ABSTRACT
Introduction: Omalizumab is a recombinant monoclonal anti-IgE antibody approved in the US as add-on treatment in moderate-to-severe allergic asthma (in severe allergic asthma [SAA] in Europe). A 2016 review of 24 real-world effectiveness studies in SAA published between 2008–2015 concluded that omalizumab was associated with significant improvements in objective and subjective outcomes with benefits extending beyond 2 years. Several new real-world studies have been published since, bringing the total to 42 studies.
Areas covered: This systematic review of 42 studies published since 2008 updates and extends the 2016 review on the real-word evidence on omalizumab in SAA. It offers greater granularity as to time windows within which outcomes are reported and includes studies extending well beyond 4 years post omalizumab initiation.
Expert commentary: This review firmly establishes the short-term effectiveness of omalizumab in adolescent and adult patients with SAA at 1 year, and provides strong evidence of long-term effectiveness up to 4 years and emergent evidence of effectiveness beyond 4 years. In the aggregate, these 42 studies underscore the long-term effectiveness of omalizumab in terms of: reducing exacerbations and symptoms, achieving asthma control, improving lung function, enhancing quality of life, decreasing emergency department visits and hospitalizations, and promoting concomitant medication-sparing.
1. Introduction
In its 2018 report, the Global Asthma Network estimated that as many as 339 million people worldwide, or 5% of the world population, suffer from asthma [Citation1], with other estimates reaching as high as 16% [Citation2]. Asthma is the 16th leading cause of years lived with disability and the 28th ranked cause of disability-adjusted life years [Citation1]. About 1000 people die of asthma daily [Citation1]. With no cure, asthma management strategies aim to: control the clinical aspects of the disease, including respiratory function; prevent exacerbations and thus reduce unscheduled medical visits, emergency room admissions, and hospitalizations; lower medication burden; and enhance quality of life.
One treatment target concerns the antibody class of immunoglobulin E (IgE), which binds to the high-affinity receptors on the surface of mast cells and causes these cells to release inflammatory mediators [Citation3]. Omalizumab (Xolair®, Novartis, Basel, Switzerland; Genentech, San Francisco, California USA) is a recombinant monoclonal anti-IgE antibody that binds to free IgE and downregulates high-affinity IgE receptors (FcεRI) on mast cells as well as basophils, eosinophils and dendritic cells [Citation4,Citation5]. Omalizumab interrupts the allergic cascade by inhibiting IgE from binding to these receptors [Citation4,Citation5], preventing IgE cross-linking [Citation6], limiting mast cell degranulation [Citation5,Citation6], and minimizing the release of mediators in the early- and late-phase of allergen response [Citation6,Citation7]. It is administered as add-on treatment to inhaled corticosteroids (ICS) and/or long-acting β2-agonists (LABA) [Citation8,Citation9]. The efficacy and safety of omalizumab were demonstrated in phase III clinical trials, including its positive impact on asthma symptom management and control, exacerbations, medication burden, and quality of life [Citation9–Citation12]; were confirmed in subsequent studies and a meta-analysis [Citation13]; and have been summarized extensively [Citation14–Citation23].
Following the approval of omalizumab for moderate-to-severe allergic asthma in the US in 2003 and for severe allergic asthma in Europe in 2005, several ‘real-world’ (also referred to as ‘real-life’) studies were initiated to evaluate the effectiveness of omalizumab add-on therapy under conditions of heterogeneity in patients, clinicians, and settings. In a prior independent systematic review of 24 such studies [Citation24–Citation47] published between 2008 and 2015 and covering 4117 unique patients with severe allergic asthma from 32 countries, we concluded that omalizumab was effective in: reducing asthma symptoms, exacerbations, and work/school days lost; improving asthma control; improving lung function; decreasing healthcare utilization; lowering the use of other asthma medications; and enhancing quality of life; while presenting a similar safety profile as that of the randomized controlled trials [Citation48]. That systematic review was the basis for an independent meta-analysis [Citation49] of these 24 studies plus one subsequently published investigation [Citation50] that synthesized results for the following outcomes: Global Evaluation of Treatment Effectiveness (GETE); FEV1 (% predicted); Asthma-related Quality of Life Questionnaire (AQLQ); Asthma Control Test (ACT); oral corticosteroid (OCS) and ICS use; asthma exacerbations; and asthma-related hospitalizations. The meta-analysis demonstrated that, quantitatively, the real-life effectiveness of pharmacotherapy with omalizumab mirrors, complements, and extends the efficacy data from randomized controlled studies.
Several new real-world studies have been reported since our initial systematic review, bringing the total to 42 studies published between 2008 and 2018 and including a total of 9377 patients from 35 countries. In the aggregate, thirty of these studies (n = 3558) included adult patients only, and twelve studies (n = 5819) comprised both adult and adolescent patients. We report here on an updated systematic review of these 42 observational studies of the real-world benefits and harms of omalizumab in the management of severe allergic asthma. Notably, our prior systematic review [Citation48] and meta-analysis [Citation49] provided strong evidence on outcomes up to 1 year with some emergent evidence beyond 1 year. This present systematic review also provides significant evidence for the long-term effectiveness of omalizumab after 2 and 3 years of treatment, with emergent evidence well beyond 4 years.
This present systematic review is part of a broader initiative to synthesize the real-world evidence on omalizumab. In addition to our earlier independent systematic review [Citation48] and meta-analysis [Citation49], two papers commissioned by Novartis have summarized the evidence on omalizumab in childhood asthma [Citation51,Citation52]. We recently published a commissioned systematic review [Citation53] and meta-analysis [Citation54] of omalizumab in the IgE-mediated condition of chronic idiopathic/spontaneous urticaria.
2. Evidence base
This is an update and extension of the Abraham et al. [Citation48] systematic review published in 2016 on the real-world effectiveness of omalizumab in adults with severe allergic asthma. The protocol for the prior systematic review, based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) [Citation55] guidance, was updated. This review extends our prior review in terms: study designs used; the patients in these studies; the effectiveness of omalizumab therapy in terms of clinical, health resource utilization, quality of life, and medication-sparing outcomes; and safety. However, it does so with greater granularity as to time points at which, and time windows within which, outcomes are reported; specifically, at the fixed time points of 16 weeks and 1 year, and the time windows of 5–9 months (including the 6-month mark), 23 to 32 months (including the 2-year and 2.5-year marks), and 36 months (or 3 years) and beyond. In addition, effectiveness and safety outcomes are presented on standardized scales of percent change; specifically, positive percent change for improvements on positive outcomes, and negative percent change for improvements on negative markers.
