ABSTRACT
Background: The 29th EADV Virtual Congress took place between the 29th-31st of October 2020. On October 29th, there was a Session on systemic treatment in which Professors Ulrich Mrowietz and Mar Llamas-Velasco presented the latest research on the efficacy of dimethyl fumarate (DMF) treatment for moderate-to-severe plaque psoriasis (BRIDGE and DIMESKIN 1 studies, respectively). The accepted DMF abstract from Professor Matthias Augustin, on the SKILL study, is also presented here. Results: Data from either prospective interventional (BRIDGE) or non-interventional (DIMESKIN 1, SKILL) studies among patients with moderate-to-severe psoriasis showed that DMF provides a positive efficacy profile in all four body regions included in the Psoriasis Area and Severity Index assessment (head and neck, trunk, upper and lower extremities) and a particularly interesting profile (strong efficacy) in the head and neck region. These findings may be of special interest to patients with scalp psoriasis who have been using topical therapies for a long time. Patient-reported outcomes (quality of life, pruritus) also improved during the 24 weeks of DMF treatment. The safety profile of DMF was similar to the previously described with fumaric acid esters. Conclusions: In summary, these results confirm the favorable efficacy and safety profile of DMF in long-term treatment.
1. Introduction
Several systemic medications are recommended for the treatment of moderate-to-severe psoriasis. Although biologic agents include the most effective therapies for moderate-to-severe psoriasis[Citation1], their use may be limited by safety concerns and costs[Citation2], evidencing unmet treatment needs in the care of psoriasis patients. Thus, novel therapies, such as oral small-molecule drugs, are also being investigated for the treatment of psoriasis[Citation3].
Fumaric acid esters (FAEs) are small molecules with anti-inflammatory and immune-modulatory effects[Citation4]. They were approved and extensively used in Germany as a first-line treatment of psoriasis since 1994[Citation5]. FAEs are a mixture of dimethyl fumarate (DMF) together with calcium, magnesium, and zinc salts of monoethyl fumarate, with DMF being the main pharmacologically active compound[Citation6]. An oral formulation containing DMF alone was approved in 2017 by the European Medicines Agency for the treatment of moderate-to-severe plaque psoriasis in adults [Citation7,Citation8]. The advantages of using DMF over FAEs are the reduced costs while maintaining flexible administration[Citation9]. Moreover, Falkvoll et al [Citation9]. showed that patients positively switched from the FAE mixture to the same dose of DMF, observing no particular differences and appreciating that recommended intervals between checkups were longer with DMF compared to the FAE mix. Available clinical data indicated that DMF was an effective and safe oral treatment option for patients with moderate-to-severe psoriasis with the maximum efficacy usually seen after 24 weeks of treatment [Citation7,Citation10].
Here, we aim to bring together data on the efficacy of DMF treatment for moderate-to-severe psoriasis presented at the 29th EADV Virtual Congress from three clinical studies in patients with moderate-to-severe plaque psoriasis (). All studies were approved by ethical committees in accordance with local legislation and regulations.
Table 1. Studies on DMF included
2. PRESENTATION EXTRACT 1
2.1. Efficacy of dimethyl fumarate per body region and per sign among patients with moderate-to-severe plaque psoriasis: post-hoc analysis through 16 weeks from the BRIDGE phase III trial
Ulrich Mrowietz, Peter Van de Kerkhof, Andreu Schoenenberger, Anna Ryzhkova, Ignasi Pau-Charles.
2.2. Objective
The BRIDGE phase III trial (NCT01726933) [Citation10] was designed to investigate the short-term efficacy and safety of dimethyl fumarate (DMF) for the treatment of adults with moderate-to-severe chronic plaque psoriasis. The objective of this post-hoc analysis was to assess the efficacy of DMF by body region in patients with moderate-to-severe plaque psoriasis from the BRIDGE trial.
2.3. Methods
This was a post-hoc analysis from the multicenter, randomized, three‐arm, double‐blind, placebo and comparator-controlled BRIDGE phase III trial in adults with moderate‐to‐severe plaque psoriasis. Patients were randomized 2:2:1 to receive DMF, active comparator (Fumaderm®, a fixed combination of fumaric acid esters) or placebo during a 16‐week treatment phase.
