ABSTRACT
Introduction: Evaluating the fertility and pregnancy outcomes in systemic sclerosis (SSc) women is challenging. Studies are still limited or subject to potential methodological biases.
Areas covered: This work is a comprehensive review of the literature. We discuss the potential impact of SSc on women’s pregnancy outcomes and the effects of pregnancy on SSc. We summarize the physiological changes during pregnancy and describe our experience.
Expert commentary: Although the miscarriage rate does not appear increased in SSc, women are exposed to a higher risk of premature birth and intrauterine growth restriction compared with the general population. Early diffuse cutaneous SSc and use of corticosteroids are risk factors, whereas folic acid use prevents against premature birth. All SSc women wishing to conceive should be counselled during a preconception visit. Physiological changes arising during pregnancy may be the source of clinical problems in SSc women with organs with limited capacities.
1. Introduction
Systemic sclerosis (SSc) is a chronic connective tissue disease including various degrees of vascular involvement, tissue fibrosis, and inflammation/autoimmunity [Citation1,Citation2]. SSc affects around nine women for one man [Citation3]. With a mean age of disease onset in the early 1940s and a tendency for couples nowadays to delay their wish to have a child, pregnancy can be a questioning situation for women with SSc and medical teams.
Evaluating the fertility and outcomes of pregnancies in SSc women is challenging as it implies to take account of numerous influencing factors: psychosocial environment, maternal age at conception, physical capability to have a sexual activity and to become pregnant, influence of SSc on pregnancy but also pregnancy on SSc, and medical team management. Furthermore, early reports were first based on small or retrospective cohorts [Citation4–Citation9]. Steen et al. published a prospective study but with self-administered questionnaire [Citation7]. For those reasons, data were limited or controversial and conclusions could not be drawn. Recent publications have provided more detailed information on maternal disease and/or have compared pregnant SSc patients with pregnant healthy women [Citation10–Citation12].
2. Sexuality and fertility of women with SSc
Physical changes associated with SSc may have a negative impact on female sexuality and sexual functioning. Indeed, as a multisystem disease, SSc can affect various organs and is associated with cardiopulmonary and musculoskeletal involvements, which limit the exercise capacity. Skin thickening around the genital tract, joint contractures, or distal vascular disease (digital ulcerations) has a potential impact on sexual functioning. Moreover, the changes in physical appearance may have emotional effects and influence interpersonal relationships [Citation13,Citation14]. Impens et al. conducted a questionnaire study about sexual activity and functioning among 101 women with SSc. Fifty-nine percent of them reported to be sexually active. The sexually inactive patients were around 10 years older. The reasons cited for sexual inactivity were lack of partner (20%), personal choice (32%), health status of the respondent’s partner (20%), and SSc itself (17%). Among sexually active patients, fatigue (60%), dyspareunia related to vaginal dryness (42%) or discomfort (38%), and body pain (40%) were the main scleroderma-related sexual problems. Others factors such as depression, heartburn, hand pain due to Raynaud’s phenomenon or digital ulcers had also a significant impact on sexual activity. Many of these problems are amenable to health interventions and should be addressed during health-care visits [Citation14,Citation15].
The evaluation of fertility in women with SSc is complex. First, it implies to collect precise data on pregnancies events before and after disease onset. Most of the studies are retrospective and may have potential memory bias from patients. Moreover, it is unknown whether SSc actually starts from the usual definition (first non-Raynaud’s phenomenon or the occurrence of Raynaud’s phenomenon) or has early consequences on pregnancies before the first symptoms of SSc are noticed in the mother. Silman et al. found an increased incidence of spontaneous abortion and infertility before disease onset. They suggested that the pregnancy losses could antedate the clinical diagnosis of SSc and have a potential etiological role. One of the hypotheses was that SSc could be a type of chronic graft-versus-host disease resulting from transplacental transfer of cells between mother and fetus [Citation4]. Englert et al. specifically studied the occurrence of pregnancies before the onset of SSc. The women with SSc were more likely to have had a delay in conception (≥12 months) than healthy women. There was a trend toward a higher infertility rate in SSc women compared to healthy controls. However, these differences were not apparent when the SSc group was compared to women with primary Raynaud’s phenomenon [Citation6]. Second, many potential factors can influence the ability and desire to conceive. Steen et al. compared the fertility and pregnancy outcomes in 214 women with SSc, 167 with rheumatoid arthritis (RA), and 105 controls. The proportion of women who had never been pregnant was higher in the SSc (21%) and RA (23%) groups than in the normal control (12%) group (p < 0.05). However, there was no significant difference when adjusting for potential influencing factors such as the number of women who had never married, who were sexually inactive, or who had chosen not to have children. Only small proportions (2–5%) of patients in each group had attempted to conceive but were unsuccessful (no difference between groups). Also, the proportion of women with at least a 1-year delay in conception was not significantly different in the three groups (12–15%). During infertility evaluations, SSc patients were more likely to be told of possible causes of infertility such as fallopian tube obstruction or endometriosis or fertility problems of the partner. The rate of successful pregnancy with treatment was less in SSc than in RA patients and healthy controls. Nevertheless, the overall successful pregnancy rate in those patients with a prior period of infertility was similar in all three groups, 37%, 40%, and 43%, respectively [Citation7]. The disease treatment can also have an impact. A recent study of a cohort of young women with rheumatologic disease showed that more women with prior cyclophosphamide than without had amenorrhea, nulliparity, and infertility. Gonadotropin-releasing hormone agonists co-therapy had a potential role in preventing these adverse effects of cyclophosphamide [Citation16].
