1. Introduction
Mounting evidence indicates that peripheral tissues and organs have inherent regulatory activity and impact on the host’s immune response; hence, they are not passive targets of inflammatory reactions. Indeed, tissue-derived homeostatic molecules can regulate the accumulation and functional plasticity of immune cells in order to maintain or restore tissue integrity [Citation1,Citation2]. This concept, which is integral to understanding tissue-specific immunity in steady-state and pathologies, is illustrated by research on the function of developmental endothelial locus-1 (DEL-1), a molecule that is secreted by tissue-resident cells (e.g., endothelial and mesenchymal stromal cells, and some macrophage subsets) [Citation2]. DEL-1 is 52-kDa protein comprising three epidermal growth factor (EGF)-like repeats at the N-terminus and two discoidin I-like domains at the C-terminus, and is encoded by the EDIL3 (EGF-like repeats and discoidin I-like domains-3) gene (). DEL-1 has the capacity to interact with distinct integrins, including β2 (e.g., αLβ2 and αMβ2) and αv (e.g., αvβ3) integrins, as well as phospholipids [Citation2]. These interactions in turn enable DEL-1 to regulate important immune functions that have significant impact in inflammatory and autoimmune disorders in preclinical models [Citation2], raising the possibility that DEL-1 may be a promising therapeutic target. DEL-1 is structurally similar with another integrin-binding protein, milk-fat-globule-EGF factor 8; although the two proteins share some functions, they are typically expressed by distinct cell subsets and regulated by different transcription factors, suggesting that they may often exert non-redundant biological activities [Citation2].
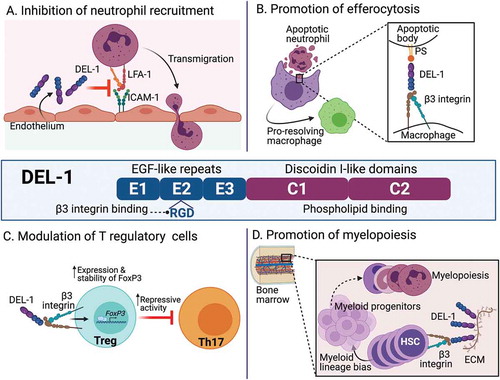
Figure 1. Structure and biological activities of DEL-1. Shown are the multi-domain structure of DEL-1 (central panel) as well as four major regulatory activities of this protein which impact innate and adaptive immune cell function (panels a–d, as indicated). For details, see text.The binding site of DEL-1 for LFA-1 is currently uncertain and was not indicated, although the E3 repeat is required for this interaction. DEL-1, Developmental endothelial locus-1; ECM, extracellular matrix; EGF, epidermal growth factor; FOXP3, forkhead box P3; HSC, hematopoietic stem cell; ICAM-1, intercellular adhesion molecule-1; LFA-1, lymphocyte function-associated antigen-1; PS, phosphatidyl serine; Th17, T helper 17 cell; Treg, T regulatory cell
2. Initiation of inflammation
By binding to αLβ2 integrin (aka LFA-1; lymphocyte function-associated antigen-1) on circulating leukocytes, endothelial cell-secreted DEL-1 inhibits the interaction of LFA-1 with intercellular adhesion molecule-1 (ICAM-1) on the vascular endothelium, thereby limiting firm adhesion and subsequent transendothelial migration of leukocytes, such as neutrophils, to inflamed sites [Citation3] ()). Consequently, DEL-1-deficient mice are susceptible to inflammatory pathologies, including the oral inflammatory disease periodontitis [Citation4,Citation5], experimental autoimmune encephalomyelitis (EAE; modeling multiple sclerosis) [Citation6], lung inflammatory pathology and fibrosis [Citation3,Citation7], and allergic asthma [Citation8]. Many of these disorders are driven by IL-17-dependent inflammation. Indeed, the increased susceptibility to periodontitis or EAE in DEL-1 deficiency is counteracted in mice that are deficient in both DEL-1 and the IL-17 receptor [Citation4,Citation6].
