KEYWORDS:
Asthma affects 25.7 million people in the United States including 7.0 million children [Citation1], and its global pharmacotherapeutic costs exceed $5 billion per year [Citation2]. Primary prevention of asthma has been identified as a key public health goal to decrease morbidity, mortality, and economic burden of disease. Recently, an Asthma Birth Cohort Workshop, jointly sponsored by the National Institute of Allergy and Infectious Disease (NIAID), the National Heart, Lung, and Blood Institute (NHLBI), and the European Commission Framework Program for Research and Technological Development 7 (Mechanisms of the Development of Allergy, MeDALL), convened to review the findings from asthma/allergy birth cohorts and identify key knowledge gaps and research priorities. In their summary, they conclude that current asthma phenotypes are not amenable to primary prevention or early intervention because ‘their natural history cannot be reliably predicted’[Citation3]. They identified that healthcare providers and researchers need better tools that reliably predict the development of asthma in young children and better align natural history with mechanisms. With this conclusion in mind, Drs. Biagini Myers and Khurana Hershey developed the Pediatric Asthma Risk Score (PARS) [Citation4] to better screen for asthma in children. Here we provide a brief review of asthma screening tools, the creation of the PARS, and the significant potential of the PARS as a reliable and accurate tool to screen for asthma development in children.
Over the last 20 years, many investigators have attempted to accurately predict asthma development in early life. The first screening tool, by Clough et al. in 1999, found that when evaluating children ≤3 years of age that his/her age and soluble serum interleukin-2 receptor (sIL2R) concentration (both from the time of evaluation) could accurately predict asthma development one year after the initial evaluation [Citation5]. Since then multiple studies have pointed to a variety of factors in early childhood and/or infancy that may accurately predict subsequent asthma development. However, many of these studies suffer from the use of multiple laboratory tests and other information not readily available in a general pediatrics clinic. Further, they often use binary (‘yes-no’) outcomes that fail to take into account the full spectrum of risk.
While multiple assessments arose around this time, Castro-Rodriguez et al. developed the first widely used tool to predict an infant’s risk for asthma, the Asthma Predictive Index (API), in 2000 [Citation6]. Studying over 1000 children from the Tucson Children’s Respiratory Study, Castro-Rodriguez et al. developed major (physician diagnosed parental asthma, physician diagnosed eczema in the child) and minor (physician diagnosed allergic rhinitis in the child, wheezing apart from colds, eosinophilia ≥4%) criteria to assess a child’s asthma risk. These criteria formed the basis for two asthma development indices. The stringent API requires that children have frequent wheezing during the first 3 years of life and have at least one of two major criteria or two minor criteria. The loose API does not require children with early wheezing to have frequent wheezing episodes and maintains the requirement for one major or two minor criteria. Castro-Rodriguez et al. found that children with a positive stringent API were between 4.3 to 9.8 times more likely to have active asthma during school years when compared with children who had a negative stringent predictive index. When applying the loose API, children who met criteria had 2.6 to 5.5 times the risk for an asthma diagnosis at school age compared with their peers who did not meet the criteria.
While the API is useful, it has some important limitations that impact its practical utility and generalizability. First, the API is better at assessing who will not go on to develop asthma, rather than who will develop asthma. The negative predictive value ranges between 93.9% at year 6 to 86.5% at the year 13 follow-up. In contrast, the positive predictive value only ranges between 26.2% (year 6 follow-up) to 31.7% (year 13 follow-up). Therefore, while the tool can detect who will develop asthma, it is much better at predicting who will not develop the disease over time. The second limitation is that the API generates either a ‘yes’ or a ‘no’ outcome for children at high risk for asthma, however, does not assess asthma risk in children who are low or moderate risk for asthma development. Third, the API criteria are not weighted so it does not account for differences in attributable risks of the individual criteria (e.g. parental asthma may cause a higher attributable risk than physician diagnosed eczema). This means that regardless of which individual criteria a child may have, any positive or ‘yes’ outcome generates the same risk. This fails to account for potential differential effects of the individual risk factors on asthma development in children.
To improve the power of the API, Guilbert et al. developed the modified API (mAPI) as part of the Prevention of Early Asthma in Kids (PEAK) investigation by incorporating objective measures of food- and aero-allergen sensitization rather than a clinical diagnosis of allergic rhinitis [Citation7]. Guilbert et al. developed the mAPI in order to enhance their ability to identify infants and young children at risk for asthma so that they could be recruited for inclusion in the PEAK intervention trial. Chang et al. subsequently used the mAPI tool to assess future asthma disease risk within the high-risk Childhood Origins of ASThma (COAST) cohort [Citation8]. The studies by Guilbert et al. and Chang et al. demonstrate the mAPI’s usefulness to predict the likelihood of asthma diagnosis at school age. However, despite the mAPI’s improvements on the API, it shares many of the same limitations as the API and loose API. For example, the mAPI, while providing an accurate screening measure for children at high risk for asthma development, does not produce a risk for children at mild to moderate disease risk. In addition, the mAPI has never been externally validated in a primarily nonwhite cohort.
