ABSTRACT
Introduction: Severe acute respiratory syndrome causing coronavirus SARS-CoV-2 (coronavirus disease 2019 (COVID-19)) has recently resulted in the recent global pandemic. As convalescent plasma (CP) therapy has been used with success in several viral infections before, it has become a treatment of choice. Medical literature is reviewed for randomized controlled studies using convalescent plasma therapy.
Areas covered: More than one type of neutralizing antibody against a specific microorganism may be found in both CP and hyperimmune globulins. To give a standard titer of a specific neutralizing antibody to a patient, a reliable antibody titration assay should be developed. It is challenging to test the efficacy of the CP and HIG therapies with double-blind studies. There is a difficulty in the standardization of the CP and HIG study groups, as patients use various additional therapies. Different amounts and titers of CP and HIG and different titers of CP are used in patients. This review discusses the current knowledge on CP and HIG therapies used in COVID-19 disease.
Expert opinion: The immune response to COVID-19 have diverse characteristics. The antibody produced after COVID-19 disease and vaccination is short-lived. Thus, CP should be an alternative especially in patients with lymphopenia and primary/secondary antibody deficiency.
1. Introduction
Coronaviruses are zoonotic viruses having an envelope and a positive-sense RNA. Human coronavirus infections are generally associated with respiratory disease. In the last decade, highly pathogenic coronaviruses – Severe Acute Respiratory Syndrome and Middle East Respiratory Syndrome causing coronaviruses (SARS-CoV and MERS-CoV, respectively) – have led to severe outbreaks, and the last member of the family, SARS-CoV-2 (coronavirus disease 2019 (COVID-19)) has recently resulted in the recent global pandemic.
The SARS-CoV-2 pandemic management has started with a comprehensive analysis of drugs to treat SARS-CoV-2 infection [Citation1].The morbidity and mortality rates were high [Citation2], and there was not yet any randomized and controlled double-blind clinical study on potential medications having efficacy. Randomized controlled studies were planned in order to show the efficacy of convalescent plasma (CP) [Citation3–9], and they have been reviewed for the efficacy analysis [Citation10,Citation11]. However, there was an old, nonspecific, but effective therapy: the convalescent or hyperimmune plasma therapies.
The use of CP in the therapy of infectious disease started in the early nineteenth century. The gammaglobulin peak in plasma protein electrophoresis was documented in electrophoresis after infections in animals (horse and rabbit) and human [Citation12], and Clemens von Pirque first used the horse CP in the therapy of children with diphtheria and rubella [Citation13]. The Serum Disease, a severe reaction that developed after serum therapy, was soon described. Cohn developed a fractionation process in the 1940s to isolate the gammaglobulins (immunoglobulins (Ig)) [Citation14]. Pepsin-treated gammaglobulin form, the form without ‘fraction of crystallization’ (Fc) part, did not have any additional immunomodulatory effect. So, the therapy with complete gammaglobulin form, including two ‘fragments of antigen-binding’ (F(ab)s) and one Fc fragment, was rather preferred [Citation15]. Consequently, gammaglobulin therapy became more reliable due to the viral inactivation and elimination procedures (solvent detergent and nanofiltration) [Citation16].
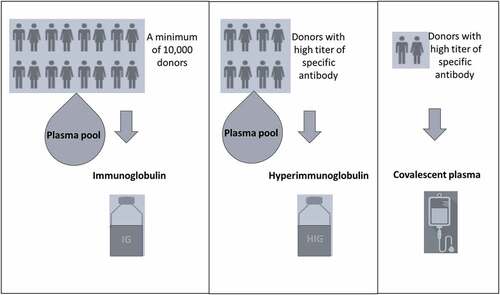
Immunoglobulin products are prepared from large pools (obtained from a minimum of 10,000 donors) of whole blood or apheresis-derived plasma [Citation17] (). It has several effects on viral infections. It can block the viral entry to an infected cell by binding to the viral antigen, leading to neutralization. After it binds to the virus, it can further activate the mononuclear cells via their Fc receptors. Fc-mediated protection has been shown to play at least some part in providing protection from several viruses. Additionally, it can activate the complement system, mediating phagocytosis by opsonization through complement fragments. Another function of the neutralizing antibodies is the blockage of the viral budding by binding to the viral glycoproteins on the infected cell surface. Thus, neutralization has roles in both blocking the entry and egress of new progeny and may lead to a decrease in viral load during infections. Convalescent plasma and hyperimmune globulin (HIG) therapies have been used with success against several severe viral infections, such as hepatitis B and rabies. Thus, it is still being used in the therapy of infectious diseases in the absence of specific antimicrobial therapies.