3. Placental pathology
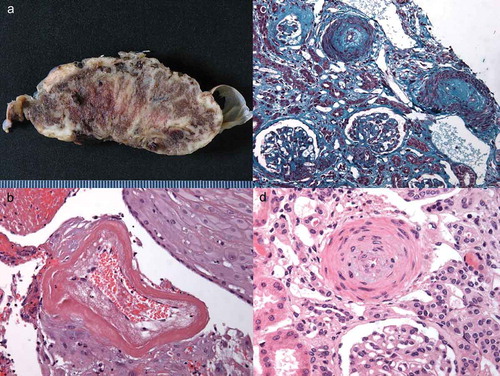
As the unique maternal–fetal blood exchange interface, the placenta can be affected by SSc vasculopathy leading to fetal suffering and premature delivery ((a,b)). Limited histopathologic studies of placentas from SSc pregnancies found normal placenta weight for gestational age [Citation17–Citation19]. Pathological findings included decidual vasculopathy with stromal fibrosis, placental mesenchymal villous dysplasia, infarcts, and reduction of uteroplacental perfusion. Chorioamnionitis was sometimes present. The decidual vascular abnormalities in SSc were similar to those observed in pregnancies complicated by hypertensive disorders. Immunohistochemistry studies demonstrated strong connective tissue growth factor expression in the vessel wall, decidual cells, and fibroblasts. α-smooth muscle actin positive myofibroblasts were found. vascular endothelial growth factor (VEGF) and VEGFR (VEGF receptor)-2 expression was stronger in SSc than in healthy placentas, while VEGFR-1 expression was similar to controls [Citation17,Citation19].
Figure 1. Pathological features of placenta (A and B) and renal biopsy (C and D) in the same patient. A. Macroscopic examination: massive perivillous fibrin deposits throughout the parenchyma. B. Microsopic examination: hyalinized decidual vessels with marked fibrinoid necrosis. C. Fibro-edematous thickening of the arteries, under the endothelial wall and glomerular ischemia (Masson’s trichrome staining). D. Arterial section showing proliferation of myocytes into the layer of the media (concentric ‘onion-skin’ hypertrophy) and edema under the endothelial wall (hematoxylin-eosin-safran staining).
4. Impact of SSc on women’s pregnancy outcomes
4.1. Terminology
The issue of pregnancy outcomes in SSc women is difficult to determine because published studies are limited. Also, the reader should keep in mind some potential methodological limitations:
- Most of the studies were retrospective and/or based on questionnaire (memory biases).
- The definitions used were different because concepts have evolved during time and depend on various terms among countries.
- Some studies have examined pregnancy outcomes before the onset of SSc, others have focused on outcomes after the occurrence of the disease, and there are some which have explored the outcomes of both.
- Proportions given for pregnancy outcomes have included all women with SSc, but sometimes only women who had already been pregnant once, or all pregnancies.
This review aims to help the clinician managing pregnant SSc women; we then assume that SSc diagnosis is already known and describe only pregnancy outcomes after SSc onset – except for miscarriage where exhaustive data before/after SSc onset are given.
4.2. Miscarriage
summarizes studies providing data about miscarriage in women with SSc. Most of the studies have defined miscarriage as a spontaneous abortion before the 20th week of gestation. The overall rate of miscarriage was comprised between 12% and 15% of pregnancies [Citation4–Citation8,Citation11,Citation12,Citation20,Citation21]. This is slightly higher than 11% of pregnancies in a prospective register linkage study of around 630,000 women (general population) in Denmark (more than 1.2 million pregnancy outcomes identified from 1978 to 1992). In this study, the approximate frequencies of clinically recognized miscarriage among the general population and according to maternal age were 9–17% between 20 and 30 years, 20% at 35 years, 40% at 40 years, and 80% at 45 years. However, only known outcomes involving admission to a hospital were recorded [Citation22]. The majority of SSc pregnancy studies used patients’ questionnaires, which could be one of the explanations of the slightly higher rate of miscarriage. A percentage of 19–33 SSc women had already presented a miscarriage. No difference was found between SSc women and controls (RA or the general population) when comparing the rates of miscarriages among all pregnancies or all women. In one study, the rate of miscarriage was identical (15%) before and after SSc onset [Citation5] while in two studies the rate seemed higher after SSc onset (11% vs. 15% and 13% vs. 19%) [Citation7,Citation8]. Two prospective studies found no difference in the rate of miscarriage according to the cutaneous subtype (limited vs. diffuse) [Citation11,Citation20]. Taraborelli et al. found a miscarriage rate of 4% of pregnancies but defined miscarriage as <10 weeks [Citation11]. In conclusion, the miscarriage rate does not appear really different between SSc women and the general population.
Table 1. Studies evaluating miscarriages in SSc women.
4.3. Premature birth
Preterm birth complications are estimated to be responsible for one-third of the neonatal deaths (death in the first 28 days of life). Additional to its contribution to mortality, preterm birth has lifelong effects on neurodevelopmental functioning and an increased risk of chronic disease in adulthood. The World Health Organization (WHO) defines preterm birth as any birth before 37 completed weeks of gestation [Citation23]. The rates of premature birth ranged from 11% to 40% of pregnancies in SSc women (). As a comparison, 11% of all live births worldwide have been estimated as preterm, ranging from about 5% in several European countries to 18% in some African countries [Citation23]. In the recent PROMISSE (Predictors of Pregnancy Outcome: Biomarkers in Antiphospholipid Antibody Syndrome and Systemic Lupus Erythematosus) study of pregnancy outcomes in patients with lupus (patients with high disease activity were excluded), preterm delivery occurred in 9% of pregnancies [Citation24].
Table 2. Maternal and pregnancy outcomes among women with SSc (only studies of outcomes after SSc onset are shown).
In the first retrospective reports, no difference was noted between SSc women and controls regarding the overall rate of premature births (between 5% and 11%) [Citation5,Citation7]. Steen et al. found an increased frequency of premature births after SSc onset (15%) than before (8%; p < 0.05). A similar difference was noted in RA patients (14% vs. 8%; p < 0.05) [Citation7]. A study of 59 SSc women prospectively followed during 10 years found a significant higher proportion of preterm births in SSc women than in controls (25% vs. 5%; relative risk [RR] 2.69 [1.94–3.56]; p < 0.05). There was no difference between lcSSc and dcSSc. However, a higher prevalence of severe lung and gastrointestinal involvement was noted in women who had preterm births. Most preterm births occurred in women with dcSSc and disease duration <4 years. In the 19 pregnancies in early dcSSc, 65% were preterm compared with 24% in other scleroderma subjects (RR 3.67 [1.61–8.33]; p < 0.01) [Citation20].
