ABSTRACT
Introduction
Oral immunotherapy (OIT) is effective at inducing desensitization in food-allergic individuals, and is a valid therapeutic option for those allergic to peanut, cow’s milk and egg. However, there is a high rate of dose-related adverse events, and at least one fatality to OIT has been reported.
Areas covered
We provide an update on the broader framework of issues which will impact on the availability and uptake of OIT.
Expert opinion
The need for standardized products remains controversial. A licensed product exists for peanut-OIT, but OIT can also be safely achieved using peanut-containing foods at much lower cost. For other allergens, OIT can only be done with non-pharma products – something which has been done safely for over 2 decades. There is a need to develop personalized protocols for OIT, particularly for the 20% of patients unable to tolerate standard OIT. Cost-effectiveness is dependent on improved quality of life, but evidence for this is currently lacking, and is a key evidence gap. OIT is likely to be cost-effective, particularly if noncommercial products are used. There may be a trade-off: in patients with lower reaction thresholds, a commercial product may be needed for initial updosing, until a level of desensitization is achieved when they can be switched to natural food products.
1. Introduction
Oral immunotherapy (OIT) offers a viable disease-modifying treatment strategy for food-allergic individuals, in contrast to traditional approaches to the management of food allergy (allergen avoidance, the provision of rescue medication for accidental reactions, and interval reassessment for possible resolution). The aim of OIT is to increase the reaction threshold to which any given food-allergic individual reacts (). It was originally hoped that OIT would offer a ‘cure’ for food allergy, however data now shows that treatment – which in general is successful in around 80% of individuals – results in an increase in reaction threshold (the amount of the food allergen which triggers a reaction) and may moderate the severity of any ensuing reaction – however, this is dependent on ongoing maintenance dosing[Citation1]. Sustained unresponsiveness, where the desensitization effect persists without the need for ongoing maintenance dosing, occurs only in the minority of treated patients, although this may be increased through longer duration of treatment[Citation2].
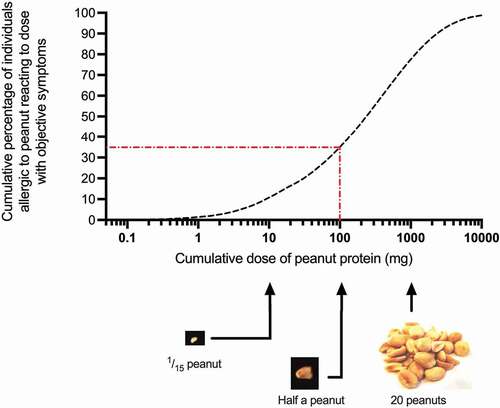
Figure 1. Population-based dose-distribution curve for reaction thresholds derived from 1306 double-blind placebo-controlled food challenges (DBPCFC) to peanut. Red dotted line shows (as an example) ED35, the eliciting dose which will trigger an objective allergic reaction in 35% of peanut-allergic individuals, in this case 100 mg which is approximately a half peanut. Data from Houben et al, Food Chem Toxicol. 2020 Dec;146:111,831. doi: 10.1016/j.fct.2020.111831.
The first product for peanut-OIT was approved in the USA and Europe in 2020. [Citation3,Citation4] However, OIT using semi-standardized protocols with food products available from the supermarket is not unusual in many countries [Citation5,Citation6]. There is ongoing debate as to whether the former option, using a food product produced to pharmaceutical-grade, according to GMP (Good Manufacturing Practice), is needed when other cheaper but less standardized non-pharma food products are available. More concerningly, existing OIT protocols fail in around 20% of patients, with the majority experiencing at least mild dose-related adverse events (AEs) at some stage [Citation1,Citation6]. Some individuals will experience anaphylaxis, and at least one death has been reported in a child undergoing OIT to cow’s milk[Citation7]. The question therefore is: is it worth it?
