1. Introduction
In recent years, numerous new genetic reasons for human inborn errors of immunity (IEI) have been identified [Citation1,Citation2]. Knowing the monogenic causes helps us predict future encountering illness, especially malignancies that determine prognosis. Regarding immune defense during diseases, the model of surveillance was first used by Burnet in 1963 [Citation3], later extended by immunoediting encompassing immunosurveillance that describes the active role of the immune system in maintaining self-protection by destroying invaders and pre-malignant cells [Citation4,Citation5]. Defective immunosurveillance poses individuals to increased risk of malignancy. Following infections, malignancy is the second leading cause of death among immunodeficient patients [Citation4,Citation6]. The type of malignancy and the mechanisms underlying this predisposition is varied among different categories of IEI [Citation7]. The combination of diagnostic delay/challenge, increased comorbidity, infections and toxicity usually results in a poorer outcome and survival compared to immunocompetent patients [Citation8–10]. Herein, we discuss the concept of malignancy in IEI by dissecting the diseases based on their molecular defects.
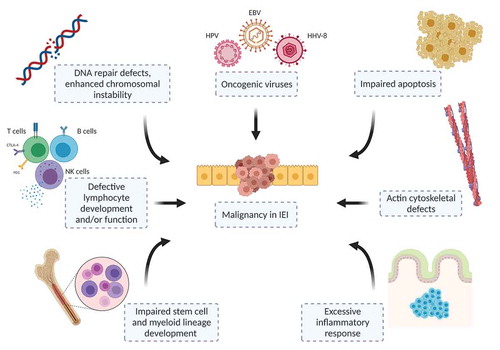
2. Etiological background of cancer susceptibility in IEI
The factors leading to cancer development in IEI patients create a hard task to understand the causality (). The recent concept of etiological evaluation includes the intrinsic and extrinsic factors that initiate and progress the malignant transformation [Citation4,Citation7,Citation8]. Intrinsic causes are errors in cell apoptosis, accelerated immune senescence in the setting of phosphatidylinositol 3-kinase pathway activation, abnormalities involving cell development and/or signaling, actin cytoskeleton, cytotoxicity, DNA repair, chromosome instability and telomere maintenance [Citation4,Citation5,Citation7,Citation11]. Extrinsic causes are chronic tissue inflammation and numerous infectious agents associated with oncogenesis such as Epstein-Barr virus (EBV) in lymphoproliferative conditions and soft tissue tumors, human papillomavirus (HPV) in epithelial tumors, and Helicobacter pylori in stomach cancer [Citation8,Citation12]. Although this concept provides an umbrella covering the possible causes of malignant transformation, generally the diagnosis necessitates vigilance and treatment is challenging, requiring multidisciplinary team approach [Citation9,Citation13]. Finally, in daily practice, pathological classification of malignancies in IEI patients is not always possible due to the aberrant lymphocyte infiltration and/or hyperactive inflammatory responses that license naturally by the IEI itself [Citation13,Citation14].
3. Epidemiology of malignancies in patients with IEI
Several national cohorts provided higher incidence for malignancy in IEIs with approximately 1.4 to 5-fold increase compared to age-adjusted general population [Citation6,Citation15,Citation16]. Among the IEI diagnoses in these reports are predominantly antibody deficiencies, especially common variable immunodeficiency (CVID), reported to have a prevalence of malignancy ranging from 88% to 21% combined immunodeficiencies (CIDs), which mainly include Ataxia-telangiectasia (AT), Bloom syndrome (BS) and Nijmegen breakage syndrome (NBS); congenital phagocytic disorders, EBV susceptibility disorders and diseases with immune dysregulation [Citation4,Citation7,Citation12]. The United States Immune Deficiency Network Registry reported 1.91-fold excess relative risk of malignancy in men compared with the age-adjusted gender population, while women showed equal rate as normal population. However, the incidence of lymphoma increased 10 times in men and 8.34 times in women [Citation6]. On the other hand, solid organ malignancies of lung, colon, breast and prostate were not found to have a higher frequency in IEI [Citation6,Citation12]. In a study with 6392 Turkish patients with IEI, prevalence of malignancy was reported as 0.9% and found to be 1.57 times more common compared with general population; mostly including CID group as DOCK8 deficiency, AT, Wiskott-Aldrich syndrome (WAS), BS, and purine nucleoside phosphorylase deficiency [Citation15].
