ABSTRACT
Introduction
More than one million breast augmentation procedures using silicone breast implants (SBI) have been performed worldwide. Adverse events of SBI include local complications such as pain, swelling, redness, infections, capsular contracture, implant rupture, and gel-bleed. Furthermore, patients experience systemic symptoms such as chronic fatigue, arthralgias, myalgias, pyrexia, sicca, and cognitive dysfunction. These symptoms received different names such as autoimmune/autoinflammatory syndrome induced by adjuvants (ASIA) due to silicone incompatibility syndrome and breast implant illness (BII). Because of chronic immune activation, BII/ASIA, allergies, autoimmune diseases, immune deficiencies, and finally lymphomas may develop in SBI patients.
Areas covered
Causality for SBI-related BII/ASIA is reviewed. To address the role of silicone implants in promoting causality, we utilized the Bradford Hill criteria, with results highlighted in this article.
Expert opinion
We conclude that there is a causal association between SBIs and BII/ASIA. Using data derived from patients with BII/ASIA and from other medically implanted devices, there appears to be clear pathogenic relationship between SBI and BII/ASIA. Breast implants cause characteristic systemic reactions in certain women, leading to symptoms of sufficient severity to warrant device removal. The morbidity suffered is variable. SBI removal resolves the symptoms in most women, and removal is the most effective treatment.
1. Introduction
Silicones are commonly used for various medical applications, including cosmetic ones (such as breast implants and rhinoplasty) and non-cosmetic medical ones, namely hydrocephalus shunts, catheter lines, intraocular implants, rhinoplasty (for non-cosmetic purposes), hearing aids, laryngotracheal stenosis, joint implants, and testicular prostheses. When they were introduced medically in the 1960s, they were initially thought to be biologically inert. However, over the past 50 years, it has become evident that they may be associated with inducing various immunological effects [Citation1].
Silicon is a natural chemical element and is the basic element of silicones, a man-made synthetic polymer with a backbone of repeated Si-O units with two organic groups attached to it. Silicones vary in their composition. By varying the Si-O chain length, side groups and extent of cross-linking extent, silicone properties change from liquid to solid. Polydimethylsiloxane is the polymer used for the mammary prosthetic devices.
One of the most common uses for silicone include the use of silicone breast implants (SBI), which were first developed by Cronin and Gerow in 1962 for cosmetic procedures [Citation2].
Breast implants are indicated for females for various reasons including
Cosmetic breast augmentation
Breast reconstruction following a mastectomy
Revision surgery to correct or improve a previous breast augmentation or breast reconstruction surgery.
Breast implants surgery should not be performed
In women with an active infection anywhere in their body at the time of surgery,
In women with existing cancer or ductal carcinoma in situ of their breast who have not received adequate treatment for those conditions
In women who are pregnant or breastfeeding their babies
In women who are younger than 22 years of age.
Dow Corning wrapped silicone gel in an impermeable silicone envelope (or capsule), which has subsequently been utilized for many women requiring SBI for either a cosmetic procedure or for reconstruction following native breast tissue removal (post-mastectomy). Later on, the breast implant industry improved during the last decade via a concerted effort aimed at better product design and manufacturing process to reduce local complications. These measures resulted in implants with a more cohesive silicone gel, a thicker (yet supple) shell, and an additional barrier layer that claimed to better ‘mimic’ the sensation of natural breast tissue [Citation3].
2. Adverse events associated with breast implants
As of 2021, more than one million breast augmentation procedures have been performed worldwide – although many consequences ensued and are becoming increasingly reported. A large number of these events have been reported to the FDA with an increasing number of women requesting surgical removal of the breast implants based on these adverse events [Citation4].
Adverse events can be categorized into several subgroups:
Local complications such as pain;
Silicone migration to the lungs, skin, and lower extremities;
Surgical complications such as rupture and capsular formation;
Allergy to components of the breast implants;
Systemic inflammatory symptoms such as those present in autoimmune rheumatic diseases and the development of lymphomas.
As early as the 1960s, local and distant complications of the SBI procedure were reported. Local complications of SBI include pain, swelling, redness, infections, capsular contracture, implant rupture, and gel-bleeding through the intact capsule.
In addition, general complications are also common. Among the systemic symptoms described from 2002 to 2020, the most frequent patient-reported symptoms noted by the FDA include fatigue, joint pain (arthralgia), hair loss, and/or a hypersensitivity/rash [Citation4]. Most of the reports received listed multiple patient problems in each report. Recently, many women also developed autoimmune inflammatory diseases, which include inflammatory arthritis, Sjögren’s syndrome, systemic sclerosis, and lupus-like syndromes [Citation5]. In Canada, Edworthy reported the results of a study of 1576 Canadian patients who underwent breast augmentation. In these patients, systemic symptoms such as cognitive impairment and myalgia’s were significantly more frequently present in these patients when compared to patients who underwent cosmetic surgery for other purposes [Citation6]. The silicones were thought to be causative as in many women (60–80%) removal of the SBI resulted in amelioration of symptoms [Citation7]. As a result, a moratorium was imposed on the sale of SBIs by the U.S. FDA and by Health Canada as of 1992, citing concerns including connective tissue disease and autoimmune disease. It is not clear if the composition of the SBIs following the moratorium changed, and if so, what the change was and whether this influenced the rate of (systemic) adverse events. However, as highlighted below, it appears that numerous concerns such as inflammatory connective tissue and autoimmune diseases were not resolved in the ‘new generation’ SBIs [Citation8].
3. Symptoms and signs of breast implant illness
Locally, about half of the SBI patients with ‘breast implant illness’ (BII) have breast pain, tenderness, and/or burning sensations. Patients with BII develop changes in breast shape, symmetry, firmness, and/or size. Intriguingly, some of these symptoms have been reported to occur after trauma to the breasts and/or after mammography [Citation9]. BII patients also experience systemic symptoms (see ) as described in chronic fatigue syndrome/myalgic encephalomyelitis (CFS/ME) [Citation10,Citation11], which are universally present. In contrast to other forms of fatigue, both BII and CFS/ME patients are severely symptomatic when they wake up, and their symptoms are not alleviated by rest. These patients have a substantial reduction in their ability to engage pre-illness levels of occupational, educational, social and/or personal activities. Importantly, most patients report post-exertional malaise, or symptom flares or ‘crash’ after physical or cognitive exertion lasting days to sometimes weeks.
Figure 1. Breast implant illness (a form of autoimmune/autoinflammatory syndrome induced by adjuvants) presents with neurological/musculoskeletal, immunological, and/or vascular manifestations.
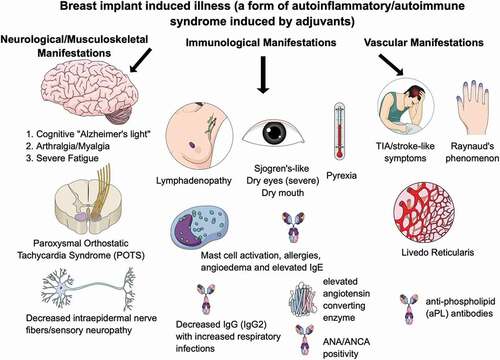
Sleep disturbances exacerbate symptoms of fatigue. These include difficulties with falling asleep and/or staying asleep. Indeed, most patients (up to 90%) of patients with BII fulfill the classification criteria for CFS/ME. Like other patients with CFS/ME, these patients also develop symptoms of cognitive impairment resulting in debilitating symptoms such as memory deficits (‘Alzheimer-light’), absent-mindedness, word-finding difficulties, and poor attention span [Citation12]. Most of all, these patients may also develop symptoms, which are indistinguishable from fibromyalgia, as up to 90% of these patients fulfill the 2016 classification criteria for fibromyalgia (see ) [Citation13]. Patients suffer from morning stiffness that sometimes may last more than an hour. Occasionally, however, patients may also develop (symmetric) polyarthritis compatible with a diagnosis of rheumatoid arthritis [Citation14,Citation15].
Table 1. Fibromyalgia 2016 criteria [Citation13]
Also, up to 90% of the patients have myalgia’s and/or muscle weakness. Weakness can be severe and may render the patient bedridden. This weakness is not only subjective as investigated in one study that included 93 patients, 53% of these patients had an abnormal EMG [Citation16], where a ‘myopathic’ pattern was described. Furthermore, two-third of the patients report subjective fever-like ‘pyrexia’ and night sweats. Occasionally, patients have strongly elevated ferritin levels and fulfill the classification criteria for (silicone-induced) Still’s disease [Citation17].
Importantly, most patients (up to 75%) have sicca symptoms such as dry eyes and dry mouth, which are typically severe. Also, impaired tear production (measured by objective tests such as Schirmer’s tests), headaches, blurred vision, and/or a keratitis sicca are frequently observed. Complaints of a dry mouth are often exacerbated by treatment with amitriptyline and may result in swallowing difficulties and dental issues such as gum disease and dental cavities. In contrast to patients with Sjögren’s syndrome, anti-SSA/SSB antibodies are only present in a minority of patients [Citation1], and salivary gland biopsies disclose mononuclear cell infiltrates, which is different from patients with Sjögren’s syndrome who typically have a lymphocytic infiltrate [Citation18,Citation19].
Also, 30–50% of the patients develop new-onset Raynaud’s phenomenon with nailfold capillaroscopy abnormalities suggestive of systemic sclerosis spectrum pattern [Citation9].