Efficacy analyses were performed for the full analysis set (FAS), which consisted of all patients who were randomized and received at least one dose of investigational medicinal product, and with at least one post-baseline Psoriasis Area and Severity Index (PASI) value. Efficacy was assessed based on the regional PASI scores, which were calculated for head and neck, trunk, upper extremities, and lower extremities as the sum of the degree of severity of erythema, induration and desquamation, multiplied by the estimated body surface area (BSA) affected. Each of the signs was rated in a 5-level scale of severity, ranging from 0 (no sign) to 4 (very marked). BSA involved was rated in a 7-level scale ranging from 0 (none) to 6 (90–100%). Mean percent improvement in regional PASI from baseline and percentage of patients achieving a severity level of 0 or 1 in signs of psoriasis (i.e., no or slight signs) per body region at week 16 were assessed. The t-test was used to compare paired data from baseline to that of 16 weeks. Patients with PASI = 0 at baseline for the body region were excluded from the assessments. Missing data were not imputed.
2.4. Results
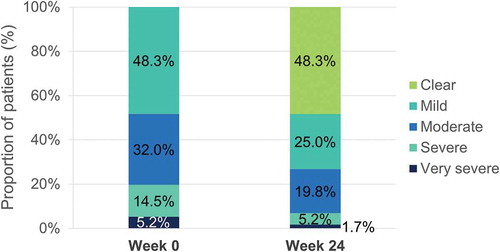
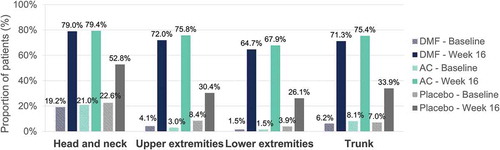
Baseline characteristics of patients have been published previously[Citation10]. A total of 671 patients were included in the FAS, from which 617, 646, 665, and 667 were assessed for head and neck, trunk, lower extremities and upper extremities signs, respectively. At week 16, mean regional PASI for patients receiving DMF was improved at all body regions compared with patients receiving placebo, with numerically higher mean percentage improvements in regional PASI with DMF in the head and neck (69.2%) and upper extremities (68.4%), followed by lower extremities (65.8%) and trunk (62.2%) ().
Table 2. Mean percentage improvements in regional PASI at week 16
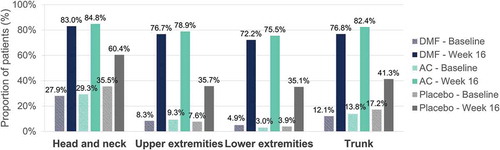
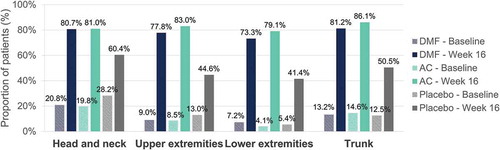
Percentage of patients achieving a severity level of 0 or 1 in erythema, induration and desquamation per body region at baseline and at week 16 are shown in , , and , respectively. Regarding head and neck assessments at week 16, 79.0%/79.4%/52.8%, 83.0%/84.8%/60.4% and 80.7%/81.0%/60.4% of patients in the DMF/active comparator/placebo groups achieved a severity level of 0 or 1 in erythema (), induration (), and desquamation (), respectively. Similar results were obtained regarding trunk, lower and upper extremities assessments (, , and ).
Figure 1. Erythema – Percentage of patients with a severity level of 0–1 (no or slight signs) at baseline and at week 16 per body region
2.5. Conclusions
In this post-hoc analysis from the BRIDGE trial, the efficacy of DMF was consistent across the different body regions assessed in the PASI score among patients with moderate-to-severe plaque psoriasis. Up to week 16, DMF provided a comparable efficacy profile to the active comparator in all body regions and a particularly interesting profile in the head and neck, with most of patients achieving no or slight signs in this body region. The strongest positive effect observed in the head and neck region would be of particular importance as involvement in this visible region have a major effect on the quality of life of the patients.
3. PRESENTATION EXTRACT 2
3.1. Analysis of efficacy per body region among patients with moderate-to-severe plaque psoriasis treated with dimethyl fumarate in clinical practice: interim analysis through 24 weeks from the DIMESKIN 1 study
Mar Llamas-Velasco, Esteban Daudén, José Manuel Carrascosa, Pablo de la Cueva, Laura Salgado-Boquete, Meritxell Guilà, Francisco Javier Fernández-Soriano.
3.2. Objective
The objective of this analysis was to report the efficacy of dimethyl fumarate (DMF) by body region in patients with moderate-to-severe plaque psoriasis through 24 weeks of DMF treatment in the DIMESKIN 1 study, designed to simulate conditions close to routine clinical practice.
