1. Introduction
Systemic lupus erythematosus (SLE) is an autoimmune disorder characterized by a complex immune dysregulation leading to diverse clinical phenotypes. The preclinical lupus phase (preclinical SLE) is defined as the time of emerging maladaptive immune responses prior to full-blown SLE, and is mainly applied in a retrospective manner [Citation1]. In contrast, incomplete or suspected SLE (iSLE) is often the term used by many rheumatologists in an attempt to identify individuals at risk for clinical SLE. Another common term used is undifferentiated connective tissue disease (UCTD), which describes more broadly people with suggestive but not entirely diagnostic features of an underlying connective tissue disease, which besides SLE may include scleroderma or rheumatoid arthritis. Studies among individuals with UCTD have shown that clinical and immunologic factors, such as female sex, ANA-homogenous pattern, anti-dsDNA, anti-Sm, and anticardiolipin antibodies, portend an increased risk to transition to clinical SLE [Citation2]. On the contrary, nonspecific markers for inflammation like the sedimentation rate and C-reactive protein are not particularly useful as predictors of disease progression [Citation3]. Efforts to understand the intrinsic mechanisms for the development of SLE revealed that many of these patients generate and accumulate pathogenic autoantibodies, probably related to a break of B and/or T cell tolerance, many years before a clinical diagnosis is made [Citation4]. Even though autoantibody profiling appears to be an interesting approach to detect individuals at risk, it is limited by the low percentage of autoantibody-positive healthy subjects who ultimately develop SLE.
Figure 1. As genetically susceptible patients under the influence of to-date incompletely understood environmental factors, progress from the pre-clinical stage to clinical SLE, various cytokines and antibodies are gradually detected in their peripheral blood. As shown in this figure, IFNγ (blue) is an early marker of immune aberration followed by specific autoantibodies (orange) and IFNα (green)
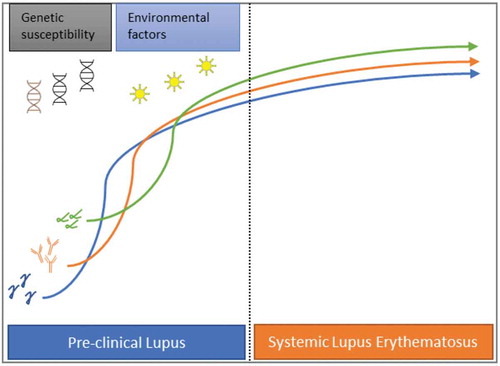
For several decades now it is known that the interferon (IFN) signaling pathway is prominently activated in SLE, with up to 50% of these patients exhibiting various IFN-inducible genes (IFIGs) linked with disease activity and severity [Citation5–7]. Although there are available direct methods that detect interferon levels in peripheral blood, these lack sensitivity, many times, IFN activity is measured simultaneously by calculating a score from the expression of a set of IFIGs, which is commonly represented as either a categorical variable (high vs low) or a continuous one. The latter have been found to be more informative though [Citation8]. The increased understanding of the important role of interferon in SLE led to therapeutic strategies targeting this cytokine. Despite the fact initial trials that evaluated a monoclonal antibody against interferon alpha were unsuccessful, there are some promising results with Anifrolumab, a human monoclonal antibody that blocks the common interferon alpha receptor subunit 1, especially among those SLE patients with active skin disease [Citation9,Citation10]. Interferons have also been found overexpressed in preclinical SLE and it is hypothesized these might be implicated in the development of clinical autoimmunity [Citation11] (). Therefore, ascertaining interferon status could be of value in distinguishing individuals at risk that may benefit from early preventive interventions. In this regard, hydroxychloroquine has been shown to delay the onset of SLE [Citation12], and this effect may be connected to type I interferon suppression. Currently, hydroxychloroquine is under investigation for the prevention of SLE (NCT03030118) [Citation13,Citation14]. Here we center our discussion on the different types of interferons, their expression, and their role in the disease, stressing their potential as diagnostic and prognostic biomarkers in suspected SLE.