2.1. Eligibility
Eligibility for inclusion was defined in PICOS terms:
Participants Male or female adolescents and adults diagnosed with severe allergic asthma.
Interventions Treated with omalizumab for severe allergic asthma.
Comparators Permissible but not required.
Outcomes At least one measure of the following: lung function, exacerbations, daytime or nighttime symptoms, asthma control (Asthma Control Test [ACT] or Asthma Control Questionnaire [ACQ]), quality of life (Juniper Asthma-related Quality of Life [AQLQ] or European Quality of Life Questionnaire 5 Dimensions [EQ-5D]), physician-rated asthma classification (Global Initiative on Asthma [GINA]), physician-rated treatment effectiveness (Global Evaluation of Treatment Effectiveness [GETE]), asthma-related emergency department visits or hospitalizations, use of concomitant anti-asthmatic treatments, or safety.
Studies Any observational method including registry, retrospective chart review, prospective observational study, or pragmatic trial reported as full-text articles.
Note that we also admitted studies that included both adult and adolescent (age 12 years or older) patients, but not children. However, to assure a predominant adult study population, the pooled weighted mean age for the adult/adolescent set of studies could not be statistically significantly lower than that for the adult-only set of studies.
2.2. Search strategy, screening, and data extraction
PubMed, Embase and Cochrane Library databases were searched from initiation on 17 July 2018 using the terms ‘IgE’, ‘immunoglobulin E’; ‘omalizumab’; ‘asthma’; ‘effectiveness’, ‘real-life’, ‘real-world’; without date, sample size, or language restrictions. Abstract-only publications were not included due to lack of sufficiently detailed data. Additional potential sources were identified through citation analysis and general monitoring of the literature. With the search period being from initiation of the databases to 17 July 2018, it was possible to validate the studies in the prior systematic review [Citation48] in addition to identifying studies reported since 2015.
If two or more publications containing the same subjects were identified (either partially or wholly), the publications were assessed for sample size and detail of reporting, and only one publication was selected for inclusion. Likewise, subsequently published pooled analyses comprising one or more samples already selected for this review were excluded. One exception is the eXpeRience Registry in which different outcomes on the entire sample were reported across three different publications [Citation39,Citation56,Citation57]. This registry study is included in this review once but the data were aggregated from the three publications.
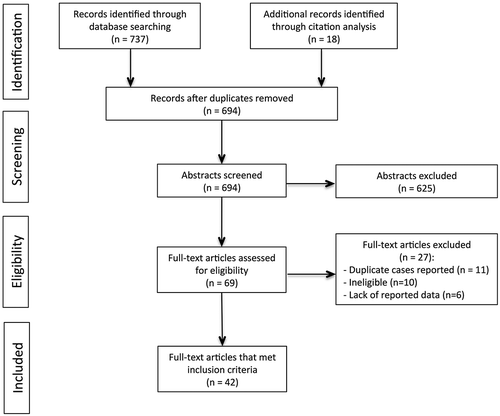
An initial pool of 755 publications was identified (). Following removal of 61 duplicate citations, 694 studies were retained and screened, of which 625 were rejected. The remaining 69 full-text articles were assessed for eligibility and 27 were excluded: 11 papers reported results on samples previously published and thus included duplicate cases, 10 were ineligible per PICOS criteria, and 6 lacked sufficiently detailed outcome data. This yielded a total of 42 studies.
Data were extracted as reported in the published studies, except where noted, and entered into master tables of evidence (Supplementary Tables S1 through S4).
2.3. Other methodological considerations
Published results in all papers were validated, data permitting, to assess data integrity and derive common metrics for comparisons across studies. Hence, results in our analysis may diverge from originally reported data if recalculations were done using a different formula to permit cross-study comparisons. Changes in outcomes between baseline and each reported time point were transformed into percentage change. The statistical significance associated with changes, if reported, was extracted from the original publications. The reported or standardized data from the individual studies were aggregated where possible to provide pooled weighted estimates (either pooled weighted percentage or pooled weighted mean±SD). To minimize bias, non-significant results reported in the original publication were included in our pooled estimates.
3. Review of studies
summarizes the 42 studies stratified by 30 adult-only studies [Citation24,Citation26,Citation27,Citation30–Citation37,Citation40–Citation47,Citation58–Citation68] (n = 3558) and 12 adult/adolescent studies [Citation25,Citation38,Citation39,Citation56,Citation57,Citation69–Citation77] (n = 5819). These 42 studies comprised a total of 9377 patients from 35 countries: 25 in Europe (incl. Israel, Russia, and Turkey), 5 in Asia-Pacific (incl. Australia), 3 in North-America, and 2 in South-America.
Table 1. Summary of designs, time points of assessments, and effectiveness outcomes reported.
Detailed data by study are presented in the Supplementary Tables S1 through S4: patient demographics, clinical status, healthcare resource utilization, and asthma treatment at enrolment (Table S1); and evolution in clinical outcomes and quality of life (Table S2), healthcare resource utilization (Table S3), and medication and safety outcomes (Table S4). These are subsequently aggregated into summary tables of demographics and baseline characteristics () and evolution of outcome measures ().
Table 2. Summary of demographics and baseline characteristics.
Table 3. Summary of outcome measures (all studies: n = 42).
Note that evolutions are presented at two fixed time points (16 weeks and 1 years) and in three time windows (5–9 months; 23–32 months; and 36 months and beyond). Not all studies reported on similar time frames, as some studies had shorter and some had longer follow-up durations. Outcomes at different time points may or may not include the same patients, the same samples, and the same studies. Trends over time should therefore be interpreted as trends across studies and their samples. Longer follow-up durations are unlikely to include all initial patients. Various causes of censoring are likely (e.g., attrition, lost-to-follow-up, discontinuation of omalizumab, or death). Analyses at a given time point or time window could be influenced by long-term responders. Findings should be interpreted as pertaining to those persisting in treatment due to, among other reasons, positive treatment response.