In this respect, DEL-1 and IL-17 not only promote opposing functions with regard to neutrophil infiltration and regulation of inflammation but also display a reciprocal regulation pattern [Citation9,Citation10]. This cross-regulation between IL-17 and DEL-1 can potentially maintain a balance between the beneficial and damaging consequences of neutrophil recruitment, hence contributing to immune homeostasis and health [Citation2]. However, in inflammatory and autoimmune disorders, the balance is skewed toward IL-17, which directly reduces DEL-1 expression and thus potentiates inflammation including neutrophil recruitment. This antagonistic relationship between DEL-1 and IL-17 may also be implicated in COVID-19-related and especially Kawasaki disease-related hyperinflammation [Citation11]. Autoantibodies to DEL-1, which are particularly highly abundant in Kawasaki disease, are associated with IL-17-mediated hyperinflammation [Citation11], suggesting that DEL-1 function may be humorally neutralized, thereby leading to unrestrained IL-17 actions.
3. Resolution of inflammation
DEL-1 is upregulated during inflammation resolution, in both mice and humans, and plays a proactive role in promoting resolution [Citation12]. The pro-resolving function of DEL-1 is attributed to its ability to facilitate efferocytosis, that is, the phagocytosis of dying cells, for example, neutrophils, by macrophages. Mechanistically, DEL-1 interacts, through its discoidin-I-like domains, with the ‘eat-me’ signal phosphatidylserine localized on the surface of apoptotic cells and, via its RGD motif in the second EGF-like repeat, with the αvβ3 integrin receptor on macrophages. In essence, therefore, DEL-1 acts as a molecular ‘bridge’ promoting efferocytosis ()). DEL-1-mediated efferocytosis in turn reprograms the macrophage to a pro-resolution phenotype, which requires liver X receptor (LXR)-dependent signaling [Citation12]. DEL-1-deficient mice fail to resolve inflammation in different settings, including experimental periodontitis, where they also fail to regenerate bone, unless they are treated locally with recombinant DEL-1 [Citation13], which also protects non-human primates against periodontitis [Citation14]. Importantly, DEL-1 promotes bone regeneration in a manner that does not depend solely on its pro-efferocytic activity. This is because a truncated version of DEL-1, specifically the N-terminal EGF-repeat-containing segment of DEL-1 that lacks the phosphatidylserine-interacting domains and is, therefore, incapable of mediating efferocytosis, is sufficient to induce bone regeneration during inflammation resolution [Citation13]. In contrast, DEL-1-mediated efferocytosis requires full-length DEL-1 [Citation12]. Mechanistically, DEL-1 induces osteogenic differentiation in osteoblast progenitors by activating, via its RGD motif, an αvβ3–FAK (focal adhesion kinase)–ERK1/2 (extracellular signal-regulated kinase1/2)–RUNX2 (Runt-related transcription factor-2) signaling pathway [Citation13].
During inflammation resolution, moreover, DEL-1 enhances the abundance and function of T regulatory (Treg) cells, while diminishing the numbers of T helper 17 (Th17) cells, a major cellular source of IL-17 in inflammatory and autoimmune diseases [Citation9]. Mechanistically, DEL-1 does not directly inhibit Th17 differentiation but rather enhances the induction, stability, and suppressive activity of Treg cells ()). The underlying mechanism involves the capacity of DEL-1 to interact with αvβ3 integrin on T cells and stably upregulate the expression of forkhead box P3 (FOXP3), which was mediated by the Runt-related transcription factor-1 (RUNX1). Similarly, this activity of DEL-1 requires an intact RGD site but not the whole molecule, since truncated DEL-1 containing only the N-terminal EGF-like repeats is adequate to upregulate FOXP3 expression [Citation9]. These findings suggest that DEL-1-based therapies may not necessarily use the entire molecule but selected segments thereof.
4. Myelopoiesis
Emergency or demand-adapted myelopoiesis to replenish myeloid cells constitutes a critical homeostatic process in immunity [Citation15]. DEL-1 is an important component of the bone marrow niche for hematopoietic stem cells (HSCs) and contributes to emergency myelopoiesis [Citation16]. Arteriolar endothelial cells, osteoblastic lineage cells and particularly CXCL12-abundant reticular cells (a specialized type of mesenchymal stromal cells with perivascular location) produce DEL-1, which binds to β3 integrin on HSCs and promotes their proliferation and preferential differentiation toward myeloid cells ()). The crucial response of the bone marrow toward enhanced myelopoiesis under stressful settings fails in mice lacking DEL-1 [Citation16]; in contrast, mice overexpressing DEL-1 in the vascular endothelium displayed an elevated pool of functional long-term HSCs with augmented capacity for myeloid-lineage regeneration [Citation17]. Overall, therefore, DEL-1 regulates not only the recruitment and disposal of neutrophils (when they undergo apoptotic cell death) in peripheral tissues, but also their generation in the bone marrow. The fact that DEL-1 regulates both central and peripheral homeostatic mechanisms further supports its importance as a potential target for novel therapies in periodontitis, multiple sclerosis and other inflammatory or autoimmune disorders.