2. Type I interferons
Interferon (IFN) I proteins are essential for human host response against viruses. Type I interferons include twelve IFN-α subtypes, IFN-β, IFN-ε, IFN-κ, and IFN-ω. Previous studies in animal models and humans have consistently shown an aberrant expression of type I interferon regulated genes in peripheral blood and affected tissues in SLE, commonly known as the IFN signature. The inciting event that triggers a persistent production of type I IFN is not completely understood but evidence shows that a combination of genetic (e.g., IRF5 haplotype, IFNK polymorphisms) and environmental factors (e.g., UVB light, infections) is implicated [Citation15,Citation16]. In addition, estrogen had been found to enhance the activation of IFN-α signaling [Citation17,Citation18]. High levels of IFN-α are induced by the interaction of immune-complexes with toll-like receptors (TLR) particularly TLR7 or TLR9, a process that takes place primarily within plasmacytoid dendritic cells (pDCs). Moreover, an increased production of interferon-α can be further amplified by a prolonged exposure to neutrophil extracellular traps (NETs). Several signaling pathways are activated after the binding of type I interferons to their receptor (IFNAR1), triggering the transcription of hundreds of type I IFN-stimulated genes, creating the interferon signature. Importantly, the interferon signature has been used as a biomarker to predict the effectiveness of interferon targeting drugs, albeit with variable success [Citation9].
The role of type I IFN, and in particular IFN-α in preclinical and suspected SLE has also been explored (see ). A longitudinal study analyzed clinical and laboratory data from 55 military recruits collected prior to, at, and after fulfilling the revised (1997) ACR classification criteria for SLE, and compared them to a matched control group (age, sex, race, and time of blood sample) [Citation19]. The majority of individuals were African American women with a mean age of 29 years old. It was noted that serum interferon-α activity score increased up to 44% in those individuals who were 2 years or less close to meet SLE classification and further increased up to 75% at classification. Additionally, a similar rise in interferon-α activity was observed with autoantibody positivity and also correlated with a higher number of SLE-specific autoantibodies. Many of these autoantibodies were detected prior to increases in IFN-α activity though. Of note, female sex, the number of autoantibodies specificities, and a number of positive IFN-associated mediators (BLyS, MIG, IP-10, IFN-γ, MIP-1α, and MCP-3) were found to be significant predictors of increased interferon-α activity. Another prospective study recruited 118 subjects referred due to suspected connective tissue disease with many of them having at least a positive ANA. From these, 14 developed SLE at 12 months. In many of these patients, it was noted higher Type I interferon scores at baseline in peripheral blood and non-lesional skin biopsy samples when compared to healthy controls, and its production was an independent predictor for progression to clinical SLE [Citation20]. Similar findings were observed in a small cross-sectional study of 29 patients with iSLE, where a high interferon score was present in half of these patients, and activity correlated positively with the number of autoantibodies [Citation21]. Another cross-sectional study investigated the interferon signature gene expression in four different groups of individuals, namely normal controls, first-degree relatives of SLE subjects, iSLE, and SLE. Incremental levels of expression correlated with the burden of the disease, being the lowest in normal controls and the highest in SLE (NC<FDR<iSLE<SLE) [Citation22]. Notably, 12 out of 24 patients with iSLE demonstrated a high interferon signature, and these subjects were more likely to be Hispanic, African American, or Native American. In line with previous studies, serum autoantibody levels against DNA or RNA binding proteins were significantly higher in this subset of patients with overexpressed interferon activity. Interestingly, recent data in preclinical autoimmunity revealed that one potential source for the high expression of type I interferon in the skin is the keratinocytes and not the pDCs as previously thought [Citation23].
Table 1. Role of interferon in preclinical SLE
These results from different cohorts suggest that interferon alpha activity is elevated prior to the development of SLE but is only one part of a wider dysregulation of the immune system.
3. Type II interferons
Similar to type I interferon, its function is to inhibit viral replication but is also important for anti-bacterial immunity. The only member of this group is IFN-γ that is mainly produced by activated Th1 CD4+ lymphocytes, CD8+ lymphocytes, and NK cells and plays a role in the innate and adaptive immune responses. It has been established that IFN-γ overactivation promotes a chronic pro-inflammatory cascade contributing to organ damage accrual in SLE patients [Citation24]. Additional evidence suggests that various genetic polymorphisms involving interferon-γ and its receptor are associated with an increased susceptibility to SLE [Citation25,Citation26]. Concerning preclinical lupus, type II interferon positivity was found, at least in one study, to precede autoantibody accrual and increased IFN-α [Citation19] (see ). High levels of IFN-γ, as far as 4 or more years before SLE classification, were observed to be predictive of elevated IFN-α activity and future SLE classification. These findings suggest that IFN-γ signaling is highly active at the preclinical SLE stage. It was hypothesized that this upregulated IFN-γ activity enhances neoantigen presentation and promotes accumulation of pathogenic autoantibodies which in turn lead to dysregulated interferon-α signaling, increasing the likelihood that preclinical lupus evolves to clinical SLE.