In the IMPRESS study (Italian Multicentric Study on Pregnancy in Systemic Sclerosis), premature births were significantly more frequent in SSc women than in the general obstetric population (25% vs. 12%; odds ratio 2.53 [1.55–4.10]; p < 0.001). Patients also had a higher prevalence of severe preterm deliveries (<34 weeks of gestation) than the general obstetric population (10% vs. 5%; p = 0.03). In univariate analysis, the factors associated with prematurity were corticosteroid use, intrauterine growth restriction (IUGR) and very-low birth weight babies (<1500 g). Use of folic acid and surprisingly anti-Scl70 positivity were protective factors. Maternal age, disease duration, Medsger severity scale, disease subtype, anti-centromere antibodies, and antiphospholipid antibodies were not associated with prematurity. In multivariable analysis, only corticosteroids were associated with preterm deliveries. Folic acid use and anti-Scl70 antibodies remained protective. The majority of the 25 preterm deliveries (72%) were iatrogenic (cesarean or vaginal), suggesting that there were probably some fetal or maternal complications underlying the obstetrician’s decision to bring delivery forward. Fifty-one cesarean sections were performed (52% of pregnancies) of which 20 as an emergency procedure (9 for obstetric reasons, 11 for fetal distress). Fourteen out of the 31 cesarean sections that were elective were ordered by the obstetric team because of concerns about the maternal disease although there was no real deterioration [Citation11].
In an Australian population-based cohort study, the frequency of preterm births was also significantly higher than in the general obstetric population (30% vs. 7%; unadjusted RR 4.80 [3.03–7.61]) [Citation12]. When adjusted for maternal age, country of birth, socioeconomic status, maternal residence, chronic hypertension, pregestational diabetes, smoking during pregnancy, parity, and multiple births, the RR remained significantly high (4.19 [2.56–6.86]). Not only medically indicated but also spontaneous preterm births were more frequent in SSc than in the general population. Interestingly, this study also examined preterm delivery rates in other autoimmune diseases. There were higher frequencies of preterm births in systemic vasculitis and polymyositis/dermatomyositis than in controls. No difference was found for Sjögren’s syndrome, Behçet’s disease, and vasculitis limited to the skin. Although numerous autoimmune diseases were associated with an increased risk of medically induced preterm births, SSc was the only one associated with spontaneous preterm births [Citation12]. In conclusion, the premature births rate seems higher in SSc than in the general population. Early dcSSc with less than 4 years of disease duration and use of corticosteroids are risk factors, whereas folic acid use prevents against premature birth.
4.4. IUGR and small for gestational age infants
Fetal growth restriction (FGR) is traditionally defined as <10th percentile weight for gestational age. Infants whose FGR is not due to constitutional factors (maternal height, weight, ethnicity, and parity) are at increased risk for significant morbidity and mortality. In SSc, the proportion of small for gestational age (SGA) infants (birth weight <10th percentile for gestational age) is comprised between 0% and 50% depending on studies () [Citation5,Citation7,Citation9,Citation11,Citation12,Citation20]. Most recent studies have found 6% in Australia and 14% in Italy, similarly to the general population [Citation11,Citation12]. As a comparison, in the PROMISSE study of lupus pregnancy outcomes, SGA neonate (<5th percentile) occurred in 10% [Citation24]. Taraborelli et al. showed a higher proportion of very-low birth weight (<1500 g) in SSc pregnancies than in controls (5% vs. 1%; p < 0.002).
IUGR rates were higher in SSc women than in controls in two recent studies: 5% versus 2% in Chakravarty et al. (IUGR defined as weight <10th percentile; p < 0.001) and 6% versus 1% in Taraborelli et al. (fetal abdominal circumference <5th percentile; p < 0.001) [Citation10,Citation11]. Obstetricians should be aware of this risk, and fetal growth should be closely followed with echography.
4.5. Perinatal morbi-mortality
Studies have used various definitions for fetal mortality (). However, no differences have been found in neonatal or perinatal deaths rates between SSc and controls [Citation5,Citation7–Citation9,Citation11,Citation12,Citation20]. In the Australian study, SSc and systemic vasculitis were the two autoimmune diseases associated with severe neonatal morbidity (life-threatening conditions and procedures associated with severe morbidity) compared to the general population after adjustment [Citation12].
5. Effects of pregnancy on SSc
Pregnancy is generally associated with a global stability of SSc. Steen showed among 133 SSc pregnancies a worsening in 7%, an improvement in 5%, and a stability in 88% of disease-related symptoms (Raynaud’s phenomenon, finger ulcers, arthralgias, and skin thickening). One pregnancy-related maternal death occurred [Citation5]. In their prospective study, the same authors found a stability of SSc in 63%, an improvement in 20%, and a worsening in 18%. Most of the women noticed no changes in their symptoms postpartum. One woman with previously known severe pulmonary fibrosis died from respiratory failure 3 months after a therapeutic abortion [Citation20]. In a Brazilian cohort of 42 pregnancies that occurred after SSc onset, disease-related symptoms were unchanged in 72%, worsened in 14%, and improved in 14%. Among the patients studied, there were no maternal deaths. The IMPRESS study confirmed the global stability of SSc during pregnancy. There was a trend for worsening of upper gastrointestinal symptoms in 10–20% of women, albeit those signs are more frequently encountered in pregnant women. Interestingly, 32% and 20% of SSc women noticed improvement in Raynaud’s phenomenon and digital ulcerations, respectively, which recurred after delivery [Citation11]. These overall good outcomes of disease-related symptoms are reassuring. However, it should be underlined that patients included in these studies may represent only a part of the whole SSc population. The most severe patients may have been discouraged to conceive by their physicians, familial environment, or themselves. Their physical conditions may also have prevented them to have sexual activity.