5. Importance of the location of DEL-1 expression
Studies utilizing different transgenic mice with cell-specific overexpression of DEL-1 demonstrated that the cellular source of DEL-1 critically determines its regulatory functions. Specifically, the anti-leukocyte-recruitment action, and hence function to counteract inflammation initiation, is associated with endothelial cell-derived DEL-1, whereas the efferocytic/pro-resolving action is attributed to macrophage-derived DEL-1 [Citation12]. Interestingly, in this context, the diffusion of secreted DEL-1 is likely restricted since the molecule has a strong propensity to associate with the surface of cells or the extracellular matrix surrounding DEL-1-secreting cells [Citation2]. These findings point to a new tissue homeostatic concept regarding the spatial regulation of the immune response. According to the so-coined ‘location principle’, DEL-1, and perhaps other secreted homeostatic molecules, can mediate distinct regulatory functions that depend on the location of their expression. In other words, functional versatility is facilitated by tissue- and cell-specific compartmentalized expression.
6. Conclusions and therapeutic implications
The above-discussed studies collectively indicate that DEL-1 regulates the host inflammatory response both centrally and peripherally. In peripheral tissues and organs, DEL-1 mediates regulatory effects through actions on mature myeloid (neutrophils, macrophages) and other cell types. In the bone marrow, DEL-1 functions as a non-redundant regulatory constituent of the HSC niche that guides emergency myelopoiesis and contributes to the replenishment of mature myeloid cells in inflammation [Citation16]. However, the expression of DEL-1 is severely diminished under inflammatory conditions (owing to downregulation by IL–17 and certain other inflammatory cytokines, e.g., TNF) and with advanced age in both mice and humans [Citation4,Citation18–20]. Therefore, in the context of age-related chronic inflammatory and/or autoimmune diseases, novel therapeutic approaches to reconstitute the levels of DEL-1 could restore the homeostatic functions attributed to this regulatory molecule. The administration and/or replacement of endogenous factors, such as DEL-1, may entail reduced adverse effects than the use of biologics. Therefore, DEL-1, or specific segments thereof, merit further investigation as potential therapeutics for the treatment of inflammatory or autoimmune disorders. In this regard, EDIL3, the DEL-1-encoding gene, is a susceptibility locus in IL-17-related diseases, like ankylosing spondylitis [Citation21] and multiple sclerosis [Citation22]. With regard to periodontitis and other inflammatory osteolytic disorders, exogenous DEL-1 administration may also restore bone lost due to these disorders. Another approach would be to increase endogenous DEL-1 protein levels through administration of positive regulators of DEL-1 expression, such as the steroid hormone dehydroepiandrosterone [Citation23], the macrolide antibiotic erythromycin, or modified versions thereof retaining DEL-1 modulatory activity but lacking antibiotic action [Citation24]. Moreover, given its role in supporting myelopoiesis, DEL-1 could additionally be harnessed in therapeutic bone marrow transplantation or in hematological disorders, e.g., involving cytopenias.
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Acknowledgments
The figure was created using Biorender.com.
Additional information
Funding
References
- Galli SJ, Borregaard N, Wynn TA. Phenotypic and functional plasticity of cells of innate immunity: macrophages, mast cells and neutrophils. Nat Immunol. 2011;12:1035–1044.
- Hajishengallis G, Chavakis T. DEL-1-regulated immune plasticity and inflammatory disorders. Trends Mol Med. 2019;25:444–459.
- Choi EY, Chavakis E, Czabanka MA, et al. Del-1, an endogenous leukocyte-endothelial adhesion inhibitor, limits inflammatory cell recruitment. Science. 2008;322:1101–1104.
- Eskan MA, Jotwani R, Abe T, et al. The leukocyte integrin antagonist Del-1 inhibits IL-17-mediated inflammatory bone loss. Nat Immunol. 2012;13:465–473.