2. Convalescent plasma and hyperimmunoglobulin therapies
Plasma includes several numbers and amount of neutralizing antibodies against several microorganisms. Convalescent plasma is the plasma collected from donors who have survived an infectious disease by producing protective antibodies [Citation11,Citation18]. Convalescent plasma is usually collected after 14–28 days of infection from generally male donors. However, females who have not been pregnant before may also serve as a donor. Polymerase chain reaction should be negative at least twice in the donor before the donation. Donations are possible in a 2-week intervals, at most three times. There is always a risk of transmission of an infectious agent by plasma. So, viral screening tests, such as hepatitis B, hepatitis C, HIV, syfilis, are applied to donors before the plasma collection.
HIG therapies are produced from plasma of a definite number of individuals with high titer of specific antibody against a microorganism. According to WHO/IUIS, HIG products should contain at least fivefold increased specific antibody titers compared with standard preparations of IVIG [Citation17]. For the preparation of HIG, a new plasma pool collection is required to produce commercial HIG products as a time-span is needed for convalescence. For example, neutralizing antibodies to an infectious agent may be present in the HIG products at least a few months after the pandemic [Citation19]. HIG products have a standard titer of an antibody with less volume compared to CP, which may be an advantage in patients with cardiovascular instability. On the other hand, CP may be physiologically more effective compared to HIG despite a relatively increased risk of microorganism inoculation, as it includes plasma factors, pro-coagulants, and anti-fibrinolytic [Citation20,Citation21]. The comparison of CP and HIG is given in .
Table 1. Comparison of the convalescent plasma and hyperimmune globulin therapies
A neutralizing antibody previously produced against an infectious agent may cross-react with other infectious agents. More than one type of neutralizing antibody against a specific microorganism may be found in both HIG and CP. To give a standard titer of a specific neutralizing antibody to a patient, a reliable antibody titration assay should be developed. For a reliable assay, the isolation of the virus is needed first. Then, the serial titers of a neutralizing antibody should be defined by evaluating the cytopathic effect with serial dilutions of the virus [Citation22]. The neutralizing antibody test will help the clinicians to measure the titer of the antibody in CP therapies. On the other hand, this is also a requirement to produce a standard commercial HIG product having a definite titer of neutralizing antibody.
It is challenging to test the efficacy of the CP and HIG therapies with double-blind studies. There is a difficulty in the standardization of the CP and HIG study groups, as patients may use various additional therapies. Different amounts and titers of CP and HIG and different titers of CP may be used in patients. The common benefit of CP and HIG therapies seems to be the reduction in mortality, based on several studies and meta-analyses performed in influenza A (H1N1) and SARS-CoV infections [Citation19, Citation23–26], despite all the challenges in studies. Hung et al. [Citation12] compared HIG with Ig treatment and reported a faster serum IL-6 decrease and decreased mortality with HIG if used within 5 days.
The outcome of the CP therapy is affected by several characteristics of the patient and the donor: clinical severity of the infectious disease, viral load in the patient, the timing of the plasma collection, the amount of the CP given, titer and durability of the antibody in CP, the time and frequency of the application, and the presence of autoantibodies, and so on. Most frequent side effects of CP and HIG are the transfusion-related acute lung injury (TRALI) [Citation27,Citation28], transfusion-associated circulatory overload (TACO) [Citation29], hemolytic transfusion reactions, and transfusion-related immunomodulation (TRIM). Another adverse reaction that is first defined in Ebola infection [Citation30], is the antibody-dependent enhancement (ADE) of infectious disease. ADE is also reported during the therapy of Dengue virus infection [Citation31]. It is explained by cross-linking with complexes of anti-virus-antibody or virus-activated complement components through interaction with cellular molecules, such as Fc receptors or complement receptors. This is suggested to lead to enhanced inflammation of susceptible cells and tissues [Citation32], and disease exacerbation. Nonetheless, it is interesting not to see this effect during the standard Ig therapy in patients with virus-induced secondary hemophagocytosis or primary immunodeficient patients with infectious diseases. To prove the presence and effect of ADE, in a sufficient number of patients with different severity of the disease, a broad application of serologic assays that quantify the amounts and titers of different neutralizing antibodies is needed. Additionally, the amount of the viral load which may be related to the phase and severity of the infectious disease may be important for ADE. Regarding ADE, a recent meta-analysis [Citation33] showed that there is no relationship between the kinetics of antibody responses to SARS-CoV, MERS-CoV, or SARS-CoV-2 and the clinical outcomes of diseases [Citation34].
