ABSTRACT
Objectives
There are few comparative data for tumor necrosis factor inhibitors in patients with rheumatoid arthritis (RA).
Methods
Historical data for reference product/biosimilar intravenous infliximab, or adalimumab and etanercept, were pooled and compared with phase 3 study results for a subcutaneous (SC) formulation of the infliximab biosimilar CT-P13, in a systematic review and meta-analysis (PROSPERO: CRD42019149621).
Results
The authors identified 13 eligible controlled trials that randomized over 5400 participants to prespecified treatments of interest. Comparison with pooled historical data suggested a numerical advantage for CT-P13 SC over intravenous infliximab for almost every prespecified efficacy outcome evaluated, including Disease Activity Score in 28 joints (C-reactive protein/erythrocyte sedimentation rate), Clinical/Simplified Disease Activity Index scores, American College of Rheumatology responses, and multiple measures of disease remission and low disease activity; for the majority of outcomes, there was no overlap in 95% confidence intervals between groups. A numerical advantage for CT-P13 SC was also observed for safety outcomes (adverse events, infections, and discontinuations). Similar, but less marked, trends were observed for comparison with historical efficacy and safety data for adalimumab/etanercept.
Conclusion
CT-P13 SC offers an improved or similar benefit-to-harm ratio compared with infliximab (intravenous) and adalimumab/etanercept, for the treatment of moderate-to-severe RA.
1. Introduction
Rheumatoid arthritis (RA) is the most common form of autoimmune arthritis, with a global, age-standardized annual incidence of 14.9 per 100,000 [Citation1]. RA predominantly affects the synovial joints, potentially involving cartilage damage, bone erosion, and systemic consequences [Citation2]. The impact of RA on health-related quality of life is substantial and can result in disability characterized by severe functional limitation and comorbidities [Citation3].
Treatment options for RA include non-steroidal anti-inflammatory drugs, glucocorticoids, and several classes of disease-modifying antirheumatic drugs (DMARDs) (conventional synthetic DMARDs [csDMARDs], biologic DMARDs [bDMARDs], and targeted synthetic DMARDs [tsDMARDs]) [Citation4–6]. The use of bDMARDs (including tumor necrosis factor [TNF] inhibitors [anti-TNFs]) or tsDMARDs is considered when disease activity remains moderate to high despite csDMARD therapy (i.e. when a treatment target is not met) [Citation4–6]. Of the five currently available anti-TNFs, infliximab, etanercept, and adalimumab are the most commonly prescribed, collectively accounting for around 90% of anti-TNF prescriptions for RA [Citation7–10]. Reference product adalimumab (Humira; AbbVie Inc., North Chicago, IL), reference product infliximab (Remicade; Janssen Biotech, Horsham, PA), and reference product etanercept (Enbrel; Immunex Corporation, Thousand Oaks, CA) are approved by the US Food and Drug Administration and the European Medicines Agency for indications including RA [Citation11–16]. For each of these anti-TNFs, several biosimilars are approved for use in Europe and other regions [Citation17].
At present, the evidence base to guide selection of bDMARDs is limited [Citation18]. Despite the growing number of available anti-TNFs, there are few comparative data on the relative efficacy and safety of the different anti-TNF treatment options. To the best of our knowledge, the EXXELERATE study is the only head-to-head randomized controlled trial (RCT) conducted to date to compare anti-TNFs (specifically, certolizumab vs. adalimumab) in patients with RA. The results showed that certolizumab pegol was not superior to adalimumab, in patients receiving concomitant methotrexate (MTX) [Citation19].
A subcutaneous (SC) formulation of CT-P13 (Remsima SC; Celltrion, Inc., Incheon, Korea), representing a new mode of administration for infliximab, received European Union marketing authorization in 2019; CT-P13 SC is currently approved in Europe for the treatment of the same indications (except juvenile) as reference infliximab, including RA [Citation20,Citation21]. In a phase 1/3 study of patients with active RA, CT-P13 SC administered every 2 weeks at a dose of 120 mg (after dose-loading with intravenous [IV] CT-P13; CT-P13 IV) was shown to have non-inferior efficacy to CT-P13 IV (3 mg/kg administered every 8 weeks) at Week 22, as assessed by change from baseline in Disease Activity Score in 28 joints (DAS28)-C-reactive protein (CRP) [Citation22,Citation23]. The two infliximab formulations demonstrated at least comparable efficacy, with post hoc analysis suggesting that the SC formulation was associated with significantly better efficacy outcomes compared with the IV formulation [Citation24].
The primary objective of this systematic review and meta-analysis was to compare the efficacy and safety of CT-P13 SC with historical clinical data for infliximab IV, adalimumab, and etanercept, and their respective biosimilars, in patients with moderate-to-severe RA.
2. Methods
The protocol for this systematic review was prospectively registered (PROSPERO number: CRD42019149621) [Citation25].
2.1. Systematic literature search
We conducted systematic electronic searches of Embase.com (comprising Embase, MEDLINE, and PubMed) and the Cochrane Library (comprising the Cochrane Central Register of Controlled Trials [CENTRAL], Cochrane Database of Systematic Reviews [CDSR], Database of Abstracts of Reviews of Effects [DARE] and the Health Technology Assessment database). Search strategies were constructed using relevant Medical Subject Headings (MeSH) and free-text terms, as presented in Supplementary Tables 1 and 2. The searches were restricted to English-language publications, human studies and dates from January 2009 to August 2019.
2.2. Criteria for considering studies for this review
2.2.1. Study design
We included parallel-group RCTs and open-label extensions of RCTs. Studies were required to be reported as full-text articles.
2.2.2. Participants
We included adults (aged ≥18 years) with moderate-to-severe RA, defined as having either ≥6 swollen and ≥6 tender joints, a DAS28-erythrocyte sedimentation rate (ESR) ≥3.2, or a DAS28-CRP ≥3.2, plus at least one of the following criteria: morning stiffness lasting ≥45 min; serum CRP >1.0 mg/dL; ESR >28 mm/h. Participants were required to be receiving background treatment with MTX.
2.2.3. Interventions
We included trials that studied infliximab IV, adalimumab, etanercept, and their biosimilars. Treatments were required to be administered at the dose and regimen specified in the relevant Summary of Product Characteristics.