4. Type III interferons
Type III interferons contribute to antiviral immunity but to a lesser extent than interferon-α. These cytokines comprise IFN-λ1, IFN-λ2, IFN-λ3, and IFN-λ4, and their production is more abundant at the level of epithelial cell barriers. Recent basic science work indicates that IFN- λ activity is upregulated in a TLR7-dependent manner in murine lupus. It was further noted that IFN-λ exerts local inflammation in murine lupus skin likely through effects on keratocytes that release pro-inflammatory cytokines inducing immune cell recruitment [Citation27]. There is also growing evidence that IFN-λ is essential in human SLE. The expression of genetic variants in the IFN-λ locus is associated with an increased risk of SLE, while serum levels of IFN-λ3 appear to correlate with SLE disease activity and renal involvement. On the other hand, IFN-λ1 expression was upregulated in patients with milder disease [Citation28]. To date, no studies have evaluated IFN-λ activity in preclinical SLE, but, like other type of interferons, a potential contribution in SLE development is possible.
5. Clinical implications of measuring interferon activity
Early recognition of SLE poses a challenge due to the heterogeneous nature of the disease with diagnostic delays being common in clinical practice. As discussed above, type I and type II interferon activity were found to be upregulated in patients during the preclinical stage of the disease. Using interferon as a biomarker for progression from preclinical to clinical disease though, is still far from being useful in practice. First, the available data come from exceedingly small sample size studies limiting the generalizability of the results: Notwithstanding publication bias toward positive studies, large longitudinal cohort studies are needed to validate these findings. Secondly, although there are a few functional assays used to assess type I interferon activity and promising new technology using a single-molecule array to detect interferon- α blood levels, there is no test standardization. To complicate matters interferon activation is not limited to preclinical SLE and has been found elevated in pre-clinical stages of other autoimmune diseases. Finally, relying on interferon activity as a sole strategy to predict progression from iSLE to SLE may miss ‘at risk’ individuals, hence combining the best predictive biomarkers will result in a better estimation of disease initiation in SLE. With that being said, interferon expression remains a valuable tool in the research field that worth continue investigating as a predictive biomarker.
6. Conclusion
Interferon signaling is thought to be a key player in all stages of SLE. Data over the past decade suggest that type I and II interferon expression may be important for progression from pre-clinical to the clinical stage of the disease as well. Quantification of interferon-related gene expression or serum levels of interferon may help identify a high-risk group of individuals who may benefit from closer monitoring or early institution of therapy to prevent progression to the disease and organ damage. This is, however, at this stage, a hypothesis that needs validation.
Declaration of interest
Dr. Kyttaris has a research grant from Exagen Diagnostics, that is not relevant to this publication. The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Additional information
Funding
References
- Bourn R, James JA. Preclinical lupus. Curr Opin Rheumatol. 2015;27:433–439.
- Mosca M, Tani C, Carli L, et al. Undifferentiated CTD: a wide spectrum of autoimmune diseases. Best Pract Res Clin Rheumatol. 2012;26:73–77.
- Dima A, Opris D, Jurcut C, et al. Is there still a place for erythrocyte sedimentation rate and C-reactive protein in systemic lupus erythematosus? Lupus. 2016;25:1173–1179.
- Arbuckle MR, McClain MT, Rubertone MV, et al. Development of autoantibodies before the clinical onset of systemic lupus erythematosus. N Engl J Med. 2003;349:1526–1533.
- Oke V, Gunnarsson I, Dorschner J, et al. High levels of circulating interferons type I, type II and type III associate with distinct clinical features of active systemic lupus erythematosus. Arthritis Res Ther. 2019;21:107.
- Kirou KA, Lee C, George S, et al. Coordinate overexpression of interferon-α–induced genes in systemic lupus erythematosus. Arthritis Rheum. 2004;50:3958–3967.
- Rönnblom L, Leonard D. Interferon pathway in SLE: one key to unlocking the mystery of the disease. Lupus Sci Med. 2019;6:e000270.
- El-Sherbiny YM, Psarras A, Md Yusof MY, et al. A novel two-score system for interferon status segregates autoimmune diseases and correlates with clinical features. Sci Rep [Internet]. 2018 [cited 2021 Mar 23];8. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5895784/
- Morand EF, Furie R, Tanaka Y, et al. Trial of anifrolumab in active systemic lupus erythematosus. N Engl J Med. 2020;382:211–221.