2.1. Convalescent plasma and hyperimmunoglobulin therapies for SARS-CoV-2
Polyclonal nature of antibody in CP and HIG therapies serves as a potential benefit [Citation35]. There is an anti-nucleocapsid antibody in addition to an antibody against viral spike protein in the plasma of patients who recovered from SARS-CoV-2 [Citation36]. It has recently been suggested that some of the Ig products in use include anti-SARS-CoV-2 antibodies [Citation37]. Although the type and titer of the neutralizing antibody were not explained, there may be cross-reactivity between anti-SARS-CoV-1 and anti-SARS-CoV-2 antibodies, as we know that 79.6% of genomic sequence is common in both viruses [Citation38].
The SARS-CoV-2 disease is shown to be associated with microvascular injury and thrombosis [Citation39]. Since homeostatic interactions between complement and extrinsic/intrinsic coagulation pathways contribute to a net procoagulant state in the microvasculature of critical organs [Citation40], the balance between the activating and regulating complement components in the CP is important. A shift in the balance toward regulation with CP may control the inflammatory response during infection. On the contrary, a shift in the balance toward activation may increase the inflammation. Reports suggest the importance of C3, C5a, and Factor D blockade in the therapy of acute inflammatory state in SARS-CoV-2 [Citation41–42].
A possible outcome of CP therapy became apparent after a study that reminded the possibility of the transfer of different types of autoantibodies from the plasma of donors to recipients [Citation43]. Recent data showed the risk of transmission of anti-interferon antibodies by CP and HIG. These antibodies are shown to be more common in male patients who are in their late adulthood and experiencing severe SARS-CoV-2 [Citation44]. With this study, Bastard et al. have described a new phenocopy of primary immunodeficiency disease (PID). If the CP includes autoantibodies against the immune response elements such as interferons, a progression in the severity of the infectious disease may be seen in the recipient. Accordingly, the characteristics of the CP donor should be meticulously defined ().
Medical literature is reviewed for the randomized controlled studies using convalescent plasma therapy for COVID-19. There is an increase in CP use in the world after these studies showing efficacy [Citation3–5,Citation9] (). The therapy is more effective when given in the first 10–14 days of the disease, possibly when given before the increase in the viral load and disease severity [Citation24]. In the study of Duan et al., viremia resolved, and clinical parameters improved in 10 severe SARS-CoV-2 patients within 3 days after CP therapy (200 mL, neutralizing antibody titer>1:640) [Citation9]. Another study in 10 SARS-CoV-2 patients reported improvements in lung injury with CP therapy on chest X-ray and computed tomography scan [Citation45]. A significant decrease in sequential organ failure assessment (SOFA) score, body temperature, ferritin, C-reactive protein, and D-dimer were observed. The CP therapy made extubation possible in some patients. Studies report less intubation need and mortality reduction in severe cases [Citation44, Citation46]. In the study of Simonovich et al. [Citation6], CP was given as a randomized trial in Covid-19 severe pneumonia. They have suggested that there were no differences in clinical status or overall mortality between patients treated with CP and those who received placebos. However, when the characteristics of the patient at baseline were evaluated, it could be seen that the ratio of chronic obstructive pulmonary disease in CP group was 10.1% while it was 1.9% in placebo group, which may be a significant bias in the study. In the study of Agarwal, mortality was reported within 6 hours of completion of convalescent plasma transfusion. Because of the severity of the disease, the authors suggest that there is a possibility of a relation with COVID-19 or convalescent plasma. Joyner et al. reviewed the studies for the safeness of the convalescent antibody use [Citation47]. Serious adverse events within 4 hours of completion of infusion were less than 1% (n = 146) of all transfusions. Thirteen mortality events were suggested to be possibly, but not definitely, related to the transfusion. Other events were transfusion associated circulatory overload (n = 37), transfusion associated acute lung injury (n = 20), and transfusion associated allergic reactions (n = 26).