3.3. Methods
This was an interim analysis at week 24 from a 52-week open-label clinical trial (DIMESKIN 1 study, EudraCT number 2017–001368-40) in adult patients with moderate-to-severe plaque psoriasis.
Efficacy analyses were performed for the intention-to-treat (ITT) population, which consisted of all patients aged ≥18 years with moderate-to-severe chronic plaque psoriasis, treated with at least one dose of DMF as per its Summary of Product Characteristics, and with at least one post-baseline Psoriasis Area and Severity Index (PASI) value. Efficacy of DMF was assessed based on the regional PASI scores, which were calculated for head and neck, trunk, upper extremities, and lower extremities as the sum of the degree of severity of erythema, induration and desquamation, multiplied by the estimated body surface area (BSA) affected. Each of the signs was rated in a 5-level scale of severity, ranging from 0 (no sign) to 4 (very marked). BSA involved was rated in a 7-level scale ranging from 0 (none) to 6 (90–100%). Mean percent improvement in regional PASI from baseline and percentage of patients achieving a severity level of 0 or 1 in signs of psoriasis (i.e., no or slight signs) per body region at week 24 were assessed. Patients with PASI = 0 at baseline for the body region were excluded from the assessments. Presented analysis was based on observed cases.
3.4. Results
A total of 175 patients were included in the ITT population. Baseline characteristics are shown in .
Table 3. Baseline characteristics
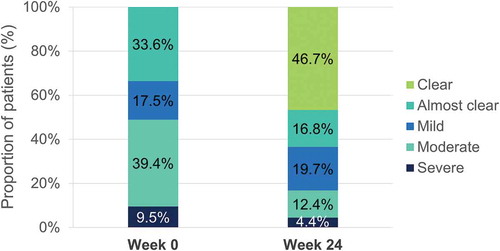
After 24 weeks of treatment with DMF, mean regional PASI was improved in all body regions, with numerically higher mean percentage improvements in regional PASI in the head and neck (78.9%), followed by trunk (73.5%), lower extremities (72.7%) and upper extremities (66.8%) ().
Table 4. Mean percentage improvements in regional PASI at week 24
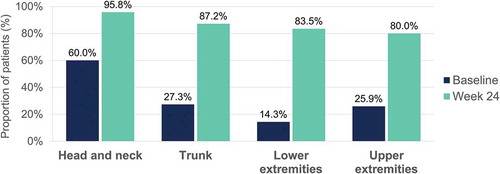
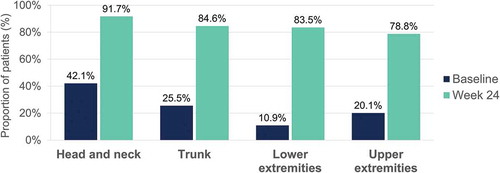
Percentage of patients achieving a severity level of 0 or 1 in erythema, induration and desquamation per body region at baseline and at week 24 are shown in , , and , respectively. Regarding head and neck assessments at week 24, 91.7%, 95.8%, and 91.7% of patients achieved a severity level of 0 or 1 in erythema (), induration (), and desquamation (), respectively. Percentage of patients with no or slight signs at week 24 was also high for the other locations, with similar results regarding trunk, lower and upper extremities assessments (, , and ).
Figure 4. Erythema – Percentage of patients with a severity level of 0–1 (no or slight signs) at baseline and at week 24 per body region
3.5. Conclusions
The results of this interim analysis suggested that DMF was an effective treatment for the four body regions and the three different signs among patients with moderate-to-severe plaque psoriasis in clinical practice, with the strongest effect observed in the head and neck region.
4. PRESENTATION EXTRACT 3
4.1. Efficacy of dimethyl fumarate in clinical practice among patients with moderate plaque psoriasis: interim analysis through 24 weeks from DIMESKIN 1 study
Mar Llamas-Velasco, Esteban Daudén, José Manuel Carrascosa, Pablo de la Cueva, Laura Salgado-Boquete, Meritxell Guilà, Francisco Javier Fernández-Soriano.
4.2. Objective
The objective of this analysis was to report the efficacy of dimethyl fumarate (DMF) in patients with moderate plaque psoriasis through 24 weeks of DMF treatment in the DIMESKIN 1 study, designed to simulate conditions close to routine clinical practice.
4.3. Methods
This was an interim analysis at week 24 from a 52-week open-label clinical trial (DIMESKIN 1 study, EudraCT number 2017–001368-40) in adult patients with moderate-to-severe plaque psoriasis.