Therefore, there is a risk of worsening of the disease for some SSc women, especially for vital organ involvements such as lungs, heart, and kidneys. More importantly, pregnancy in SSc is a high-risk situation mainly because of the multisystem nature of the disease. Whether counseling SSc women who wish to conceive or facing a pregnancy that has already started, the clinician should take in consideration the basal status of the disease and the potential effect of pregnancy on organs with limited capacities.
5.1. Physiological changes during pregnancy
Many organ systems undergo profound changes as a result of the maternal adaptation to pregnancy (). They contribute to optimal growth and development of the fetus and help to prepare the mother to delivery. Blood volume increases by 50% during normal pregnancy. Early after conception, major hemodynamic changes begin, reaching a peak during the second trimester. Until delivery, cardiac parameters remain relatively stable. Systemic vascular resistances tend to decrease while heart rate accelerates. Cardiac output (CO) increases by 40% during pregnancy, and by 10–40% during labor reaching around 200% of basal value of nonpregnant women. Each contraction expels 300–500 ml of blood into the maternal circulation, increasing venous return and therefore CO by up to 20%. Data on pulmonary hemodynamics are lacking but it seems that pulmonary vascular resistance decreases by 20% during pregnancy [Citation25,Citation26]. While minute ventilation augments significantly during pregnancy and labor, vital capacity and forced expiratory volume seem stable [Citation27]. As a consequence of the relative hyperventilation, there is a decrease in arterial carbon dioxide levels (PaCO2) [Citation26]. Some studies have shown a slight decrease in diffusing capacity of the lung for carbon monoxide [Citation28]. Finally, there is a significant augmentation in renal perfusion and glomerular filtration rate [Citation26,Citation29]. These physiological changes are generally well tolerated in healthy women, but may be source of clinical problems when arising in SSc women with limited capacities organs.
Table 3. Physiological changes during normal pregnancy.
5.2. Renal disease
Renal involvement, as manifested by proteinuria, elevated serum creatinine concentration, and/or hypertension, has been observed in up to 50% of patients with SSc [Citation30]. Many of these findings are due to prerenal disease associated with pulmonary hypertension or heart disease and/or drugs toxicity. Scleroderma renal crisis (SRC) is the most serious renal complication, occurring in 5–20% of SSc patients. Risk factors include diffuse skin involvement, use of glucocorticoids (≥15 mg/day of prednisone or equivalent), and presence of anti-RNA polymerase III antibodies or absence of anticentromere antibodies. Usual signs of presentation are an abrupt onset of hypertension, acute renal failure, headaches, fevers, malaise, hypertensive retinopathy, encephalopathy, and pulmonary oedema [Citation31]. It can be difficult to distinguish between SRC and preeclampsia. In two different studies by Steen et al. (retro- and prospective), SRC onset during pregnancy has been described in two (2%) and two (2%) cases. All occurred in early diffuse SSc patients, between 16 and 28 weeks of gestation [Citation5,Citation20]. The frequency of SRC during pregnancy did not seem increased regarding the known risk in early diffuse SSc. Of note, normotensive SRC has been described [Citation32].
Preeclampsia is a multisystem disorder characterized by the new onset of hypertension (systolic blood pressure [BP] ≥140 mmHg or diastolic BP ≥90 mmHg) and proteinuria or end organ dysfunction or both after 20 weeks of gestation. Eclampsia corresponds to the development of grand mal seizures in a woman with preeclampsia. These complications are not uncommon in pregnant women; the worldwide prevalence of preeclampsia and eclampsia has been estimated at 4.6% (95% uncertainty range 2.7–8.2) and 1.4% (1.0–2.0) of all deliveries, respectively [Citation33]. Prevalence of pregnancy hypertension during SSc pregnancies has been estimated between 2% and 23% [Citation10–Citation12]. In Chakravarty et al., the proportion of hypertensive disorders (23%) in SSc patients was significantly higher than in controls (8%) [Citation10]. Preeclampsia occurred in 8% of SSc pregnancies in a small study [Citation9].
Patients with preeclampsia can present with hemolysis and microangiopathic hemolytic anemia, elevated liver enzymes, and a thrombocytopenia, especially in the HELLP syndrome. These clinical manifestations are very similar to those of SRC. Other causes of hypertension, proteinuria, thrombocytopenia, liver and kidney abnormalities include thrombotic thrombocytopenic purpura, hemolytic uremic syndrome, antiphospholipid syndrome, or an overlap with another connective tissue disease such as systemic lupus erythematosus (SLE). Distinguishing among these different causes may be challenging. Renal biopsy can be helpful and guide the treatment ((c,d)). A recent systematic review found heterogeneous evidence on kidney biopsy in pregnancy, but a significantly higher risk of complications (relative to postpartum biopsy), with a possible peak at around 25 gestational weeks [Citation34]. Moreover, uncontrolled hypertension during the procedure increases the risk of complications. Some authors recommend to avoid renal biopsy after 28 or 32 weeks of gestation, but there is a global consensus around performing biopsy only if it may change management prior to delivery [Citation34,Citation35]. Measurement of plasma renin might be useful. In preeclampsia, the serum renin is low-to-normal, whereas in SRC plasma, renin is elevated as a result of renocortical ischemia [Citation36,Citation37]. In the future, new biomarkers distinguishing preeclampsia from other hypertensive-proteinuric disorders such as sFLT1 (soluble fms-like tyrosine kinase-1)/PlGF (placental growth factor) ratio should become available [Citation38]. Their potential to rule out SRC remains unknown.
Among patients with a known renal disease, pregnancy can cause a decline in renal function. Increased risk for this decline is conferred by estimated glomerular filtration rate (GFR) <40 ml/min/1.73 m2, proteinuria >1 g/24 h and hypertension [Citation29,Citation39].