Table 2. Randomized controlled studies using convalescent plasma therapy in patients with SARS-CoV-2
There are a few reports on SARS-CoV-2 in patients with PID, especially primary antibody deficiency [Citation49, Citation50]. A positive effect of CP therapy was reported in the latter days of COVID-19 infection in X-linked agammaglobulinemia patients [Citation51]. There may be an efficacy of standard Ig therapies due to the cross-reactive antibodies they may contain or their immunomodulatory function on monocytes and macrophages. Nowadays, unfortunately, we see that undiagnosed PID patients have severe infections, and some patients get their PID diagnoses after the SARS-CoV-2 infection. CP may alternatively be used instead of HIG in different types of PID as it includes both immunoglobulins and several complement components.
Convalescent plasma may also be collected from vaccinated individuals instead of patients who have recovered from infections. However, CP collected from these two groups may differ in the specificity and the clonality of the neutralizing and non-neutralizing antibodies. Additionally, the production of neutralizing antibodies as a commercial product in the therapy of infections is on the way. SARS-CoV-2 spike (S) protein has been suggested to be an important target for neutralizing antibodies since SARS-CoV-2 binding and fusion is through this protein [Citation52].
3. Conclusion
The development of specific neutralizing antibody assays is necessary to titrate the antibody in CP and HIG therapies. This will help clinicians to organize proper clinical trials leading to comparison and precise results. Increased CP use due to the absence of specific therapy for SARS-CoV-2 may show clinicians the spectrum of the side effects.
An extreme therapy may be the plasma exchange therapy by using convalescent plasma therapy in the most severely affected patients. The need for more plasma amount is a restriction for this kind of therapy. However, it can be tested in the future studies.
We now know that we should meticulously define the characteristics of the donor from whom we collect the plasma. However, there is a possibility that the presence of autoantibodies across active immune response elements, such as interferons, which may increase the severity of the infections. In this circumstance, clinicians may use the options of high dose Ig, plasmapheresis or plasma exchange therapies instead of convalescent plasma therapy. Production of standardized tests for the determination of neutralizing antibody amount and for autoantibody screening, including anti-HLA or anti-interferon antibodies, will ease and increase the use of convalescent plasma in the clinical settings.
As the antibody produced after COVID-19 disease and vaccination are reported to be short-lived, CP should be an alternative especially in patients with lymphopenia and primary or secondary antibody deficiency.
4. Expert opinion
Convalescent plasma and hyperimmunoglobulin therapy are used in the first line in a pandemic if there are serious morbid and mortal consequences of an infectious agent, despite the advances in the infectious disease treatment. Thus, it has become a treatment of choice, especially in the first months of the SARS-CoV-2 pandemic. As the patients are in an inflammatory state, and each has a different genetic background with inflammatory susceptibility genes, their inflammatory response is diverse, and they need additional therapies during their infectious disease treatment. The standardization of the convalescent plasma (CP) and hyperimmune globulin (HIG) therapy in the patient groups is difficult in the studies. Beyond the patient characteristics, antibody characteristics in CP and HIG need to be standardized in studies. These are primary challenges to test the efficacy of the CP and HIG therapies with double-blind studies.
Antibodies are the vital humoral components of the plasma and their production is the result of the B cell’s adaptation to antigens. Being an adaptive immune mechanism, antibodies are important, especially in the clearance of the bacterial agents. Thus, the consequence of antibody deficiency is the susceptibility to bacterial infections. However, their presence is shown to prevent the development of viral infections. Antigen-specific antibodies are usually measured to see the antigen-specific response to infections in a bacterial or viral infectious disease. A specific immune response is induced, resulting in the active protection of the individual in vaccination, similar to infections. Although both the specific antibody responses and specific T cell responses should be measured in monitoring the active immune response, the antibody response is usually measured as it is easier. Many antibodies and specific T cells against many different antigenic epitopes are produced in case of an infectious disease. Despite the technological advancements, there are limitations in the in vitro measurement of specific antibodies and specific T cells. This leads to the limitations of the standard use in the global era. This is a secondary challenge in the studies.