Efficacy analyses were performed for the subgroup of patients with moderate psoriasis from the intention-to-treat (ITT) population. ITT population consisted of all patients aged ≥18 years with moderate-to-severe chronic plaque psoriasis, treated with at least one dose of DMF as per its Summary of Product Characteristics, and with at least one post-baseline Psoriasis Area and Severity Index (PASI) value. Moderate psoriasis was defined according to PASI and Dermatology Life Quality Index (DLQI) thresholds proposed by Llamas-Velasco et al [Citation11].: DLQI <5 and absolute PASI 7–15 or DLQI ≥5 and absolute PASI ≤15. The Wilcoxon test was used to compare paired data from baseline to that of 24 weeks. Presented analysis was based on observed cases (OC) and last-observation-carried-forward (LOCF).
4.4. Results
A total of 130 adult patients with moderate psoriasis at baseline were included. Baseline characteristics are shown in .
Table 5. Baseline characteristics
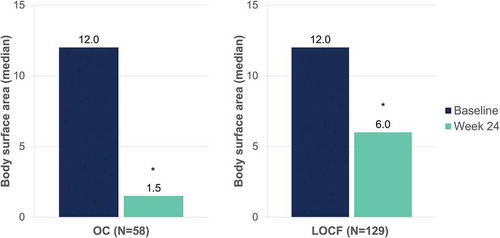
After 24 weeks of DMF treatment, median absolute PASI decreased from 10.8 to 1.8 (OC) and from 10.6 to 5.3 (LOCF) (p < 0.001, both) (). Median body surface area affected decreased from 12.0 to 1.5 (OC) and from 12.0 to 6.0 (LOCF) after 24 weeks of DMF treatment (p < 0.001, both) ().
Figure 7. Evolution of median absolute PASI after 24 weeks of dimethyl fumarate treatment
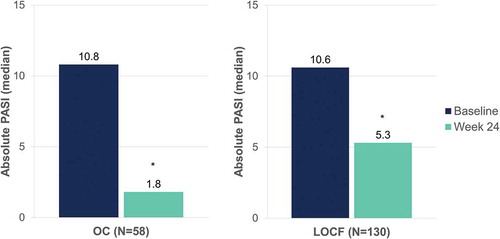
Figure 8. Evolution of median body surface area affected after 24 weeks of dimethyl fumarate treatment
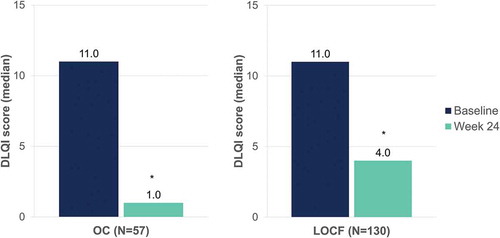
Median pruritus visual analogue scale scores decreased from 7.0 to 1.0 (OC) and from 7.0 to 4.0 (LOCF) after 24 weeks of DMF treatment (p < 0.001, both) (). Median DLQI scores decreased from 11.0 to 1.0 (OC) and from 11.0 to 4.0 (LOCF) at week 24 (p < 0.001, both) ().
Figure 9. Evolution of median pruritus VAS scores after 24 weeks of dimethyl fumarate treatment
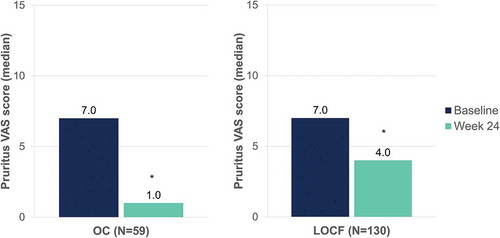
Figure 10. Evolution of median DLQI scores after 24 weeks of dimethyl fumarate treatment
The safety profile of DMF was similar to that previously described with fumarates.
4.5. Conclusions
Preliminary data through 24 weeks suggested that DMF significantly improves psoriasis severity as well as psoriasis’ related patient-reported outcomes such as quality of life and pruritus in patients with moderate psoriasis.
5. PRESENTATION EXTRACT 4
5.1. Interim analysis of the non-interventional study SKILL: Dimethyl fumarate (DMF) as long-term treatment for moderate-to-severe plaque psoriasis and its impact on sensitive areas affected by psoriasis
Matthias Augustin, Christine Loew-Juettner, Astrid Kirsch, Sebastian Diemert, Ina Hadshiew.
