Anaphylaxis is currently recognized as a severe, life-threatening systemic hypersensitivity reaction characterized by rapid onset and the potential to endanger life. Since anaphylaxis is characterized by rapidly developing life-threatening airway and/or breathing and/or circulation problems, the need of rapid management of this condition is well-known. Epinephrine (adrenaline), injected by the intramuscular route, is the medication of choice for the first-aid treatment of anaphylaxis [Citation1–Citation3].
Regional epidemiological data cite anaphylaxis incidence rates ranging from 1.5 to 7.9 per 100 000 person-years in European countries [Citation4] and estimated in at 5.1% (95% CI, 3.4% to 6.8%) in the United States [Citation5]. In all countries, epidemiological and health services research can serve as a baseline for quality improvement, prioritization of anaphylaxis programs, and eventual reduction in morbidity and mortality. However, the global epidemiological morbidity [Citation6] and mortality [Citation7] data for anaphylaxis remain unclear due to the lack of standardized tools for capturing harmonized and accurate data, particularly in the International Classification of Disease (ICD) (). As an example, we confirmed in 2012, the under notification of anaphylaxis deaths due to difficult coding under the ICD-10 [Citation7] and documented previously [Citation8,Citation9].
Table 1. Anaphylaxis classification and coding in ICD-10 and in ICD-11.
The ICD is an international standard diagnostic classification for mortality and morbidity statistics (MMS) maintained by the World Health organization (WHO). Uses include monitoring of the incidence and prevalence of diseases, observing reimbursements and resource allocation trends, and keeping track of safety and quality guidelines. In use by more than 100 countries, the ICD also includes the counting of deaths as well as diseases, injuries, symptoms, reasons for encounter, factors that influence health status, and external causes of disease [Citation10]. The weak identity of disorders in the ICD contributes to the lack of ascertainment and recognition of their importance for health care planning and resource allocation, and prevents clinical research from being performed. Regarding mortality statistics, unless the data have been compiled using the same methods and according to the same standards, comparisons potentially yield misleading results. For these reasons, the WHO issued international instructions on data collection, coding and classification, and statistical presentation of causes of death. In most countries, mortality statistics are routinely compiled according to regulations and recommendations adopted by the World Health Assembly [Citation10]. The international mortality coding instructions presuppose that data have been collected with a death certificate conforming to the International form of medical certificate of cause of death. On current death certificates, a limited number of ICD codes are valid as underlying causes of death, and death certificates do not include the word anaphylaxis per se [Citation7].
Anaphylaxis resides in ICD-10 under the nonspecific ‘Chapter XIX Injury, poisoning and certain other consequences of external causes’ which the main references are ‘Anaphylactic shock due to adverse food reaction’ (T78.0) and ‘Anaphylactic shock, unspecified’ (T78.2) (). Moreover, the term ‘shock’, which means the drop of blood pressure, is one of the anaphylaxis manifestations, however, should not be taken as synonymous or always together. It suggests the need of implementing a severity degree classification for anaphylaxis into the ICD [Citation6], currently implemented for ICD-11.
Understanding the specifications of more accurate anaphylaxis morbidity and mortality statistics can support the identification of patients at highest risk, facilitating the implementation of prevention measures. In theory, such data could be used to support national and international public health strategies such as mapping emerging triggers, assessing the safety of marketed products, and establishing the mandatory declaration of most relevant allergens. Besides, these data can support decision-making actions and advocate for a better management of anaphylaxis through the global affordable availability of auto-injectable adrenaline (AAI), as first-line medications in the treatment of this condition.