2.2.4. Outcome measures
We included trials that reported one or more of the following outcome measures between Weeks 22 and 54 (inclusive). Efficacy outcomes: change from baseline in DAS28-CRP/ESR; low disease activity (LDA) and remission based on DAS28-CRP/ESR criteria; European League Against Rheumatism (EULAR) response (based on CRP/ESR criteria); 20%, 50% or 70% improvement in American College of Rheumatology (ACR) response (ACR20, ACR50, and ACR70, respectively); change from baseline in Clinical Disease Activity Index (CDAI) and Simplified Disease Activity Index (SDAI); LDA and remission based on CDAI and SDAI; and remission based on ACR/EULAR criteria and Boolean clinical criteria. Safety outcomes included rates of adverse events (AEs; any or serious), infections (any or serious), and treatment discontinuations due to AEs or lack of efficacy.
2.3. Selection of studies for inclusion in the review
Two reviewers (TSK, JSC) independently screened titles and abstracts of the records (retrieved through the electronic searches) for potential inclusion in the review. These reviewers then independently conducted an assessment of the full-text articles to determine whether to ‘include’ or ‘exclude,’ recording reasons for exclusion. A third reviewer (MinYoung Jang, Celltrion Healthcare) arbitrated in the event of disagreement between the two independent screenings. A randomly selected sample of excluded studies was considered by the third reviewer to verify appropriate application of the exclusion criteria. Multiple reports of the same study were identified and collated so that each study was the unit of interest in the review. The selection process was recorded in sufficient detail to complete a PRISMA flow diagram [Citation26].
2.4. Data extraction and management
Study characteristics and outcome data were collected using a Microsoft Excel template. The following study characteristics were extracted: study design (randomization method, blinding, duration, number of centers, dates performed; geographic location); population (number of participants randomized, demographics, prior anti-TNF use, concomitant medication, baseline disease activity); intervention and comparators (type, dose, regimen); and outcome measures (prespecified as of interest to this review, as previously described).
2.5. Measures of treatment effect and data synthesis
Data from multiple infliximab studies and data from multiple adalimumab and etanercept studies were combined in two separate analyses. The relatively low number of adalimumab and etanercept RCTs conducted in RA during the previous 10 years (i.e. the inclusion timeframe) prevented meaningful meta-analysis of adalimumab and etanercept data separately. For binary outcomes, a generalized linear mixed model was used for pooling with logit transformation. For continuous outcomes, the inverse variance method was used for pooling without transformation. For completeness, both random-effect and fixed-effects models were conducted, and the results are presented together in the forest plots.
Heterogeneity was assessed using the Chi-squared-based Q-test [Citation27]. In the presence of significant heterogeneity (i.e. the p-value of the Q-test < 0.05 or I2 >50%), the random-effects model [Citation28] was used to calculate pooled odds ratios and 95% confidence intervals (CI). Otherwise, the fixed-effects model was used [Citation29].
Pooled historical data for (i) infliximab IV and (ii) adalimumab and etanercept were compared with recent data for CT-P13 SC [Citation24]. All statistical analyses were performed using R software version 3.6.1 [Citation30] and the ‘meta’ package for R, version 4.9–7 [Citation31].
2.6. Quality assessment
The included studies were assessed for risk of bias (i.e. internal validity) according to National Institute for Health and Care Excellence guideline criteria [Citation32]. Assessments were conducted by one author with independent verification by a second author.
3. Results
3.1. Results of the search
A PRISMA diagram summarizing the flow of information through this systematic review is presented in . We identified 821 records through the electronic searches. After removal of duplicates, 809 records were screened (778 records excluded) and a total of 31 full-text articles were assessed for eligibility. Fifteen articles were excluded for the following reasons: study population did not meet eligibility criteria (n = 6); reported time points did not meet eligibility criteria (n = 5); duplicate of results (n = 3); and other (n = 1) [Citation33–47].
Figure 1. Flow diagram of rheumatoid arthritis studies, illustrated per PRISMA guidelines [Citation26]
![Figure 1. Flow diagram of rheumatoid arthritis studies, illustrated per PRISMA guidelines [Citation26]](/cms/asset/f44f0d2e-28b8-42f5-8a8a-3225d578b183/ierm_a_1858803_f0001_b.gif)
Thirteen RCTs (reported in 16 publications) were included in the qualitative and quantitative syntheses, as follows. Infliximab (n = 6 studies): NCT01936181 [Citation48,Citation49]; NCT02222493 [Citation50,Citation51]; NCT01217086 (PLANETRA) [Citation52,Citation53]; JapicCTI-111620 [Citation54]; NCT00202852 and open-label extension (OLE; NCT00732875) [Citation55]; NCT00691028 (RISING) [Citation56]. Adalimumab (n = 4 studies): NCT02167139 [Citation57]; NCT02137226 (VOLTAIRE-RA) [Citation58]; NCT03052322 (AURIEL-RA) [Citation59]; NCT01710358 (RA-BEAM) [Citation60]. Etanercept (n = 3 studies): NCT01895309 [Citation61]; NCT02357069 [Citation62]; NCT01270997 (HERA) [Citation63].
3.2. Description of the included studies
3.2.1. Infliximab IV or infliximab biosimilars
The design and eligibility criteria of the six included studies were generally consistent () [Citation48–56]. All six studies were RCTs, included a double-blind period, and had a duration of ≥54 weeks. In two of the six studies, open-label treatment was administered from either Weeks 0–14 or from Week 30 onwards. One of the six studies included a switching phase where participants receiving the reference product anti-TNF were re-randomized to remain on their current treatment or switch to the biosimilar treatment (NCT02222493 [Citation50,Citation51]). Two of the studies (JapicCTI-111620 [Citation54]; NCT00691028 [Citation56]) were conducted in Japan, and one study (NCT00202852 and OLE NCT00732875 [Citation55]) was conducted in Korea; the other studies were multinational [Citation48–53].
