study on 300 women with pregestational and gestational DM consisting of 109 T1D women, Voormolen et al. reported no significant difference in the primary outcome of fetal macrosomia but lower rates of PIH and PET in women using blinded CGM compared CBG monitoring. However, CGM was used for 5–7 days every six weeks to monitor women in the CGM group [Citation16]. Perea et al. reported on 300 women with T1D, and found higher rates of neonatal hypoglycemia in offspring of women monitored with CGM throughout pregnancy [Citation29]. On the other hand, Murphy et al. reported improved glycemic control, birth weight, and reduced risk of macrosomia in 71 pregnant women with diabetes mellitus (46 T1D women) using CGM compared to the standard CBG [Citation15]. The CONCEPT trial also reported significant improvement in time spent in TIR, TAR, lower incidence of LGA, neonatal intensive care unit (NICU) admissions, neonatal hypoglycemia, and shorter infant length of hospital stay in pregnant T1DM women with the use of CGM [Citation14]. Such variation in results of different studies is possibly due to the effect of other factors on feto-maternal outcomes, such as lipids. Total cholesterol and triglyceride levels have been associated with developing feto-maternal complications, including LGA, SGA and PIT [Citation30]. Nevertheless, further evidence is required to ascertain the beneficial effects of CGM use in pregnant T1D women regarding feto-maternal outcomes.
An interesting finding in our study is the higher proportion of macrosomia in CGM group compared to the CBG group. This points toward the contribution of several factors to the development of fetal macrosomia in addition to the hyperglycemia. Some of the factors include glycemic variability, maternal lipids, GWG and maternal obesity [Citation31]. In our study, there were no differences between baseline BMI and GWG between the 2 groups. However, data regarding several factors including maternal lipids and glycemic variability during pregnancy was not available that might explain the higher proportion of macrosomia in the CGM group. Further studies are needed to explore the effect of these findings
Our study is one of the region’s largest observational studies comparing CGM use to routine care in pregnant women with T1DM. All women in the CGM group used CGM consistently throughout the pregnancy, adding to the authenticity of the outcomes. However, due to its retrospective design, there might have been some effect of confounders on the study results. This limitation is inherent to the retrospective study design, and further prospective studies are required to validate the results. Another important limitation of our study is the selection bias which is a critical limitation of all retrospective studies examining the use of technology in T1DM. In general, people with T1DM who utilize equipments like continuous subcutaneous insulin infusion and CGM can afford them and have the necessary educational background to use the technology, and our study is no exception to that. However, as pointed out in the methodology section, we are trying hard to ensure that all pregnant women with T1DM have access to CGM.
5. Conclusion
In this retrospective study on 265 pregnant women with T1D comparing CGM use to routine CBG monitoring, except for the lower insulin needs in the CGM group, there were no significant differences in last trimester HbA1c, GWG, PIH, eclampsia/preeclampsia, preterm labor, primary C-section, fetal weight percentiles and birth injury between the two groups. Women in the CGM group increased TIR from 44.2% in the first trimester to 53.8% in the third trimester without a concomitant rise in the risk for hypoglycemia. Overall, despite higher baseline and last trimester HbA1c levels, the proportion of macrosomia and LGA was lower in our study population than reported in other studies. We believe CGM is a valuable tool in managing T1DM during pregnancy. However, further studies are needed to develop better management strategies to achieve and sustain adequate glycemic control at an earlier gestational age.
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Ethics
The study was conducted in full compliance with the principles of the ‘Declaration of Helsinki,’ Good Clinical Practice (GCP), and other relevant guidelines. The study was approved by the Medical Research Centre (MRC) of Hamad Medical Corporation, Qatar (Protocol ID: MRC-01-21-1035). Due to the study’s retrospective design, informed consent was waived by the Medical Research Centre (MRC) at Hamad Medical Corporation, Qatar.
Author contributions
Adeel Ahmad Khan: Principal Investigator, Study design, Interpretation of results, Manuscript writing (original draft and revision). Fateen Ata: Literature review, manuscript writing and review
Naglaa Abdelaleem, Al Sayed Alsharkawy, Eman Mahmoud Mohamed Othman, Ifrah Mohamed Hassan, Faten Altaher Mohd Taha: Data collection and manuscript writing. Khaled Baagar, Hamda Ali, Jutin C Konje, Abdul Badi Abou-Samra: Manuscript review and writing. Mohammed Bashir: Study conceptualization and study design, Manuscript writing and review, Statistical analysis, Overall study supervisor.