In practice, a renal biopsy can be helpful distinguishing between SRC and differential diagnoses. However, due to a potential maternal–fetal morbidity, we would recommend to avoid or defer the procedure until the postpartum period unless it may change management prior to delivery. A pregnancy project should be reconsidered in women with chronic kidney disease and GFR < 30–40 ml/min/1.73 m2, proteinuria > 1 g/24 h, and/or uncontrolled hypertension.
5.3. Interstitial lung disease and cardiac involvement
There are few studies reporting a relative stability of interstitial lung disease and cardiac involvement during pregnancy [Citation11,Citation20]. Women with restrictive lung disease (parenchymal abnormalities, chest wall involvement) are exposed to hypoxic or hypercapnic respiratory failure during pregnancy. Close monitoring of spirometry and oxygen saturation is therefore mandatory, particularly from the second trimester, and oxygen therapy must be introduced if needed. Some reports indicate that patients with restrictive lung disease tolerate pregnancy reasonably well, but many have premature delivery [Citation40,Citation41]. Although there is no validated cutoff to contraindicate pregnancy, it seems reasonable to avoid a pregnancy if the forced vital capacity (FVC) is below 50% of predicted. Regarding heart involvement, the known risk factors associated with an adverse cardiac event during pregnancy include prior cardiac event (heart failure, transient ischemia attach, or stroke) or arrhythmia, baseline New York Heart Association (NYHA) class III or IV or cyanosis, and reduced left ventricular ejection fraction (LVEF) < 40%. A B-type natriuretic peptide level < 100 pg/ml could have a good negative predictive value for identifying cardiac events during pregnancy, albeit this has not been validated in SSc [Citation42].
In practice, pregnancy should be contraindicated in case of cardiac failure with NYHA class III/IV and/or LVEF <40% or interstitial lung disease with FVC <50% of predicted.
5.4. Pulmonary arterial hypertension
Pulmonary arterial hypertension (PAH) is a severe complication of SSc, affecting around 10% of patients [Citation43]. Usually, diagnosed in patients older than 50, PAH has been classically associated with long-standing lcSSc with anticentromere antibodies [Citation44]. However, younger patients can also be affected, especially those with anti-U1 RNP antibodies [Citation45]. According to the 2015 European Society of Cardiology/European Respiratory Society (ESC/ERS) Guidelines for the diagnosis and treatment of pulmonary hypertension, it is recommended that PAH patients avoid pregnancy (class of recommendation I, level of evidence C) [Citation46]. Indeed, patients with PAH have very limited capacities to tolerate the increased CO and blood volume during pregnancy. There are substantial risks of right heart failure and maternal mortality especially during the early postpartum period [Citation47]. Recent studies indicate that the outcome of pregnancy in PAH has improved in the modern management era, at least when PAH is well controlled. However, these data must be confirmed by larger series, especially in SSc-PAH [Citation47].
We recommend to consider PAH as a contraindication of pregnancy in accordance with ESC/ERS recent guidelines [Citation46].
6. Pregnancy management
summarizes general recommendations for management of pregnancy during SSc. Preconceptional counseling should include a complete assessment of the disease and vital functions in order to evaluate the risk of starting a pregnancy. Dyspnea scale, 6-min walking distance, renal function, cardiac echography, and pulmonary function tests should be updated. Early diffuse SSc (less than 4 years) and presence of anti-topoisomerase I or anti-RNA polymerase III antibodies are associated factors of a more active disease. Patients with antiphospholipid antibodies are at higher risk of materno-fetal complications – introduction of low-dose aspirin and low-weight molecular heparin should be discussed. Anti-Ro/SSA and anti-La/SSB antibodies have been associated with the development of heart block in the fetus or newborn and are not uncommon antibodies in SSc [Citation48]. A close fetal surveillance is therefore recommended for these patients.
Precise information on the potential complications should be given to the couple. Patients can be reassured on the risk of hereditary neonatal scleroderma. The risks of IUGR, prematurity, and cesarean delivery should be explained. One should educate our patients: explaining the potential occurrence of symptoms (dyspnea, hypertension, swelling edema, etc.) and when/where to seek help if needed; then check if patients have understood/reformulate. The preconception visit also includes the evaluation of teratogenicity of drugs prescribed for SSc. One should discontinue/switch those with teratogenic potential at least 3 months before pregnancy. Expert information about exposures during pregnancy or breastfeeding can be found on dedicated websites e.g. http://www.mothertobaby.org (USA), http://www.medicinesinpregnancy.org (UK), http://www.lecrat.fr (France). A EULAR (European League Against Rheumatism) task force recently developed statements on the compatibility of antirheumatic drugs during pregnancy and lactation [Citation50]. Use of corticosteroids is allowed during pregnancy, to the minimal possible dose. Proton pump inhibitors, histamine blockers, or calcium channel blockers are classical medications in SSc patients (gastrointestinal and vascular involvements) and are usually allowed during pregnancy.
Early during the pregnancy, a multidisciplinary team should be involved in the management of a SSc pregnancy. It includes a close follow-up with an obstetrician experienced in high-risk pregnancies with periodic assessments of fetal growth and Doppler velocimetry of uterine and/or umbilical arteries. Regular BP monitoring (plus oxygen saturation and spirometry in case of restrictive lung disease) is recommended. If systemic hypertension (systolic BP ≥ 140 mmHg or diastolic BP ≥ 90 mmHg) occurs, it is recommended to treat aggressively. We usually ask promptly for serum creatinin, liver function, uric acid, complete blood count with red cells indices, platelets count and examination of blood smear, reticulocyte count, lactate dehydrogenase, haptoglobin, and proteinuria. SRC should be considered in priority, along with preeclampsia and differential diagnosis (thrombocytopenic purpura, hemolytic uremic syndrome, antiphospholipid syndrome, or an overlap with another connective tissue disease such as SLE). General drugs to control BP are usually allowed during pregnancy. If BP remains uncontrolled, or serum creatinin increases, or there is a significant probability of SRC, the patient should be transferred in intensive care unit (ICU). An angiotensin-converting enzyme (ACE) inhibitor should be started at low doses and increased as needed, despite the risk of fetal toxicity (see specific section later) [Citation14]. During the third trimester of pregnancy, the neonatal team must be involved in the management. A representative case history is presented .