2. Historical and current context
Adverse reactions to food have been reported for at least 2 millennia. The Greek philosopher Hippocrates (circa 400 BC) described individuals who were unable to tolerate cheese, in contrast to the majority[Citation8]; whether this was related to what we now classify as allergy is unknown – cheese contains histamine which can mimic allergic symptoms in some individuals. Moses Maimonides, a Jewish physician, scholar and philosopher in 12th century Spain, provided written medical advice to the son of Al-Nasir Salah al-Din Yusuf ibn Ayyub (also known as Saladin), the first sultan of both Egypt and Syria), advising him to avoid milk, nuts and legumes which had been causing food-related asthma[Citation9]. However, it was only in the last century that there has been recognition of food allergy as an immune-mediated disease, as opposed to a nonimmune mediated adverse reaction for food. In fact, wider recognition of food allergy is relatively recent: it was only in 2000 that mandatory labeling of foods containing allergens was introduced in Europe[Citation10], with equivalent legislation passed in the USA through the Food Allergen Labeling and Consumer Protection Act (FALCPA) in 2004. [Citation11]
Food immunotherapy is not a new concept. The first successful report of OIT was published in 1908, and described desensitization in a 13-year-old boy with egg allergy[Citation12]. However, OIT has only been evaluated as a potential treatment modality in food allergy for the last 20 years, despite allergen immunotherapy being established for the treatment of venom allergy and allergic rhinitis for many more decades. In general, this has been due to a paucity of large clinical trials evaluating allergen immunotherapy for food allergy, although in some countries (Italy, Spain), OIT (for food allergy) has been undertaken for some years on the basis of small case series rather than extensive clinical trials data [Citation13,Citation14]. This might be because of an initial focus on using injected allergen extracts (in the same way these are used to treat allergic disease due to venom and aeroallergens), rather than considering the oral route as an valid means of administering immunotherapy. In 1997, Nelson et al. reported a small, double-blind, placebo-controlled trial using subcutaneous injection of peanut extract in 12 peanut-allergic adults; while the intervention did result in a level of desensitization, systemic reactions were common and it was concluded that the protocol would not be suitable for future use[Citation15]. This is likely to have led to a switch to research using the oral route as the preferred means of administration, which in turn has led to the establishment of OIT in some countries (notably, Italy and Spain) as part of routine clinical care, for some allergens (e.g. cow’s milk, egg), with national guidelines published from national specialist societies[Citation16]. In Japan, OIT was still regarded as a research intervention in national guidelines from 2011, but the latest 2020 guideline provides detailed guidance as to the implementation of OIT in clinical practice[Citation17]. The European Academy of Allergy and Clinical Immunology (EAACI) Food Allergy guideline did not recommend OIT for routine clinical use in 2014, [Citation18] but did publish a specific guideline recommending OIT could ‘be performed in research centers or in clinical centers with an extensive experience’ in 2018. [Citation19] National guidelines in Canada were published in 2020 which also promote the safe use of OIT in routine clinical practice[Citation20].
The drive toward larger clinical trials has come from the availability of more research funding (for example, through the CoFAR initiative) or due to the potential for commercialization (and thus profit-generation) of a standardized product (AR101) for OIT (Aimmune Therapeutics, CAM-Allergy, Prota Therapeutics, Alladapt, among others). This has resulted in larger datasets which have now formally demonstrated the efficacy (and relative safety) of oral immunotherapy in the clinical trials setting. The first systematic review of OIT were published in 2010, [Citation21] but as more trials (with more robust methodology) have been published, the quality and quantity of evidence has also increased (). [Citation22–27] In 2020, the Food and Drug Administration (FDA) approved AR101 (now called Palforzia®) for peanut-OIT, [Citation3] with the European marketing authorization following later that year. [Citation4] In 2021, Palforzia was approved by the National Institute for Health and Care Excellence in the United Kingdom (UK) for use in the state-funded health system – making the UK the first country to offer a commercial product for OIT through a state-funded healthcare system[Citation28]. However, there remains significant uncertainty in implementation and the treatment is not yet widely available in the UK.
Figure 2. Number of participants (receiving OIT) in randomized controlled trials (RCTs) of OIT reported in the literature since 2010. [Citation26,Citation27] The publication of various commercially-funded multicenter Phase 3 RCTs after 2017 for peanut have significantly increased the evidence base for OIT.