In 30–40% of the patients, severe neurological manifestations such as ischemic cerebral disease or multiple sclerosis-like symptoms are present [Citation1]. In patients with ischemic cerebral disease, anti-cardiolipin antibodies, anti-beta-2-glycoprotein antibodies, and/or lupus anticoagulant are detected in only a minority of the patients. This suggests that many of these patients have so-called ‘seronegative antiphospholipid syndrome’ [Citation20].
Atopic symptoms, such as sneezing, a runny nose, itchy eyes, red eyes, nasal congestion and post nasal drip, are also common and are reported in 50–80% of patients [Citation21]. Although allergies may be preexistent, many patients report exacerbation of their symptoms that were minimal prior to implantation. Allergies that are frequently observed are pollen allergy, pet allergy, mold allergy, latex allergy, and food allergy.
Remarkably frequently (about 50% of patients) metal-allergy is documented presenting as an itchy rash after exposure to nickel-containing substances
About 10–20% of the patients experience the occurrence of hives and/or Quincke’s edema (or isolated uvular angioedema).
In addition, some patients present with symptoms suggestive of a diagnosis of mast cell activation syndrome [Citation22,Citation23] and/or with multiple chemical sensitivity [Citation24].
Shortness of breath in SBI patients can be a result of (severe) asthma, pulmonary nodules, interstitial lung disease, and/or pulmonary silicone embolism [Citation25–28].
Furthermore, 20–40% of patients suffer from severe and/or recurrent (upper respiratory tract) infections associated with hypogammaglobulinemia (particularly in association with IgG2 subclass deficiency) – suggesting a humoral immune deficiency [Citation29]. Like other patients with humoral immune dysfunction and Sjögren’s syndrome, lymph nodes (axillary, cervical, and inguinal) are also often enlarged and tender (70–80% of patients).
Cardiovascular complaints include signs of orthostatic intolerance such as dizziness, disturbed balance, irregular heartbeat, and sometimes chest pain. Tilt testing may reveal a diagnosis of POTS (Postural Orthostatic Tachycardia Syndrome). A mitral valve prolapse and/or joint hypermobility is found in about half of the patients [Citation30]. In the study by Watad et al., 24,651 SBI recipients were matched to 98,604 SBI-free women. SBI recipients were more likely to be ever smokers and had more often cardiovascular disease (6.55%) in comparison with the SBI-free group (5.64%) [Citation31]. In addition, it can be postulated that there is an increased risk of cardiotoxicity when breast cancer patients with SBIs are being treated with immune checkpoint inhibitors [Citation32–34].
A large proportion of patients (20–40%) have gastrointestinal symptoms such as cramping, abdominal pain, bloating, gas, and changes in bowel movement patterns such as found in irritable bowel syndrome. Swallowing difficulties are found in most patients due to their mouth dryness.
A substantial amount of patients (10–20%) have interstitial cystitis. These patients report bladder pressure, bladder pain, and sometimes pelvic pain often accompanied by urinary symptoms, such as urgency or frequency.
The skin may be painful. Patients often describe unpleasant tingling sensations or burning pain suggestive of (atypical) small fiber neuropathy [Citation35]. Also, unusual sensations such as ‘pins-and-needles’ and numbness occurs.
At physical examination, livedo reticularis is often observed; severe livedo reticularis occurs in about 20–30% of patients, whereas mild livedo reticularis is present in another 30–40% of patients.
Some patients have tender subcutaneous nodules in their arms, legs, abdominal wall and/or elsewhere in the body. Histologically, these nodules consists of granulomatous inflammation (i.e. migratory silicone granulomas) [Citation26,Citation36].
In about 20–40% of patients, ill-defined skin rashes are present and/or unexplained (sometimes severe) pruritus, whereas hair loss is reported in up to 10% of patients.
4. Laboratory and radiological findings and other diagnostic procedures in BII
Patients with BII undertake many investigations prior to obtaining a diagnosis. This is because the testing required confirming BII is not commonly understood by front-line medical practitioners. Generally, CRP levels are normal. Angiotensin-converting enzyme and soluble interleukin-2 receptor levels are, however, elevated in up to 50% of patients. Antinuclear antibodies are present in 20% of patients, whereas various other antibodies such as SSA/SSB, anti-dsDNA, anti-Scl-70, anti-polymerase III, anti-cardiolipin, anti-CCP antibodies, IgM-rheumatoid factor, ANCA, and/or cryoglobulins may be found – albeit less commonly [Citation8,Citation29]. Furthermore, anti-polymer antibodies have been described but their diagnostic value is at present uncertain [Citation37]. Vitamin D insufficiency and/or deficiency is a frequent finding [Citation38] and 20–50% of patients have decreased levels of IgG and/or IgG subclasses [Citation8,Citation29,Citation38]. Recently, it was reported that women with BII had a significant reduction in the sera level of anti-β1 adrenergic receptor, anti-angiotensin II type 1 receptor and anti-endothelin receptor type A autoantibodies as compared with aged matched healthy women [Citation39] – suggesting that these autoantibodies may be engaging tissue targets.
To screen for implant rupture it is recommended to perform periodic imaging (ultrasound or magnetic resonance imaging (MRI)). MRI is the examination of choice. Mammograms are relatively contraindicated in patients with SBI, since there is a slight risk that compression may induce and/or exacerbate (intracapsular) ruptures [Citation9]. Also, MRI investigations may detect capsular contractures, seromas, anaplastic large cell lymphoma (ALCL), and silicone-induced granuloma of the breast [Citation40]. In addition, silicone-containing granulomas in lymph nodes can be detected by MRI. The method of choice to detect this silicone lymphadenopathy is, however, ultrasound imaging, which typically shows a ‘snowstorm sign’ representing free silicone droplets mixed with breast tissue [Citation41].
Although not routinely performed, there are several other diagnostic procedures that are useful to objectively assess patients with suspected BII. Examples include cardiopulmonary exercise test by cycling till maximal exertion that is repeated after 24 hours to objectify the post-exertional malaise [Citation42], overnight polysomnography to ascertain objectively poor sleep quality, capillaroscopy to detect nailfold abnormalities [Citation11], and/or ocular surface evaluation including Schirmer testing, tear breakup time, and staining of the cornea and conjunctiva to confirm the impaired tear production.
To detect small fiber neuropathy, a punch biopsy of the skin can be performed that shows reduced intraepidermal nerve fiber density. Otherwise, temperature threshold testing can be done to confirm small fiber neuropathy [Citation35]. A labial salivary gland biopsy will show mild lymphocytic infiltration, which differentiates silicone-induced sicca symptoms from Sjögren’s syndrome [Citation19]. Testing for breast-implant-associated ALCL involves ultrasound to detect seroma so that fresh fluid can be obtained for further examinations by immunohistochemistry and/or flow cytometry.
Finally, explanted SBI and lymph nodes should be histologically examined to confirm granulomatous silicone inflammation and/or ALCL.
Migrated silicones from implant ‘bleeds’ and/or from implant ruptures can be determined by the histological examination of lymph nodes and/or other tissues that show a granulomatous ‘sarcoid-like’ foreign body reaction. Using confocal Raman microprobe analysis, the material can be confirmed as silicones [Citation43], whereas energy dispersive X-ray microanalysis (EDX) can be used to measure elemental Silicon (Si) [Citation44].
5. ASIA, autoimmune diseases, and ALCL
The wide range of symptoms that may develop after a breast reconstruction and/or a cosmetic breast augmentation received during the last 50 years several different names: human adjuvant disease, siliconosis, silicone incompatibility syndrome, silicone-induced toxicity, and autoimmune/autoinflammatory syndrome induced by adjuvants (ASIA) due to silicone incompatibility syndrome (Citation11,Citation29,Citation45; ). In the USA, however, the term Breast Implant Illness (‘BII’) is used when patients develop these symptoms.
Table 2. Criteria for the diagnosis of autoimmune/autoinflammatory syndrome induced by adjuvants (ASIA) [Citation8,Citation45]
Because of the silicone-induced immune activation, ASIA, allergies, autoantibodies, autoimmune diseases, IgG and/or IgG subclass deficiencies, and finally lymphomas may develop in patients with breast implants.
In 2013, we reported 32 patients with ASIA due to silicone incompatibility syndrome [Citation29]. Median time between start of complaints and time of breast implant was 10 years (2–24 years). Fifty-three percent of the ASIA patients had an established systemic autoimmune disease, 22% of patients had an organ-specific autoimmune disease, 47% of patients a humoral immunodeficiency (either hypogammaglobulinemia or IgG1 or IgG2 subclass deficiency), and 6% had a lymphoma. Subsequently, many patients with self-reported symptoms were evaluated in the Netherlands [Citation8,Citation21,Citation38,Citation46]. From these patients, about 95% fulfilled the criteria for ASIA (). Most of these patients had (a) fatigue and/or cognitive symptoms, (b) arthralgias and/or myalgia’s, and (c) sicca complants and/or pyrexia. Most (70–80%) of these ASIA patients had cosmetic breast augmentation whereas 20–30% of these patients had breast reconstruction after mastectomy for breast cancer. More than 99% of the patients were women, the remaining being (transgender) males.