If some of the maternal vital functions are severely involved – severe restrictive lung disease with FVC <50% predicted or respiratory failure, severe cardiomyopathy with LVEF <30–40%, renal insufficiency with GFR <30–40 ml/min/1.73 m2, or pulmonary hypertension – the decision of pregnancy termination should be discussed considering the risks to the mother and the fetus. This should be a multidisciplinary decision involving the couple.
6.1. In case of a renal crisis
As previously discussed, when hypertension, renal insufficiency, or thrombocytopenia occur, a SRC should be suspected in priority. Differential diagnoses including preeclampsia should be discussed. SRC is an emergency condition where the vital prognosis of mother and infant are threatened. The prognosis of patients with SRC has dramatically improved with the use of ACE inhibitors [Citation51]. ACE inhibitors have been associated with fetal toxicity (anhydramnios, renal atresia, pulmonary hypoplasia, and fetal death). However, regarding the fact that maternal complications exceed the fetal risk, it is generally advised to start ACE inhibitor promptly using high doses. The treatment must not wait for diagnostic confirmation or a cesarean delivery [Citation14,Citation20,Citation49].
After a SRC, ACE inhibitors are given indefinitely to maintain BP and renal function. If a woman with a prior history of SRC wishes to conceive, it is advisable to try switching progressively to another antihypertensive treatment. However, if the BP is not controlled with other antihypertensive drugs, ACE inhibitors should be restarted. Then the patient should be advised on the potential teratogenicity and a multidisciplinary decision, involving the patient and her partner, should be taken whether to become or continue a pregnancy [Citation20,Citation49].
6.2. In case of pulmonary hypertension
According to the 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension, pregnant patients with PAH should be informed of the high risk of pregnancy and termination of the pregnancy should be discussed. Those patients who choose to continue pregnancy should be treated with disease-targeted therapies, planned elective delivery, and effective close collaboration between obstetricians and the PAH team [Citation46].
6.3. Delivery and anesthesia
Every aspect of anesthesia care in a patient with SSc may be altered by the pathogenesis of the disease [Citation14,Citation52]. Peripheral IV access, endotracheal intubation, ventilation management, pharmacologic choices, fluid management, coagulation profiles, and postoperative extubation all may be affected [Citation53]. These need to be discussed before delivery in a multidisciplinary assessment including the anesthesia and intensive care team. Skin thickening or joint contractures may induce technical difficulties to get peripheral venous access, perform regional anesthesia, monitor vital signs, or install the patient for delivery. Epidural block provides adequate anesthesia and allows peripheral vasodilatation and increased skin perfusion. General anesthesia should be avoided whenever possible because of the risk of difficult intubation and gastric liquid aspiration [Citation52]. It is generally advised a special warming of the delivery room, intravenous fluids, and patients themselves (e.g. extra blankets, thermal socks, gloves). There is no concern regarding wound healing; therefore, cesarean or episiotomy can be considered [Citation14,Citation49]. Because of the peripartum stress, hydrocortisone hemisuccinate should be infused in patients who received continuous corticotherapy before delivery.
6.4. After delivery
A careful postpartum monitoring is mandatory, including BP and blood tests (renal function, full blood count). Patients are usually admitted in ICU during 24–72 h. Several cases of SRC after delivery have been described [Citation11,Citation20]. In the early postpartum period, previous medication for SSc should be progressively reintroduced, with special care if the patient is breastfeeding [Citation14]. Expert information about exposures during breastfeeding can be found on dedicated websites, e.g. http://www.mothertobaby.org (USA), http://www.medicinesinpregnancy.org (UK), http://www.lecrat.fr (France). A EULAR task force recently provided statements on the compatibility of antirheumatic drugs during lactation [Citation48]. If the mother does not breastfeed, drugs suppressing lactation should be avoided given potential thromboembolic side effects observed with the dopamine agonist bromocriptine [Citation54]. The clinical team should enquire into the potential limitations of the mother to provide care of the newborn, which can arise from physical incapacities and fatigue linked to SSc. There is no evidence that the risk of postpartum depression could be greater in SSc patients.
7. Expert commentary
Before the 1990s, the knowledge on SSc pregnancies was based on historical cases reporting severe outcomes until the studies listed in this review provided evidence of a global more favorable denouement. Despite the fact that published studies are limited, we have now relevant tools to inform and counsel patients who wish to conceive and take care of those who are pregnant. However, there are potential methodological limitations that still limit the interpretation of some outcomes. It emphasizes the need for more prospective studies. Reports should provide clear and consensual definition of outcomes to improve comparability between studies and compilation of data.
The increasing number of registries collecting data on pregnancies in patients with autoimmune diseases will be a valuable help to build future prospective observational studies. By building networks, registries enable to increase the knowledge of the community and to homogenize management.
8. Five-year view
Promising markers of preeclampsia (sFLT1 and PlGF) should become available soon in clinical practice. Their potential to distinguish between preeclampsia and SRC should be evaluated. Recent reports indicate successful pregnancies in some PAH patients. These data should be confirmed by larger studies especially in SSc patients. The role of specific drug therapy in these patients should be assessed.
Box 2. Representative case history
Key issues
Evaluating the fertility in systemic sclerosis (SSc) women is challenging as it implies to take account of numerous influencing factors: psychosocial environment, maternal age at conception, physical capability to have a sexual activity and to become pregnant, influence of SSc on pregnancy but also pregnancy on SSc, and medical team management.
Studies evaluating pregnancy outcomes in SSc are limited and most of them are retrospective and/or based on questionnaire. The miscarriage rate does not appear really different between SSc women and the general population.
There is a higher risk of premature birth in SSc than in the general population. Early diffuse cutaneous SSc with less than 4 years of disease duration, use of corticosteroids are risk factors whereas folic acid use prevents against premature birth.