Data availability statement
Data sharing requires permission from the Ministry of Public Health, Qatar. Any request for datasets requestions can be made to the Medical Research Center (MRC) Qatar at Hamad Medical Corporation, which will seek legal permission form the MOPH before data sharing. [email protected]. The corresponding author Adeel Ahmad Khan can be contacted at akhan40@http://[email protected] to initiate data availability request.
Additional information
Funding
References
- Maresh MJ, Holmes VA, Patterson CC, et al. Glycemic targets in the second and third trimester of pregnancy for women with type 1 diabetes. Diabetes Care. 2015 Jan;38(1):34–42. doi: 10.2337/dc14-1755
- McGrath RT, Glastras SJ, Hocking SL, et al. Large-for-gestational-age neonates in type 1 diabetes and pregnancy: contribution of factors beyond hyperglycemia. Diabetes Care. 2018;41(8):1821. doi: 10.2337/dc18-0551
- Clausen TD, Mathiesen ER, Hansen T, et al. High prevalence of type 2 diabetes and pre-diabetes in adult offspring of women with gestational diabetes mellitus or type 1 diabetes: the role of intrauterine hyperglycemia. Diabetes Care. 2008 Feb;31(2):340–346. doi: 10.2337/dc07-1596
- Holmes VA, Young IS, Patterson CC, et al. Optimal glycemic control, pre-eclampsia, and gestational hypertension in women with type 1 diabetes in the diabetes and pre-eclampsia intervention trial. Diabetes Care. 2011 Aug;34(8):1683–1688. doi: 10.2337/dc11-0244
- American Diabetes A. 14. management of diabetes in pregnancy: standards of medical care in diabetes—2021. Diabetes Care. 2021;44(Supplement 1):S200. doi: 10.2337/dc21-S014
- Excellence NIfHa C. Diabetes in pregnancy: management from preconception to the postnatal period 2020. [cited 2020 Dec 16]. Available from: https://www.nice.org.uk/guidance/ng3/resources
- Goudie RJB, Lunn D, Hovorka R, et al. Pharmacokinetics of insulin aspart in pregnant women with type 1 diabetes: every day is different. Diabetes Care. 2014;37(6):e121. doi: 10.2337/dc13-2535
- Ringholm L, Pedersen-Bjergaard U, Thorsteinsson B, et al. Hypoglycaemia during pregnancy in women with type 1 diabetes. Diabet Med. 2012 May;29(5):558–566. doi: 10.1111/j.1464-5491.2012.03604.x
- Bumbu A, Moutairou A, Matar O, et al. Non-severe hypoglycaemia is associated with weight gain in patients with type 1 diabetes: results from the diabetes control and complication trial. Diabetes Obesity Metab. 2018;20(5):1289–1292. doi: 10.1111/dom.13197
- Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study G, Tamborlane WV, Beck RW, et al. Continuous glucose monitoring and intensive treatment of type 1 diabetes. N Engl J Med. 2008 Oct 2;359(14):1464–1476.