3.1. Study designs
Of the 42 studies, 23 (55%) were designed as prospective and 19 (45%) as retrospective studies (). The prospective studies included 17 (74%) adult-only studies and 6 (26%) adult/adolescent studies. Of the 19 retrospective studies, 13 (68%) studied only adults and 6 (32%) a mixed adult-adolescent sample. Most (36/42; 86%) studies reported retrospective data from prior to the initiation of omalizumab therapy. The majority of these (30/36; 83%) went back 1 year, including 19/24 (79%) adult-only and 11/12 (92%) adult/adolescent studies reporting pre-omalizumab data.
Evaluable sample sizes ranged from 4 to 3593 patients, including from 4 to 723 patients in the adult-only and from 16 to 359 in the adult/adolescent studies. Though only 28/42 (67%) of studies stated the number of physicians or centers contributing patients, the observed range was from 1 to 1001, including 1 to 134 for the adult-only studies and 1 to 1001 for the adult/adolescent studies.
Sixteen weeks is the indicated time point to initially assess treatment response and decide about continuation of omalizumab therapy. Only 11/30 (37%) adult-only and 6/12 (50%) adult/adolescent studies reported fixed 16-week data. A few studies 7/42 (17%) reported fixed 6-month data and 18/42 (43%) reported fixed 1-year data, including the majority (8/12 or 67%) of adult/adolescent but only 10/30 or 33% of adult-only studies. In total, 26/42 (62%) of studies reported data at various other time points ranging from fixed 2 months to 4 years or at averages ranging from 20 to 60.7 months.
In descending order, the clinical outcome variables and instruments most consistently evaluated – across time points but not necessarily at each time point or in each time window – were exacerbations (74% of studies), FEV1 (% predicted) (71%), concomitant corticosteroid use (69%), hospitalizations (55%), emergency department visits (45%), ACT (43%), concomitant medications other than corticosteroids (36%), GETE (31%), and AQLQ (26%). All other outcomes were evaluated in fewer than 20% of studies. Adverse events were recorded in 55% of the studies.
3.2. Baseline characteristics of patients
In pooled weighted analyses, mean (±SD) age of patients at enrolment across all studies was 52.3 ± 14.6 years across (see ). Mean age was 48.8 years in adult-only versus 54.4 years in adult/adolescent studies, showing that mean age in the adult-adolescent sample was not suppressed by the inclusion of small sub-cohorts of adolescents. Unless marked differences are noted between the adult-only and adult/adolescent studies, we focus on the all-studies results.
About two-thirds (63.2%) of patients were female and 70.9% of patients had never smoked. At baseline, mean weight was 74.8 kg, mean serum total IgE was 374.3 IU/mL, and mean FEV1 was 64.7% predicted. In terms of asthma control at baseline, mean ACT scores were 14.1 (reported in n = 13 studies) and mean ACQ scores were 3.0 (n = 7 studies). Quality of life was significantly impaired, as indicated by mean AQLQ scores of 3.8 (n = 8 studies). In the year preceding the start of omalizumab therapy, 93.8% of patients had experienced exacerbations (reported in n = 14 studies) and the weighted mean number of exacerbations per patients in the preceding year was 4.0 (n = 25 studies); 28.6% had required at least one emergency room visit (n = 5 studies) and the weighted mean number of ER visits per patient was 2.0 (n = 7 studies); and 26.2% were hospitalized at least once (n = 10) with a mean of 0.9 hospitalizations per person (n = 16). Daytime (90.5%) and nighttime symptoms (79.5%) in the year prior to enrolment were reported in a large majority of patients, though this information was collected in only a small number of studies (n = 4 and n = 5, respectively). Summarized, patients who comprised the samples in these studies had the hallmarks of severe allergic asthma including high serum concentrations of IgE, poor predicted FEV1, poor asthma control, frequent emergency department visits and hospitalizations, and impaired quality of life.
Virtually all patients were previously treated with ICSs (98.8%) and LABAs (96.7%), and many (73.2%) with leukotriene receptor antagonists (LTRA). Compared to the adult-only studies, the adult/adolescent studies reported proportionately higher rates of treatment with LTRAs (80.1% vs. 58.0%) and theophylline derivatives (59.1% vs. 25.8%).
Weighted mean omalizumab dose, as reported in 21 studies (n = 6,084), was 308 mg/month. Eight studies reported that an aggregated 20.4% of patients were initiated on an omalizumab dose inconsistent with the recommended dosing schedule.
3.3. Effectiveness
summarizes the time course of outcomes measures from baseline to the fixed time points of 16 weeks and 12 months, and from baseline to the time windows of 5–9 months, 23–32 months, and through ≥ 36 months. As the first 16 weeks are a trial period to evaluate whether patients respond to omalizumab, and a decision to continue of discontinue treatment is made at this time, we focus mainly on outcomes in patients in whom treatment is continued.
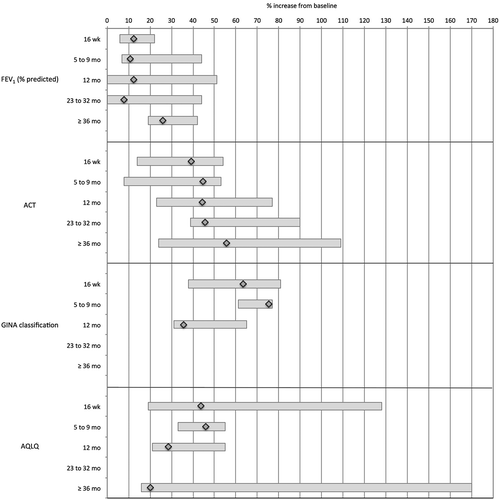
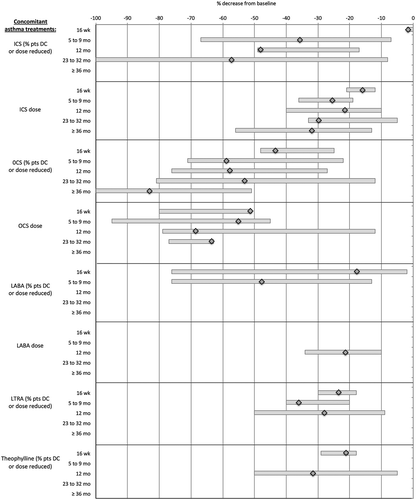
depicts the standardized outcomes where improvement is expressed as a positive percentage from baseline. and present the standardized outcomes where improvement is expressed as a negative percentage from baseline. For a given outcome, the diamond represents the point estimate of the pooled weighted mean percent of change over baseline. The bar denotes the lowest and the highest percent change reported by singular studies and also reflects the relative variation in rates reported across studies.