![Figure 2. Number of participants (receiving OIT) in randomized controlled trials (RCTs) of OIT reported in the literature since 2010. [Citation26,Citation27] The publication of various commercially-funded multicenter Phase 3 RCTs after 2017 for peanut have significantly increased the evidence base for OIT.](/cms/asset/f5ad341f-1e0b-4934-9b43-a9978933b8eb/ierm_a_2053675_f0002_oc.jpg)
Currently, Palforzia is the only commercial product available for OIT, specifically for peanut allergy. Palforzia is essentially defatted peanut flour which is produced, characterized and aliquoted according to GMP. As might be expected, some clinicians, predominantly in North America but also in the UK, have begun to offer their patients peanut-OIT using peanut flour at the same time as Palforzia was being considered by regulatory authorities. Data published from the USA has demonstrated that this approach is entirely feasible[Citation5]. This has resulted in significant debate as to whether a cheaper, non-pharma grade product or even food should be used for OIT when a commercial, but significantly more expensive pharma-grade product is available (see section 6).
3. Food ladders and OIT
The recent fatality reported in Canada [Citation7] brings a spotlight to the issue of whether the use of ‘food-ladders’ is a form of immunotherapy. ‘Food-ladders’ refer to ‘the process of gradually reintroducing food allergens into an individual’s diet,’ with ‘a step-wise progression from extensively heated/processed to less processed food’ which also takes into consideration the actual dose of allergen presented[Citation29]. Food-ladders were originally proposed for use in egg- and milk-allergic children with non-IgE-mediated food allergy (and thus not at risk of anaphylaxis), on the basis that many children with this form of food allergy outgrow the allergy over time, and often tolerate more processed forms of the allergen e.g. baked milk, baked egg[Citation29]. However, over time, they have also been used in children with IgE-mediated food allergy and thus at risk of anaphylaxis [Citation30–32]. Such an approach in younger children may well be justified when undertaken by experienced staff with appropriate patient supervision, and is supported by epidemiological data which shows that infants and preschool children are relatively protected from severe outcomes in anaphylaxis [Citation33,Citation34].
Up to 70% of children with food allergy to egg or cow’s milk are able to tolerate the allergen when presented in a baked food matrix, such as a cake or biscuit [Citation35,Citation36]. This does not seem to be related to the dose of allergen, but rather, due to heat-processing in the presence of a matrix such as wheat, which alters the allergenicity of the protein[Citation37]. The introduction of baked egg or milk into the diet of a child who is shown to tolerate it is not immunotherapy (although potentially, doing so may hasten resolution of that food allergy – but such data are currently lacking). [Citation36]
However, the slow and gradual introduction of foods using a ‘food-ladder’ which are not otherwise tolerated in normal serving amounts at the time of introduction is arguably a form of OIT[Citation32]. After all, food is being introduced by the oral route to induce desensitization in an individual who is not already tolerant to that food. Indeed, a joint statement from the Canadian and British national allergy societies following the death of a 9 year old child undergoing OIT using ‘baked milk’ implies that the egg/milk ladder is an alternative to OIT in younger children with IgE-mediated disease[Citation38]. Those allergic to ‘baked egg’ or ‘baked milk’ often report delayed and more severe reactions[Citation39], perhaps because the matrix also slows allergen absorption which allows more allergen to be eaten prior to symptoms. This highlights the inherent risks of using baked foods for OIT compared to native allergen where absorption is more predictable.