The WHO lists adrenaline as an essential medication for the treatment of anaphylaxis [Citation9]. However, it is not readily available in the form of auto-injectors in more than 65% of world countries [Citation11] in which best management of patients with anaphylaxis is therefore impossible. The key issues leading to the lack of availability of AAI include cost but also national regulations, lack of regional evidence about the value of epinephrine and the limited accurate data about the epidemiology of anaphylaxis morbidity and mortality data. For these reasons, regional and international allergy academies support the initiatives to narrow these gaps. Our WHO Collaborating Center is deeply involved in this process and our first key goal is to ensure the availability of essential medications for patients, therefore the AAI in low and middle-income countries and to promote better management and education in high-income countries, with the support of the WHO-Family of International Classifications, academic, and scientific networks, the Joint Allergy Academies, stakeholders and patients’ organizations. The WHO Collaborating Center leadership is working in an education program in order to support the transition for the ICD-11.
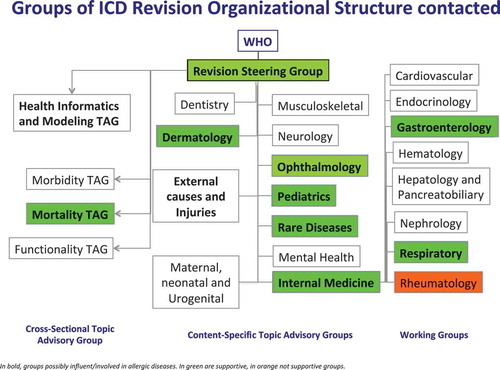
With the aim of having a better representation of allergic and hypersensitivity conditions, including anaphylaxis, in international classification systems and taking the window of opportunity presented by the ICD-11 revision, the documented anaphylaxis deaths under-notification data [Citation7] triggered a cascade of strategic international actions acknowledged by a Joint Allergy Academies’ consortium composed by six international regional allergy academies, the ICD WHO governance [Citation6,Citation7,Citation12–Citation20] in order to update the classifications of allergic and hypersensitivity conditions for the new ICD edition. The design of the anaphylaxis subsection was based on the Allergic and hypersensitivity diseases proposal, which had been validated by crowd-sourcing [Citation13] and simplified [Citation15] according to ICD Revision Steering Group (RSG) guidance. The construction of the new Allergic and hypersensitivity conditions section of the ICD-11 resulted by intensive scientific, academic and technical discussions to ensure comparability and consistency. The development of the ‘Anaphylaxis’ sub-section involved strong academic input and extensive consultation and agreement from the relevant Topic Advisory Groups and Expert Working Groups (). The intensive exchange of e-mails and teleconference/videoconferences started in February 2014 and was the basis for the submission of proposals into the online ICD-11 beta draft platform [Citation21]. All the actions of the Allergy Working Group have so far been undertaken with RSG guidance. All the terms of the ICD-11 count with definitions.
Figure 1. Framework of groups in charge of the international classification of diseases (ICD)-11 revision process.
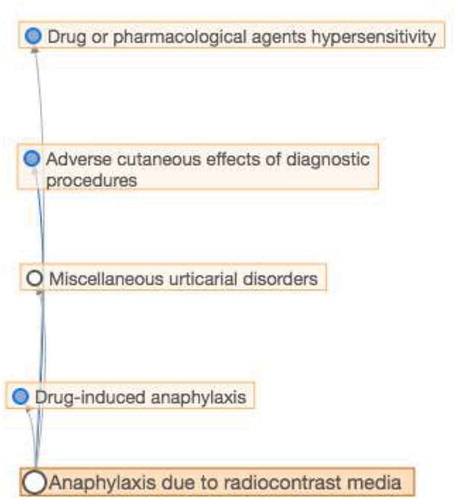
These efforts have resulted in the construction of the pioneer ‘Allergic and hypersensitivity conditions’ section under the ‘Disorders of the Immune system’ chapter of the online ICD-11 Beta draft [Citation21,Citation22]. In the new ‘Allergic and hypersensitivity conditions’ section of ICD-11, it was possible to build a sub-section specifically addressed to anaphylaxis. For the first time, anaphylaxis is elected as individualized conditions into the ICD-11 frame, receiving a sub-section addressed to this condition. The new ‘Anaphylaxis’ sub-section was constructed, with 11 entities classified under 7 main headings: Anaphylaxis due to allergic reaction to food, Drug-induced anaphylaxis, Anaphylaxis due to insect venom, Anaphylaxis provoked by physical factors, Anaphylaxis due to inhaled allergens, Anaphylaxis due to contact with allergens and Anaphylaxis secondary to mast cell disorders (), and followed a multi-hierarchical framework. As an example, ‘anaphylaxis due to contrast media’ is multi-parented by ‘drug or pharmaceutical agent hypersensitivity’, ‘adverse cutaneous effects of diagnostic procedures’, ‘miscellaneous urticarial disorders’ and ‘drug-induced anaphylaxis’ (). The construction of the new section dealing with anaphylaxis means that the latter will now be recognized as a clinical condition requiring specific documentation and management.