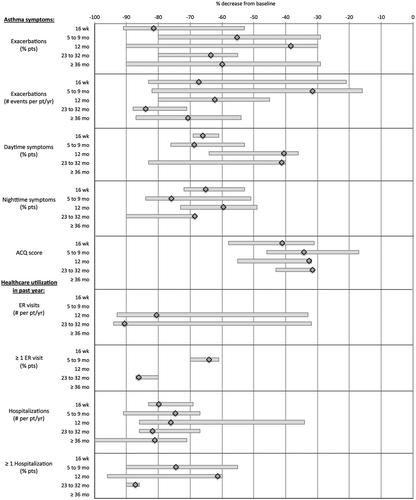
Figure 3. Effectiveness outcomes: % decrease from baseline for asthma symptoms and healthcare utilization.
3.3.1. Clinical outcomes
In terms of objective lung function, FEV1 (% predicted) improved consistently from baseline by an average of 8.5% to 12.4% between 5 and 32 months, and by an average of 26.0% from baseline to ≥ 36 months. Some studies reported improvements from baseline as high as 42% and 51%.
Major reductions in exacerbations were identified. At the 5–9 month time window, exacerbations declined by an average of 55.3% compared to the year prior to omalizumab treatment. At 12 months, the rate was 38.4% lower than the 12 months pre-omalizumab and this trend continued over time to eventually reach declining averages of 60%. Singular studies reported decreases in exacerbations beyond the initial 16 weeks as high as 80% and 90%. In parallel, the annual number of exacerbations per patient declined on average by 31.5% at 5–9 months, and this decline continued steadily to 62.2% at 12 months and 83.9% at 23–32 months; to settle at a 70.7% decline at 36 months and beyond. Singular studies reported percentages of decline in exacerbation events between 80% and 88%.
Whereas daytime symptoms had decreased by an average 68.7% by the 5–9 month time window, later on the reported decreases averaged slightly above 40%. Singular studies reported declines as high as 83%. In contrast, nighttime symptoms declined initially by 75.9% (5–9 months) over baseline. At 12 months, the decline in nighttime symptoms was 59.6% but improved to 68.6% in the 23–32 month time window. Improvements in nighttime symptoms by as much as 84% (5–9 months) and 90% (23–32 months) were reported in singular analyses.
As to asthma control, ACT scores improved by about 45% over baseline between 5 and 32 months, with an improvement by 55.6% noted at 36 months and beyond. Individual studies related changes as high as 77% at 12 months, 90% at 23–32 months, and 109% at ≥ 36 months. ACQ scores showed consistent average improvements by about one-third over baseline from the short-term (5–9 months) to the relatively long-term (23–32 months). Average improvements in GINA classification varied between 35.7% (12 months) and 75.4% (5–9 months); with the dual caveat that the GINA classification is limited to 4 levels and therefore yields relatively little variation in general, and that only 1 or 2 studies reported useable data further limiting variability.
On average, about two-thirds of patients were classified as good or excellent on the GETE scale at 16 weeks, 5–9 months, and 1 year after the start of omalizumab treatment. This increased to an average of 80.8% by 23–32 months.
3.3.2. Quality of life
AQLQ scores increased significantly to an average 46.1% over baseline over the first 9 months, to settle at average increases of 28.4% at 12 months and, based however on only two studies, 20.2% at 36 months and beyond. Improvements in quality of life, as measured by the AQLQ, reached as high as 55% between 16 weeks and 1 year. Though included in , EQ5D data should be considered with reservation because of the limited number of patients in the 1–2 studies using this method of measuring quality of life.
3.3.3. Healthcare resource utilization
Compared with the year before initiation of omalizumab therapy, emergency room visit events declined by an average of 80.6% in the first year of treatment and 90.6% in the 23–32 month time window. Singular studies reported decline rates of 93% and 94% at these respective times. The mean percentage of patients requiring one or more emergency room visits declined by an average annualized rate of 64.1% in the 5–9 month time window, 55% in the first year of omalizumab treatment, and 86.1% by 23–32 months since start of treatment; yet these results require reservation considering only 1 or 2 studies reported this metric.
In parallel, hospitalization events decreased by an average annualized rate 74.7% by the 5–9 month time window, 76.1% in the first year of treatment, and about 81% beyond 23 months. Most studies reported hospitalization rate decreases of 67% and higher at all time points and in all time windows, with top rates of decline ranging from 86% to 100%. The mean percentage of patients admitted once or more to the hospital declined by an average annualized rate of 74.5% in the 5–9 month time window, 61.4%% in the first year of omalizumab treatment, and 87.2% by 23–32 months since start of treatment; with top decline rates ranging from 90% to 96% reported in singular studies.
3.3.4. Concomitant medication sparing
The average percentage of patients who were discontinued on ICSs or whose ICS dose was reduced, varied between 35.7% in the 5–9 month time window and declining further to 48.1% at 12 months and further to 57.2% in the 23–32 month window; with reported singular decline rates across these time points of 49% to 100%. Mean ICS dose reductions ranged from 21.5% at 12 months to 31.9% in the ≥ 36 months period.
As to OCSs, the average percentage of patients discontinued or with dose reductions over baseline declined by about 58% in the 5–9 month window and at 12 months, by 53.1% in the 23–32 month time window, and by 83.1% in the ≥36 months period. Mean OCS dose reductions varied from 55.1% at 5–9 months to 68.6% at 12 months and 63.6% at 23–32 months.
Data on LABA use are limited. The reduction in the average percentage of patients prescribed LABAs and the average LABA dose are, at best, indicative of a trend of lower concomitant LABA treatment. Similarly, the reductions in patients discontinued from LTRAs or theophylline, or with dose adjustments on these agents, should be considered as indicative as well.
3.4. Safety
The estimated adverse event rate at any level of seriousness and severity and irrespective of follow-up duration was 26.8% (range 0% to 55.6% reported in n = 20 studies, n = 5877 patients). The estimate for serious adverse event rate irrespective of follow-up duration was 12.6% (range 0% to 23.9% from n = 17 studies, n = 6289 patients).