Table 1. Characteristics of the included studies (infliximab IV and infliximab biosimilars)
Across studies, inclusion criteria required participants to have active RA according to ACR (1987) [Citation64] or EULAR/ACR (2010) criteria [Citation65] and to have received a stable dose of MTX prior to baseline (permitted doses ranged from 6 to 25 mg/week). Prior bDMARD use was not permitted in two studies (NCT01936181 [Citation48,Citation49]; NCT01217086 [Citation52,Citation53]), and required a washout period in two studies (NCT02222493 [Citation50,Citation51]; NCT00202852 [Citation55]).
All six studies included a treatment arm where reference product infliximab was administered IV at a dose of 3 mg/kg at Weeks 0, 2, and 6, and every 8 weeks thereafter. The comparator arms included the biosimilars SB2 (NCT01936181 [Citation48,Citation49]), GP1111 (NCT02222493 [Citation50,Citation51]), and CT-P13 (NCT01217086 [Citation52,Citation53], JapicCTI-111620 [Citation54]). These agents were administered at the same dose and dosing interval as the reference product infliximab (i.e. IV at an initial dose of 3 mg/kg at Weeks 0, 2, and 6, and every 8 weeks thereafter).
A total of 2342 participants were initially randomized/enrolled to relevant treatment arms in the included studies (Supplementary Table 3). Where reported, the mean or median age of participants ranged from 49.3 to 54.5 years, 79–90% of participants were female, mean/median body weight ranged from 53.4 to 74.2 kg, and mean/median disease duration ranged from 6.3 to 8.2 years.
3.2.2. Reference product/biosimilar adalimumab or etanercept
The design and eligibility criteria of the seven included studies were generally consistent () [Citation57–63]. All seven studies were RCTs, included a double-blind period, and had a duration of ≥48 weeks. Additionally, two studies included an open-label extension and two studies included a switching period where participants were re-randomized to either remain on the current treatment or switch to the biosimilar (NCT02167139 [Citation57] and NCT02137226 [Citation58]). Of the etanercept studies, one (NCT01270997 [Citation63]) was conducted in Korea and one (NCT02357069 [Citation62]) was conducted in Japan and Korea; the other etanercept [Citation61] and adalimumab [Citation57–60] studies were multinational.
Table 2. Characteristics of the included studies for reference product or biosimilar adalimumab and etanercept
Across studies, inclusion criteria required participants to have active RA according to ACR (1987) [Citation64] or EULAR/ACR (2010) criteria [Citation65] and to have received a stable dose of MTX prior to baseline; permitted doses ranged from 6 to 25 mg/week. Prior bDMARD use was not permitted in three of the studies (NCT02167139 [Citation57]; NCT01710358 [Citation60]; NCT01895309 [Citation61]), one prior anti-TNF was permitted in one study (NCT03052322 [Citation59]), and one prior bDMARD was permitted in one study (NCT02137226 [Citation58]).
Four of the studies included a treatment arm where reference product adalimumab was administered SC at a dose of 40 mg every other week. Comparator arms included SB5 (NCT02167139 [Citation57]), BI-695501 (NCT02137226 [Citation58]) and MSB11022 (NCT03052322 [Citation59]) and placebo/baricitinib (NCT01710358 [Citation60]). Comparators were also administered SC at a dose of 40 mg every other week. Three of the studies included a treatment arm where reference product etanercept was administered SC at a dose of 50 mg once weekly or 25 mg twice weekly; the comparator arms included the etanercept biosimilars SB4 (NCT01895309 [Citation61]), LBEC0101 (NCT02357069 [Citation62]), and HD203 (NCT01270997 [Citation63]), administered SC at doses of 50 mg weekly or 25 mg twice weekly (HD203).
A total of 3069 participants were initially randomized/enrolled to relevant treatment arms in the included studies (Supplementary Table 4). Where reported, the mean age of participants ranged from 49.8 to 55.5 years, 76–89% of participants were female, mean body weight ranged from 56.3 to 75.1 kg and mean disease duration ranged from 5.4 to 10 years.
3.2.3. CT-P13 SC
Participants were required to have active RA according to ACR/EULAR 2010 criteria [Citation65]. A total of 167 individuals were randomized to receive CT-P13 SC at a dose of 120 mg every 2 weeks; participants were aged 19 to 74 years old, 77.8% were female and mean (standard deviation) disease duration was 6.82 (7.15) years [Citation23].
3.3. Risk of bias in the included studies
All studies included in this systematic literature review were rated as having a low risk of selection, performance, attrition, and detection bias (). Internal and external validity were confirmed for all included studies ().
Table 3. Quality assessment results
3.4. Effects of the interventions
3.4.1. Infliximab versus CT-P13 SC
A summary of findings for the meta-analyses and comparison with CT-P13 SC is presented in . Selected forest plots for the meta-analyses of the historical data are presented in Supplementary Figures 1 to 9. A numerical advantage (with non-overlapping 95% CIs) was observed for CT-P13 SC over infliximab IV at the 30-week and 54-week time points for most outcomes: mean change from baseline in DAS28-CRP score; DAS28-CRP remission rates; mean change from baseline in DAS28-ESR score; DAS28-ESR remission rate (54 weeks only); the proportion of patients achieving a EULAR good response (CRP criteria); the proportion of patients achieving ACR20, ACR50 and ACR70 responses; mean change from baseline in CDAI score; CDAI remission rate (54 weeks only); mean change from baseline in SDAI score; SDAI remission rate; Boolean clinical remission rate; ACR/EULAR remission rate, the proportion of patients experiencing any AE; the proportion of patients experiencing any serious adverse event (SAE); the proportion of patients who discontinued due to AEs; and the proportion of patients who discontinued due to lack of efficacy (no patients discontinued due to lack of efficacy for CT-P13 SC at either time point); data for these outcomes are presented in . A visual presentation of the results for selected outcomes is shown in .