The PARS team sought to address the need for an improved screening tool that identify children at risk of developing asthma using a continuous outcome scale and personalized demographic and biomarker risk factors[Citation4]. The team also wanted the PARS to be easily used within the general pediatric setting without the need for multiple blood tests so that the risk evaluation could be completed in the same, initial visit in which the family or provider’s concern arises.
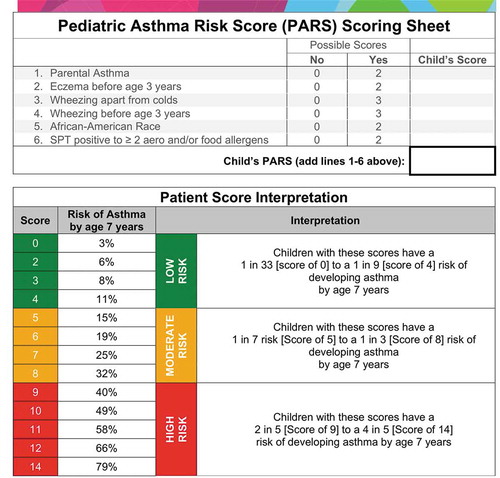
Drs. Biagini Myers and Khurana Hershey generated the PARS using known predictors in children ≤3 that were predictive of asthma at age 7 in the Cincinnati Childhood Allergy and Air Pollution Study (CCAAPS), a longitudinal birth cohort study[Citation9]. The final, significant factors included parental asthma, eczema (atopic dermatitis) before the age of three years old, wheezing apart from colds, wheezing before the age of three years old, African-American Race, and polysensitization (≥2 positive SPT responses to aeroallergens or food allergens, ). The team determined each individual factor’s odds ratio (OR) for asthma development. The ORs were then rounded to the nearest whole number to weight their contribution to the final score. To calculate the PARS for a given patient, the healthcare provider simply sums the weights to generate the child’s personalized PARS. The possible scores in the PARS range from 0 to 14; a PARS of 0 represents a 3% risk of a child having asthma at age seven, whereas a PARS of 14 represents a 79% risk.
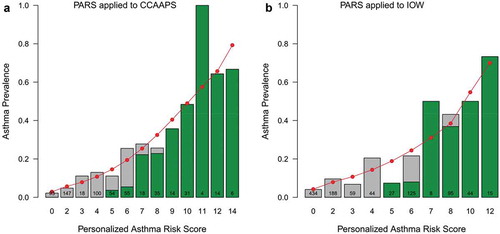
To determine the PARS’s screening accuracy, the group first determined the screening accuracy of the three validated API versions (loose API, stringent API, and mAPI) in the CCAAPS birth cohort. When applied to CCAAPS, each API version generated similar or identical findings compared with their original published results[Citation4]. Of the three tools, the loose API criteria in the Tucson Children’s Respiratory Study had the best sensitivity and AUC. Thus, all comparisons between the PARS and the API were performed with the loose API definition. When applied to the CCAAPS cohort, the PARS was able to accurately predict which children went on to develop asthma with an 11% increase in sensitivity over the loose API. Children with a PARS of 7 to 14 showed a strong concordance with the API generated risk (). However, the PARS performed better than the API at predicting a child’s asthma risk at age seven if they had low/moderate scores (0 to 7). There was weak concordance with the API for a PARS of less than 7. Likewise, the PARS discriminatory power was superior to that of the API in the CCAAPS cohort, meaning that the PARS was better able to discern overall who would and would not develop asthma compared with the API in the cohort. The PARS also underwent independent replication in the United Kingdom’s Isle of Wight birth cohort (), which supports its potential for use in other screening non-US populations for asthma. In addition, the PARS assessment outperformed 30 published models – lower AUC, sensitivity, or PPV – and/or was less invasive – less biologic sampling, no spirometry, no blood draw[Citation4]. Indeed, when compared with other risk scores, the PARS directly uses skin prick testing to assess for sensitization as the preferred method by both the Joint Task Force and the European Academy of Allergy and Clinical Immunology [Citation10,Citation11].
Figure 2. Comparison of PARS to loose API in the CCAAPS and Isle of Wight (IOW) cohort. The green shading indicates the proportion of at-risk children using the loose API (API) in each cohort. The red circles represent the predicted asthma prevalence using the PARS within each cohort, the grey bars represent the observed asthma prevalence in each cohort. Note that the predicted asthma prevalence (red dots) is more accurate than the loose API (green bars) for children with low- and moderate-risks of asthma. The two scoring systems perform equally well in children with a high-risk of asthma.