Figure 2. Taking the example from ‘anaphylaxis due to radiocontrast media’ to demonstrate the multi-hierarchical framework of ICD-11.
From the public health perspective, by allowing all the relevant diagnostic terms for anaphylaxis to be included in the ICD-11 MMS, WHO has recognized their importance not only to clinicians but also to epidemiologists, statisticians, health-care planners, and other stakeholders. Importantly the new classification will enable the collection of more accurate epidemiological data to support quality management of patients with allergies, better health care planning and gathering public health measures to reduce the morbidity and mortality attributable to allergy. Examples are the availability of AAI in all countries for patients at risk, the provision of resuscitation kits in public places and the implementation of prevention campaigns in surgical and radiology departments.
Overall, the WHO indicates that the ICD is currently responsible for allocating about 70% of the world’s health expenditures, meaning by USD 2.3 trillion in 2013 and USD 2.6 trillion in 2014 according to the National Center for Health Statistics [Citation10,Citation22]. Therefore, every modification into the ICD framework may have a potential impact in health finance and economy. Greater specificity regarding clinical conditions and services delivered will provide payers, policy makers, and providers with better information to make major refinements to payment and reimbursement systems, including the design and implementation of pay-for-performance program [Citation22].
Increasing the specification of conditions will help clarify the connection between a provider’s performance and the patient’s condition. Accurate and updated diagnostic, and procedure codes will improve data on the outcomes, efficacy, and costs of new medical technology and facilitate fair reimbursement policies for the use of this system. It will help payers and providers more easily identify patients in need of disease management and more effectively tailor disease management programs [Citation22].
Although the WHO’s ICD-11 is still some time away to be officially implemented in diverse health systems for final use, the new classification will enable the collection of more accurate epidemiological data to support quality management of patients with allergies, and better facilitate health-care planning and decision-making and public health measures to prevent and reduce the morbidity and mortality attributable to allergic diseases. The improved logic and standardized definitions through the ICD-11 will also facilitate international comparisons of quality care and the sharing of best practice globally.
Declaration of interest
Pascal Demoly and Luciana Kase Tanno received an unrestricted Novartis and MEDA/Mylan Pharma grants through CHRUM administration. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose
Additional information
Funding
References
- Sampson HA, Munoz-Furlong A, Campbell RL, et al. Second symposium on the definition and management of anaphylaxis: summary report – second National Institute of allergy and infectious disease/food allergy and anaphylaxis network symposium. J Allergy Clin Immunol. 2006;117(2):391–397.
- Simons FER, Ardusso LR, Bilò MB, et al. International consensus on (ICON) anaphylaxis. World Allergy Organ J. 2014;30:7: 9.
- Muraro A, Roberts G, Worm M, et al. on behalf of EAACI food allergy and anaphylaxis guidelines group. Anaphylaxis: guidelines from the European academy of allergy and clinical immunology. Allergy. 2014. DOI:10.1111/all.12437
- Panesar SS, Javad S, de Silva D, et al. Sheikh A on behalf of the EAACI food allergy and anaphylaxis group. The Epidemiology of Anaphylaxis in Europe: a Systematic Review. Allergy. 2013;68:1353–1361.