At present, there are few epidemiologic studies performed to calculate the risk of ASIA in SBI patients. In 1994, Giltay et al. reported a study comparing 235 patients who had breast implants and 210 patients who underwent another esthetic surgical procedure. Eighty-eight (37%) cases and 44 (21%) controls (p < 0.001) presented with at least one complaint with onset after surgery. 20% of SBI patients had at least three months painful joints (controls 9%) and 16% burning eyes (controls 7%) [Citation47]. In a pilot study, we calculated that about one quarter of unselected patients with silicone breast implants had symptoms compatible with ASIA; four times more often than healthy age-matched controls (see ). More epidemiological studies on the association between ASIA and SBI are, however, needed to fully assess the impact of breast implants on ASIA symptoms.
Table 3. Frequency of autoimmune/autoinflammatory syndrome induced by adjuvants (ASIA) symptoms in unselected patients with breast implants, healthy controls, and patients who registered themselves as having breast implant illness (BII)
Patients with BII/ASIA and fatigue often fulfill the criteria for CFS/ME [Citation48], whereas patient with joint and muscle complaints fulfill the criteria for fibromyalgia [Citation13] and/or undifferentiated connective tissue disease [Citation11]. Sarcoidosis or sarcoid-like disease may be diagnosed based on the granulomatous inflammation as detected in lymph nodes, lungs, and various other tissues that are infiltrated with silicones. Histopathological findings are, in these cases, very difficult to differentiate from ‘idiopathic’ sarcoidosis. Finally, a substantial number of patients have well-defined systemic autoimmune diseases such as Sjögren’s syndrome, rheumatoid arthritis (RA), systemic sclerosis, systemic lupus erythematosus, anti-phospholipid syndrome, eosinophilic granulomatosis with polyangiitis, and/or different other forms of vasculitis [Citation8,Citation9,Citation25,Citation29,Citation49].
Epidemiologic evidence for an increased occurrence of these autoimmune diseases has been reported [Citation15]. In a meta-analysis published in 2016, increased risks for rheumatoid arthritis and Sjögren syndrome were found. Importantly, the systematic review concluded that studies that were performed between 1980 and 2016 did not provide conclusive evidence regarding safety of SBI and that further investigations were required to determine whether increased occurrences exist between silicone gel implants and autoimmune diseases [Citation15].
Recent epidemiological evidence quantifies the increased risk of autoimmune diseases in women with silicone breast implants [Citation31]. The Watad study, which included 24,651 women with silicone breast implants and 98,604 matched SBI-free women, found that 26.4% of women with breast implants had an autoimmune/rheumatic disorder. They calculated that women with breast implants had a 45% increased risk for being diagnosed with at least one autoimmune/rheumatic disorder, compared to those without breast implants [Citation31]. The strongest association was recorded for Sjögren’s syndrome, systemic sclerosis, and sarcoidosis, but also for less rare conditions such as rheumatoid arthritis and fibromyalgia, a significant increased risk was observed.
Recently, also a large postapproval cohort study concluded that silicone breast implants are associated with a clear increase in risk in rare autoimmune diseases such as systemic sclerosis, Sjögren syndrome, and dermatomyositis/polymyositis [Citation50]. It should be mentioned, however, that geographical differences exist in the occurrence of autoimmune diseases [Citation51], which may stem from the frequency of HLA haplotypes present in a given population, and/or vitamin D insufficiency [Citation11]
In addition, SBI patients have an increased risk to develop lymphomas [Citation52]. Especially, the risk to develop an anaplastic large T-cell lymphoma (ALCL) of the breast negative for anaplastic lymphoma kinase-1 (ALK-1) but positive for CD30 is strongly increased. This lymphoma occurs mainly but not exclusively in SBIs with a macrotextured device. Macrotextured implants from Allergan have the highest risk [Citation53].
The first case of breast implant-associated anaplastic large cell lymphoma was described in 1997 [Citation54]. In 2008, de Jong et al. reported 11 Dutch patients with ALCL due to breast implants. Furthermore, they convincingly demonstrated that breast implants were to blame [Citation55]. In 2018, in a more extensive Dutch study, it was calculated that the cumulative risks of breast-ALCL in women with implants were 29 per million at 50 years and 82 per million at 70 years, whereas the observed odds ratio was 421.8; 95% CI, 52.6–3385.2 [Citation53]. More recently, the same group demonstrated 20q13.13 loss and deregulation of the IL6-JAK1-STAT3 pathway in breast implant associated ALCL (BIA-ALCL). As a result of these observations, it can be concluded that the characteristic loss of chromosome 20 in BIA-ALCL provides further justification to recognize BIA-ALCL as a separate disease entity [Citation56].
Although significant barriers exist to estimate accurately both the number of women with implants (denominator) and the number of cases of BIA-ALCL (numerator), including poor registries, underreporting, lack of awareness, and cosmetic tourism, it has been suggested that the incidence and risk of BIA-ALCL have increased dramatically from initial reports of 1 per million to current estimates of 1 in 2,832 women. It must be realized, however, that this estimate is largely dependent on the ‘population’ (implant type and characteristics) examined and increased awareness of the disease [Citation57].
6. ASIA: autoimmune/autoinflammatory syndrome induced by adjuvants
In 2011, a syndrome entitled ASIA (autoimmune/autoinflammatory syndrome induced by adjuvants) was first described [Citation45], but the idea of such an immune-mediated disease was not a new one. Evidence has been accumulating, since silicones were introduced in medical applications in the 1960s, that (auto)immune symptoms can be triggered by exposure to environmental immune stimulatory factors that act as an adjuvant in susceptible individuals [Citation11,Citation58]. Adjuvants are compounds that, when introduced into the body, enhance a specific immune reaction resulting in higher titers of antibodies, for instance against specific pathogens [Citation59]. Well-known examples of adjuvants are aluminum hydroxide, squalene, and silica [Citation60]. During the last decade, it became clear that implanted medical implants, including injectables such as silicones and polypropylene meshes, may act as adjuvants [Citation61,Citation62].
As the lead author of the 2011 paper describing ASIA, Shoenfeld stated: ‘The role of various environmental factors in the pathogenesis of immune mediated diseases is well established’ [Citation45]. Shoenfeld did not describe a new phenomenon with ASIA, but instead, aimed to organize under a single umbrella the existing evidence regarding certain environmental factors that possess immune stimulatory properties, in order to shed light on a common pathway of autoimmune pathogenesis. Such environmental immune stimulators, or adjuvants, include among others, injectable silicones, aluminum salts, various implants, as well as various infectious agents. There is support in the literature demonstrating that susceptible patients may develop ASIA after breast or testicular implantation, rhinoplasty, polypropylene mesh implantation for hernia repair or for reinforcement of a weak pelvic floor, tension-free vaginal tape implantation for stress incontinence, and/or implantation of prosthetic materials for arthroplasty [Citation11,Citation62].
While most of these individual adjuvants have already been linked to the development of autoimmunity, ASIA suggests an underlying shared mechanism of adjuvant-induced autoimmunity, in genetically or otherwise susceptible individuals.
At present, it is unknown which patients are more susceptible to development of ASIA after implantation of medical devices of various materials. Several factors, however, have been postulated. For example, patients with a history of allergy are at risk of developing ASIA after implantation [Citation11]. Furthermore, patients with an established autoimmune disease or a familial predisposition to autoimmune disease are at risk of developing symptoms after silicone breast implantation (‘SBI’). It is important to note that not only immunogenetic (i.e. human leukocyte antigens ‘HLA’) factors play a role in the development of SBI-induced ASIA but also environmental factors such as smoking and obesity may play a role.
All patients with SBI should be monitored for the development of chronic inflammation, the development of autoimmunity, ASIA/BII and BIA-ALCL. Recently, Caravantes-Cortes et al. proposed an algorithm using determination of biomarkers, assessment by Rheumatology, and breast ultrasound examination for follow-up of patients with SBI [Citation63].
6.1. Pathophysiology of ASIA/BII
Biomaterials that are used for implantation are – in general – non-immunogenic and nontoxic. Implanted biomaterials may, however, trigger a foreign body reaction resulting in granulomatous inflammation [Citation64,Citation65]. Furthermore, microbial biofilms may form on implants [Citation66–68], and contribute to the chronic inflammatory response. Importantly, implanted biomaterials act as an adjuvant resulting in the enhancement of the adaptive immune response to an (auto) antigen [Citation69].
When a biomaterial is implanted in a human body, a layer of host proteins is absorbed, resulting in the attraction of phagocytes. Further analysis of these phagocytes showed that these are predominantly macrophages of the pro-inflammatory M1 subtype [Citation70]. This process is critically dependent on the presence of activated mast cells and histamine [Citation71] (). Histamine also plays a pivotal role in the (often severe) pain that these patients may develop at the site of implantation, since histamine may sensitize the transient reporter potential channel V1 (TRPV1), one of the nociceptors [Citation72].
Figure 2. Pathophysiology of breast implant illness (a form of autoimmuneinflammatory/autoimmunityinflammatory syndrome induced by adjuvants).
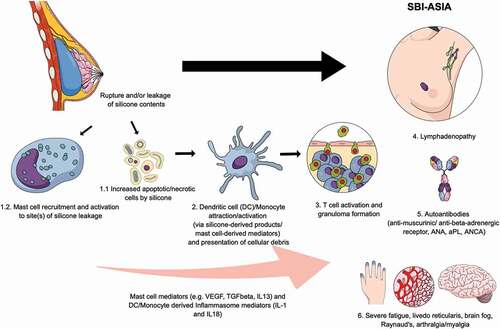
Foreign implanted materials such as silicone breast implants, silicones in other materials, polypropylene mesh, and prosthetic materials used for arthroplasty are all known to cause a systemic disease with clinical features as seen in ASIA. Importantly, virtually all implanted materials may induce a foreign body giant cell reaction [Citation73]. Furthermore, the biomaterial may deliver a ‘danger signal’ to the immune system and subsequently result in an enhanced immune response acting as an adjuvant [Citation69,Citation74,Citation75].