4. Expert commentary
The benefit of real-world effectiveness studies is the insight such studies provide about the effect of drugs, shown in clinical trials to be efficacious and safe, across variations in patients, clinicians, clinical settings, and treatment regimens. In the case of omalizumab in severe asthma, such studies complement and extend the findings of efficacy trials, which are focused on the pharmacological effect of omalizumab. The real-world studies reviewed here enable a better understanding of the pharmacotherapeutic effect of omalizumab as part of treatment regimens in broad cross-sections of the general population of patients with severe asthma.
The present systematic review of 42 real-world omalizumab studies covering 9377 patients from 35 countries worldwide confirms and extends the conclusions of our systematic review published in 2016 [Citation48], which covered studies published up to 2015 (and likely concluded in 2014 at the latest). Our prior review provided strong evidence for the short-term (16 weeks to 1 year) effectiveness of omalizumab, and emergent yet still tentative evidence that the effectiveness may extend up to 2 to 4 years. This present review firmly establishes the short-term effectiveness of omalizumab at 16 weeks and 1 year, and provides strong evidence of its long-term effectiveness: seven studies reported outcomes at 2 years [Citation37,Citation39,Citation43,Citation45,Citation60,Citation70,Citation76], seven studies at 3 years or more [Citation36,Citation47,Citation58,Citation63,Citation64,Citation66,Citation70,Citation73], one study at 4 years [Citation36], and one study with a mean follow-up of 60.7 months followed patients for as long as 121 months [Citation66]. Thus, our review provides strong evidence on the major outcomes of interest for up to 2 to 4 years; and emergent evidence reaching well beyond 4 years. Note in this regard that Menzella et al. [Citation79,Citation80] report on a subsample of patients from the Bousquet et al. [Citation30] study already included in this review who had been on omalizumab for, respectively, four (n = 11) and nine (n = 9) years. Significant improvements over baseline in AQLQ and FEV1 found at four years were maintained after nine years of treatment; reduction in serious exacerbations, emergency department visits and hospitalizations were also observed with no safety concerns in this small cohort of patients on long-term therapy.
Focusing on the studies that reported outcomes in either the 23–32 month time window or at 36 months and beyond (and taking the latest of the two; ), there is convincing evidence to infer the long-term effectiveness of omalizumab in the management of severe allergic asthma.
In terms of lung function,
FEV1, the only objective marker of lung function included in the studies, improved by 26%.
As to exacerbations and symptoms,
the rate of patients experiencing exacerbations declined by 60%, and the average number of events per patient per year by 71%; and
the proportion of patients reporting daytime symptoms decreased by 41%, while the proportion relating nighttime symptoms declined by 69%.
Regarding asthma control,
ACT scores increased by 56%; and
ACQ scores decreased by 32%.
In terms of overall assessment,
the GINA classification score improved for 69% of patients; and
81% of patients were classified at good or excellent on the GETE scale.
As to quality of life,
AQLQ quality of life scores rose by 20%.
In terms of healthcare resource utilization,
emergency department visits (per patient per year) declined on average by 91%, and the proportion of patients with one or more emergency department visits annually by 86%; and
in parallel, hospitalizations (per patient per year) dropped an average of 81%, and the proportion of patients with one or more hospitalizations annually by 87%.
As to medication use,
for 57% of patients on ICS, this treatment was discontinued or the ICS dose was reduced, with an average dose reduction of 32%;
for 83% of patients on OCS, this treatment was discontinued or the OCS dose was reduced, with average dose reduction of 64%;
for 66% of patients treated with LTRAs and for 69% of patients prescribed theophylline, therapy was discontinued or dose was reduced; but
the percentage of patients who were discontinued on LABAs or whose dose was reduced varied over time with no discernible pattern.
Safety data tended to be reported rather inconsistently across the studies detailing AEs and SAEs. Over-reporting of some and under-reporting of other AEs and SAEs are likely given the lack of harmonization in how safety data were collected across studies.
Although outside of the scope of the current review on severe allergic asthma, a number of real-world studies have been published on cohorts of patients with moderate-to-severe allergic asthma. Findings on this body of evidence of over 6,000 patients, with treatment durations ranging from six months to five years, are qualitatively similar to our findings on patients with severe asthma. Significant improvements in these moderate-to-severe allergic asthma patients were found for: FEV1 [Citation81], exacerbations [Citation81–Citation83], asthma symptom scores [Citation84], ACT scores [Citation50,Citation81,Citation83], ACT classification of well-controlled (ACT >20) [Citation85], ACQ sores [Citation82], AQLQ scores [Citation82], emergency department visits [Citation81,Citation82,Citation86], hospitalizations [Citation81–Citation83,Citation86], ICS use [Citation86] and dose [Citation87], OCS use [Citation81,Citation82,Citation85,Citation86] and dose [Citation82], SABA puffs/day [Citation87], use of high-dose ICS [Citation85], symptoms of coughing, shortness of breath, and wheezing [Citation85], and asthma-affected work, school and regular daily activities [Citation50]. Improvements were also found in a subgroup of moderate-to-severe allergic asthma patients with IgE levels above 700 IU/mL for ACT, emergency department visits, and OCS use [Citation88]. Likewise, our findings are in line with real-world practice in children with severe allergic asthma [Citation51,Citation52].
The published body of evidence of real-world omalizumab use in severe allergic asthma demonstrates: 1) the short-term treatment effectiveness, mirroring the efficacy found in clinical trials, and 2) long-term on-treatment effectiveness up to and beyond 4 years. However, data on any sustained effect following treatment discontinuation are still needed.
In conclusion, there is strong long-term evidence that omalizumab improves lung function and asthma control, enhances quality of life, decreases emergency department visits and hospitalizations, and lowers the need for corticosteroid treatment and other therapies. This translates into marked reductions in suffering and significant health care savings. In both the short- and long-term, omalizumab is highly effective in managing severe allergic asthma in adults as well as adolescents in routine clinical practice and heterogeneity in patients, clinicians, and settings.
Article highlights
42 real-world studies published between 2008 and 2018 were reviewed to confirm the short-term and to evaluate the long-term effectiveness and safety of omalizumab in the treatment of severe allergic asthma.
Omalizumab treatment is associated with significant short- and long-term improvements in clinical outcomes, quality of life, healthcare resource utilization, and medication-sparing.