It is worth to note that the survival rate after malignancies is inferior in IEI patients when compared with immunocompetent persons [Citation17]. Overall survival after lymphoma in IEI was detected to be 62%, whereas non-IEI patients exhibit event-free survival rates of up to 90% [Citation9,Citation17]. The less favorable survival results have been attributed to advanced disease stage with more extranodal sites of involvement, chronic organ dysfunctions, increased infectious comorbidities, and severe side effects following cytotoxic cancer therapies [Citation4,Citation7,Citation13].
4. Common malignancies in IEI cohorts
In most IEI cohorts, non-Hodgkin lymphoma (NHL), leukemia, skin, gastrointestinal, genitourinary, breast malignancies and thymoma were commonly observed [Citation6,Citation12,Citation15,Citation16]. The NHL was the predominant malignancy in IEI and mostly reported in CVID and CIDs with syndromic immunodeficiencies, whereas leukemia was higher in patients with disorders of immune dysregulation and IgG subclass deficiency [Citation12]. Types of lymphoma in IEI were mostly unspecified NHL and diffuse large B-cell lymphoma followed by Hodgkin lymphoma (HL), delineating the aggressiveness and atypical morphology in this population [Citation10]. Malignancies and associated IEI disorders are presented in .
Table 1. Types of IEI and reported malignancies
CVID patients were reported to have increased gastric cancers, NHL and mucosa-associated lymphoid tissue lymphoma [Citation4,Citation8]. The NHL location is frequently extranodal, shows rapid progression with inferior survival and approximately 30% is associated with EBV [Citation9,Citation10].
The plausible mechanisms such as chronic insufficient antigenic clearance, defective DNA repairing and other drivers (EBV infections) make patients with DNA repair defects (Artemis, DNA Ligase IV, AT, BS, NBS) vulnerable to lymphoma and leukemic transformation. Specifically, WASP-deficient patients strikingly illustrate susceptibility for lymphoma, leukemia and myelodysplasia, accounting for 90% of malignancies observed in this syndrome [Citation7,Citation8,Citation15].
Susceptibility to EBV is associated with IEI, presenting with malignant B-cell lymphoproliferative disorder, hemophagocytic syndrome and EBV-driven lymphoproliferation. X-linked lymphoproliferative syndrome type 1 and 2, interleukin-2-inducible T-cell kinase deficiency and CD70-CD27 deficiencies are the most reported disorders [Citation1]. Other rare defective genes characterized by RASGRP1, CTPS1, CD137, MAGT1 and PRKCD are also attributed to this group [Citation1]. Overall, several mechanisms have been demonstrated to facilitate malignancy in these disorders, which impair NK-cell function and reduce invariant NKT cells, which are important for immunity against EBV [Citation7].
Lymphomas can also be observed in the course of other IEI characterized by autoimmune lymphoproliferative syndromes, patients with defective check point inhibitors like CTLA-4 and LRBA deficiencies, activated phosphoinositide 3-kinase Delta syndrome type 1 and 2 and hyper-IgE syndromes, especially DOCK8 deficiency [Citation1,Citation4,Citation7,Citation18,Citation19]. Chronic mucocutaneous candidiasis accompanied by impaired IL-17-mediated immunity is associated with oral and esophageal squamous cell malignancies, which were described in patients with autoimmune polyglandular syndrome type 1 and STAT1 gain-of-function [Citation4]. Due to the chronic HPV infection, DOCK8-deficient patients are prone to develop squamous cell carcinomas [Citation9].
The patients with severe congenital neutropenia have intrinsic development defects prone to leukemic transformation. They usually require granulocyte colony-stimulating factor to maintain normal neutrophil counts and avoid severe infections, but the long-term use of this treatment has been associated with an increased risk of acute myeloid leukemia (AML) and myelodysplastic syndrome (MDS) because of the exaggerated stimulation [Citation4,Citation7,Citation12].