4. Outcome measures used to evaluate OIT
Different studies have utilized different outcome measures in defining successful OIT. [Citation1,Citation40] These range from ‘tolerance’ to a specific dose of allergen to composite outcomes which include a certain-fold increase on ‘tolerance’ due to treatment. In addition, there are significant data gaps relating to longer-term outcomes and the impact of ceasing regular maintenance dosing (i.e. presence of sustained unresponsiveness). As a result, those undertaking systematic reviews and meta-analyses often group ‘desensitization’ outcomes together, even though success may be defined in some studies as tolerance to 300 mg peanut protein (about 1½ peanuts), while in others, a far more stringent definition may be used e.g. tolerance to 5000 mg peanut protein. There is also no consensus as to what ‘tolerance’ means, with most studies defining a tolerated dose as one in which no objective symptoms occur; thus, a patient experiencing pruritus, nausea and abdominal pain to a dose would often be considered to have ‘tolerated’ that dose. Of note, the first approved commercial preparation for peanut-OIT (Palforzia) has been perceived by some as allowing patients to become ‘bite-proof’ to peanut exposure (a fairly ambiguous term, since a ‘bite’ of a food that does not contain peanut as an ingredient is very different from a bite of a confectionery item containing peanut butter). In the studies evaluating Palforzia, this translates to a ‘tolerated’ dose of 1000 mg peanut protein, which is likely to be more peanut than one might be exposed to in a ‘bite.’ However, there are instances when more peanut might be present, as evidenced by a case of fatal anaphylaxis to a curry which had been intentionally adulterated with peanut flour instead of almond[Citation41]. Such cases are fortunately rare, and hopefully becoming less likely given the successful prosecutions for manslaughter of those responsible.
There is a concern that in the presence of ‘cofactors’ (such as an intercurrent infection, use of medicines such as non-steroidal anti-inflammatory medication or exercise), a person’s reaction threshold may fall by an average of 50% and more in some individuals; thus, tolerance to 1000 mg on a ‘good day’ in the absence of co-factors may be insufficient protection when co-factors are present[Citation42]. We recently reported results from a systematic review and individual participant data meta-analysis of studies, summarizing challenge data from over 3151 peanut-allergic participants who underwent double-blind, placebo-controlled food challenge (DBPCFC) to peanut, including 534 of whom subsequently underwent a second peanut challenge without any disease-modifying intervention[Citation43]. Reaction thresholds within individuals varied by up to 3 logs, although this variation was limited to only a half-log change in 71.2% (95% CI, 56.2% to 82.6%) of individuals (). Thus, the change in threshold reported due to cofactors is well-within the inherent variability reported for reaction thresholds during DBPCFC, with the possible exception of food-dependent, exercise-induced anaphylaxis. In addition, these data provide a rationale for a minimum 10-fold increase in reaction threshold in OIT studies, since any change less than this could be due to inherent variability in the reaction threshold determined at food challenge.
Figure 3. Reproducibility of reaction thresholds determined at food challenge. Violin plot of the distributions of log change in reaction thresholds (from initial DBPCFC to repeat FC) for study participants within each included cohort. A half-log change in ED is equivalent to a shift in reaction threshold by 2 dosing increments when a PRACTALL-based semilog regimen is used. The red dashed line represents the median, and the red dotted line represents the interquartile range. Reproduced from Patel et al [Citation43] under the terms of the Creative Commons Attribution 4.0 International License.
![Figure 3. Reproducibility of reaction thresholds determined at food challenge. Violin plot of the distributions of log change in reaction thresholds (from initial DBPCFC to repeat FC) for study participants within each included cohort. A half-log change in ED is equivalent to a shift in reaction threshold by 2 dosing increments when a PRACTALL-based semilog regimen is used. The red dashed line represents the median, and the red dotted line represents the interquartile range. Reproduced from Patel et al [Citation43] under the terms of the Creative Commons Attribution 4.0 International License.](/cms/asset/e4e97b7f-2da9-47da-8fa8-27dbb9da1901/ierm_a_2053675_f0003_oc.jpg)
Whether this also holds true in the context of OIT is unknown. Reactions in patients established on maintenance OIT for peanut allergy have often been associated with co-factors such as concomitant viral illness, tiredness and menstruation as well as exercise [Citation44,Citation45], and the degree to which these cofactors alter reaction thresholds resulting in reactions to normally-tolerated OIT doses is unclear[Citation46]. This scenario highlights the need for longer-term, real-world efficacy data following OIT, to evaluate the impact of cofactors on accidental reactions and whether this changes after OIT.