Another challenge is the risk of the presence of autoantibodies in the CP or HIG. These autoantibodies may be the antibodies against the components of the immune response. Thus, donor selection, according to individual characteristics, will be important.
Individualized therapies are increasingly being used in the therapy of diseases when there is a genetic or biochemical characteristic. Biomarker research in the diseases, also in the SARS-CoV2 disease, is ongoing. Soon, the characterization of patients with specific biomarkers seems to be used more frequently in the set-up of clinical studies.
Despite these challenges in the CP or HIG use, these therapies decrease morbidity and mortality in many infectious diseases in most of the studies. As the antibody produced after COVID-19 disease and vaccination are reported to be short-lived, CP should be an alternative therapy, especially in patients with lymphopenia and primary/secondary antibody deficiency. The development of specific neutralizing antibody assays is necessary to titrate the antibody in CP and HIG therapies. This will help clinicians to organize proper clinical trials leading to comparison and precise results.
Article highlights
The experience of the convalescent plasma (CP) in the therapy of infectious diseases is older than a century. It was started in the early nineteenth century.
Convalescent plasma (CP) therapy has been used with success in several viral infections before, and it has become a treatment of choice, especially in the first months of the pandemic.
It is challenging to test the efficacy of the CP and HIG therapies with double-blind studies. This is because there is a difficulty in the standardization of the CP and HIG study groups using various additional therapies.
The antibody produced after COVID-19 disease and vaccination are reported to be short-lived. Thus, CP should be an alternative especially in patients with lymphopenia and primary/secondary antibody deficiency.
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Additional information
Funding
References
- Nitulescu GM, Paunescu H, Moschos SA, et al. Comprehensive analysis of drugs to treat SARS‑CoV‑2 infection: mechanistic insights into current COVID‑19 therapies. Int J Mol Med. 2020;46:467–488.
- Piroth L, Cottenet J, Mariet AS, et al. Comparison of the characteristics, morbidity, and mortality of COVID-19 and seasonal influenza: a nationwide, population-based retrospective cohort study. Lancet Respir Med. 2021;9:251-259.
- Agarwal A, Mukherjee A, Kumar G, et al. Convalescent plasma in the management of moderate covid-19 in adults in India: open label phase II multicentre randomised controlled trial (PLACID Trial). bmj 2020;371:m3939.
- Li L, Zhang W, Hu Y, et al. Effect of convalescent plasma therapy on time to clinical improvement in patients with severe and life-threatening COVID-19: a randomized clinical trial. Jama. 2020;324:460–470.
- Liu ST, Lin H-M, Baine I, et al. Convalescent plasma treatment of severe COVID-19: a propensity score-matched control study. Nat Med. 2020 Nov;26(11):1708-1713.
- Simonovich VA, Burgos Pratx LD, Scibona P, et al. A randomized trial of convalescent plasma in Covid-19 severe pneumonia. N Engl J Med. 2021;384:619-629.
- Salazar E, Christensen PA, Graviss EA, et al. Treatment of coronavirus disease 2019 patients with convalescent plasma reveals a signal of significantly decreased mortality. Am J Pathol. 2020;190:2290–2303.
- Libster R, Pérez Marc G, Wappner D, et al. Early high-titer plasma therapy to prevent severe covid-19 in older adults. N Engl J Med. 2021;384:610–618.
- Duan K, Liu B, Li C, et al. Effectiveness of convalescent plasma therapy in severe COVID-19 patients. Proc Nat Acad Sci. 2020;117:9490–9496.
- Valk SJ, Piechotta V, Chai KL, et al. Convalescent plasma or hyperimmune immunoglobulin for people with COVID‐19: a rapid review. Cochrane Database Syst Rev. 2020;5.