Omalizumab is associated with reduced exacerbations and symptoms; better asthma control; improved lung function; enhanced quality of life; fewer emergency room visits and hospitalizations; and lower use of corticosteroids and other asthma medications.
Our review establishes the short-term (up to 1 year of treatment) effectiveness of omalizumab, provides strong evidence for its long-term (up to 4 years) effectiveness, and presents emergent evidence for its effectiveness over 4 and more years of treatment.
The reductions in healthcare resource utilization and concomitant asthma medications are likely to translate into significant cost savings.
This box summarizes key points contained in the article.
Declaration of interest
K MacDonald, I Abraham and CS Lee are associated with and K MacDonald and I Abraham hold equity in Matrix45. By company policy Matrix45 associates are prohibited from owning equity in sponsor organizations (except through mutual funds or other independently administered investment instruments) or contracting independently with sponsor organizations. Matrix45 provides similar services to those described in this paper to other biopharmaceutical companies on a non-exclusivity basis. A Kavati and B Ortiz are both an employee and shareholder of Novartis. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Supplemental Material
Download MS Excel (672.2 KB)Supplementary materaial
Supplemental data for this article can be accessed here.
Additional information
Funding
References
- The Global Asthma Report 2018. Auckland, New Zealand: Global Asthma Network; 2018.
- Martinez FD, Vercelli D. Asthma. Lancet. 2013;382:1360–1372.
- Gould H, Sutton B, Beavil A, et al. The biology of IGE and the basis of allergic disease. Annu Rev Immunol. 2003;21:579–628.
- Shin JS, Greer AM. The role of FcεRI expressed in dendritic cells and monocytes. Cell Mol Life Sci. 2015;72(12):2349–2360.
- Licari A, Marseglia G, Castagnoli R, et al. The discovery and development of omalizumab for the treatment of asthma. Expert Opin Drug Discov. 2015;10(9):1033–1042.
- Storms W. Allergens in the pathogenesis of asthma: potential role of anti-immunoglobulin E therapy. Am J Respir Med. 2002;1(5):361–368.
- Fahy JV, Fleming HE, Wong HH, et al. The effect of an anti-IgE monoclonal antibody on the early- and late-phase responses to allergen inhalation in asthmatic subjects. Am J Respir Crit Care Med. 1997;155(6):1828–1834.
- Solèr M, Matz J, Townley R, et al. The anti-IgE antibody omalizumab reduces exacerbations and steroid requirement in allergic asthmatics. Eur Respir J. 2001;18:254–261.
- Bousquet J, Wenzel S, Holgate S, et al. Predicting response to omalizumab, an anti-IgE antibody, in patients with allergic asthma. Chest. 2004;125:1378–1386.
- Humbert M, Beasley R, Ayres J, et al. Benefits of omalizumab as add-on therapy in patients with severe persistent asthma who are inadequately controlled despite best available therapy (GINA 2002 step 4 treatment): INNOVATE. Allergy. 2005;60:309–316.
- Soler M, Matz J, Townley R, et al. The anti-IgE anti- body omalizumab reduces exacerbations and steroid requirement in allergic asthmatics. Eur Respir J. 2001;18:254–261.
- Buhl R, Hanf G, Soler M, et al. The anti-IgE antibody omalizumab improves asthma-related quality of life in patients with allergic asthma. Eur Respir J. 2002;20:1088–1094.
- Rodrigo G, Neffen H, Castro-Rodriguez J. Efficacy and safety of subcutaneous omalizumab vs. placebo as add-on therapy to corticosteroids for children and adults with asthma: a systematic review. Chest. 2011;139:28–35.
- D’Amato G, Bucchioni E, Oldani V, et al. Treating moderate-to-severe allergic asthma with a recombinant humanized anti-IgE monoclonal antibody (omalizumab). Treat Respir Med. 2006;5:392–398.
- D’Amato G, Perticone M, Bucchioni E, et al. Treating moderate-to-severe allergic asthma with anti-IgE monoclonal antibody (omalizumab) An update. Eur Ann Allergy Clin Immunol. 2010;42:135–140.
- Holgate S, Buhl R, Bousquet J, et al. The use of omalizumab in the treatment of severe allergic asthma: a clinical experience update. Respir Med. 2009;103:1098–1113.
- Kuhl K, Hanania N. Targeting IgE in asthma. Curr Opin Pulm Med. 2012;18:1–5.
- Pelaia G, Gallelli L, Renda T, et al. Update on optimal use of omalizumab in management of asthma. J Asthma Allergy. 2011;4:49–59.
- Menzies-Gow A, Fan Chung K. Omalizumab for the treatment of severe allergic asthma. Expert Rev Clin Immunol. 2008;4:543–548.
- Babu K, Arshad S, Holgate S. Omalizumab, a novel anti-IgE therapy in allergic disorders. Expert Opin Biol Ther. 2001;1:1049–1058.
- Ledford D. Omalizumab: overview of pharmacology and efficacy in asthma. Expert Opin Biol Ther. 2009;9:933–943.
- Corren J, Casala T, Lanier B, et al. Safety and tolerability of omalizumab. Clin Exp Allergy. 2009;39:788–797.
- Tan R, Corren J. Safety of omalizumab in asthma. Expert Opin Drug Saf. 2011;10:463–471.
- Molimard M, de Blay F, Didier A, et al. Effectiveness of omalizumab (Xolair) in the first patients treated in real-life practice in France. Respir Med. 2008;102:71–76. .
- Brusselle G, Michils A, Louis R, et al. “Real-life” effectiveness of omalizumab in patients with severe persistent allergic asthma: the PERSIST study. Respir Med. 2009;103:1633–1642.
- Korn S, Thielen A, Seyfried S, et al. Omalizumab in patients with severe persistent allergic asthma in a real-life setting in Germany. Respir Med. 2009;103:1725–1731.
- Cazzola M, Camiciottoli G, Bonavia M, et al. Italian real-life experience of omalizumab. Respir Med. 2010;104:1410–1416.
- Korn S, Schumann C, Kropf C, et al. Effectiveness of omalizumab in patients 50 years and older with severe persistent allergic asthma. Ann Allergy Asthma Immunol. 2010;105:313–319.
- Molimard M, Buhl R, Niven R, et al. Omalizumab reduces oral corticosteroid use in patients with severe allergic asthma: real-life data. Respir Med. 2010;104:1381–1385.