Intrauterine growth restriction (IUGR) rates were higher in SSc women than in controls in two recent studies. Obstetricians should be aware of this risk, and fetal growth should be closely followed with echography.
No clear difference has been found in neonatal or perinatal deaths rates between SSc and controls. However, studies have used various definitions for fetal mortality.
Pregnancy is generally associated with a global stability of SSc. Nevertheless, some patients are exposed to a risk of worsening of the disease, especially for vital organ involvements such as lungs, heart and kidneys. Early diffuse SSc, presence of anti-topoisomerase I or anti-RNA polymerase III antibodies are associated factors of a more active disease.
All SSc women wishing to conceive should be counselled during a preconception visit. Patients should be carefully evaluated and organ involvement assessed.
Physiological changes arising during pregnancy are generally well tolerated in healthy women, but may be source of clinical problems in SSc women with limited capacities organs. Pregnancy should be contra-indicated in women with significant kidney involvement, cardiac failure with NYHA class III/IV and/or ejection fraction <40%, interstitial lung disease with forced vital capacity <50% of predicted or pulmonary arterial hypertension.
Pregnancy management involves a multidisciplinary team including at least obstetrician familiar with high-risk pregnancies, anesthesiologist trained to the specificities of SSc and neonatologist.
If systemic hypertension, renal insufficiency or thrombocytopenia occur, a scleroderma renal crisis (SRC) should be suspected in priority (along with preeclampsia). Urgent management is required.
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Acknowledgments
We are very grateful to Dr. Louise Devisme and Dr. Sarah Humez (Institute of Pathology, CHRU Lille, Université Lille 2) for providing histological pictures.
References
- Gabrielli A, Avvedimento EV, Krieg T. Scleroderma. N Engl J Med. 2009;360(19):1989–2003.
- Sanges S, Guerrier T, Launay D, et al. Role of B cells in the pathogenesis of systemic sclerosis. Rev Med Interne. Forthcoming 2016.
- Meier FMP, Frommer KW, Dinser R, et al. Update on the profile of the EUSTAR cohort: an analysis of the EULAR Scleroderma Trials and Research group database. Ann Rheum Dis. 2012;71(8):1355–1360.
- Silman AJ, Black C. Increased incidence of spontaneous abortion and infertility in women with scleroderma before disease onset: a controlled study. Ann Rheum Dis. 1988;47(6):441–444.
- Steen VD, Conte C, Day N, et al. Pregnancy in women with systemic sclerosis. Arthritis Rheum. 1989;32(2):151–157.
- Englert H, Brennan P, McNeil D, et al. Reproductive function prior to disease onset in women with scleroderma. J Rheumatol. 1992;19(10):1575–1579.
- Steen VD, Medsger TA. Fertility and pregnancy outcome in women with systemic sclerosis. Arthritis Rheum. 1999;42(4):763–768.
- Sampaio-Barros PD, Samara AM, Marques Neto JF. Gynaecologic history in systemic sclerosis. Clin Rheumatol. 2000;19(3):184–187.
- Chung L, Flyckt RLR, Colón I, et al. Outcome of pregnancies complicated by systemic sclerosis and mixed connective tissue disease. Lupus. 2006;15(9):595–599.
- Chakravarty EF, Khanna D, Chung L. Pregnancy outcomes in systemic sclerosis, primary pulmonary hypertension, and sickle cell disease. Obstet Gynecol. 2008;111(4):927–934.
- Taraborelli M, Ramoni V, Brucato A, et al. Brief report: successful pregnancies but a higher risk of preterm births in patients with systemic sclerosis: an Italian multicenter study. Arthritis Rheum. 1970–1977;64(6):2012.
- Chen JS, Roberts CL, Simpson JM, et al. Pregnancy outcomes in women with rare autoimmune diseases. Arthritis Rheumatol. 2015;67(12):3314–3323.
- Bruni C, Raja J, Denton CP, et al. The clinical relevance of sexual dysfunction in systemic sclerosis. Autoimmun Rev. 2015;14(12):1111–1115.
- Steen VD. Pregnancy in scleroderma. Rheum Dis Clin America. 2007;33(2):345–358.
- Impens AJ, Rothman J, Schiopu E, et al. Sexual activity and functioning in female scleroderma patients. Clin Exp Rheumatol. 2009;27(3 Suppl 54):38–43.
- Harward L, Mitchell K, Pieper C, et al. The impact of cyclophosphamide on menstruation and pregnancy in women with rheumatologic disease. Lupus. 2012;22(1):81–86.
- Ibba-Manneschi L, Manetti M, Milia AF, et al. Severe fibrotic changes and altered expression of angiogenic factors in maternal scleroderma: placental findings. Ann Rheum Dis. 2010;69(2):458–461.
- Papakonstantinou K, Hasiakos D, Kondi-Paphiti A. Clinicopathology of maternal scleroderma. Int J Gynecol Obstetrics. 2007;99(3):248–249.
- Doss BJ, Jacques SM, Mayes MD, et al. Maternal scleroderma: placental findings and perinatal outcome. Hum Pathol. 1998;29(12):1524–1530.
- Steen VD. Pregnancy in women with systemic sclerosis. Obstet Gynecol. 1999;94(1):15–20.
- Van Wyk L, Van Der Marel J, Schuerwegh AJ, et al. Increased incidence of pregnancy complications in women who later develop scleroderma: a case control study. Arthritis Res Ther. 2011;13(6):R183.
- Nybo Andersen AM, Wohlfahrt J, Christens P, et al. Maternal age and fetal loss: population based register linkage study. BMJ (Clinical Research Ed.). 2000;320(7251):1708–1712.
- Blencowe H, Cousens S, Oestergaard MZ, et al. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: a systematic analysis and implications. Lancet. 2012;379(9832):2162–2172.
- Buyon JP, Kim MY, Guerra MM, et al. Predictors of pregnancy outcomes in patients with lupus. Ann Intern Med. 2015;163(3):153–15.