Figure 2. A comparison of infliximab IV historical data with CT-P13 SC results in terms of remission rates (a), ACR response rates (b), and key safety outcomes (c)
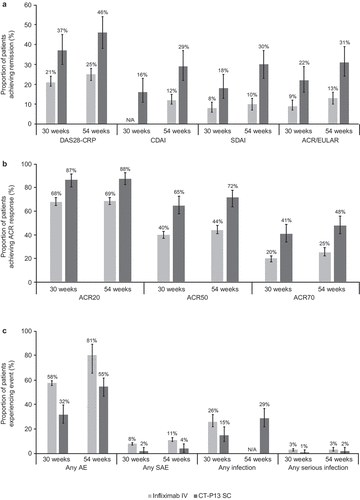
Table 4. Summary of 30-week and 54-week findings for infliximab IV, infliximab biosimilars, and CT-P13 SC
A numerical advantage for CT-P13 SC over infliximab IV (with overlapping 95% CI) was observed for the proportion of patients achieving/reporting DAS28-ESR remission (30 weeks), DAS28 LDA (ESR criteria; 30 and 54 weeks), EULAR good response (ESR criteria; 30 weeks), any infection (30 weeks) or any serious infection (30 weeks and 54 weeks); data for these outcomes are presented in .
3.4.2. Adalimumab and etanercept versus CT-P13 SC
A summary of the findings for the meta-analyses and comparison with CT-P13 SC is presented in . Selected forest plots for the meta-analyses are presented in Supplementary Figure 1 to 9. A numerical advantage (with non-overlapping 95% CIs) was observed for CT-P13 SC at 54 weeks over adalimumab/etanercept at 1 year for most outcomes: mean change from baseline in DAS28-CRP score; mean change from baseline in DAS28-ESR score; the proportion of patients achieving ACR50 and ACR70 responses; mean change from baseline in CDAI score; mean change from baseline in SDAI score; Boolean clinical remission rate; the proportion of patients experiencing any AE; the proportion of patients experiencing any infection and the proportion of patients who discontinued due to lack of efficacy (no patients discontinued due to lack of efficacy with CT-P13 SC); data for these outcomes are presented in . A visual presentation of the results for selected outcomes is presented in .
Figure 3. A comparison of adalimumab and etanercept historical data at 1 year with CT-P13 SC results at 54 weeks in terms of remission rates (a), ACR response rates (b), and key safety outcomes (c)
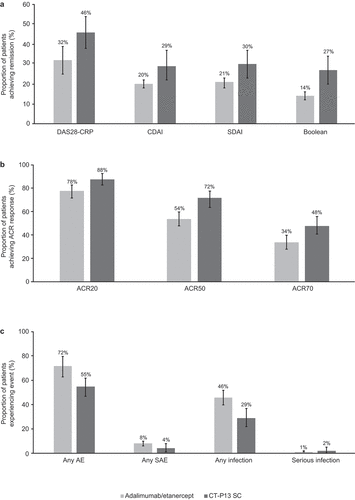
Table 5. Summary of findings for reference product or biosimilar adalimumab and etanercept (at 1 year) and CT-P13 SC (at 54 weeks)
A numerical advantage for CT-P13 SC over adalimumab/etanercept (with overlapping 95% CI) was observed for the proportion of patients achieving/reporting DAS28 remission (CRP/ESR criteria), EULAR good response (CRP/ESR criteria), ACR20, CDAI remission; CDAI LDA, SDAI remission, any SAE, and discontinuation due to AEs. Data for these outcomes are presented in .
4. Discussion
This is the first systematic review comparing the safety and efficacy of infliximab IV, adalimumab or etanercept versus CT-P13 SC. Our study identified 13 relevant RCTs that evaluated the efficacy and safety of infliximab IV, adalimumab, or etanercept and their respective biosimilars. Pooled data from these studies were compared with data from a pivotal study of CT-P13 SC in patients with RA [Citation24].
Previous analyses have demonstrated comparable efficacy between infliximab, etanercept, adalimumab, certolizumab, and golimumab [Citation66,Citation67]; however, golimumab and certolizumab were not considered in the present review as they account for less than 10% of anti-TNFs prescribed for RA [Citation9,Citation10]. Aaltonen and colleagues conducted a systematic literature review and analysis of 26 RCTs and concluded that no single agent clearly rose above the others in terms of efficacy [Citation67]. In agreement, a more recent network meta-analysis showed that infliximab, etanercept, adalimumab, certolizumab, and golimumab demonstrated a highly significant inhibitory effect on joint destruction versus placebo; results for infliximab, etanercept, adalimumab, and certolizumab did not differ significantly from each other [Citation66]. However, the study concluded that high infliximab doses (infliximab 10 mg/kg every 8 weeks) resulted in better efficacy than the standard dose [Citation66]. Since SC formulations may offer similar effects of dose escalation, further investigation into the potential benefits of SC infliximab formulations is warranted.
In our analysis, a numerical advantage for CT-P13 SC over infliximab IV was observed for almost every efficacy outcome evaluated, including DAS28, CDAI, and SDAI scores, ACR response, and measures of disease remission (including DAS28-CRP, DAS28-ESR, CDAI, SDAI, Boolean, and ACR/EULAR definitions) and LDA. This advantage was observed at both the 30-week and 54-week time points, and, in most instances, there was no overlap in 95% CIs between the groups. The statistical significance of the advantage was not assessed using p-values. The present findings can potentially be explained by the pharmacokinetic profile of CT-P13 SC, which maintains trough serum concentrations that are higher than the therapeutic threshold [Citation20,Citation52]. Considering the improved efficacy reported with high infliximab doses, compared with standard doses, in a previous meta-analysis [Citation66], it follows that any numerical improvements in the efficacy profile of infliximab SC compared with infliximab IV might be plausibly explained by increased trough infliximab concentrations. Indeed, several studies have demonstrated a correlation between clinical efficacy and trough concentration levels of anti-TNFs [Citation56,Citation68]. Similarly, a numerical advantage was observed for CT-P13 SC for every safety outcome evaluated, including the proportions of participants experiencing AEs or infections, or discontinuing study treatment. Although generally less marked, similar numerical trends were observed for efficacy and safety outcomes based on comparison of the historical data for adalimumab and etanercept with the data for CT-P13 SC.