To explain how adjuvants interact with the immune system several mechanisms have been proposed:
Induction of a progressive release of the antigen (Ag) and blocking its clearance resulting in a longer exposure to antigen-presenting cells (APCs);
Promotion of the translocation of Ag to lymph nodes where Ag can be recognized by T cells;
Conversion from soluble Ag into a particulate form, which is subsequently phagocytosed by APCs such as macrophages, dendritic cells (DCs), and B cells;
Stimulating danger signals;
Induction of the release of inflammatory cytokines;
Interaction with Toll-like receptors (TLRs) and nucleotide oligomerization domain-like receptors including the NALP3 inflammasome.
Most importantly, adjuvants stimulate the NALP3 inflammasome pathway. The NALP3 inflammasome pathway comprises a multiprotein signaling complex containing NALP3 and ASC, an adaptor protein, responsible for the activation of intracellular enzymes that result in the production of inflammatory cytokines from immune cells, including IL1β and IL18 through activation of caspase 1. The NALP3 inflammasome is a primary sensing mechanism by which metal debris, aluminum salts, silicones, and other adjuvants induce the secretion of pro-inflammatory cytokines and recruit myeloid-lineage cells [Citation76].
Together, these lines of evidence indicate that these adjuvants are capable of evoking immune ‘memory.’ T cells and B cells, contain the amnestic, ‘memory’ features of acquired immunity through selective programming and dating of specific lymphocyte clones. Moreover, these cells play a pivotal role in orchestrating the recruitment, activation, and effector functions of other cells of the immune system, in an ongoing process through cellular and molecular crosstalk. Lymphocytes often dominate implant-associated ectopic lymphoid structures. In joint implants, this is called aseptic lymphocytic (lymphocyte-dominated) vasculitis-associated lesions (ALVAL) [Citation77].
Pattern recognition receptors, such as Toll-like receptor 4 (TLR4) serve as critical triggers of inflammation following recognition of endogenous ‘alarmin’ molecules released by injured tissues such as heat-shock proteins, biglycan fragments, and heparan sulfates [Citation78]. Adjuvants have been shown to directly activate TLR4, promoting local inflammation and tissue remodeling through NFkB-mediated cytokine production [Citation79].
Release of alarm signals from injured host tissues couple initial implantation to immediate inflammatory and innate immune responses; however, tissue injury, presenting as necrosis in association with failed or compromised implants may perpetuate these maladaptive pathways [Citation80].
The association between implants and ASIA likely results in the following scenario: mast cells, macrophages and, subsequently, inflammasomes are activated, resulting in the production of cytokines such as interleukin-1β. Reactive oxygen species (ROS) and reactive nitrogen species are also produced. Subsequently, apoptosis of macrophages occurs, and neutrophils are attracted. These neutrophils are activated, produce ROS, and release enzymes such as myeloperoxidase. Additionally, materials from implants are transported to the regional lymph nodes, resulting in a pronounced adjuvant effect. In animal models, it has been shown that implants such as silicone breast implants and polypropylene meshes induce an adjuvant effect and increase the susceptibility to and/or exacerbate autoimmune diseases. In non-susceptible animals, however, autoimmunity could not be induced [Citation81].
6.2. Pathophysiologic findings in SBI patients
Silicones trigger a histiocytic reaction with giant foreign body cells that form granulomas. In addition, Wolfram et al. [Citation82] demonstrated that silicone breast implants trigger locally an immune response through activated TH1/TH17 cells, suggesting that ensuing fibrosis is promoted by the production of inflammatory cytokines such as IL-17, IL-6, IL-8, and other growth factors as a consequence of faltering function of local T regulatory cells.
ASIA/BII patients often have humoral immune deficiencies [Citation29] and a suppressed natural killer cell activity [Citation83].
It has been recently demonstrated that exposure of small silicone particles can induce – in vitro – cell death as was found for human cultured Jurkat cells [Citation84] suggesting that immune cells may be killed by silicones.
Silicones are not only deposited around the capsule but also migrate through the periprosthetic capsule and can be demonstrated at distant sites [Citation40,Citation85]. Recently, it was demonstrated that this so-called ‘gel bleed’ occurs not only in women who have ‘noncohesive’ implants but also in patients with ‘cohesive’ silicone gel breast implants [Citation86] suggesting that systemic immune responses may be triggered by migrated silicones as well.
Furthermore, Lee et al. demonstrated biofilm formation with Propionibacterium acnes and/or Staphylococcus epidermidis in 36% of their 50 patients with ASIA/BII, whereas a biofilm was only observed in 6% of 50 SBI patients without ASIA/BII that underwent an explantation [Citation68]. The authors suggested that this chronic indolent infection can cause systemic symptoms as well. Otherwise, it has been suggested that platinum that is released from the SBI may cause systemic symptoms [Citation87].
6.3. Evidence that Breast Implants Can Cause ASIA
Patients are considered to have ASIA when either two major or one major and two minor clinical criteria are present (Citation45; ). Breast implant patients with autoimmune symptoms generally satisfy at least two of these major clinical criteria.
The exposure to breast implants precedes onset or worsening of symptoms, typical clinical manifestations appear, and removal of breast implants induces improvement. The latter criterion is somewhat unique to ASIA, and is key on causation, as these clinical manifestations generally do not spontaneously resolve, and these autoimmune symptoms would not be expected to resolve after surgery, absent the removal of some triggering stimulus. We will discuss each of these criteria in detail.
6.4. Exposure to breast implants precedes the onset or worsening of symptoms
Apart from the clinical trials, which did not systematically assess adverse events, most research on adverse events associated with breast implants relies upon self-report as to when the symptoms began. Although this introduces a minor element of uncertainty, as women may have faulty memories, or even biases, regarding the timing of the onset of symptoms, the consistency in the types of complaints women report as occurring after breast implants provides some reassurance as to the validity of their recollections.
6.5. The appearance of ‘typical’ clinical manifestations
As discussed above, patients suffering from ASIA tend to present with a cluster of similar clinical characteristics including chronic fatigue, myalgia, arthralgia, neurological and skin manifestations, sicca (dry eyes and dry mouth), cognitive impairment (‘brain fog’), and fever. This same diverse but predictable cluster of symptoms is observed in breast implant patients [Citation1,Citation29]. Due to chronic activation of the immune system, genetically prone patients may progress from experiencing ASIA to developing full-blown autoimmune diseases, immune deficiency, allergy, and/or lymphomas (ALCL). While adjuvant removal (at least where complete removal with capsulectomy is possible) often resolves all ASIA symptoms (as will be discussed in the next section), once the person’s autoimmune condition progresses to autoimmune disease (e.g. rheumatoid arthritis), it is rare for full recovery after removal of the adjuvant and in these cases additional treatment (e.g. with immune-suppressing drugs) is needed.
6.6. Removal of inciting agent induces improvement
Another major criterion of ASIA is that the symptoms and signs that began after implantation (such as chronic fatigue or widespread pain) improve after explantation of the inciting medical device. The cessation or reduction of symptoms after removal (also called ‘dechallenge’) is an extremely important observation in diagnosing ASIA, and in determining causation. Such improvement has been well documented in patients with silicone breast implants [Citation1,Citation7,Citation88], and surgical mesh implants [Citation62].
In summary, from these studies, it can be concluded that many women who developed symptoms after placement of breast implants, including symptoms such as fatigue, arthralgias, myalgia’s, hair loss, and cognitive impairment, had a significant improvement in symptoms and quality of life when the breast implants were removed. All of the above studies support the diagnosis of ASIA, caused by breast implants in patients with breast implants.
Improvement in the ASIA symptoms (fatigue, arthralgia, muscle pain and weakness, cognitive impairment, alopecia, widespread pain, and allergies) after removal of breast implants also provides confirming evidence of causation. These symptoms would not be expected to improve spontaneously after removal of breast implants if they were not caused by the device. Similar symptoms are seen in idiopathic chronic fatigue syndrome and fibromyalgia, but spontaneous improvements are extremely rare. Improvement upon explantation of breast implants points to a different pathophysiology for the autoimmune-like symptoms seen in patients with breast implants.
7. Biological plausibility of breast implants as a cause of ASIA
Apart from the general mechanisms by which implants induce a foreign body reaction, described above, specific components of the breast implants may exacerbate the above-described processes.
Breast implants cause a foreign body reaction (‘FBR’) creating a chronic inflammatory response that results in scarring around the device. This FBR occurs quickly after implantation, and within a few days after implantation granulomatous inflammation is observed with the formation of foreign body giant cells around the device [Citation89]. The chronic inflammation is characterized by an increased turnover of cells even years after implantation. When breast implants are explanted, these foreign body reactions are histologically observed.
7.1. Approaches to causation
While arguably the strongest evidence [Citation90], randomized controlled trials are not always feasible or ethical when attempting to assess whether certain exposures causally alter disease risk. For studies of etiology, a systematic review of prospective cohort studies is considered the highest level of evidence, but again, such studies may not be feasible, for example, for rare diseases. Instead, the assessment of causation may need to be inferred from the ‘harmony of evidence’ – the accumulation of evidence from different, including multidisciplinary, approaches that consistently tells the same story.” [Citation91].