- Hajishengallis G, Chavakis T. Local and systemic mechanisms linking periodontal disease and inflammatory comorbidities. Nat Rev Immunol. 2021. Online ahead of print. DOI:10.1038/s41577-020-00488-6
- Choi EY, Lim JH, Neuwirth A, et al. Developmental endothelial locus-1 is a homeostatic factor in the central nervous system limiting neuroinflammation and demyelination. Mol Psychiatry. 2015;20:880–888.
- Kim DY, Lee SH, Fu Y, et al. Del-1, an endogenous inhibitor of TGF-beta activation, attenuates fibrosis. Front Immunol. 2020;11:68.
- Yan S, Chen L, Zhao Q, et al. Developmental endothelial locus-1 (Del-1) antagonizes interleukin-17-mediated allergic asthma. Immunol Cell Biol. 2018;96:526–535.
- Li X, Colamatteo A, Kalafati L, et al. The DEL-1/β3 integrin axis promotes regulatory T cell responses during inflammation resolution. J Clin Invest. 2020;130:6261–6277.
- Maekawa T, Hosur K, Abe T, et al. Antagonistic effects of IL-17 and D-resolvins on endothelial Del-1 expression through a GSK-3beta-C/EBPbeta pathway. Nat Commun. 2015;6:8272.
- Consiglio CR, Cotugno N, Sardh F, et al. The immunology of multisystem inflammatory syndrome in children with COVID-19. Cell. 2020;183:968–81.e7.
- Kourtzelis I, Li X, Mitroulis I, et al. DEL-1 promotes macrophage efferocytosis and clearance of inflammation. Nat Immunol. 2019;20:40–49.
- Yuh DY, Maekawa T, Li X, et al. The secreted protein DEL-1 activates a β3 integrin-FAK-ERK1/2-RUNX2 pathway and promotes osteogenic differentiation and bone regeneration. J Biol Chem. 2020;295:7261–7273.
- Shin J, Maekawa T, Abe T, et al. DEL-1 restrains osteoclastogenesis and inhibits inflammatory bone loss in nonhuman primates. Sci Transl Med. 2015;7:307ra155.
- Chavakis T, Mitroulis I, Hajishengallis G. Hematopoietic progenitor cells as integrative hubs for adaptation to and fine-tuning of inflammation. Nat Immunol. 2019;20:802–811.
- Mitroulis I, Chen L-S, Singh RP, et al. Secreted protein Del-1 regulates myelopoiesis in the hematopoietic stem cell niche. J Clin Invest. 2017;127:3624–3639.
- Chen LS, Kourtzelis I, Singh RP, et al. Endothelial cell-specific overexpression of Del-1 drives expansion of haematopoietic progenitor cells in the bone marrow. Thromb Haemost. 2018;118:613–616.
- Saxena S, Venugopal R, Chandrayan Rao R, et al. Association of chronic periodontitis and type 2 diabetes mellitus with salivary Del-1 and IL-17 levels. J Oral Biol Craniofac Res. 2020;10:529–534.
- Folwaczny M, Karnesi E, Berger T, et al. Clinical association between chronic periodontitis and the leukocyte extravasation inhibitors developmental endothelial locus-1 and pentraxin-3. Eur J Oral Sci. 2017;125:258–264.
- Inonu E, Kayis SA, Eskan MA, et al. Salivary Del-1, IL-17, and LFA-1 levels in periodontal health and disease. J Periodontal Res. 2020;55:511–518.
- Lin Z, Bei JX, Shen M, et al. A genome-wide association study in Han Chinese identifies new susceptibility loci for ankylosing spondylitis. Nat Genet. 2012;44:73–77.
- Goris A, Sawcer S, Vandenbroeck K, et al. New candidate loci for multiple sclerosis susceptibility revealed by a whole genome association screen in a Belgian population. J Neuroimmunol. 2003;143:65–69.
- Ziogas A, Maekawa T, Wiessner JR, et al. DHEA inhibits leukocyte recruitment through regulation of the integrin antagonist DEL-1. J Immunol. 2020;204:1214–1224.
- Maekawa T, Tamura H, Domon H, et al. Erythromycin inhibits neutrophilic inflammation and mucosal disease by upregulating DEL-1. JCI Insight. 2020;5:e136706.