- Bousquet J, Siergiejko Z, Swiebocka E, et al. Persistency of response to omalizumab therapy in severe allergic (IgE-mediated) asthma. Allergy. 2011;66:671–678.
- Costello RW, Long DA, Gaine S, et al. Therapy with omalizumab for patients with severe allergic asthma improves asthma control and reduces overall healthcare costs. Ir J Med Sci. 2011;180:637–641.
- Rottem M. Omalizumab reduces corticosteroid use in patients with severe allergic asthma: real-life experience in Israel. J Asthma. 2012;49:78–82.
- Rubin S, Souza-Machado A, Andradre-Lima M, et al. Effect of omalizumab as add-on therapy on asthma-related quality of life in severe allergic asthma: a Brazilian study (QUALITX). J Asthma. 2012;49:288–293.
- Schumann C, Kropf C, Wibmer T, et al. Omalizumab in patients with severe asthma: the XCLUSIVE study. Clin Respir J. 2012;6:215–227.
- Sweeney J, Brightling CE, Menzies-Gow A, et al. Clinical management and outcome of refractory asthma in the UK from the British thoracic society difficult asthma registry. Thorax. 2012;67:754–756.
- Tzortzaki EG, Georgiou A, Kampas D, et al. Long-term omalizumab treatment in severe allergic asthma: the south-eastern Mediterranean “real-life” experience. Pulm Pharmacol Ther. 2012;25:77–82.
- Vennera Mdel C, Pérez de Llano L, Bardagí S, et al. Omalizumab therapy in severe asthma: experience from the Spanish registry — some new approaches. J Asthma. 2012;49:416–422.
- Barnes N, Menzies-Gow A, Mansur AH, et al. Effectiveness of omalizumab in severe allergic asthma: a retrospective UK real-world study. J Asthma. 2013;50:529–536.
- Braunstahl G, Chlumský J, Peachey G, et al. Reduction in oral corticosteroid use in patients receiving omalizumab for allergic asthma in the real-world setting. Allergy Asthma Clin Immunol. 2013;9:47.
- Grimaldi-Bensouda L, Zureik M, Aubier M, et al. Does omalizumab make a difference to the real-life treatment of asthma exacerbations? Results from a large cohort of patients with severe uncontrolled asthma. Chest. 2013;143:398–405.
- Kelmenson DA, Kelly VJ, Winkler T, et al. The effect of omalizumab on ventilation and perfusion in adults with allergic asthma. Am J Nucl Med Mol Imaging. 2013;3:350–360.
- Subramaniam A, Al-Alawi M, Hamad S, et al. A study into efficacy of omalizumab therapy in patients with severe persistent allergic asthma at a tertiary referral center for asthma in Ireland. QJM. 2013;106:631–634.
- Alfarroba S, Videira W, Galvão-Lucas C, et al. Clinical experience with omalizumab in a Portuguese severe asthma unit. Rev Port Pneumol. 2014;20:78–83.
- Tajiri T, Niimi A, Matsumoto H, et al. Comprehensive efficacy of omalizumab for severe refractory asthma: a time-series observational study. Ann Allergy Asthma Immunol. 2014;113:470–475.
- Vieira T, Oliveira J, Castel-Branco M. Short and long-term quality of life and asthma control with omalizumab therapy in a real life setting in Portugal. Allergol Immunopathol (Madr). 2014;42:3–10.
- Novelli F, Latorre M, Vergura L, et al. Asthma control in severe asthmatics under treatment with omalizumab: a cross-sectional observational study in Italy. Pulm Pharmacol Ther. 2015;31:123–129.
- Tiro J, Contreras E, Pozo M, et al. Real life study of three years omalizumab in patients with difficult-to-control asthma. Allergol Immunopathol (Madr). 2015;43:120–126.
- Abraham I, Alhossan A, Lee CS, et al. ‘Real-life’ effectiveness studies of omalizumab in adult patients with severe allergic asthma: systematic review. Allergy. 2016;71:593–610.
- Alhossan A, Lee CS, MacDonald K, et al. “Real-life” effectiveness studies of omalizumab in adult patients with severe allergic asthma: meta-analysis. J Allergy Clin Immunol Pract. 2017;5(5):1362–1370.
- Zazzali JL, Raimundo KP, Trzaskoma B, et al. Changes in asthma control, work productivity, and impairment with omalizumab: 5-year EXCELS study results. Allergy Asthma Proc. 2015;36:283–292.
- Chipps BE, Lanier B, Milgrom H, et al. Omalizumab in children with uncontrolled allergic asthma: review of clinical trial and real-world experience. J Allergy Clin Immunol. 2017;139(5):1431–1444.
- Tortajada-Girbés M, Bousquet R, Bosque M, et al. Efficacy and effectiveness of omalizumab in the treatment of childhood asthma. Expert Rev Resp Med. 2018;12(9):745–754. .
- Bernstein JA, Kavati A, Tharp MD, et al. Effectiveness of omalizumab in adolescent and adult patients with chronic idiopathic/spontaneous urticaria: a systematic review of ‘real-world’ evidence. Expert Opin Biol Ther. 2018;18(4):425–448.
- Tharp MD, Bernstein JA, Kavati A, et al. Effectiveness of omalizumab in adolescent and adult patients with chronic idiopathic (spontaneous) urticaria: a meta-analysis of “real-world” evidence. JAMA Dermatol. 2018;155(1):29–38.
- Moher D, Shamseer L, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4(1):1–9.
- Braunstahl GJ, Chen CW, Maykut R, et al. The eXpeRience registry: the ‘real-world’ effectiveness of omalizumab in allergic asthma. Respir Med. 2013;107(8):1141–1151.
- Braunstahl GJ, Canvin J, Peachey G, et al. Healthcare resource utilization in patients receiving omalizumab for allergic asthma in a real-world setting. Biol Ther. 2014;4(1–2):57–67.
- Dal Negro RW, Tognella S, Pradelli L. A 36-month study on the cost/utility of add-on omalizumab in persistent difficult-to-treat atopic asthma in Italy. J Asthma. 2012;49:843–848.
- Wittchen H-U, Mühlig S, Klotsche J, et al. Omalizumab versus ‘usual care’: results from a naturalistic longitudinal study in routine care. Int Arch Allergy Immunol. 2012;159:83–93.