7.2. Koch’s Postulates are overly rigorous
Koch’s postulates are rigorous criteria that were proposed to assess the causal relationship between a microbe and a disease in 1884. In 2002, we adapted the Koch postulates in an attempt to assess which diseases with a suspected autoimmune etiology are caused by an autoimmune reaction [Citation92]. The adapted postulates are as follows:
The specific adaptive immune response is directed to the affected organ or tissue;
Autoreactive T cells and/or autoantibodies are present in the affected organ or tissue;
Autoreactive T cells and/or autoantibodies can transfer the disease to healthy individuals or animals;
Immunization with the autoantigen induces the disease in animal models;
Elimination or suppression of the autoimmune response prevents disease progression or even ameliorates the clinical manifestation.
However, when well-known autoimmune diseases such as multiple sclerosis, celiac disease, myasthenia gravis, and granulomatosis with polyangiitis were evaluated using these postulates, none were found to fully fulfill demands of the postulates. One reason is that these conditions involve co-factors, such as infectious or triggering agents (such as S. aureus in granulomatosis with polyangiitis, gluten in celiac disease, or a foreign body in ASIA). From this exercise, we concluded that Koch’s postulates are far too stringent, and thus cannot reasonably be employed to determine causation for the wide array of diseases with a suspected autoimmune etiology.
7.2.1. Bradford Hill criteria
In determining whether an association between a triggering event and an outcome is causal, scientists often consider the Bradford Hill criteria [Citation93].
The Bradford Hill criteria are consistent in many ways with the ASIA criteria described in detail above.
7.2.2. Causality assessment
Determination of whether there is reasonable possibility that the product is etiologically related to the adverse event. Causality assessment includes, for example, assessment of temporal relationships, dechallenge/rechallenge information, association with (or lack of association with) underlying disease, presence (or absence) of a more likely cause, plausibility, etc. [Citation94].
Not all of the causation criteria need to be met to draw reliable causal conclusions.
The Bradford Hill criteria and relevant evidence regarding breast implants and autoimmune symptoms are as follows:
7.2.3. Strength of association
Where available, randomized controlled studies that are properly powered to study an outcome of interest are a strong method for demonstrating an association between an exposure and an outcome. However, if not conducted during the pre-approval stage for a drug or medical device, it is unusual for such studies to be conducted, for ethical and practical reasons [Citation95]. For breast implants, no randomized, double-blind, controlled studies prior to FDA approval are performed, and none have been conducted since approval.
In some circumstances, epidemiological studies of large populations can be used to show an association between an exposure and an outcome, by quantifying the relative risk of exposure and non-exposure. But epidemiological calculation of relative risk is not always necessary to demonstrate an association.
Some rarer and longer term effects of a drug, such as diabetes or birth defects, may require signal detection methods operating on large databases. But, for most adverse effects, seasoned clinicians allied to increasingly health literate patients are better placed than RCTs [Randomized Controlled Trials] to determine causality. [Citation90]
Cohort studies are a well-accepted method of demonstrating an association. Cohort studies that have looked at breast implants and autoimmune-like symptoms found an association [Citation7,Citation8,Citation21,Citation29,Citation46,Citation47,Citation96–109].
In addition, scientists give great weight to challenge–dechallenge evidence in circumstances where removal of the suspected cause of symptoms may result in relief from those symptoms.
7.2.4. When epidemiology studies of large populations are needed
In some circumstances, epidemiology studies of large populations are necessary evidence of causation. For example, to demonstrate that smoking increases the risk of lung cancer, the rate at which large groups of smokers versus nonsmokers develop the condition was studied, and, where possible, it was ensured that the groups were well-matched for potentially confounding factors like age, gender, overall health, work environment, etc. Challenge–dechallenge evidence to demonstrate the association in this example cannot be used, because once cancer develops, cessation of smoking will have no impact on the development of the disease. In such a context, it is necessary to study large populations of individuals and demonstrate that smokers have an increased risk of lung cancer compared to nonsmokers.
Large-population epidemiological studies of breast implants that have looked at autoimmune conditions are performed [Citation1,Citation15,Citation31]. Recent studies did find a statistically significant association between breast implants and ICD-9 codes indicating treatment for the autoimmune diseases such as systemic scleroderma, rheumatoid arthritis, Sjögren syndrome, and sarcoidosis [Citation31,Citation49].
7.2.5. When challenge–dechallenge–rechallenge evidence is valuable
If a woman with a breast implant is experiencing breast pain, and that pain disappears upon removal of the device, challenge–dechallenge evidence of a causal association between the breast pain and the implants is present. If the woman wished to try a second breast implant, and the pain reappeared, that ‘rechallenge’ would provide additional evidence of causation. If large numbers of women show this same pattern, broader conclusions about whether the breast implant is causally associated with the symptoms described can be drawn.
Bradford Hill himself acknowledged the role of challenge–dechallenge–rechallenge evidence in determining causality [Citation90] and the FDA mentions it in its definition of causal assessment [Citation94]. Unfortunately, however, there is often no rechallenge evidence, as requiring an individual to undergo re-exposure to a drug or device that is believed to have caused harm, simply to confirm causation, would be unethical. Once an association is suspected based upon challenge and dechallenge, a rechallenge should be undertaken to collect further evidence of causation only with great caution and a compelling need, because a rechallenge test might cause severe or even fatal reactions. Contraindications and ethical considerations must be taken into account and an individual risk–benefit calculation must be performed in every case. Where rechallenge would be unethical, challenge–dechallenge information can still be persuasive even in individual cases, but it becomes more persuasive when the challenge–dechallenge response is observed in many patients and by many doctors or researchers.
Challenge–dechallenge can provide evidence of general causality, even in a case study of one patient. Case studies have historically been viewed as a lower level of causality that view is beginning to change. In 2008, for instance, the publication of a few case reports revolutionized the treatment for severe hemangioma in infants [Citation110].
We have seen accumulating and persuasive challenge–dechallenge evidence in the case of breast implants, as described above. Reports in the FDA’s MAUDE database, and the published literature on breast implants, which includes both case reports and larger observational studies, demonstrate that symptoms begin after breast implant placement, and resolve in a great many patients after removal. The fact that multiple researchers have documented that autoimmune-like symptoms begin or worsen after breast implants are placed, and often decrease or resolve when breast implants are removed, strongly suggests a causal association.
7.3. Consistency
Consistency refers to a result that is replicated by multiple researchers. A consistent cluster of autoimmune-like symptoms, as well as a consistent pattern of challenge–dechallenge evidence, has been reported by several researchers independently studying breast implants in different populations of women using different methods, as described above.
7.4. Specificity of association
Specificity is the idea that an exposure causes one disease or syndrome, not a number of diverse diseases or syndromes. In breast implant patients, it is common to see a cluster of symptoms consistent with the diagnostic criteria for ASIA. It is not the case that SBIs are associated with sicca symptoms in some patients, fatigue in others, joint pain in still others, but rather that patients tend to have a predictable cluster of symptoms consistent with an ASIA diagnosis [Citation11]. This is what we call specificity of association.
The concept of specificity also involves ruling out alternative explanations (the medical concept of differential diagnosis). As discussed above, alternative causes of autoimmune disease can easily be ruled out when a patient’s symptoms begin after breast implants are implanted, and cease when the implants are removed.
The published research has demonstrated that autoimmune diseases are resistant to treatment and rarely resolve spontaneously since once immunological tolerance is broken, immunological memory will persist. So, when breast implants are removed, symptoms such as fatigue and arthralgias/myalgia’s disappear but autoimmune diseases such as rheumatoid arthritis and/or systemic sclerosis – if they have developed – still need to be treated according to our international guidelines.
7.5. Temporal relationship
Temporality is the idea that for an exposure to be considered causal, it must precede the onset of the disease. The published literature supports the conclusion that new autoimmune symptoms often appear for the first time after breast implants are implanted. This is also an ASIA criterion and was discussed above.
7.6. Dose response
This criterion is applicable to drugs and not devices.
7.7. Biological plausibility
The plausible biological mechanism by which breast implants may cause ASIA has been discussed at length above.
7.8. Coherence
Coherence is related to biological plausibility, and often involves a coherent set of findings from animal and human research. There is in vitro and animal evidence with regard to silicones and autoimmune symptoms, as described above.
7.9. Experimental evidence (where available)
Evidence from controlled experimental studies, which attempt to isolate and manipulate single variables, can be strong causation evidence, although only if the study is designed and powered to study a particular outcome [Citation90]. However, in the case of breast implants and autoimmune disease, no such studies have been conducted, in animals or in humans. This is not unusual, which is why experimental evidence is just one factor among many Bradford Hill outlined. That being said, breast implant removal (‘dechallenge’) creates a ‘natural experiment’ in that a single, possibly contributing variable is manipulated, while other factors remain the same for the individual suffering the autoimmune symptoms.
7.10. Consistency with other scientific knowledge (Analogy)
Hill implied that when there is strong evidence of a causal relationship between a particular agent and a specific disease, researchers should be more accepting of weaker evidence that a similar agent may cause a similar disease. Analogy has been interpreted to mean that when one causal agent is known, the standards of evidence are lowered for a second causal agent that is similar in some way. [Citation93]
After breast implants, peer-reviewed research demonstrates that patients may develop systemic autoimmune like symptoms [Citation11].