Across the studies included in our analysis, demographics of the participants were representative of a population with moderate-to-severe RA. Participants were aged 50–55 years, had active disease according to ACR or EULAR criteria despite prior treatment with stable doses of MTX, and had an average disease duration of 5–10 years. Consistent with the epidemiology of RA, the included studies enrolled a greater proportion of women. The baseline clinical and demographic characteristics of individuals receiving CT-P13 SC (mean age 50.9 years; 78% female; mean disease duration 6.8 years) were comparable to those observed in the included historical studies. Importantly, eligibility criteria for inclusion of studies in the review were reflective of the population of interest and usual care pathways for that population [Citation4,Citation5]. Strengths of the review process include the comprehensive nature of the electronic searches conducted and independent screening of the search results by two reviewers to determine relevant studies for inclusion. Therefore, our findings are applicable to current populations of patients with active, moderate-to-severe RA and an inadequate response to MTX.
In total, the included studies randomized over 2300 participants to receive either infliximab IV or infliximab biosimilars, and over 3000 participants to receive reference product or biosimilar adalimumab or etanercept, resulting in a large overall sample size. Half of the contributing studies enrolled over 500 participants, and most of the meta-analyses conducted included more than 500 participants in total. All prespecified outcomes of interest were well reported across the included studies, and 52/54-week data were available for most outcomes evaluated. The findings of our analysis can be considered contemporary, as the included studies were generally conducted in the previous decade and most patients were recruited to the studies from 2015 onwards. Furthermore, the included studies contributing to the historical dataset were assessed for risk of bias and determined to have a low risk of bias for all domains assessed. Based on these findings, it is acceptable to conclude that pooling studies with internal validity yields generalizable results.
The present review has several limitations. Firstly, for some of the meta-analyses the p-values for Cochran’s Q-test ‘may be representative’ of substantial heterogeneity. We used both fixed and random-effects meta-analysis models, the latter of which assumes that the effects being estimated in the different studies are not identical, but follow a normal distribution [Citation69]. Thus, random-effects models assist in controlling for heterogeneity that cannot be readily explained by other factors. It remains possible, however, that the observed statistical heterogeneity may be a consequence of clinical or methodological diversity among the included studies. For example, permitted prior or concomitant treatments varied across the included studies (possibly depending on the year that the study was performed and available treatments at that time). Secondly, two studies employing placebo as the sole control group (NCT00202852; reference product infliximab vs. placebo [Citation55]), or as one of the control groups (NCT01710358 [RA-BEAM] [Citation60]; reference adalimumab vs. baricitinib or placebo), may have had relatively unfavorable results in terms of the observed effectiveness or safety of the active comparator, compared with the trials evaluating a biosimilar and its reference drug. This is because in clinical trials involving placebo, the psychological impact of the possibility of receiving placebo can lead to a negative effect on observed efficacy and safety of the drug under evaluation [Citation70]. Besides these two studies, all included studies employed active controls, whereby a reference product was compared with either a biosimilar or an alternative dose of the reference product. In contrast to placebo-controlled trials, clinical trials using a reference drug that has already been demonstrated to be effective and well tolerated as an active control may result in relatively more favorable findings for the drug under evaluation, in terms of the observed effectiveness or safety. For this reason, biosimilar studies have shown better efficacy compared to those from historical studies with placebo. Thirdly, while most of the included studies were multinational, several infliximab and etanercept studies were locally focused in Korea and/or Japan. There were some numerical differences in efficacy findings between these types of study. However, it is important to note that the number of patients included in the locally conducted studies represents a relatively small proportion of the total number of patients included in this analysis. Taken together, we believe that the inclusion of the locally focused studies will not have significantly impacted the overall conclusions of this analysis. A fourth limitation of the present review is that data were pooled for adalimumab and etanercept. Interestingly, despite the pooling of data for two different drugs, heterogeneity within the associated meta-analyses did not appear to be consistently greater than that observed for the infliximab analyses; this finding is consistent with the results of a meta-analysis suggesting similar clinical efficacy between etanercept and adalimumab [Citation67]. Fifth, the time points reported varied across the adalimumab and etanercept studies (48–58 weeks) and were compared with the longest-term, 54-week data currently available for CT-P13 SC. However, we are satisfied that the comparison is robust and reflective of long-term treatment with anti-TNFs. In addition, outcomes relating to structural damage measurements and immunogenicity were not included in this review. Finally, as the historical data were compared with findings for CT-P13 SC originating from a single study, the comparative findings might not be applicable to any other infliximab SC formulations that become available in the future.
In the context of the limitations discussed above, our findings suggest that CT-P13 SC administration offers a similar or improved benefit-to-harm ratio compared with infliximab IV or adalimumab/etanercept, for the treatment of moderate-to-severe RA. In terms of clinical practice, adalimumab and etanercept are commonly used in RA because SC is preferred by patients and physicians. However, the clinical benefit of IV infliximab is well demonstrated and is thus still commonly used. The present meta-analysis suggests that infliximab SC displays an improved (or, at least similar) clinical profile compared with infliximab IV, etanercept, and adalimumab. Therefore, CT-P13 SC may bring improved patient outcomes in a given clinical context.
5. Conclusions
The present systematic literature review and meta-analyses have shown that CT-P13 SC offers an improved, or at least similar, benefit-to-harm ratio for all outcome measures compared with infliximab IV and adalimumab/etanercept, for the treatment of moderate-to-severe RA.
Declaration of interest
R Caporali has received speaker’s fees and consultation fees from AbbVie, Bristol-Myers Squibb, Celltrion, Fresenius-Kabi, Gilead-Galapagos, Lilly, Merck Sharp & Dohme, Pfizer, Roche, Samsung-Bioepis, Sanofi, and UCB. Y Allanore has no competing interests to disclose. R Alten has received honoraria from AbbVie, Bristol-Myers Squibb, Celltrion, Gilead, Janssen, Lilly, Merck, Novartis, Pfizer, Roche-Chugai and UCB; and research grants from Novartis, Pfizer, and Roche. B Combe has received honoraria from AbbVie, Bristol-Myers Squibb, Gilead, Janssen, Lilly, Merck, Novartis, Pfizer, Roche-Chugai, Sanofi and UCB; and research grants from Novartis, Pfizer, and Roche. P Durez has received speaker’s fees from AbbVie, Bristol-Myers Squibb, Galapagos, Lilly, and Sanofi. F Iannone has received speaker’s fees and consultation grants from AbbVie, Actelion, Bristol-Myers Squibb, Biogen, Lilly, Merck Sharp & Dohme, Pfizer, Roche, Sanofi, and UCB. MT Nurmohamed has received consulting fees from AbbVie, Celgene, Celltrion, Eli Lilly, Janssen, and Sanofi; speaker’s fees from AbbVie, Bristol-Myers Squibb, Eli Lilly, Roche, and Sanofi; and research funding from AbbVie, Bristol-Myers Squibb, Celgene, Eli Lilly, Janssen, Merck Sharp & Dohme, Mundipharma, Novartis, Pfizer, Roche, and Sanofi. SJ Lee and G Park are employed full time by Celltrion Inc. TS Kwon and JS Choi are employed full time by Celltrion Healthcare. DH Yoo has received speaker’s fees and consultation fees from Celltrion Healthcare and Celltrion Inc.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
RC, YA, RA, BC, PD, FI, MTN, SJL, GP and DHY contributed to data analysis or interpretation. TSK and JSC contributed to study design, data collection and data analysis or interpretation. All authors reviewed and critically revised drafts of the manuscript, approved the final draft for submission, and are accountable for the accuracy and integrity of the article.