- Convalescent Plasma SV, Challenging A. Tool to treat COVID-19 patients—A lesson from the past and new perspectives. Biol Med Res Int. 2020;5:CD013600.
- Heidelberger M, Pedersen KO. The molecular weight of antibodies. J Exp Med. 1937;65:393–414.
- Von Pirquet C. Allergy. Arch Internal Med. 1911;7:383–436.
- Cohn EJ Protein product and process. Google Patents; 1945.
- Fehr J, Hofmann V, Kappeler U. Transient reversal of thrombocytopenia in idiopathic thrombocytopenic purpura by high-dose intravenous gamma globulin. N Engl J Med. ;306:1254–1258.
- Duhem C, Dicato M, Ries F. Side-effects of intravenous immune globulins. Clin Exp Immunol. 1994;97:79.
- Restivo JS, Karafin MS Human Immunoglobulin Preparations. Transfusion Medicine and Hemostasis: Elsevier; 2019. p. 235–246.
- Zhang J, Xie B, Hashimoto K. Current status of potential therapeutic candidates for the COVID-19 crisis. Brain Behav Immun. 2020;87:59–73.
- Hung IFN, To KKW, Lee CK, et al. Hyperimmune IV immunoglobulin treatment: a multicenter double-blind randomized controlled trial for patients with severe 2009 influenza A(H1N1) infection. Chest. 2013 ;144:464–473.
- Wolberg AS, Aleman MM, Leiderman K, et al. Procoagulant activity in hemostasis and thrombosis: virchow’s triad revisited. Anesthesia Analg. 2012;114:275–285.
- Wolberg AS, Aleman MM. Influence of cellular and plasma procoagulant activity on the fibrin network. Thromb Res. 2010;125:S35–7.
- Nurtop E, Villarroel PMS, Pastorino B, et al. Combination of ELISA screening and seroneutralisation tests to expedite Zika virus seroprevalence studies. Virol J. 2018;15:1–6.
- Yeh KM, Chiueh TS, Siu LK, et al. Experience of using convalescent plasma for severe acute respiratory syndrome among healthcare workers in a Taiwan hospital. J Antimicrob Chemother. 2005 ;56:919–922.
- Cheng Y, Wong R, Soo YO, et al. Use of convalescent plasma therapy in SARS patients in Hong Kong. Eur J Clin Microbiol Infect Dis. 2005;24:44–46.
- Soo YO, Cheng Y, Wong R, et al. Retrospective comparison of convalescent plasma with continuing high-dose methylprednisolone treatment in SARS patients. Clin Microbiol Infect. 2004;10:676–678.
- Mair-Jenkins J, Saavedra-Campos M, Baillie JK, et al. The effectiveness of convalescent plasma and hyperimmune immunoglobulin for the treatment of severe acute respiratory infections of viral etiology: a systematic review and exploratory meta-analysis. J Infect Dis. 2015;211:80–90.
- Mora-Rillo M, Arsuaga M, Ramírez-Olivencia G, et al. Acute respiratory distress syndrome after convalescent plasma use: treatment of a patient with Ebola virus disease contracted in Madrid, Spain. Lancet Respir Med. 2015;3:554–562.
- Chun S, Chung CR, Ha YE, et al. Possible transfusion-related acute lung injury following convalescent plasma transfusion in a patient with middle east respiratory syndrome. Ann Lab Med. 2016 ;36:393–395.
- Okazaki H. [Transfusion-related acute lung injury (TRALI) and transfusion-associated circulatory overload (TACO)]. Rinsho Byori Jap J Clin Pathol. 2013;61:399–406.
- Takada A, Feldmann H, Ksiazek TG, et al. Antibody-dependent enhancement of Ebola virus infection. J Virol. 2003;77:7539–7544.
- Kliks SC, Nisalak A, Brandt WE, et al. Antibody-dependent enhancement of dengue virus growth in human monocytes as a risk factor for dengue hemorrhagic fever. Am J Trop Med Hyg. 1989 ;40:444–451.
- Takada A, Kawaoka Y. Antibody-dependent enhancement of viral infection: molecular mechanisms and in vivo implications. Rev Med Virol. 2003;13:387–398.