While the PARS has significant potential for asthma prediction in children and generalized use in primary healthcare settings, it has some important limitations. First, PARS is a screening not diagnostic tool, thus not all children identified at risk would be expected to develop asthma. Second, the PARS needs validation in other racial/ethnic groups other than white and African American children. In particular, the PARS needs evaluation within Latino populations since these children have higher asthma rates than non-Latino (white or African American) children. Third, a potential for recall bias exists when determining the child’s wheezing history. This can be minimized by clinicians asking about wheezing or respiratory problems related to asthma at each well-child visit. However, this is a common limitation found amongst all questionnaire-based tools that use subjective data, including the different API versions. Fourth, there is no agreement on when to institute preventative strategies or what those strategies should include in a child at risk for asthma. Thus, the precise management strategies that should be implemented in a child based on their PARS score remain to be determined. Similarly, PARS is not a diagnostic tool and should not be used to establish an asthma diagnosis in children. Finally, the PARS does not include several known environmental risk factors (e.g. secondhand smoke exposure). While these are important in asthma risk, neither a uniform exposure estimate nor a generalizable intervention to minimize a child’s burden to these exposures exists. Even with these limitations, the PARS is a robust and accurate tool to evaluate a child’s asthma risk.
In summary, the PARS provides a simple, effective, and personalized screening tool to estimate asthma risk in children, which can be easily implemented in a point of care clinical setting. PARS uses food- and aeroallergen-sensitization in determining risk, which are known risk factors for asthma development. While sensitization and skin prick testing are not generally available within primary care clinics, the other factors are easily obtained. A major advantage of the PARS is the continuous scale, which enables a more accurate and personalized risk assessment for each child. Finally, the PARS outperforms currently available asthma predictive screening assessments including the current standards, the original API and mAPI. Notably, it is much better at identifying children with moderate/low risk for asthma development; 43.2% of asthmatics in CCAAPS missed by the API had scores <9, indicating a mild- to moderate-risk of asthma. In addition, some factors within the PARS may ultimately prove to be modifiable and a means to mitigate risk, highlighting the need for intervention studies to screen for asthma using the PARS. This is critical because the API and the mAPI have been used to populate asthma prevention trials, and the children put into these trials have had very high risk and may be already too far down the road to asthma for primary prevention. The PARS may more prove useful in the future as it screens for a wider variety of asthma risk, as opposed to only high-risk children. The PARS should be considered by all practitioners who treat children to help provide parents with the most accurate risk of asthma development in their children. The PARS screening tool can be downloaded to your smart device from either the iOS or Google Play app stores for ease of use within the clinic. The tool can also be accessed online at https://pars.research.cchmc.org.
Reviewer disclosures
One of the peer reviewers has declared that they have served on an advisory board for AstraZeneca. Peer reviewers on this manuscript have no other relevant financial relationships or otherwise to disclose.
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Additional information
Funding
References
- Akinbami LJ, Moorman JE, Bailey C, et al. Trends in asthma prevalence, health care use, and mortality in the United States, 2001–2010. NCHS Data Brief. 2012May;(94):1–8.
- Palmer LJ, Cookson WO. Genomic approaches to understanding asthma. Genome Res. 2000;10:1280–1287.
- Savenije OE, Kerkhof M, Koppelman GH, et al. Predicting who will have asthma at school age among preschool children. J Allergy Clin Immunol. 2012;130:325–331.
- Biagini Myers JM, Schauberger E, He H, et al. A pediatric asthma risk score to better predict asthma development in young children. J Allergy Clin Immunol. 2019 May;143(5):1803–1810.
- Clough JB, Keeping KA, Edwards LC, et al. Can we predict which wheezy infants will continue to wheeze? Am J Respir Crit Care Med. 1999;160:1473–1480.
- Castro-Rodriguez JA, Holberg CJ, Wright AL, et al. A clinical index to define risk of asthma in young children with recurrent wheezing. Am J Respir Crit Care Med. 2000;162:1403–1406.
- Guilbert TW, Morgan WJ, Krawiec M, et al. The prevention of early asthma in kids study: design, rationale and methods for the childhood asthma research and education network. Controlled Clin Trials. 2004;25:286–310.
- Chang TS, Lemanske RF Jr., Guilbert TW, et al. Evaluation of the modified asthma predictive index in high-risk preschool children. J Allergy Clin Immunol Pract. 2013;1:152–156.
- LeMasters GK, Wilson K, Levin L, et al. High prevalence of aeroallergen sensitization among infants of atopic parents. J Pediatr. 2006;149:505–511.
- Bernstein IL, Li JT, Bernstein DI, et al. Allergy diagnostic testing: an updated practice parameter. Ann Allergy Asthma Immunol. 2008;100:S1–148.
- Scadding G, Hellings P, Alobid I, et al. Diagnostic tools in rhinology EAACI position paper. Clin Transl Allergy. 2011;1:2.