5.2. Objective
The 24-month, prospective, multicenter, non-interventional SKILL study was designed to assess the effectiveness and safety during long-term dimethyl fumarate (DMF) treatment in routine clinical practice in Germany. This interim analysis at week 24 focused on the impact of DMF on sensitive areas affected by psoriasis in adult patients with moderate-to-severe plaque psoriasis.
5.3. Methods
This was an interim analysis at week 24 from the SKILL study. The treating physician took the decision for treatment with DMF independently and prior to patient enrollment in the study.
Parameters assessed included absolute PASI scores <3 and <5, scalp-, nail- and palmoplantar-Physician’s Global Assessment (PGA) 0 or 1 (clear or almost clear), itch (Numeric Rating Scale [NRS] ranging from 0 [no itch] to 10 [maximum itch]) <3 (no or minor itch)[Citation12], treatment satisfaction (4-level scale, ranging from very good to poor), and adverse events (AEs). Medical Dictionary for Regulatory Activities (version 22.0) preferred terms were assigned to AEs. Efficacy analyses were based on observed cases (OC) and last-observation-carried-forward (LOCF). Safety analyses were conducted on all of the DMF-treated patients.
5.4. Results
Overall, a total of 673 patients were included in SKILL. For this interim analysis data at week 24 was available for 343 patients, 28% of patients (n = 187) had dropped out by that time. Baseline characteristics are shown in .
Table 6. Baseline characteristics
The average maintenance dose of DMF at week 24 was 313 mg/day with approximately 70% of patients receiving a dose between 120 and 480 mg of DMF per day.
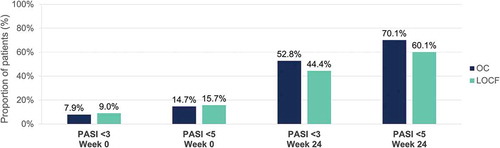
At week 24, PASI <3 was reported in 52.8% (OC) and 44.4% (LOCF), and PASI <5 in 70.1% (OC) and 60.1% of patients (LOCF), respectively ().
Figure 11. Evolution of absolute PASI <3 and <5 after 24 weeks of dimethyl fumarate treatment
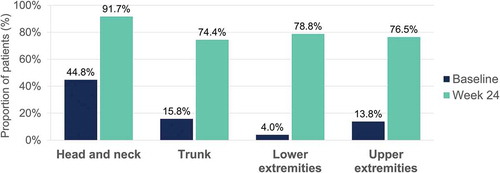
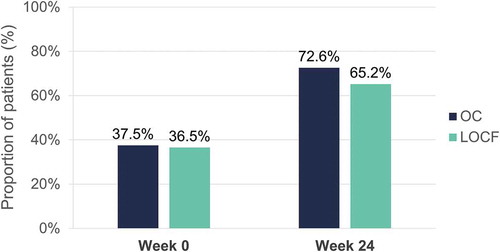
At week 24, scalp PGA 0 or 1 was reported by 72.6% (OC) and 65.2% (LOCF) of patients ().
Figure 12. Evolution of scalp PGA 0 or 1 (clear or almost clear) after 24 weeks of dimethyl fumarate treatment
In patients with nail psoriasis PGA >0 at baseline (n = 172), 65.7% experienced an improvement of nail psoriasis at week 24. In addition, 48.3% of patients reported a PGA 0 (clear) at week 24 (LOCF) ().
In patients with palmoplantar psoriasis PGA >0 at baseline (n = 137), 70.1% experienced an improvement of palmoplantar psoriasis at week 24. Additionally, 46.7% of patients reported a PGA 0 (clear) at week 24 (LOCF) ().
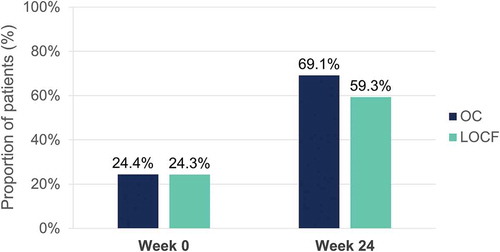
Mean itch at baseline was 5.0 (OC) and 4.5 (LOCF) on the NRS. At week 24, 69.1% (OC) and 59.3% (LOCF) of patients reported no or only minor itch (NRS <3) ().
Figure 15. Evolution of itch (NRS <3) after 24 weeks of dimethyl fumarate treatment
Treatment satisfaction was rated as good or very good by 91.2% of the treating physicians and by 86.1% of patients. Regarding safety, 432 AEs occurred in 33.4% of patients (n = 225). The most frequent AE was diarrhea occurring in 11.1% of patients ().