Silicone breast implants are the prototype of implant-related autoimmune complaints, with a huge body of published literature and medical experience, spanning over 50 years, supporting a causal link between the implants and autoimmune-like symptoms in susceptible individuals. Well-designed animal studies provide additional support [Citation81,Citation111,Citation112]. In the study by McDonald et al., researchers used an animal model, and put breast implants into some mice that were genetically predisposed to autoimmune disease, and other mice that were not predisposed in the predisposed mice, the breast implants accelerated the development of autoimmune disease and made it more severe than it would otherwise be. No autoimmune disease developed in mice that were not predisposed [Citation81]. In humans, a history of extensive allergies or an autoimmune condition, or a family history of autoimmune disease, should be a contraindication to the use of silicone breast implants [Citation11].
In autoimmune diseases, the immune system attacks normal cells and tissues in the body that are generally recognized as ‘self’ and do not normally trigger immune responses. In allergy, the immune system overreacts to a harmless substance such as dust, mold, or pollen. The inappropriate immune response in both conditions results from a deficient regulation of the immune response. This defect is partially genetically and partially environmentally determined. An increased occurrence of both disorders has been observed in families with these disorders.
In conclusion, we propose that the causal link between silicone breast implants and systemic symptoms should be accepted by the scientific community. As of September 2020, the FDA has suggested Labeling Recommendations to Improve Patient Communication for breast implants (‘a black box warning’) that breast implants have been associated with systemic symptoms.
Scientific consensus sometimes develops slowly on the issue of medical causation [Citation91]. Evidence has accumulated that implantable medical devices like silicone breast implants can create a foreign body reaction and are able to induce a chronic activation of the immune system, resulting in ASIA or hypersensitivity reactions in certain patients. Although there are differences in the components of these devices, all are foreign bodies and the patients at issue experience similar clinical characteristics after the foreign body implantation. With all of these implants, complete explantation helps to alleviate autoimmune symptoms, even without other treatment. This evidence of analogous autoimmune symptom activation in women with breast implants and surgical mesh further supports the conclusion that breast implants can cause ‘breast implant illness’.
8. Conclusion
There is a causal association between SBIs and systemic reactions such as those observed in breast implant illness/ASIA (autoimmune/autoinflammatory syndrome induced by adjuvants).
Breast implants cause characteristic systemic reactions in certain women, leading to symptoms of sufficient severity to warrant device removal. The morbidity suffered is variable. Pathophysiology is established and data from other medical implants support this causal relationship as well. Removal resolves the symptoms in most women and removal is the most effective treatment.
9. Expert opinion
(1) SBI women often present to surgeons with a self-diagnosis of BII requesting explantation. Plastic surgeons often state ‘that they do not believe in BII’. Currently, two hypotheses have been postulated for the development of BII:
(a) ‘Adjuvant hypothesis’: activation of the immune system by silicones that are leaked (known as silicone ‘bleeding’) and/or spread into the body after rupture of the implant.
(b) The ‘psychosomatic illness hypothesis’. Patients have mental health issues where SBI act as a nociceptive stimulator.
Based on current clinical and pathophysiological evidence, we conclude that BII is causally related to SBI. Hence, the ‘adjuvant hypothesis’ explains BII. Therefore, one should use the term ‘ASIA (autoimmune/autoinflammatory syndrome induced by adjuvants) due to silicone incompatibility syndrome’ rather than the nonspecific term ‘BII’ for this syndrome.
(2) Mental health issues in SBI patients are secondary to their BII/ASIA and are not the primary cause of symptoms
(3) In patients with fibromyalgia and in patients with chronic fatigue syndrome/myalgic encephalomyelitis (CFS/ME), physicians should always search for environmental triggers. Important triggers are implants that induce a foreign body reaction. SBI, mesh as used for hernia repair, and metal implants as used for arthroplasty are important triggers of these syndromes.
(4) Patients with a history of allergy are at risk of developing BII/ASIA. Also, patients with an established autoimmune disease or a familial predisposition to autoimmune disease are at risk of developing BII/ASIA. Before considering SBI, women should receive information regarding these risk factors. In addition, we advise plastic surgeons to perform preoperative measurement of immunoglobulins (IgG, IgA, IgM, IgE, and IgG subclasses) and referral to an internal medicine and/or rheumatology specialist on suspicion of abnormal clinical and/or biochemical findings.
(5) About one-quarter of women develop systemic symptoms after SBI. Before considering SBI, women should receive information that these symptoms may be caused by the SBI.
(6) Explantation with capsulectomy should be performed when SBI patients develop BII/ASIA. The longer the plastic surgeon waits, the more likely it is that patients will not recover from this explantation.
(7) Explantation with capsulectomy should be reimbursed when a diagnosis of BII/ASIA is made.
(8) Nonspecific laboratory findings such as elevated angiotensin-converting enzyme levels and decreased immunoglobulin levels are often present in patients with BII/ASIA. Better biomarkers should be developed for BII/ASIA
(9) In the future, the use of SBI should be replaced by using autologous material for breast augmentation or reconstruction. Otherwise, non-leaching elastomers should be further examined.
(10) Since long-term safety of SBI is still not convincingly established, women should give informed consent before they undergo SBI surgery. SBI surgery should be considered an experimental treatment for breast augmentation and/or reconstruction.
Article highlights
Synopsis of the role of silicone breast implants as triggers of systemic symptoms
The pathogenesis of breast implant illness is discussed.
Bradford Hill criteria for causality are applied to evaluate whether silicone breast implants cause breast implant illness.
Removal resolves the symptoms of breast implant illness in most women. Breast explantation is the most effective treatment of breast implant illness
Declaration of interest
J W Cohen Tervaert reports serving as the chair of the IDMC for InflaRx; he also reports lecture fees from Sanofi and Pfizer. He is a Member of the European Commission expert panel on medical devices. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Disclosure statement
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Additional information
Funding
References
- Cohen Tervaert JW, Colaris M, van der Hulst RR, et al. Silicone breast implants and autoimmune rheumatic diseases: myth or reality. Curr Opin Rheumatol. 2017 Jul;29(4):348–354.
- Deva AK, Cuss A, Magnusson M, et al. The “Game of Implants”: a perspective on the crisis-prone history of breast implants. Aesth Surg J. 2019;39(S1):S55–S65.
- [cited 2021 Jul 8]. Available from: www.natrelle.ca/en/qualitty
- [cited 2021 Jul 8]. Available from: https://www.fda.gov/medical-devices/breast-implants/medical-device-reports-systemic-symptoms-women-breast-implants
- [cited 2021 Jul 8]. Available from: https://www.fda.gov/consumers/consumer-updates/what-know-about-breast-implants
- Edworthy SM, Martin L, Barr SG, et al. A clinical study of the relationship between silicone breast implants and connective tissue disease. J Rheumatol. 1998 Feb;25(2):254–260.
- de Boer M, Colaris M, van der Hulst RR, et al. Is explantation of silicone breast implants useful in patients with complaints? Immunol Res. 2017 Feb;65(1):25–36.
- Colaris MJ, de Boer M, van der Hulst RR, et al. Two hundreds cases of ASIA syndrome following silicone implants: a comparative study of 30 years and a review of current literature. Immunol Res. 2017 Feb;65(1):120–128.
- Meijs J, de Vries-Bouwstra JK, Cohen Tervaert JW, et al. A case of late-onset systemic sclerosis with ruptured silicone breast implants. Neth J Med. 2018 Jul;76(5):243–248.
- Carruthers BM, van de Sande MI, De Meirleir KL, et al. Myalgic encephalomyelitis: international consensus criteria: review: ME: intl. Consensus criteria. J Intern Med. 2011;270(4):327–338.
- Cohen Tervaert JW. Autoinflammatory/autoimmunity syndrome induced by adjuvants (ASIA; Shoenfeld’s syndrome): a new flame. Autoimmun Rev. 2018;17(12):1259–1264.
- Colaris MJL, Cohen Tervaert JW, Ponds RWHM, et al. Subjective cognitive functioning in silicone breast implant patients: a cohort study. Plast Reconstr Surg Glob Open. 2021 Feb 17;9(2):e3394.
- Wolfe F, Clauw DJ, Fitzcharles MA, et al. Revisions to the 2010/2011 fibromyalgia diagnostic criteria. Semin Arthritis Rheum. 2016;46(3):319–329.
- Meier LG, Barthel HR, Seidl C. Development of polyarthritis after insertion of silicone breast implants followed by remission after implant removal in 2 HLA-identical sisters bearing rheumatoid arthritis susceptibility genes. J Rheumatol. 1997;24(9):1838–1841.
- Balk EM, Earley A, Avendano EA, et al. Long-term health outcomes in women with silicone gel breast implants: a systematic review. Ann Intern Med. 2016;164(3):164–175.
- Shoaib BO, Patten BM, Calkins DS. Adjuvant breast disease: an evaluation of 100 symptomatic women with breast implants or silicone fluid injections. Keio J Med. 1994;43(2):79–87.
- Dagan A, Kogan M, Shoenfeld Y, et al. When uncommon and common coalesce: adult onset Still’s disease associated with breast augmentation as part of autoimmune syndrome induced by adjuvants (ASIA). Clin Rheumatol. 2016;35(6):1643–1648.
- Freundlich B, Altman C, Snadorfi N, et al. A profile of symptomatic patients with silicone breast implants: a Sjögrens-like syndrome. Semin Arthritis Rheum. 1994;24(1 Suppl 1):44–53.