- Pickup JC, Freeman SC, Sutton AJ. Glycaemic control in type 1 diabetes during real time continuous glucose monitoring compared with self monitoring of blood glucose: meta-analysis of randomised controlled trials using individual patient data. BMJ. [2011 Jul 7];343(jul07 1):d3805. doi: 10.1136/bmj.d3805
- Yu F, Lv L, Liang Z, et al. Continuous glucose monitoring effects on maternal glycemic control and pregnancy outcomes in patients with gestational diabetes mellitus: a prospective cohort study. J Clin Endocrinol Metab. 2014 Dec;99(12):4674–4682. doi: 10.1210/jc.2013-4332
- Paramasivam SS, Chinna K, Singh AKK, et al. Continuous glucose monitoring results in lower HbA1c in Malaysian women with insulin-treated gestational diabetes: a randomized controlled trial. Diabet Med. 2018 Aug;35(8):1118–1129. doi: 10.1111/dme.13649
- Feig DS, Donovan LE, Corcoy R, et al. Continuous glucose monitoring in pregnant women with type 1 diabetes (CONCEPTT): a multicentre international randomised controlled trial. Lancet. 2017 Nov 25;390(10110):2347–2359. doi: 10.1016/S0140-6736(17)32400-5
- Murphy HR, Rayman G, Lewis K, et al. Effectiveness of continuous glucose monitoring in pregnant women with diabetes: randomised clinical trial. BMJ. 2008;337(sep25 2):a1680. doi: 10.1136/bmj.a1680
- Voormolen DN, DeVries JH, Sanson RME, et al. Continuous glucose monitoring during diabetic pregnancy (GlucoMOMS): a multicentre randomized controlled trial. Diabetes Obesity Metab. 2018;20(8):1894–1902. doi: 10.1111/dom.13310
- Battelino T, Danne T, Bergenstal RM, et al. Clinical targets for continuous glucose monitoring data interpretation: recommendations from the International Consensus on Time in range. Diabetes Care. 2019 Aug;42(8):1593–1603. doi: 10.2337/dci19-0028
- Allen K, Wallace SVF. Fetal macrosomia. Obstet Gynaecol Reprod Med. 2013 Jun 01;23(6):185–188. doi: 10.1016/j.ogrm.2013.03.012
- Schlaudecker EP, Munoz FM, Bardají A, et al. Small for gestational age: case definition & guidelines for data collection, analysis, and presentation of maternal immunisation safety data. Vaccine. 2017 Dec 4;35(48):6518–6528. doi: 10.1016/j.vaccine.2017.01.040
- Weight gain during pregnancy. Washington DCNAP. doi: 10.17226/12584
- ACOG Practice Bulletin No. 202: gestational hypertension and preeclampsia. Obstet Gynecol. 2019 Jan;133(1):1.
- American Diabetes A. 8. Obesity management for the treatment of type 2 diabetes: standards of medical care in diabetes—2020. Diabetes Care. 2019;43(Supplement_1):S89–S97.
- Lemaitre M, Ternynck C, Bourry J, et al. Association between HbA1c levels on adverse pregnancy outcomes during pregnancy in patients with type 1 diabetes. J Clin Endocrinol Metab. 1125 2022 Feb 17;107(3):e1117–e. doi: 10.1210/clinem/dgab769
- Bashir M, Naem E, Taha F, et al. Outcomes of type 1 diabetes mellitus in pregnancy; effect of excessive gestational weight gain and hyperglycaemia on fetal growth. Diabetes Metab Syndr. 2019 Jan;13(1):84–88. doi: 10.1016/j.dsx.2018.08.030
- Secher AL, Ringholm L, Andersen HU, et al. The effect of real-time continuous glucose monitoring in pregnant women with diabetes: a randomized controlled trial. Diabetes Care. 2013 Jul;36(7):1877–1883. doi: 10.2337/dc12-2360
- Murphy HR, Howgate C, O’Keefe J, et al. Characteristics and outcomes of pregnant women with type 1 or type 2 diabetes: a 5-year national population-based cohort study. Lancet Diabetes Endocrinol. 2021 Mar;9(3):153–164. doi: 10.1016/S2213-8587(20)30406-X
- Evers IM, de Valk HW, Mol BW, et al. Macrosomia despite good glycaemic control in type I diabetic pregnancy; results of a nationwide study in the Netherlands. Diabetologia. 2002 Nov;45(11):1484–1489. doi: 10.1007/s00125-002-0958-7
- Ladfors L, Shaat N, Wiberg N, et al. Fetal overgrowth in women with type 1 and type 2 diabetes mellitus. PLOS ONE. 2017;12(11):e0187917. doi: 10.1371/journal.pone.0187917
- Perea V, Picón MJ, Megia A, et al. Addition of intermittently scanned continuous glucose monitoring to standard care in a cohort of pregnant women with type 1 diabetes: effect on glycaemic control and pregnancy outcomes. Diabetologia. 2022 Aug;65(8):1302–1314. doi: 10.1007/s00125-022-05717-2
- Bashir M, Navti OB, Ahmed B, et al. Hyperlipidaemia and severe hypertriglyceridaemia in pregnancy. Obstet Gynaecol. 2023 Sep 01;25(3):196–209. doi: 10.1111/tog.12887
- McGrath RT, Glastras SJ, Hocking SL, et al. Large-for-gestational-age neonates in type 1 diabetes and pregnancy: contribution of factors beyond hyperglycemia. Diabetes Care. 2018;41(8):1821–1828. doi: 10.2337/dc18-0551