There is also a lack of consistency in the defining and reporting dose-related AEs, which prevents a meaningful pooled analysis of treatment safety across studies [Citation1,Citation6]. For example, in the STOP-2 study, a UK Research Council-funded study of peanut OIT which has now resulted in the commercialization of peanut immunotherapy in at least one UK center, the authors did not report rates of anaphylaxis[Citation47]. One study participant self-administered intramuscular adrenaline on two occasions due to dose-related wheezing, and wheezing as an AE occurred after 0.41% of doses in 22% of (49) participants. Yet in a subsequent meta-analysis, it was reported that only a single participant in the study had dose-related anaphylaxis[Citation48].
The outcomes reported in clinical trials may not correspond to what patients (and their carers) want (). Rather than achieve a specific level of desensitization, patients may prefer to increase protection from accidental exposures to allergen, both in relation to any reaction and reducing the risk of a severe reaction. However, longer-term data is lacking in terms of OIT resulting in a reduction of symptoms due to accidental reactions to the trigger food following OIT, as highlighted in 2 recent cost-effectiveness analyses [Citation28,Citation49]. Given up to 50% of patients who undergo OIT report significant and ongoing taste aversion to the food they are allergic to (even during maintenance, following initial OIT)[Citation5], sustained unresponsiveness may arguably also be a key outcome for patients following OIT.
Table 1. Challenges associated with patient-desired outcome measures for food allergy desensitization. Reproduced from Duca et al [Citation1] under the terms of the Creative Commons Attribution 4.0 International License
Only a relative minority of studies have evaluated changes in health-related quality of life (HRQL) outcomes in participants undergoing food OIT, and those that have done tend to use surrogate reports e.g. parent-reported outcomes, rather than direct-evaluation of HRQL in the participants undergoing OIT – in whom HRQL may be impacted by low grade but persisting treatment-related AEs and the need to take a daily dose of the food they are aversive to[Citation1]. This tendency to evaluate HRQL in parents rather than young people was highlighted as a criticism by the UK’s National Institute for Health and Care Excellence in their recent appraisal of Palforzia[Citation28]. Indeed, it has been previously reported that there is discordance between changes in HRQL following food challenge in parents versus that reported by the young person themselves[Citation50]. The lack of current consensus on outcomes – including patient-reported outcome measures (PROMs) – is something currently being addressed through an EAACI taskforce (CO-FAITH, Clinical Outcomes in food-AIT trials Harmonization project). [Citation51]
5. Outcomes from systematic reviews of OIT
The most recent systematic review of OIT was undertaken by the GA2LEN Food Allergy Guidelines Group and published in December 2021. [Citation27] A summary of the analysis appears in . Following peanut-OIT, treated patients are six times more likely than controls to tolerate a single dose of 300 mg peanut protein (approximately 1½ peanuts, number needed to treat (NNT 2) and 17 times more likely to tolerate 1000 mg peanut protein (about 5 peanuts, NNT 2). Similar success for desensitization was found for OIT to egg and cow’s milk (also NNT 2). Thus, for every 2 patients treated with OIT, 1 patient will achieve successful desensitization. However, 1 patient (not necessarily the same individual) will experience adverse reactions, at least with OIT to hen’s egg or cow’s milk.
Table 2. Summary of OIT outcomes evaluated by the GA2LEN [Citation27] and PACE [Citation48] systematic reviews/meta-analyses
Rates of AEs are affected by the way studies report data; if different studies report AEs differently, this hampers comparisons between studies. In the PALISADE study, patients receiving the top dose of active peanut OIT (but not yet established on maintenance) were five times as likely to have systemic allergic reactions (8.7% vs. 1.7%) as those on placebo. [Citation52] However, anaphylaxis events in PALISADE were recategorized as ‘systemic allergic reactions’ unless symptoms included documented hypotension or hypoxia. Therefore, it is not so straightforward to compare rates of anaphylaxis in PALISADE with other studies which have reported anaphylaxis more explicitly, while some studies (e.g. STOP-II) do not report anaphylaxis at all[Citation47].