5. Rare malignancies in IEI
T-cell originated lymphomas are uncommon in IEI when compared with B cells; and mostly found in patients with predominantly antibody deficiencies or CIDs [Citation10].
Other rare solid tumors were reported in patients with CD40 ligand defect such as liver, biliary tree and pancreas tumors probably due to the impaired biliary epithelium defense system against intracellular pathogens; especially cryptosporidium [Citation8]. On the other hand, medulloblastoma, neuroblastoma, dysgerminoma and thyroid malignancies were observed in patients with NBS, Wilms tumor in BS and stomach, pancreas, bladder and ovary cancers in AT [Citation4]. Colorectal carcinoma is seldomly observed in IEI, especially in X-linked agammaglobulinemia, which is reported to be 30-times more frequently, linked to the low IgA and chronic mucosal irritation [Citation8]. Thyroid, breast, bladder, pancreas related malignancies and cholangiocarcinoma were described rarely in CVID patients [Citation4].
Recently, EBV-related smooth muscle tumors (SMT) were described in special IEI patients with CARMIL-2 and GATA2 deficiencies [Citation20]. The EBV+SMTs were also observed in CID patients, particularly in adenosine deaminase, IL2RG, ZAP70 and AT deficiencies [Citation20]. GATA2-deficient patients may present with various hematopoetic malignancies, such as MDS, AML, chronic myelomonocytic leukemia, HPV- and EBV-positive tumors [Citation7].
6. Treatment options of malignancies in patients with IEI
Nowadays, the treatment of malignancies in IEI generally is similar to the non-IEI patients. The most efficient therapeutic options should deliver a weighted balance for protection in normal cells, while unleashing more killing of malignant cells. Treatment modalities must be adjusted on an individual basis for patient, as optimal treatment approaches are not yet determined [Citation13]. In patients with DNA repair defects, radiomimetic agents should not be preferred and alkylating substances, daunorubicin, etoposide and methotrexate should be used with reduced dose [Citation9,Citation10]. In B-cell lymphomas, regimens that include anti-CD20 monoclonal antibody (rituximab) for short intervals can yield advantageous outcome with less toxicity such as infections, mucositis and bone marrow suppression, which are highly observed in IEI patients [Citation10,Citation17]. Importantly, infectious complications should be prevented by antimicrobial prophylaxis and immunoglobulin replacement therapy. Hematopoetic stem cell transplantation (HSCT) seems the ultimate curative therapy for many IEI patients before developing malignancy. Early HSCT in particular groups, especially in CID patients, can be preventive therapy with more desirable survival [Citation7,Citation13,Citation18].
A special issue observed in IEI patients during follow-up is lymphoproliferative disease (LPD), which is usually aggressive in nature and displays poor prognosis [Citation9,Citation14]. The LPD is usually associated with poor T-cell function, and also can be driven by pathogens, mostly EBV. Generally, LPD demonstrates histopathologic findings that mimic lymphoma, which complicates the pathological evaluation of patients. Targeted therapies like rituximab and selective mechanistic target of rapamycin inhibitors (sirolimus) have been administered to control lymphoproliferation [Citation9]. Yet, the restorative therapy is HSCT.
Potential mechanistic targeted therapies such as anti-CTLA-4 and anti-PD1 agents can be leveraged in establishing more effective control of malignancy than classical therapies but need further evidence for use in IEI patients [Citation10].
7. Conclusion
The diagnosis of malignancies in IEI requires special focus based on distinct multidisciplinary team approaches to provide early diagnosis and better outcome. Clinical trials that address appropriate doses and treatment toxicity in different types of IEI would provide more optimal regimens for the disease control. Finally, understanding the molecular mechanisms of oncogenesis in IEI may pave a way for novel tailored therapies, which can be less toxic compared to conventional therapies.
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Additional information
Funding
References
- Tangye SG, Al-Herz W, Bousfiha A, et al. Human inborn errors of immunity: 2019 update on the classification from the International Union of Immunological Societies Expert Committee. J Clin Immunol. 2020;40(1):24–64.