- Koh JWH, Ng CH, Tay SH. Biologics targeting type I interferons in SLE: a meta-analysis and systematic review of randomised controlled trials. Lupus. 2020;29:1845–1853.
- Lambers WM, Westra J, Bootsma H, et al. From incomplete to complete systemic lupus erythematosus; A review of the predictive serological immune markers. Semin Arthritis Rheum. 2021;51:43–48.
- James J, Kim-Howard X, Bruner B, et al. Hydroxychloroquine sulfate treatment is associated with later onset of systemic lupus erythematosus. Lupus. 2007;16:401–409.
- Lambers WM, Westra J, Bootsma H, et al. Hydroxychloroquine suppresses interferon-inducible genes and B cell activating factor in patients with incomplete and new-onset systemic lupus erythematosus. J Rheumatol. 2020:jrheum.200726. DOI:10.3899/jrheum.200726
- Olsen N Study of anti-malarials in incomplete lupus erythematosus [Internet]. clinicaltrials.gov; 2020 [cited 2021 Mar 18]. Report No.: NCT03030118. Available from: https://clinicaltrials.gov/ct2/show/NCT03030118.
- Niewold TB, Kelly JA, Kariuki SN, et al. IRF5 haplotypes demonstrate diverse serological associations which predict serum interferon alpha activity and explain the majority of the genetic association with systemic lupus erythematosus. Ann Rheum Dis. 2012;71:463–468.
- Niewold TB, Hua J, Lehman TJA, et al. High serum IFN-alpha activity is a heritable risk factor for systemic lupus erythematosus. Genes Immun. 2007;8:492–502.
- Niewold TB, Adler JE, Glenn SB, et al. Age- and sex-related patterns of serum interferon-α activity in lupus families. Arthritis Rheum. 2008;58:2113–2119.
- Dong G, Fan H, Yang Y, et al. 17β-Estradiol enhances the activation of IFN-α signaling in B cells by down-regulating the expression of let-7e-5p, miR-98-5p and miR-145a-5p that target IKKε. Biochim Biophys Acta BBA - Mol Basis Dis. 2015;1852:1585–1598.
- Munroe ME, Lu R, Zhao YD, et al. Altered type II interferon precedes autoantibody accrual and elevated type I interferon activity prior to systemic lupus erythematosus classification. Ann Rheum Dis. 2016;75:2014–2021.
- Md Yusof MY, Psarras A, El-Sherbiny YM, et al. Prediction of autoimmune connective tissue disease in an at-risk cohort: prognostic value of a novel two-score system for interferon status. Ann Rheum Dis. 2018;77:1432–1439.
- Lambers WM, De Leeuw K, Doornbos-van Der Meer B, et al. Interferon score is increased in incomplete systemic lupus erythematosus and correlates with myxovirus-resistance protein A in blood and skin. Arthritis Res Ther. 2019;21:260.
- Li Q-Z, Zhou J, Lian Y, et al. Interferon signature gene expression is correlated with autoantibody profiles in patients with incomplete lupus syndromes. Clin Exp Immunol. 2010;159:281–291.
- Psarras A, Alase A, Antanaviciute A, et al. Functionally impaired plasmacytoid dendritic cells and non-haematopoietic sources of type I interferon characterize human autoimmunity. Nat Commun. 2020;11:6149.
- Karonitsch T, Feierl E, Steiner CW, et al. Activation of the interferon-γ signaling pathway in systemic lupus erythematosus peripheral blood mononuclear cells. Arthritis Rheum. 2009;60(5):1463–1471.
- Kim K, Cho S-K, Sestak A, et al. Interferon-gamma gene polymorphisms associated with susceptibility to systemic lupus erythematosus. Ann Rheum Dis. 2010;69:1247–1250.
- Nakashima H, Inoue H, Akahoshi M, et al. The combination of polymorphisms within interferon-γ receptor 1 and receptor 2 associated with the risk of systemic lupus erythematosus. FEBS Lett. 1999;453:187–190.
- Goel RR, Wang X, O’Neil LJ, et al. Interferon lambda promotes immune dysregulation and tissue inflammation in TLR7-induced lupus. Proc Natl Acad Sci. 2020;117:5409–5419.
- Zickert A, Oke V, Parodis I, et al. Interferon (IFN)-λ is a potential mediator in lupus nephritis. Lupus Sci Med. 2016;3:e000170.