The GA2LEN systematic review reported that peanut-OIT may not increase the rate of adverse reactions or severe adverse reactions compared to control. This may seem odd, given that the 2 seminal studies which evaluated Palforzia reported that 99% of participants receiving OIT has AEs: however, there was similarly a very high rate of AEs in the control groups too (). [Citation52,Citation53] In those reporting AEs in the active OIT group, the most common symptoms were gastrointestinal (abdominal pain, nausea, vomiting), occurring in around half of treated subjects. Around 1 in 5 patients on OIT withdrew during both studies, with about half of those withdrawing due to dose-related AEs – a rate substantially higher than those in the control groups. Systemic allergic reactions and use of rescue adrenaline were 4 and 2 times higher in the active versus control groups. These rates are not clearly reflected in the GA2LEN analysis, since the authors reported the proportion of participants reported at least one treatment-related AE: thus, someone reporting just a single AE to placebo during a study is equivalent to a participant on OIT who experienced frequent adverse reactions during OIT. This accounts for the discordant conclusions between the GA2LEN review and a systematic review and meta-analysis by Chu et al, who reported high-certainty evidence for a three-fold greater risk of anaphylaxis (risk ratio, RR, 3.1 [95%CI 1 · 8–5 · 6]) and two-fold higher rate of rescue adrenaline use (RR 2.2 [95%CI 1.3–3.8] high-certainty) in patients undergoing peanut-OIT. OIT also doubled the rate of non-anaphylaxis reactions reported[Citation48].
Table 3. Adverse events in people aged 4 to 17 years participating in the PALISADE [Citation52] and ARTEMIS [Citation53] studies evaluating Palforzia in peanut-allergic children
The GA2LEN analysis also reported peanut OIT to be associated with fewer AEs compared to OIT with egg or cow’s milk[Citation27]. Whether this is due to the use of standardized allergen formulations for peanut but not egg/milk is not known, although this claim may be made by clinicians with potential financial conflicts of interest. Arguably, the protein concentration of fresh cow’s milk is as standardized as the commercial products used for peanut-OIT, yet OIT to cow’s milk results in a 4-fold increase in likelihood of AEs and 7-fold increase in anaphylaxis compared to controls[Citation27].
Due to a relatively small number of studies reporting HRQL measures (and often only parent-proxy measures), there remains an absence of high-quality evidence relating to the impact of OIT on HRQL. In a systematic review by the EAACI guidelines group[Citation26], only one study was included with respect to HRQL outcomes. The most recent systematic review published (GA2LEN) also highlighted a lack of data relating to outcomes on quality of life as a major shortcoming[Citation27].
6. Cost-effectiveness analyses
Two cost-effectiveness analyses have been published for peanut-OIT, specifically for Palforzia (Aimmune Therapeutics): one by the Institute for Clinical and Economic Review (ICER) in 2019 [Citation49,Citation54] and another by the UK’s National Institute for Health and Care Excellence (NICE). [Citation28] In addition, Shaker and Greenhawt published a cost-effective analysis of Palforzia (then called AR101) independent of industry funding in 2019. [Citation55]
The ICER analysis concluded that “the long-term cost-effectiveness of AR101 is
dependent on the prices at which [this] come to market.” Using an estimated price of $4,200/year for Palforzia, the incremental cost-effectiveness ratio was $88,000/QALY. [Citation49] The report also highlighted the very significant evidence gap in terms of longer-term outcomes, something which is an issue with all OIT studies, concluding that while ‘there is hope that the rates of systemic allergic reactions, [adrenaline] use, and reactions to accidental exposure will decrease with continued therapy, this remains to be demonstrated’[Citation54].