- Tangye SG, Al-Herz W, Bousfiha A, et al. The ever-increasing array of novel inborn errors of immunity: an interim update by the IUIS Committee. J Clin Immunol. 2021;41(3):666–679.
- Burnet FM. The evolution of bodily defence. Med J Aust. 1963;2:817–821.
- Mortaz E, Tabarsi P, Mansouri D, et al. Cancers related to immunodeficiencies: update and perspectives. Front Immunol. 2016;7:365.
- Haas OA. Primary immunodeficiency and cancer predisposition revisited: embedding two closely related concepts into an integrative conceptual framework. Front Immunol. 2018;9:3136.
- Mayor PC, Eng KH, Singel KL, et al., Cancer in primary immunodeficiency diseases: cancer incidence in the United States Immune Deficiency Network Registry. J Allergy Clin Immunol. 2018;141(3):1028–1035.
- Hauck F, Voss R, Urban C, et al. Intrinsic and extrinsic causes of malignancies in patients with primary immunodeficiency disorders. J Allergy Clin Immunol. 2018;141(1):59–68e54.
- Satge D, Tumor A. Profile in Primary Immune Deficiencies Challenges the Cancer Immune Surveillance Concept. Front Immunol. 2018;9:1149.
- Kiykim A, Eker N, Surekli O, et al. Malignancy and lymphoid proliferation in primary immune deficiencies; hard to define, hard to treat. Pediatr Blood Cancer. 2020;67(2):e28091.
- Riaz IB, Faridi W, Patnaik MM, et al. A systematic review on predisposition to Lymphoid (B and T cell) neoplasias in patients with primary immunodeficiencies and immune dysregulatory disorders (Inborn Errors of Immunity). Front Immunol. 2019;10:777.
- Ogulur I, Ertuzun T, Kocamis B, et al. Parents of ataxia-telangiectasia patients display a distinct cellular immune phenotype mimicking ATM-mutated patients. Pediatr Allergy Immunol. 2021;32(2):349–357.
- Vajdic CM, Mao L, Van Leeuwen MT, et al. Are antibody deficiency disorders associated with a narrower range of cancers than other forms of immunodeficiency? Blood. 2010;116(8):1228–1234.
- Bomken S, Van Der Werff Ten Bosch J, Attarbaschi A, et al. Current understanding and future research priorities in malignancy associated with inborn errors of immunity and DNA repair disorders: the perspective of an Interdisciplinary Working Group. Front Immunol. 2018;9:2912.
- Cohen JM, Sebire NJ, Harvey J, et al. Successful treatment of lymphoproliferative disease complicating primary immunodeficiency/immunodysregulatory disorders with reduced-intensity allogeneic stem-cell transplantation. Blood. 2007;110(6):2209–2214.
- Cekic S, Metin A, Aytekin C, et al. The evaluation of malignancies in Turkish primary immunodeficiency patients; a multicenter study. Pediatr Allergy Immunol. 2020;31(5):528–536.
- Mellemkjaer L, Hammarstrom L, Andersen V, et al. Cancer risk among patients with IgA deficiency or common variable immunodeficiency and their relatives: a combined Danish and Swedish study. Clin Exp Immunol. 2002;130(3):495–500.
- Tanyildiz HG, Dincaslan H, Yavuz G, et al., Lymphoma secondary to congenital and acquired immunodeficiency syndromes at a Turkish Pediatric Oncology Center. J Clin Immunol. 2016;36(7):667–676.
- Kiykim A, Ogulur I, Dursun E, et al. Abatacept as a long-term targeted therapy for LRBA deficiency. J Allergy Clin Immunol Pract. 2019;7(8):2790–2800 e2715.
- Kolukisa B, Baris S. Primary immune regulatory disorders and targeted therapies. Turk J Haematol. 2021;38(1):1–14.
- Magg T, Schober T, Walz C, et al. Epstein-Barr Virus(+) smooth muscle tumors as manifestation of primary immunodeficiency disorders. Front Immunol. 2018;9:368.