Table 7. Most frequent adverse events (>5 events)
5.5. Conclusions
This interim analysis confirmed the positive efficacy and favorable safety profile of DMF in prospective long-term treatment. Patients with disease manifestation in sensitive areas including scalp, nails, palms, and/or soles or itch showed a relevant improvement in response to DMF.
6. Discussion
The data presented herein describe four presentations displayed at the 29th EADV Congress related to the interventional BRIDGE study and the non-interventional DIMESKIN 1 and SKILL studies. These studies aimed at investigating the efficacy and safety profile of DMF treatment for moderate-to-severe plaque psoriasis.
The BRIDGE trial evaluated the short-term efficacy and safety of DMF for the treatment of adult patients with moderate-to-severe chronic plaque psoriasis[Citation10]. For 16 weeks, randomized patients receive DMF, FAE mixture, or placebo. In this post-hoc analysis, efficacy was assessed on the regional PASI scores. This analysis revealed that DMF successfully improved mean regional PASI in different body regions among patients with moderate-to-severe plaque psoriasis at week 16, with numerically higher mean percentage improvements in regional PASI in the head and neck area. Similar results were obtained with the interim analysis at week 24 of the open-label DIMESKIN 1 study. This analysis, aiming to report DMF efficacy by body region through 24 weeks, showed effectiveness on four body regions with numerically higher mean percentage improvements in regional PASI in the head and neck region and reduced severity of erythema, induration, and desquamation after 24 weeks. The second interim analysis at week 24, also conducted from the DIMESKIN 1 study, was shown in Presentation Extract 3. This analysis focused on the subgroup of patients with moderate psoriasis [Citation11] and showed the efficacy of DMF according to absolute PASI value, which significantly decreased after 24 weeks of treatment. Moreover, this analysis showed that DMF significantly reduced body surface area affected by psoriasis, pruritus score, and improved patients’ quality of life assessed by DLQI. Presentation Extract 4 showed the results from the interim analysis of the non-interventional SKILL study, designed to assess the effectiveness and safety of long-DMF treatment in routine clinical practice. Here, PASI score, scalp-, nail- and palmoplantar-PGA, and itch were reduced already after 24 weeks. In summary, these analyses endorse the long-term efficacy of DMF for the treatment of adult patients affected by moderate-to-severe plaque psoriasis. Dimethyl fumarate resulted in successful treatment for all four body regions (head and neck, trunk, upper and lower extremities), with a strong efficacy profile in the head and neck region. These findings may be of special interest to patients with scalp psoriasis who undergo prolonged discouraging topical therapies.
Treatment of psoriasis nowadays also includes the use of biologics that have demonstrated high efficacy for patients with moderate-to-severe psoriasis. Approved biologic agents for psoriasis treatment include tumor necrosis factor α inhibitors, interleukin (IL)-23 inhibitors, IL-12/23 inhibitors or IL-17 inhibitors[Citation13]. Safety concern about biologics is no exception as side effects such as serious infections, cardiovascular events, and malignancy were associated with their usage, mainly with first-generation biologic drugs[Citation14]. In this respect, DMF may be advantageous over biologic treatments, as FAEs are not associated with increased risk of infections or malignancies[Citation7]. In addition, there is no evidence of drug-drug interactions with FAEs as with other systemic treatments[Citation15]. Thus, psoriasis therapy should be individually based and consider comorbidities, concomitant medications, or contraindications[Citation16]. Licensed FAEs contain DMF, which has been identified as the main active component [Citation17,Citation18] exerting anti-inflammatory properties against several molecular targets including, HCA2 and Nrf2 pathways [Citation19,Citation20]. Whereby, DMF has resulted in a valuable treatment option for inflammatory diseases, including psoriasis[Citation21]. Besides therapeutic benefits, DMF side effects similar to the previously described with FAEs have been reported. These include flushing, lymphopenia, and gastrointestinal symptoms [Citation4,Citation22–24]. The SKILL study presented herein reported diarrhea as the most frequent AE occurring in 11% of patients, followed by lymphopenia (8%).
Limitations of this work arise as the data presented here derive from different study designs, including interventional and non-interventional studies. More limitations are represented by subgroup post-hoc analysis and interim analysis. Thus, results would have benefited of a prespecified statistical approach, final analysis, and higher statistical power. However, the results presented here strengthen the effectiveness of DMF for the treatment of moderate-to-severe psoriasis while reporting AEs related to DMF long-term use. In conclusion, these data support the therapeutic benefit of DMF alone against psoriasis and underline the efficacy and safety profile of long-term DMF treatment.