- Neugut AI, Ghatak AT, Miller RL. Anaphylaxis in United States: an investigation into its epidemiology. Arch Intern Med. 2001 Jan 8;161(1):15–21.
- Tanno LK, Calderon MA, Goldberg BJ, et al. Categorization of allergic disorders in the new World Health Organization international classification of diseases. CTA. 2014;4:42–49.
- Tanno LK, Ganem F, Demoly P, et al. Undernotification of anaphylaxis deaths in Brazil due to difficult coding under the ICD-10. Allergy. 2012;67:783–789.
- Lieberman P, Camargo CA Jr, Bohlke K, et al. Epidemiology of anaphylaxis: findings of the American college of allergy, asthma and immunology epidemiology of anaphylaxis working group. Ann Allergy Asthma Immunol. 2006 Nov;97(5):596–602.
- Clark S, Gaeta TJ, Kamarthi GS, et al. ICD-9-CM coding of emergency department visits for food and insect sting allergy. Ann Epidemiol. 2006 Sep;16(9):696–700.
- World Health Organization [cited 2018 April]; available from: http://www.who.int/classifications/icd/en/
- World Health Organization website for WHO model list of essential medicines [cited 2018 April]; available from: http://www.who.int/medicines/publications/essentialmedicines/en/,
- Tanno LK, Simons FER, Cardona V, et al. Applying prevention concepts to anaphylaxis: a call for worldwide availability of adrenaline auto-injectors. Clin Exp Allergy. 2017 Sep;47(9):1108–1114.
- Tanno LK, Calderon MA, Goldberg BJ, et al. Constructing a classification of hypersensitivity/allergic diseases for ICD-11 by crowdsourcing the allergist community. Allergy. 2015;70:609–615.
- Tanno LK, Calderon M, Papadopoulos NG, et al. Mapping hypersensitivity/allergic diseases in the international classification of diseases (ICD)-11: cross-linking terms and unmet needs. Clin Transl Allergy. 2015;5:20.
- Tanno LK, Calderon MA, Demoly P. on behalf the joint allergy academies. Optimization and simplification of the allergic and hypersensitivity conditions classification for the ICD-11. Allergy. 2016 May;71(5):671–676. .
- Tanno LK, Calderon MA, Demoly P. on behalf the joint allergy academies. New allergic and hypersensitivity conditions section in the international classification of diseases-11. Allergy Asthma Immunol Res. 2016 Jul 8;(4):383–388. DOI:10.4168/aair.2016.8.4.383.
- Tanno LK, Calderon MA, Papadopoulos NG, et al. Joint allergy academies. Surveying the new allergic and hypersensitivity conditions chapter of the international classification of diseases (ICD)-11. Allergy. 2016 Sep;71(9):1235–1240.
- Tanno LK, Calderon M, Demoly P. Joint allergy academies. Supporting the validation of the new allergic and hypersensitivity conditions section of the World Health Organization international classification of diseases-11. Asia Pac Allergy. 2016 Jul 6;(3):149–156.
- Tanno LK, Molinari N, Bruel S, et al. Joint allergy academies. Field-testing the new anaphylaxis’ classification for the WHO International Classification of Diseases-11 revision. Allergy. 2017 May;72(5):820–826.
- Tanno LK, Bierrenbach AL, Calderon MA, et al. Joint allergy academies. decreasing the undernotification of anaphylaxis deaths in Brazil through the International Classification of Diseases (ICD)-11 revision. Allergy. 2017 Jan;72(1):120–125.
- World Health Organization, ICD-11 beta draft website.[cited 2018 April]; available from: https://icd.who.int/dev11/l-m/en
- Tanno LK, Sublett JL, Meadows JA, et al. Perspectives of the International Classification of Diseases (ICD)-11 in Allergy Clinical Practice in the United Staes of America. Ann Allergy Asthma Immunol. 2017 Feb;118(2):127–132.