- Silversides CK, Colman JM. Physiological changes in pregnancy. In: Oakley C, Warnes CA, editors. Heart disease in pregnancy. 2nd ed. Malden (MA): Blackwell; 2007.
- Carlin A, Alfirevic Z. Physiological changes of pregnancy and monitoring. Best Pract Res Clin Obstetrics Gynaecol. 2008;22(5):801–823.
- Gazioglu K, Kaltreider NL, Rosen M, et al. Pulmonary function during pregnancy in normal women and in patients with cardiopulmonary disease. Thorax. 1970;25(4):445–450.
- Zavorsky GS, Blood AB, Power GG, et al. CO and NO pulmonary diffusing capacity during pregnancy: safety and diagnostic potential. Respir Physiol Neurobiol. 2010;170(3):215–225.
- Williams D, Davison J. Chronic kidney disease in pregnancy. BMJ. 2008;336(7637):211–215.
- Steen VD, Syzd A, Johnson JP, et al. Kidney disease other than renal crisis in patients with diffuse scleroderma. J Rheumatol. 2005;32(4):649–655.
- Denton CP, Lapadula G, Mouthon L, et al. Renal complications and scleroderma renal crisis. Rheumatology (Oxford, England). 2009;48(Suppl 3):iii32–5.
- Hudson M, Baron M, Tatibouet S, et al. International Scleroderma Renal Crisis Study Investigators. Exposure to ACE inhibitors prior to the onset of scleroderma renal crisis-results from the International Scleroderma Renal Crisis Survey. Semin Arthritis Rheum. 2014;43(5):666–672.
- Abalos E, Cuesta C, Grosso AL, et al. Global and regional estimates of preeclampsia and eclampsia: a systematic review. Eur J Obstetrics Gynecol Reprod Biol. 2013;170(1):1–7.
- Piccoli GB, Daidola G, Attini R, et al. Kidney biopsy in pregnancy: evidence for counselling? A systematic narrative review. BJOG: Int J Obs Gyn. 2013;120(4):412–427.
- Day C, Hewins P, Hildebrand S, et al. The role of renal biopsy in women with kidney disease identified in pregnancy. Nephrol Dial, Transpl: Off Publ Eur Dial Transpl Assoc Eur Ren Assoc. 2008;23(1):201–206.
- Lidar M, Langevitz P. Pregnancy issues in scleroderma. Autoimmun Rev. 2012;11(6–7):A515–9.
- Launay D, Hebbar M, Valat AS, et al. Systemic sclerosis and pregnancy. Rev Med Interne. 2002;23(7):607–621.
- Zeisler H, Llurba E, Chantraine F, et al. Predictive value of the sFlt-1: PlGFRatio in women with suspected preeclampsia. N Engl J Med. 2016;374(1):13–22.
- Soh MC, Nelson-Piercy C. High-risk pregnancy and the rheumatologist. Rheumatology. 2015;54(4):572–587.
- Lapinsky SE, Tram C, Mehta S, et al. Restrictive lung disease in pregnancy. Chest. 2014;145(2):394–398.
- Boggess KA, Easterling TR, Raghu G. Management and outcome of pregnant women with interstitial and restrictive lung disease. Am J Obstet Gynecol. 1995;173(4):1007–1014.
- Lewey J, Haythe J. Cardiomyopathy in pregnancy. Semin Perinatol. 2014;38(5):309–317.
- Sobanski V, Launay D, Hachulla E, et al. Current approaches to the treatment of systemic-sclerosis-associated pulmonary arterial hypertension (SSc-PAH). Curr Rheumatol Rep. 2016;18(2):10–14.
- Lefèvre G, Dauchet L, Hachulla E, et al. Survival and prognostic factors in systemic sclerosis–associated pulmonary hypertension: a systematic review and meta‐analysis. Arthritis Rheum. 2013;65(9):2412–2423.
- Sobanski V, Giovannelli J, Lynch BM, et al. Characteristics and survival of anti-u1 rnp antibody-positive patients with connective tissue disease-associated pulmonary arterial hypertension. Arthritis Rheumatol. 2016;68(2):484–493.
- Galiè N, Humbert M, Vachiery J-L, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: the joint task force for the diagnosis and treatment of pulmonary hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Respir J. 2015;46(4):903–975.
- Jaïs X, Olsson KM, Barberà JA, et al. Pregnancy outcomes in pulmonary arterial hypertension in the modern management era. Eur Respir J. 2012;40(4):881–885.
- Brucato A, Cimaz R, Caporali R, et al. Pregnancy outcomes in patients with autoimmune diseases and anti-Ro/SSA antibodies. Clin Rev Allergy Immunol. 2011;40(1):27–41.
- De León Aguirre AR, Calvo JAR, Reyna TSR. Comprehensive approach to systemic sclerosis patients during pregnancy. Reumatología Clínica (English Edition). 2015;11(2):99–107.
- Götestam Skorpen C, Hoeltzenbein M, Tincani A, et al. The EULAR points to consider for use of antirheumatic drugs before pregnancy, and during pregnancy and lactation. Ann Rheum Dis. 2016;75(5):795–810.
- Steen VD, Medsger TA. Long-term outcomes of scleroderma renal crisis. Ann Intern Med. 2000;133(8):600–603.
- Downloads | Systemic sclerosis | Published Guidelines | Rare Diseases [Internet]. orphananesthesia.eu. Available from: http://www.orphananesthesia.eu/en/rare-diseases/published-guidelines/cat_view/61-rare-diseases/60-published-guidelines/143-systemic-sclerosis.html.
- Roberts JG, Sabar R, Gianoli JA, et al. Progressive systemic sclerosis: clinical manifestations and anesthetic considerations. J Clin Anesth. 2002;14(6):474–477.
- Bernard N, Jantzem H, Becker M, et al. Severe adverse effects of bromocriptine in lactation inhibition: a pharmacovigilance survey. BJOG: Int J Obs Gyn. 2015;122(9):1244–1251.