Supplemental Material
Download MS Word (4.4 MB)Acknowledgments
We would like to thank MinYoung Jang (Celltrion Healthcare), who acted as an independent reviewer for the selection of studies included in the review. Medical writing support (including development of a draft outline and subsequent drafts in consultation with the authors, assembling tables and figures, collating author comments, copyediting, fact checking and referencing) was provided by Beatrice Tyrrell, DPhil, at Aspire Scientific Limited (Bollington, UK), and funded by Celltrion Healthcare (Incheon, Republic of Korea).
Supplemental material
Supplemental data for this article can be accessed on the publisher’s website.
Data availability statement
The data that support the findings of this analysis are available from TSK upon reasonable request.
Additional information
Funding
References
- Safiri S, Kolahi AA, Hoy D, et al. Global, regional and national burden of rheumatoid arthritis 1990-2017: a systematic analysis of the Global Burden of Disease study 2017. Ann Rheum Dis. 2019;78(11):1463–1471.
- Guo Q, Wang Y, Xu D, et al. Rheumatoid arthritis: pathological mechanisms and modern pharmacologic therapies. Bone Res. 2018;6:15.
- Katchamart W, Narongroeknawin P, Chanapai W, et al. Health-related quality of life in patients with rheumatoid arthritis. BMC Rheumatol. 2019;3:34.
- Smolen JS, Landewe RBM, Bijlsma JWJ, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2019 update. Ann Rheum Dis. 2020;79(6):685–699.
- Singh JA, Saag KG, Bridges SL Jr., et al. 2015 American College of Rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Rheumatol. 2016;68(1):1–26.
- Lau CS, Chia F, Dans L, et al. 2018 update of the APLAR recommendations for treatment of rheumatoid arthritis. Int J Rheum Dis. 2019;22(3):357–375.
- Brodszky V, Biro A, Szekanecz Z, et al. Determinants of biological drug survival in rheumatoid arthritis: evidence from a Hungarian rheumatology center over 8 years of retrospective data. Clinicoecon Outcomes Res. 2017;9:139–147.
- Sugiyama N, Kawahito Y, Fujii T, et al. Treatment patterns, direct cost of biologics, and direct medical costs for rheumatoid arthritis patients: A real-world analysis of nationwide Japanese claims data. Clin Ther. 2016;38(6):1359–1375.e1.
- Silvagni E, Bortoluzzi A, Carrara G, et al. Comparative effectiveness of first-line biological monotherapy use in rheumatoid arthritis: a retrospective analysis of the RECord-linkage On Rheumatic Diseases study on health care administrative databases. BMJ Open. 2018;8(9):e021447.
- Sullivan E, Kershaw J, Blackburn S, et al. Biologic disease-modifying antirheumatic drug prescription patterns for rheumatoid arthritis among United States physicians. Rheumatol Ther. 2020;7(2):383–400.
- European Medicines Agency. Humira summary of product characteristics [Internet]. 2009 cited 2020 May 26. Available from: https://www.ema.europa.eu/en/documents/product-information/humira-epar-product-information_en.pdf.
- European Medicines Agency. Remicade summary of product characteristics [Internet]. 2009 cited 2020 May 26. Available from: https://www.ema.europa.eu/en/documents/product-information/remicade-epar-product-information_en.pdf.
- European Medicines Agency. Enbrel summary of product characteristics [Internet]. 2009 cited 2020 May 26. Available from: https://www.ema.europa.eu/en/documents/product-information/enbrel-epar-product-information_en.pdf.
- US Food and Drug Administration. Humira prescribing information [Internet]. 2002 cited 2020 May 26. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/125057Orig1s411lbl.pdf.
- US Food and Drug Administration. Enbrel prescribing information [Internet]. 2012 cited 2020 May 26. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/103795s5503lbl.pdf.
- US Food and Drug Administration. Remicade prescribing information [Internet]. 2013 cited 2020 May 26. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2013/103772s5359lbl.pdf.
- European Medicines Agency. European Medicines Agency database [Internet]. 2020 cited 2020 May 26. Available from: https://www.ema.europa.eu/en/medicines.
- Favalli EG, Bugatti S, Biggioggero M, et al. Treatment comparison in rheumatoid arthritis: head-to-head trials and innovative study designs. Biomed Res Int. 2014;831603:2014.
- Smolen JS, Burmester GR, Combe B, et al. Head-to-head comparison of certolizumab pegol versus adalimumab in rheumatoid arthritis: 2-year efficacy and safety results from the randomised EXXELERATE study. Lancet. 2016;388(10061):2763–2774.
- European Medicines Agency. Remsima summary of product characteristics [Internet]. 2020 cited 2020 Dec 7. Available from: https://www.ema.europa.eu/en/documents/product-information/remsima-epar-product-information_en.pdf.
- European Medicines Agency. Assessment report on extension(s) of marketing authorisation: Remsima [Internet]. 2019 cited 2020 Jun 26. Available from: https://www.ema.europa.eu/en/documents/variation-report/remsima-h-c-2576-x-0062-epar-assessment-report-variation_en.pdf.