- Huang AT, Garcia-Carreras B, Hitchings MDT, et al. A systematic review of antibody mediated immunity to coronaviruses: antibody kinetics, correlates of protection, and associationwith severity of disease. Nat Commun. 2020;11:4704. doi: 10.1038/s41467-020-18450-4.
- Arvin AM, Fink K, Schmid MA, et al. A perspective on potential antibody-dependent enhancement of SARS-CoV-2. Nature. 2020;584:353–363.
- Krause I, Wu R, Sherer Y, et al. In vitro antiviral and antibacterial activity of commercial intravenous immunoglobulin preparations–a potential role for adjuvant intravenous immunoglobulin therapy in infectious diseases. Transfusion Med (Oxford, England). 2002;12:133–139.
- Burbelo PD, Riedo FX, Morishima C, et al. Sensitivity in Detection of Antibodies to Nucleocapsid and Spike Proteins of Severe Acute Respiratory Syndrome Coronavirus 2 in Patients With Coronavirus Disease 2019. J Infect Dis. 2020;222:206-213.
- Díez JM, Romero C, Gajardo R. Currently available intravenous immunoglobulin contains antibodies reacting against severe acute respiratory syndrome coronavirus 2 antigens. Immunotherapy 2020;12:571–576.
- Zhou P, Yang XL, Wang XG, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020;579:270–273.
- Magro C, Mulvey JJ, Berlin D, et al. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases. Transl Res. 2020;220:1–13.
- Chauhan AJ, Wiffen LJ, Brown TPCOVID. 19: a collision of complement, coagulation and inflammatory pathways. J Thromb Haemost. 2020;18:2110–2117.
- Jiang Y, Zhao G, Song N, et al. Blockade of the C5a–C5aR axis alleviates lung damage in hDPP4-transgenic mice infected with MERS-CoV. Emerg Microbes Infect. 2018;7:1–12.
- Yu J, Yuan X, Chen H, et al. Direct activation of the alternative complement pathway by SARS-CoV-2 spike proteins is blocked by factor D inhibition. Blood. 2020;136:2080–2089.
- Bastard P, Rosen LB, Zhang Q, et al. Auto-antibodies against type I IFNs in patients with life-threatening COVID-19. Science. 2020;370:eabd4585..
- Abolghasemi H, Eshghi P, Cheraghali AM, et al. Clinical efficacy of convalescent plasma for treatment of COVID-19 infections: results of a multicenter clinical study. Transfus Apher Sci. 2020;15:102875.
- Olivares-Gazca JC, Priesca-Marín JM, Ojeda-Laguna M, et al.Infusion of convalescent plasma is associated with clinical improvement in critically ill patients with COVID-19: a pilot study. Rev Invest Clin. 2020;72:159–164.
- Perotti C, Baldanti F, Bruno R, et al.Mortality reduction in 46 severe Covid-19 patients treated with hyperimmune plasma. A proof of concept single arm multicenter trial. Haematologica. 2020;105:2834-2840.
- Joyner MJ, Bruno KA, Klassen SA, et al.Safety update: COVID-19 convalescent plasma in 20,000 hospitalized patients. Mayo Clinic Proceedings. 2020;95:1888-1897.
- Shenoy AG, Hettinger AZ, Fernandez SJ, et al.Early mortality benefit with COVID-19 convalescent plasma: a matched control study. Br J Haematol. 2021;192:706–713.
- Quinti I, Lougaris V, Milito C, et al. A possible role for B cells in COVID-19? Lesson from patients with agammaglobulinemia. J Allergy Clin Immunol. 2020;146:211–213.
- Soresina A, Moratto D, Chiarini M, et al.Two X-linked agammaglobulinemia patients develop pneumonia as COVID-19 manifestation but recover. Pediatr Allergy Immunol. 2020;31:565–569.
- Jin H, Reed JC, Liu STH, et al.Three patients with X-linked agammaglobulinemia hospitalized for COVID-19 improved with convalescent plasma. J Allergy Clin Immunol Pract. 2020;8:3594–3596.
- Zhou G, Zhao Q.Perspectives on therapeutic neutralizing antibodies against the Novel Coronavirus SARS-CoV-2. Int J Biol Sci. 2020;16:1718–1723.