- Mavromatis BH, Tzioufas AG, Moutsopoulos HM. Sjögren-like disease and silicone implants: a Greek experience. J Clin Rheumatol. 1998;4(3):147–150.
- Nayfe R, Uthman I, Aoun J, et al. Seronegative antiphospholipid syndrome. Rheumatology (Oxford). 2013;52(8):1358–1367.
- Maijers MC, de Blok CJ, Niessen FB, et al. Women with silicone breast implants and unexplained systemic symptoms: a descriptive cohort study. Neth J Med. 2013;71(10):534–540.
- Frieri M, Patel R, Celestin J. Mast cell activation syndrome: a review. Curr Allergy Asthma Rep. 2013;13(1):27–32.
- Maharaj S. An atypical immune-inflammatory disorder secondary to breast implant exposure. J Long Term Eff Med Implants. 2012;22(1):33–48.
- Spencer TR, Schur PM. The challenge of multiple chemical sensitivity. J Environ Health. 2008;70(10):24–27.
- David PR, Dagan A, Colaris M, et al. Churg-Strauss syndrome: singulair or silicone (or both?). Isr Med Assoc J. 2016;18(3–4):168–170.
- Dragu A, Theegarten D, Bach AD, et al. Intrapulmonary and cutaneous siliconomas after silent silicone breast implant failure. Breast J. 2009;15(5):496–499.
- Gopinath PP, Ali A, Van Tornout F, et al. Chronic silicone embolism syndrome due to PIP breast implant leakage: a new entity? Histopathology. 2015;66(6):904–906.
- Bois MC, Hu X, Roden AC, et al. Increasing pulmonary infiltrates in a 72-year-old woman with metastatic breast cancer. Chest. 2014 Dec;146(6):e208–e211.
- Cohen Tervaert JW, Kappel RM. Silicone implant incompatibility syndrome (SIIS): a frequent cause of ASIA (Shoenfeld’s syndrome). Immunol Res. 2013;56(2–3):293–298.
- Grahame R, Bird HA, Child A. The revised (Brighton 1998) criteria for the diagnosis of benign joi hypermobility syndrome (BJHS). J Rheumatol. 2000;27(7):1777–1779.
- Watad A, Rosenberg V, Tiosano S, et al. Silicone breast implants and the risk of autoimmune/rheumatic disorders: a real-world analysis. Int J Epidemiol. 2018 Dec 1;47(6):1846–1854.
- Martins F, Sofiya L, Sykiotis GP, et al. Adverse effects of immune-checkpoint inhibitors: epidemiology, management and surveillance. Nat Rev Clin Oncol. 2019 Sep;16(9):563–580.
- Quagliariello V, Passariello M, Coppola C, et al. Cardiotoxicity and pro-inflammatory effects of the immune checkpoint inhibitor Pembrolizumab associated to Trastuzumab. Int J Cardiol. 2019 Oct 1;292:171–179.
- Quagliariello V, De Laurentiis M, Cocco S, et al. NLRP3 as putative marker of ipilimumab-induced cardiotoxicity in the presence of hyperglycemia in estrogen-responsive and triple-negative breast cancer cells. Int J Mol Sci. 2020 Oct 21;21(20):7802.
- Clauw DJ. What is the meaning of ‘small fiber neuropathy’ in fibromyalgia? Pain. 2015;156(11):2115–2116.
- Teuber SS, Reilly DA, Howell L, et al. Severe migratory granulomatous reactions to silicone gel in 3 patients. J Rheumatol. 1999;26(3):699–704.
- Wolfram D, Oberreiter B, Mayerl C, et al. Altered systemic serologic parameters in patients with silicone mammary implants. Immunol Lett. 2008;118(1):96–100.
- Colaris MJL, van der Hulst RR, Tervaert JWC. Vitamin D deficiency as a risk factor for the development of autoantibodies in patients with ASIA and silicone breast implants: a cohort study and review of the literature. Clin Rheumatol. 2017 May;36(5):981–993.
- Halpert G, Watad A, Tsur AM, et al. Autoimmune dysautonomia in women with silicone breast implants. J Autoimmun. 2021 Jun;120:102631. Epub 2021 Mar 31.
- Castro C, Fernandes D, Mendonça M, et al. Silicone-induced granuloma of breast implant capsule mimicking anaplastic large cell lymphoma. Breast J. 2020;26(5):1028–1030.
- Kim JR, Chang M-C, Kim YM, et al. Usefulness of sonography for diagnosis of siliconomas mimicking metastatic lymphadenopathy on computed tomography. J Ultrasound Med. 2015;34(1):167–169.
- Vermeulen RC, Kurk RM, Visser FC, et al. Patients with chronic fatigue syndrome performed worse than controls in a controlled repeated exercise study despite a normal oxidative phosphorylation capacity. J Transl Med. 2010 Oct 11;8(1):93.
- Centeno JA, Mullick FG, Panos RG, et al. Laser-Raman microprobe identification of inclusions in capsules associated with silicone gel breast implants. Mod Pathol. 1999 Jul;12(7):714–721.
- Kappel RM, Boer LL, Dijkman H. Gel bleed and rupture of silicone breast implants investigated by light-, electron microscopy and energy dispersive X-ray analysis of internal organs and nervous tissue. Clin Med Rev Case Rep. 2016;3(3):087.
- Shoenfeld Y, Agmon-Levin N. ASIA’-autoimmune/inflammatory syndrome induced by adjuvants. J Autoimmun. 2011;36(1):4–8.
- Miseré RML, Colaris MJL, Tervaert JWC, et al. The prevalence of self-reported health complaints and health-related quality of life in women with breast implants. Aesthet Surg J. 2021 May 18;41(6):661–668.
- Giltay EJ, Bernelot Moens HJ, Riley AH, et al. Silicone breast prostheses and rheumatic symptoms: a retrospective follow up study. Ann Rheum Dis. 1994;53(3):194–196.
- Sunnquist M, Jason LA, Nehrke P, et al. A comparison of case definitions for myalgic encephalomyelitis and chronic fatigue syndrome. J Chronic Dis Manag. 2017;2(2):1013.
- Hughes GR, Khamashta MA. Seronegative antiphospholipid syndrome. Ann Rheum Dis. 2003;62(12):1127.
- Coroneos CJ, Selber JC, Offodile AC 2nd, Butler CE, Clemens MW. US FDA breast implant postapproval studies: long-term outcomes in 99,993 patients. Ann Surg. 2019 Jan;269(1):30–36.
- Cooper GS, Bynum ML, Somers EC. Recent insights in the epidemiology of autoimmune diseases: improved prevalence estimates and understanding of clustering of diseases. J Autoimmun. 2009 Nov-Dec;33(3–4):197–207.
- Miranda RN, Medeiros LJ, Ferrufino-Schmidt MC, et al. Pioneers of breast implant–associated anaplastic large cell lymphoma: history from case report to global recognition. Plast Reconstr Surg. 2019 Mar;143(3S):7S–14S.
- de Boer M, van Leeuwen FE, Hauptmann M, et al. Breast implants and the risk of anaplastic large-cell lymphoma in the breast. JAMA Oncol. 2018 Mar 1;4(3):335–341.
- Keech JA Jr, Creech BJ. Anaplastic T-cell lymphoma in proximity to a saline-filled breast implant. Plast Reconstr Surg. 1997;100(2):554–555.
- de Jong D, Vasmel WLE, de Boer JP, et al. Anaplastic large-cell lymphoma in women with breast implants. JAMA. 2008 Nov 5;300(17):2030.
- Los-de Vries GT, de Boer M, van Dijk E, et al. Chromosome 20 loss is characteristic for breast implant-associated anaplastic large cell lymphoma. Blood. 2020 Sep 8;136(25):2927–2932. blood.2020005372. blood.2020005372.
- Collett DJ, Rakhorst H, Lennox P, et al. Current risk estimate of breast implant-associated anaplastic large cell lymphoma in textured breast implants. Plast Reconstr Surg. 2019 March;143(3S):30S–40S.
- Segal Y, Dahan S, Sharif K, et al. The value of Autoimmune Syndrome Induced by Adjuvant (ASIA) - Shedding light on orphan diseases in autoimmunity. Autoimmun Rev. 2018 May;17(5):440–448.
- Coffman R, Sher A, Seder R. Vaccine adjuvants: putting innate immunity to work. Immunity. 2010;33(4):492–503.
- Ruiz JT, Lujan L, Blank M, et al. Adjuvants-and vaccines-induced autoimmunity: animal models. Immunol Res. 2017;65(1):55–65.
- See FDA report on breast implant special topics from advisory panel, March 25th and 26th, 2019. [cited 2021 Jul 8]. Available from: https://www.fda.gov/media/122956/download
- Cohen Tervaert JW. Autoinflammatory/autoimmunity syndrome induced by adjuvants (Shoenfeld’s syndrome) in patients after a polypropylene mesh implantation. Best Prac Res Clin Rheumatol. 2018 Aug;32(4):511–520.
- Caravantes-Cortes MI, Roldan-Valadez E, Zwojewski-Martinez RD, et al. Breast prosthesis syndrome: pathophysiology and management algorithm. Aesthetic Plast Surg. 2020 Oct;44(5):1423–1437.
- Major MR, Wong VW, Nelson ER, et al. The foreign body response: at the interface of surgery and bioengineering. Plast Reconstr Surg. 2015 May;135(5):1489–1498.