Reviewer disclosures
A peer reviewer co-authored one of the abstracts included in the manuscript and is collaborating with Almirall. Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Declaration of interest
AK, AR, IP-C, MG, FJF-S, and SD were employees of Almirall; AS served as a statistical consultant for Almirall; CL-J did not have relevant financial disclosure to declare; ED has the following conflict of interests: Advisory Board member, consultant, grants, research support, participation in clinical trials, honorarium for speaking, research support, with the following pharmaceutical companies: AbbVie/Abbott, Almirall, Amgen, Janssen-Cilag, Leo Pharma, Novartis, Pfizer, MSD-Schering-Plough, Celgene, Lilly, and UCB; IH did not have relevant financial disclosure to declare; JMC has participated as a PI/SI and/or invited speaker and/or advisor for Almirall, Gebro, Janssen, Amgen, AbbVie, Leo Pharma, Mylan, Sandoz, Novartis, and Lilly; LS-B has the following conflict of interests: Advisory Board member, consultant, grants, research support, participation in clinical trials, honorarium for speaking, research support, with the following pharmaceutical companies: AbbVie, Almirall, Janssen-Cilag, Leo Pharma, Novartis, Pfizer, MSD-Schering-Plough, Celgene, and Lilly; MA has served as a consultant and/or researcher and/or received research grants from: Abbvie, ALK-Abello, Almirall, Altrazeal, Amgen GmbH, Astellas, Bayer Healthcare, Beiersdorf, Biogen, Birken, Boehringer Ingelheim, BSN, Celgene, Centocor, Coloplast, Flen Pharma, Galderma, Gerromed, GlaxoSmithKline, Heigel.com, Janssen-Cilag, Johnson&Johnson, Leo, Lilly, Medac, Medi, Medovent, Mölnlycke, MSD, Novartis, Pfizer, Pharmafacts, Pohl-Boskamp, Sandoz, Sanofi-Aventis, Söring, Stallergenes, Stiefel, Systagenix Wound Management, Tissue therapies, Urgo, and Xenoport; ML-V has potential conflicts of interests (advisory board member, consultant, research support, participation in clinical trials and honorary for speaking) with the following pharmaceutical companies: AbbVie, Almirall, Amgen, Boehringer, Celgene, Janssen, Leo Pharma, Lilly, Novartis, and UCB, not related with the submitted work; PdlC has been a consultant and advisor and/or received speaking fees and/or grants and/or served as an investigator in clinical trials for the following companies: AbbVie, Almirall, Astellas, Biogen, Boehringer, Celgene, Janssen, Leo Pharma, Lilly, MSD, Novartis, Pfizer, Roche, Sanofi, and UCB; PvdK received fees for consultancy service or lecturerships from Celgene, Centocor, Allmirall, Amgen, Pfizer, Philips, Abbott, Eli Lilly, Galderma, Novartis, Jansen Pharmaceutica, Leo Pharma, Sandoz, Bristol Mayer Squib, and Dermavant; UM has been an advisor and/or received speaker honoraria and/or received grants and/or participated in clinical trials for Abbott, AbbVie, Almirall Hermal, Amgen, Biogen, Boehringer Ingelheim, Celgene, Centocor, Foamix, Forward Pharma, Galderma, Janssen, Leo Pharma, Lilly, Medac, Miltenyi Biotec, MSD, Novartis, Pfizer, Teva, UCB, BBL, and XenoPort. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Acknowledgments
The authors would like to thank E. Mateu, PhD from TFS S.L., for their writing and editorial assistance.
Additional information
Funding
References
- Armstrong AW, Puig L, Joshi A, et al. Comparison of biologics and oral treatments for plaque psoriasis: a Meta-analysis. JAMA Dermatol. 2020;156(3):258–269. .
- Van De Kerkhof PCM, Reich K, Kavanaugh A, et al. Physician perspectives in the management of psoriasis and psoriatic arthritis: results from the population-based multinational assessment of psoriasis and psoriatic arthritis survey. J Eur Acad Dermatol Venereol. 2015;29(10):2002–2010.
- Balogh EA, Bashyam AM, Ghamrawi RI, et al. Emerging systemic drugs in the treatment of plaque psoriasis. Expert Opin Emerg Drugs. 2020;25(2):89–100.