- Westhovens R, Wiland P, Zawadzki M, et al. A novel formulation of CT-P13 for subcutaneous administration: 30 week results from a Part 2 of Phase I/III randomized controlled trial in patients with rheumatoid arthritis [Abstract SAT0170]. Ann Rheum Dis. 2019;78(Suppl 2):1158–1159.
- Westhovens R, Wiland P, Zawadzki M, et al. Efficacy, pharmacokinetics and safety of subcutaneous versus intravenous CT-P13 in rheumatoid arthritis: a randomized phase I/III trial. Rheumatology. 2020 Nov 23 [Epub ahead of print]. doi: https://doi.org/10.1093/rheumatology/keaa580.
- Combe B, Allanore Y, Alten R, et al. Comparative efficacy of subcutaneous (CT-P13) and intravenous infliximab in adult patients with rheumatoid arthritis: a network meta-regression of individual patient data from two randomised trials. 2020.
- McCabe S, Watson J, Rubikaite A. Systematic review of the comparative efficacy of anti-TNFα therapies in rheumatoid arthritis, ulcerative colitis and Crohn’s disease. PROSPERO 2019 CRD42019149621 [Internet]. 2019 cited 2020 May 26. Available from: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42019149621.
- Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097.
- Lau J, Ioannidis JP, Schmid CH. Quantitative synthesis in systematic reviews. Ann Intern Med. 1997;127(9):820–826.
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–188.
- Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959;22(4):719–748.
- R Core Team. R: A language and environment for statistical computing [Internet]. 2019 cited 2020 May 26. Available from: https://www.R-project.org/.
- Schwarzer G. Meta: An R package for meta-analysis. R News. 2007;7(3):40–45.
- National Institute for Health and Care Excellence. NICE technology appraisal submission templates: user guide for submission template (Word). 2018 cited 2020 Aug 9. Available from: https://www.nice.org.uk/about/what-we-do/our-programmes/nice-guidance/nice-technology-appraisal-guidance.
- Takeuchi T, Miyasaka N, Tatsuki Y, et al. Baseline tumour necrosis factor alpha levels predict the necessity for dose escalation of infliximab therapy in patients with rheumatoid arthritis. Ann Rheum Dis. 2011;70(7):1208–1215.
- Smolen JS, Choe JY, Prodanovic N, et al. Comparing biosimilar SB2 with reference infliximab after 54 weeks of a double-blind trial: clinical, structural and safety results. Rheumatology (Oxford). 2017;56(10):1771–1779.
- Cohen S, Genovese MC, Choy E, et al. Efficacy and safety of the biosimilar ABP 501 compared with adalimumab in patients with moderate to severe rheumatoid arthritis: a randomised, double-blind, phase III equivalence study. Ann Rheum Dis. 2017;76(10):1679–1687.
- Weinblatt ME, Baranauskaite A, Dokoupilova E, et al. Switching from reference adalimumab to SB5 (adalimumab biosimilar) in patients with rheumatoid arthritis: fifty-two-week phase III randomized study results. Arthritis Rheumatol. 2018;70(6):832–840.
- Fleischmann RM, Alten R, Pileckyte M, et al. A comparative clinical study of PF-06410293, a candidate adalimumab biosimilar, and adalimumab reference product (Humira®) in the treatment of active rheumatoid arthritis. Arthritis Res Ther. 2018;20(1):178.
- Yamanaka H, Ishiguro N, Takeuchi T, et al. Recovery of clinical but not radiographic outcomes by the delayed addition of adalimumab to methotrexate-treated Japanese patients with early rheumatoid arthritis: 52-week results of the HOPEFUL-1 trial. Rheumatology (Oxford). 2014;53(5):904–913.
- Smolen JS, van der Heijde DM, Keystone EC, et al. Association of joint space narrowing with impairment of physical function and work ability in patients with early rheumatoid arthritis: protection beyond disease control by adalimumab plus methotrexate. Ann Rheum Dis. 2013;72(7):1156–1162.
- Detert J, Bastian H, Listing J, et al. Induction therapy with adalimumab plus methotrexate for 24 weeks followed by methotrexate monotherapy up to week 48 versus methotrexate therapy alone for DMARD-naive patients with early rheumatoid arthritis: HIT HARD, an investigator-initiated study. Ann Rheum Dis. 2013;72(6):844–850.
- Kavanaugh A, Fleischmann RM, Emery P, et al. Clinical, functional and radiographic consequences of achieving stable low disease activity and remission with adalimumab plus methotrexate or methotrexate alone in early rheumatoid arthritis: 26-week results from the randomised, controlled OPTIMA study. Ann Rheum Dis. 2013;72(1):64–71.
- Matucci-Cerinic M, Allanore Y, Kavanaugh A, et al. Efficacy, safety and immunogenicity of GP2015, an etanercept biosimilar, compared with the reference etanercept in patients with moderate-to-severe rheumatoid arthritis: 24-week results from the comparative phase III, randomised, double-blind EQUIRA study. RMD Open. 2018;4(2):e000757.
- Dougados MR, van der Heijde DM, Brault Y, et al. When to adjust therapy in patients with rheumatoid arthritis after initiation of etanercept plus methotrexate or methotrexate alone: findings from a randomized study (COMET). J Rheumatol. 2014;41(10):1922–1934.
- Emery P, Vencovsky J, Sylwestrzak A, et al. A phase III randomised, double-blind, parallel-group study comparing SB4 with etanercept reference product in patients with active rheumatoid arthritis despite methotrexate therapy. Ann Rheum Dis. 2017;76(1):51–57.
- Emery P, Vencovsky J, Sylwestrzak A, et al. Long-term efficacy and safety in patients with rheumatoid arthritis continuing on SB4 or switching from reference etanercept to SB4. Ann Rheum Dis. 2017;76(12):1986–1991.
- Yamanaka H, Nagaoka S, Lee SK, et al. Discontinuation of etanercept after achievement of sustained remission in patients with rheumatoid arthritis who initially had moderate disease activity-results from the ENCOURAGE study, a prospective, international, multicenter randomized study. Mod Rheumatol. 2016;26(5):651–661.
- Matsuno H, Matsubara T. A randomized double-blind parallel-group phase III study to compare the efficacy and safety of NI-071 and infliximab reference product in Japanese patients with active rheumatoid arthritis refractory to methotrexate. Mod Rheumatol. 2019;29(6):919–927.