- Caminati M, Senna G, Chieco Bianchi F, et al. Omalizumab management beyond clinical trials: the added value of a network model. Pulm Pharmacol Ther. 2014;29(1):74–79.
- Gouder C, West LM, Montefort S. The real-life clinical effects of 52 weeks of omalizumab therapy for severe persistent allergic asthma. Int J Clin Pharm. 2015;37(1):36–43.
- Kupryś-Lipińska I, Majak P, Molinska J, et al. Effectiveness of the polish program for the treatment of severe allergic asthma with omalizumab: a single-center experience. BMC Pulm Med. 2016;16(1):61.
- Sposato B, Scalese M, Latorre M, et al. Effects of omalizumab in severe asthmatics across ages: a real life Italian experience. Respir Med. 2016;119:141–149.
- Tat TS, Cilli A. Evaluation of long-term safety and efficacy of omalizumab in elderly patients with uncontrolled allergic asthma. Ann Allergy Asthma Immunol. 2016;117(5):546–549.
- de Carvalho-Pinto RM, Agondi RC, Giavina-Bianchi P, et al. Omalizumab in patients with severe uncontrolled asthma: well-defined eligibility criteria to promote asthma control. J Bras Pneumol. 2017;43(6):487–489.
- Mansur AH, Srivastava S, Mitchell V, et al. Longterm clinical outcomes of omalizumab therapy in severe allergic asthma: study of efficacy and safety. Respir Med. 2017;124:36–43.
- Nygaard L, Henriksen DP, H M, et al. Appropriate selection for omalizumab treatment in patients with severe asthma? Eur Clin Respir J. 2017;4(1):1359477.
- Humbert M, Taillé C, Mala L, et al. Omalizumab effectiveness in patients with severe allergic asthma according to blood eosinophil count: the STELLAIR study. Eur Respir J. 2018;51(5):1702523.
- Pelaia G, Gallelli L, Romeo P, et al. Omalizumab decreases exacerbation frequency, oral intake of corticosteroids and peripheral blood eosinophils in atopic patients with uncontrolled asthma. Int J Clin Pharmacol Ther. 2011;49(12):713–721.
- Özgür ES, Özge C, Ïlvan A, et al. Assessment of long-term omalizumab treatment in patients with severe allergic asthma long-term omalizumab treatment in severe asthma. J Asthma. 2013;50(6):687–694.
- Ancochea J, Chivato T, Casan P, et al. Profile of patients treated with omalizumab in routine clinical practice in Spain. Allergol Immunopathol (Madr). 2014;42(2):102–108.
- Niven RM, Saralaya D, Chaudhuri R, et al. Impact of omalizumab on treatment of severe allergic asthma in UK clinical practice: a UK multicentre observational study (the APEX II study). BMJ Open. 2016;6(8):e011857.
- DiBona D, Fiorino I, Taurino M, et al. Long-term “real-life” safety of omalizumab in patients with severe uncontrolled asthma: a nine-year study. Resp Med. 2017;130:55–60.
- Kawamatawong T, Poachanukoon O, Boonsiri C, et al. Long-term effectiveness of omalizumab treatment in Thai severe asthmatic patients: a real-life experience. Asian Pac J Allergy Immunol. 2017 Nov 22. DOI:10.12932/AP0872
- Maltby S, Gibson PG, Powell H, et al. Omalizumab treatment response in a population with severe allergic asthma and overlapping COPD. Chest. 2017;151(1):78–89.
- Paganin F, Mangiapan G, Proust A, et al. Lung function parameters in omalizumab responder patients: an interesting tool? Allergy. 2017;72(12):1953–1961.
- Adachi M, Kozawa M, Yoshisue H, et al. Real-world safety and efficacy of omalizumab in patients with severe allergic asthma: a long-term post-marketing study in Japan. Resp Med. 2018;141:56–63. .
- Gibson PG, Reddel H, McDonald VM, et al. Effectiveness and response predictors of omalizumab in a severe allergic asthma population with a high prevalence of comorbidities: the Australian Xolair Registry. Intern Med J. 2016 Sep;46(9):1054–1062.
- Menzella F, Facciolongo N, Piro R, et al. Clinical and pharmacoeconomic aspects of omalizumab: a 4-year follow-up. Ther Adv Resp Dis. 2012;6(2):87–95.
- Menzella F, Galeone C, Formisano D, et al. Real-life efficacy of omalizumab after 9 years of follow-up. Allergy Asthma Immunol Res. 2017;9(4):368–372. .
- Storms W, Bowdish MS, Farrar JR. Omalizumab and asthma control in patients with moderate-to-severe allergic asthma: a 6-year pragmatic data review. Allergy Asthma Proc. 2012;33:172–177. .
- Bhutani M, Yang WH, Hébert J, et al. The real world effect of omalizumab add on therapy for patients with moderate to severe allergic asthma: the ASTERIX observational study. PloS One. 2017;12(8):e0183869.
- Casale TB, Luskin AT, Busse W, et al. Omalizumab effectiveness by biomarker status in patients with asthma: evidence from PROSPERO, a prospective real-world study. J Allergy Clin Immunol Pract. 2018 May 22. pii: S2213-2198(18)30323-4. DOI:10.1016/j.jaip.2018.04.043
- Ohta K, Yamamoto M, Sato N, et al. One year treatment with omalizumab is effective and well tolerated in Japanese patients with moderate-to-severe persistent asthma. Allergol Int. 2010;59(2):167–174.
- Pilon D, Kavati A, Ortiz B, et al. Asthma control, lung function, symptoms, and corticosteroid sparing after omalizumab initiation in patients with allergic asthma. Allergy Asthma Proc. 2018;39(2):127–135.
- Chen HC, Huang CD, Chang E, et al. Efficacy of omalizumab (Xolair®) in patients with moderate to severe predominately chronic oral steroid dependent asthma in Taiwan: a retrospective, population-based database cohort study. BMC Pulm Med. 2016;16:3.
- Chen H, Eisner MD, Haselkorn T, et al. Concomitant asthma medications in moderate-to-severe allergic asthma treated with omalizumab. Respir Med. 2013;107(1):60–67.
- Maselli DJ, Singh H, Diaz J, et al. Efficacy of omalizumab in asthmatic patients with IgE levels above 700 IU/mL: a retrospective study. Ann Allergy Asthma Immunol. 2013;110(6):457–461.