- Brück J, Dringen R, Amasuno A, et al. A review of the mechanisms of action of dimethylfumarate in the treatment of psoriasis. Exp Dermatol. 2018;27(6):611–624.
- Mrowietz U, Rostami-Yazdi M, Neureither M, et al. [15 years of fumaderm: fumaric acid esters for the systemic treatment of moderately severe and severe psoriasis vulgaris]. J Dtsch Dermatol Ges. 2009;7(Suppl 2):S3–16.
- Nieboer C, De Hoop D, Van Loenen AC, et al. Systemic therapy with fumaric acid derivates: new possibilities in the treatment of psoriasis. J Am Acad Dermatol. 1989;20(4):601–608.
- Mrowietz U, Barker J, Boehncke W-H, et al. Clinical use of dimethyl fumarate in moderate-to-severe plaque-type psoriasis: a European expert consensus. J Eur Acad Dermatol Venereol. 2018;32(Suppl 3):3–14.
- Blair HA. Dimethyl fumarate: a review in moderate to severe Plaque Psoriasis. Drugs. 2018;78(1):123–130.
- Falkvoll S, Gerdes S, Mrowietz U. Switch of psoriasis therapy from a fumaric acid ester mixture to dimethyl fumarate monotherapy: results of a prospective study. J Dtsch Dermatol Ges. 2019;17(9):906–912.
- Mrowietz U, Szepietowski JC, Loewe R, et al. Efficacy and safety of LAS41008 (dimethyl fumarate) in adults with moderate-to-severe chronic plaque psoriasis: a randomized, double-blind, Fumaderm® - and placebo-controlled trial (BRIDGE). Br J Dermatol. 2017;176(3):615–623.
- Llamas-Velasco M, De La Cueva P, Notario J, et al. Moderate Psoriasis: a Proposed Definition. Actas Dermosifiliogr. 2017;108(10):911–917.
- Reich A, Chatzigeorkidis E, Zeidler C, et al. Tailoring the Cut-off values of the visual analogue scale and numeric rating scale in itch assessment. Acta Derm Venereol. 2017;97(6):759–760.
- Kamata M, Tada Y. Efficacy and safety of biologics for psoriasis and psoriatic arthritis and their impact on comorbidities: a literature review. Int J Mol Sci. 2020;21(5):5.
- Kamata M, Tada Y. Safety of biologics in psoriasis. J Dermatol. 2018;45(3):279–286.
- Reich K, Thaci D, Mrowietz U, et al. Efficacy and safety of fumaric acid esters in the long-term treatment of psoriasis–a retrospective study (FUTURE). J Dtsch Dermatol Ges. 2009;7(7):603–611.
- Di Caprio R, Caiazzo G, Cacciapuoti S, et al. Safety concerns with current treatments for psoriasis in the elderly. Expert Opin Drug Saf. 2020;19(4):523–531.
- Mrowietz U, Morrison PJ, Suhrkamp I, et al. The pharmacokinetics of fumaric acid esters reveal their in Vivo effects. Trends Pharmacol Sci. 2018;39(1):1–12.
- Schweckendiek W. [Treatment of psoriasis vulgaris]. Med Monatsschr. 1959;13(2):103–104.
- Linker RA, Lee D-H, Ryan S, et al. Fumaric acid esters exert neuroprotective effects in neuroinflammation via activation of the Nrf2 antioxidant pathway. Brain. 2011;134(3):678–692.
- Hanson J, Gille A, Offermanns S. Role of HCA₂ (GPR109A) in nicotinic acid and fumaric acid ester-induced effects on the skin. Pharmacol Ther. 2012;136(1):1–7.
- European Medicines Agency. Skilarence [Internet]. European Medicines Agency. 2018 [ cited 2021 Mar 17]. Available from: https://www.ema.europa.eu/en/medicines/human/EPAR/skilarence
- Van Hezik DFC, Bovenschen HJ. Association of lymphopenia and eosinophilia with dimethylfumarate treatment efficacy and tolerability in psoriasis: a retrospective study. J Dermatolog Treat. 2020;31(4):378–381.
- Wain EM, Darling MI, Pleass RD, et al. Treatment of severe, recalcitrant, chronic plaque psoriasis with fumaric acid esters: a prospective study. Br J Dermatol. 2010;162(2):427–434.
- Reszke R, Szepietowski JC. A safety evaluation of dimethyl fumarate in moderate-to-severe psoriasis. Expert Opin Drug Saf. 2020;19(4):373–380.