- Choe JY, Prodanovic N, Niebrzydowski J, et al. A randomised, double-blind, phase III study comparing SB2, an infliximab biosimilar, to the infliximab reference product Remicade in patients with moderate to severe rheumatoid arthritis despite methotrexate therapy. Ann Rheum Dis. 2017;76(1):58–64.
- Smolen JS, Choe JY, Prodanovic N, et al. Safety, immunogenicity and efficacy after switching from reference infliximab to biosimilar SB2 compared with continuing reference infliximab and SB2 in patients with rheumatoid arthritis: results of a randomised, double-blind, phase III transition study. Ann Rheum Dis. 2018;77(2):234–240.
- Alten R, Batko B, Hala T, et al. Randomised, double-blind, phase III study comparing the infliximab biosimilar, PF-06438179/GP1111, with reference infliximab: efficacy, safety and immunogenicity from week 30 to week 54. RMD Open. 2019;5(1):e000876.
- Cohen SB, Alten R, Kameda H, et al. A randomized controlled trial comparing PF-06438179/GP1111 (an infliximab biosimilar) and infliximab reference product for treatment of moderate to severe active rheumatoid arthritis despite methotrexate therapy. Arthritis Res Ther. 2018;20(1):155.
- Yoo DH, Hrycaj P, Miranda P, et al. A randomised, double-blind, parallel-group study to demonstrate equivalence in efficacy and safety of CT-P13 compared with innovator infliximab when coadministered with methotrexate in patients with active rheumatoid arthritis: the PLANETRA study. Ann Rheum Dis. 2013;72(10):1613–1620.
- Yoo DH, Racewicz A, Brzezicki J, et al. A phase III randomized study to evaluate the efficacy and safety of CT-P13 compared with reference infliximab in patients with active rheumatoid arthritis: 54-week results from the PLANETRA study. Arthritis Res Ther. 2016;18:82.
- Takeuchi T, Yamanaka H, Tanaka Y, et al. Evaluation of the pharmacokinetic equivalence and 54-week efficacy and safety of CT-P13 and innovator infliximab in Japanese patients with rheumatoid arthritis. Mod Rheumatol. 2015;25(6):817–824.
- Kim J, Ryu H, Yoo DH, et al. A clinical trial and extension study of infliximab in Korean patients with active rheumatoid arthritis despite methotrexate treatment. J Korean Med Sci. 2013;28(12):1716–1722.
- Takeuchi T, Miyasaka N, Inoue K, et al. Impact of trough serum level on radiographic and clinical response to infliximab plus methotrexate in patients with rheumatoid arthritis: results from the RISING study. Mod Rheumatol. 2009;19(5):478–487.
- Weinblatt ME, Baranauskaite A, Niebrzydowski J, et al. Phase III randomized study of SB5, an adalimumab biosimilar, versus reference adalimumab in patients with moderate-to-severe rheumatoid arthritis. Arthritis Rheumatol. 2018;70(1):40–48.
- Cohen SB, Alonso-Ruiz A, Klimiuk PA, et al. Similar efficacy, safety and immunogenicity of adalimumab biosimilar BI 695501 and Humira reference product in patients with moderately to severely active rheumatoid arthritis: results from the phase III randomised VOLTAIRE-RA equivalence study. Ann Rheum Dis. 2018;77(6):914–921.
- Edwards CJ, Monnet J, Ullmann M, et al. Safety of adalimumab biosimilar MSB11022 (acetate-buffered formulation) in patients with moderately-to-severely active rheumatoid arthritis. Clin Rheumatol. 2019;38(12):3381–3390.
- Taylor PC, Keystone EC, van der Heijde D, et al. Baricitinib versus placebo or adalimumab in rheumatoid arthritis. N Engl J Med. 2017;376(7):652–662.
- Emery P, Vencovsky J, Sylwestrzak A, et al. 52-week results of the phase 3 randomized study comparing SB4 with reference etanercept in patients with active rheumatoid arthritis. Rheumatology (Oxford). 2017;56(12):2093–2101.
- Matsuno H, Tomomitsu M, Hagino A, et al. Phase III, multicentre, double-blind, randomised, parallel-group study to evaluate the similarities between LBEC0101 and etanercept reference product in terms of efficacy and safety in patients with active rheumatoid arthritis inadequately responding to methotrexate. Ann Rheum Dis. 2018;77(4):488–494.
- Bae SC, Kim J, Choe JY, et al. A phase III, multicentre, randomised, double-blind, active-controlled, parallel-group trial comparing safety and efficacy of HD203, with innovator etanercept, in combination with methotrexate, in patients with rheumatoid arthritis: the HERA study. Ann Rheum Dis. 2017;76(1):65–71.
- Arnett FC, Edworthy SM, Bloch DA, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31(3):315–324.
- Aletaha D, Neogi T, Silman AJ, et al. 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. 2010;62(9):2569–2581.
- Graudal N, Kaas-Hansen BS, Guski L, et al. Different original and biosimilar TNF inhibitors similarly reduce joint destruction in rheumatoid arthritis–A network meta-analysis of 36 randomized controlled trials. Int J Mol Sci. 2019;20(18):4350.
- Aaltonen KJ, Virkki LM, Malmivaara A, et al. Systematic review and meta-analysis of the efficacy and safety of existing TNF blocking agents in treatment of rheumatoid arthritis. PLoS One. 2012;7(1):e30275.
- Wolbink GJ, Voskuyl AE, Lems WF, et al. Relationship between serum trough infliximab levels, pretreatment C reactive protein levels, and clinical response to infliximab treatment in patients with rheumatoid arthritis. Ann Rheum Dis. 2005;64(5):704–707.
- Higgins JPT, Thomas J, Chandler J, et al. Cochrane handbook for systematic reviews of interventions, version 6.0 [Internet]. 2019 cited 2020 May 26. Available from: https://training.cochrane.org/cochrane-handbook-systematic-reviews-interventions.
- Kravvariti E, Kitas GD, Mitsikostas DD, et al. Nocebos in rheumatology: emerging concepts and their implications for clinical practice. Nat Rev Rheumatol. 2018;14(12):727–740.