- Anderson M, Rodriguez A, Chang DT. Foreign body reaction to biomaterials. Semin Immunol. 2008 Apr;20(2):86–100.
- Ajdic D, Zoghbi Y, Gerth D, et al. The relationship of bacterial biofilms and capsular contracture in breast implants. Aesthet Surg J. 2016 Mar;36(3):297–309.
- Langbach O, Kristoffersen AK, Abesha-Belay E, et al. Oral, intestinal, and skin bacteria in ventral hernia mesh implants. J Oral Microbiol. 2016 Jul 29;8(1):31854.
- Lee M, Ponraja G, McLeod K, et al. Breast Implant Illness: a Biofilm Hypothesis. Plast Reconstr Surg Glob Open. 2020 Apr 30;8(4):e2755.
- Babensee JE. Interaction of dendritic cells with biomaterials. Semin Immunol. 2008;20(2):101–108.
- Nolfi AL, Brown BN, Liang R, et al. Host response to synthetic mesh in women with mesh complications. Am J Obstet Gynecol. 2016 Aug;215(2):206.e1–206.e8.
- Zdolsek JWE, Tang L, Tang L. Histamine release and fibrinogen adsorption mediate acute inflammatory responses to biomaterial implants in humans. J Transl Med. 2007;5(1):31.
- Wouters MM, Balemans D, Van Wanrooy S, et al. Histamine receptor H1-mediated sensitization of TRPV1 mediates visceral hypersensitivity and symptoms in patients with irritable bowel syndrome. Gastroenterology. 2016 Apr;150(4):875–887.
- Junge K, Binnebösel M, Von Trotha KT, et al. Mesh biocompatibility: effects of cellular inflammation and tissue remodelling. Langenbecks Arch Surg. 2012 Feb;397(2):255–270.
- Matzelle MM, Babensee JE. Humoral immune responses to model antigen co-delivered with biomaterials used in tissue engineering. Biomaterials. 2004 Jan;25(2):295–304.
- Bennewitz NL, Babensee JE. The effect of the physical form of poly(lactic-co-glycolic acid) carriers on the humoral immune response to co-delivered antigen. Biomaterials. 2005 Jun;26(16):2991–2999.
- Kool M, Pétrilli V, De Smedt T. Cutting edge: alum adjuvant stimulates inflammatory dendritic cells through activation of the NALP3 inflammasome. J Immunol. 2008 Sep 15;181(6):3755–3759.
- [cited 2021 Jul 8]. Available from: https://radiopaedia.org/articles/metal-on-metal-pseudotumour?lang=us
- Cobelli N, Scharf B, et al. Mediators of the inflammatory response to joint replacement devices. Nat Rev Rheumatol. 2011 Sep 6;7(10):600–608.
- Dowling J, Mansell A. Toll‐like receptors: the Swiss army knife of immunity and vaccine development. Clin Transl Immunology. 2016;5(5):e85.
- Grammatopoulos G, Munemoto M, Inagaki Y, et al. The diagnosis of infection in metal-on-metal hip arthroplasties. J Arthroplasty. 2016 Nov;31(11):2569–2573.
- McDonald AH, Sanger JR, Schneider M, et al. Silicone gel enhances the development of autoimmune disease in New Zealand black mice but fails to induce it in BALB/cAnPt mice. Clin Immunol Immunopathol. 1998;87(3):248–255.
- Wolfram D, Rabensteiner E, Grundtman C, et al. T regulatory cells and TH17 cells in peri-silicone implant capsular fibrosis. Plast Reconstr Surg. 2012 Feb;129(2):327e–337e.
- Campbell A, Brautbar N, Vojdani A. Suppressed natural killer cell activity in patients with silicone breast implants: reversal upon explantation. Toxicol Ind Health. 1994 May-Jun;10(3):149–154.
- Onnekink C, Kappel RM, Boelens WC, et al. Low molecular weight silicones induce cell death in cultured cells. Sci Rep. 2020 Jun 12;10(1):9558.
- Todorov TI, de Bakker E, Smith D, et al. A case of silicone and sarcoid granulomas in a patient with “Highly Cohesive” silicone breast implants: a histopathologic and Laser Raman microprobe analysis. Int J Environ Res Public Health. 2021 Apr 24;18(9):4526.
- Dijkman HBPM, Slaats I, Bult P. Assessment of silicone particle migration among women undergoing removal or revision of silicone breast implants in the Netherlands. JAMA Network Open. 2021 Sep 1;4(9):e2125381.
- Lykissa ED, Maharaj SV. Total platinum concentration and platinum oxidation states in body fluids, tissue, and explants from women exposed to silicone and saline breast implants by IC-ICPMS. Anal Chem. 2006 May 1;78(9):2925–2933.
- Wee CE, Younis J, Isbester K, et al. Understanding breast implant illness, before and after explantation: a patient-reported outcomes study. Ann Plast Surg. 2020 Jul;85(S1 Suppl 1):S82–S86.
- See FDA Report: Biological Responses to Metal Implants September 2019. [cited 2021 Jul 8]. Available from: https://www.fda.gov/media/131150/download
- Healy D, Mangin D. Clinical Judgments, not algorithms, are key to patient safety. BMJ. 2019;367:5777.
- Lucas R, Rodney Harris R. On the nature of evidence and ‘Proving’ causality: smoking and lung cancer vs. sun exposure, vitamin D and multiple sclerosis. Int J Environ Res Public Health. 2018 Aug 12;15(8):ii: E1726.
- Damoiseaux JGMC, Cohen Tervaert JW. The definition of autoimmune disease: are Koch’s postulates applicable? Netherlands J Med. 2002 Aug;60(7):266–268.
- Fedak KM, Bernal A, et al. Applying the Bradford Hill criteria in the 21st century: how data integration has changed causal inference in molecular epidemiology. Emerg Themes Epidemiol. 2015 Sep 30;12(1):14. eCollection 2015.
- Davidovici BB, Wolf R. The challenge of drug-rechallenge: Facts and controversies. Clinics Derm. 2010;28(3):349–353 .
- Song JW, Chung KC. Observational studies: cohort and case-control studies. Plast Reconstr Surg. 2010;126(6):2234–2242.
- Cuellar ML, Gluck O, Molina JF, et al. Silicone breast implant–associated musculoskeletal manifestations. Clin Rheumatol. 1995;14(6):667–672.
- Contant CM, Swaak AJ, Obdeijn AI, et al. A prospective study on silicone breast implants and the silicone-related symptom complex. Clin Rheumatol. 2002;21(3):215–219.
- Shoaib BO, Patten BM. Human adjuvant disease: presentation as a multiple sclerosis-like syndrome. South Med J. 1996;89(2):179–188.
- Gaubitz M, Jackisch C, Domschke W, et al. Silicone breast implants: correlation between implant ruptures, magnetic resonance spectroscopically estimated silicone presence in the liver, antibody status and clinical symptoms. Rheumatol (Oxford). 2002;41(2):129–135.
- De Jong WH, Goldhoorn CA, Kallewaard M, et al. Study to determine the presence of antipolymer antibodies in a group of Dutch women with a silicone breast implant. Clin Exp Rheumatol. 2002;20(2):151–160.
- Englert H, Joyner E, Thompson M, et al. Augmentation mammoplasty and “silicone-osis. Intern Med J. 2004;34(12):668–676.
- Vasey FB, Havice DL, Bocanegra TS, et al. Clinical findings in symptomatic women with silicone breast implants. Semin Arthritis Rheum. 1994;24(1 Suppl 1):22–28.
- Vermeulen RC, Scholte HR. Rupture of silicone gel breast implants and symptoms of pain and fatigue. J Rheumatol. 2003;30(10):2263–2267.
- Bridges AJ, Conley C, Wang G, et al. A clinical and immunologic evaluation of women with silicone breast implants and symptoms of rheumatic disease. Ann Intern Med. 1993;118(12):929–936.
- Freundlich B, Altman C, Snadorfi N, et al. A profile of symptomatic patients with silicone breast implants: a Sjögrens-like syndrome. Semin Arthritis Rheum. 1994;24(1 Suppl 1):44–53.
- Solomon G. A clinical and laboratory profile of symptomatic women with silicone breast implants. Semin Arthritis Rheum. 1994;24(1 Suppl 1):29–37.
- Peters W, Smith D, Fornasier V, et al. An outcome analysis of 100 women after explantation of silicone gel breast implants. Ann Plast Surg. 1997;39(1):9–19.
- Melmed EP. A review of explantation in 240 symptomatic women: a description of explantation and capsulectomy with reconstruction using a periareolar technique. Plast Reconstr Surg. 1998;101(5):1364–1373.
- Wells KE, Roberts C, Daniels SM, et al. Psychological and rheumatic symptoms of women requesting silicone breast implant removal. Ann Plast Surg. 1995;34(6):572–577.
- Kiene H, Hamre HJ, Kienle GS, et al. In support of clinical case reports: a system of causality assessment. Glob Adv Health Med. 2013 Mar;2(2):64–75.
- Schaefer CJ, Lawrence WD, Wooley PH. Influence of long term silicone implantation on type II collagen induced arthritis in mice. Ann Rheum Dis. 1999;58(8):503–509.
- Schaefer CJ, Wooley PH. The influence of silicone implantation on murine lupus in MRL lpr/lpr mice. J Rheumatol. 1999 Oct;26(10):2215–2221.