The NICE assessment, commissioned by the UK Departments of Health and Social Care, concluded that ‘Palforzia is likely to be a cost-effective use of [UK Health] resources, and therefore recommended it for routine NHS use’ on the basis that the ‘most plausible incremental cost-effectiveness ratios … would be around £20,000 per QALY gained’ – less than the threshold used by NICE to recommend treatments[Citation28]. However, this evaluation did not include the cost of setting up OIT in the UK healthcare system. In addition, the process used to evaluate HRQL impact differed from that normally used: due to the absence of appropriate longitudinal HRQL data from intervention studies (evaluating the change in HRQL over time), NICE instead reviewed cross-sectional data evaluating HRQL in OIT-treated vs non-treated individuals. This would have skewed outcomes in favor of OIT for several reasons: first, data would only have been collected from individuals in whom OIT was successful. Second, such a methodology cannot control for the benefits of participating in a clinical trial. These can be considerable, particularly for trials of OIT. We recently reported that around one third of the improvement in HRQL with OIT can be ascribed to the screening process (including experiencing a reaction during a controlled food challenge under medical supervision) [Citation56] – something which is consistent with other published data relating to the impact of a positive food challenge on HRQL. [Citation57,Citation58]
The cost of Palforzia was not initially disclosed in the public record, but subsequently reported as GBP £10.12 per day (irrespective of dose). [Citation28] This is not lower than the estimate used by ICER, [Citation54] and therefore does not explain the lower incremental cost-effectiveness ratio for Palforzia in the NICE assessment compared to that undertaken by ICER. The more likely reason is an overestimate by NICE of the impact of OIT on HRQL. In reaching its conclusion, NICE has paved the way for the UK became the first country in the world where Palforzia is recommended for use within a state-funded health system (albeit with a numerical cap on the number of patients who can be treated) – although OIT with noncommercial products through publicly-funded health systems has long been established in countries such as Spain, Italy and Israel. Unsurprisingly, using food products rather than commercial products offers significantly greater cost-effectiveness in the modeling, with a cost-effectiveness analyses reporting an incremental cost-effectiveness ratio of $2463 per QALY. [Citation59] In a further analysis, introducing OIT to preschool-aged peanut-allergic children was cost-effectiveness compared to the conventional approach of dietary avoidance and provision of rescue medication[Citation60].
7. Benefits versus risks
We have summarized the benefits and risks of OIT in , using a ‘SWOT’ analysis. There remain a number of unresolved challenges:
Patients likely to gain most from OIT are also those likely to experience AEs including anaphylaxis: studies generally indicate that the individuals most likely to achieve desensitization and experience a relatively adverse event-free treatment course are those with lower levels of IgE-sensitization and higher clinical reaction thresholds at food challenge [Citation1,Citation2,Citation6,Citation61]. In contrast, OIT studies in children with allergy to cow’s milk and hen’s egg demonstrate that those with more frequent and severe reactions to OIT tend to have higher IgE-sensitization and lower clinical reaction thresholds [Citation62–64]. Thus, the very individuals most likely to benefit from OIT (due to a higher risk of anaphylaxis from accidental reactions) are those less likely to tolerate OIT and more likely to withdraw due to adverse reactions. This might, in practice, result in a skewing of patients considered for OIT toward ‘easier’ patients, particularly if there is an incentive to deliver OIT based on numbers alone rather than to target those patients most likely to benefit from OIT, impacting adversely on equity of access to OIT.
Optimal duration of treatment: for other forms of allergen immunotherapy (e.g. to venom or aeroallergens), it is well established that a treatment duration of 3–5 years is required to achieve longer-term tolerance. Few studies have assessed this for OIT, and those that have report disappointing outcomes[Citation2]. There is very little data looking at longer-term adherence to treatment (affected by taste aversion) in young people after the first year of OIT, but our experience is that taste aversion is a common issue and there is a real demand to reduce the frequency of maintenance dosing to a minimum. Put simply, young people do not want to have to take a dose of a food they have a taste aversion to, every day for 3+ years. Longer-term data are needed to evaluate strategies to reduce the risk of withdrawing from OIT (with possible recurrence of food allergy with even higher levels of IgE-sensitization due to the OIT, which might result in an even greater risk of anaphylaxis compared to that present before starting OIT).
As demand for OIT increases, it is likely that there will be a gap between supply and demand. In addition, there will be a learning curve as more practitioners undertake OIT, but without the benefit of prior experience. To an extent, the use of standardized protocols (and perhaps standardized products) might mitigate against this. However, given that severe reactions to food are unpredictable, this does little to address the learning curve faced by the majority of clinicians who do not currently have experience of managing patients on OIT. It is therefore not an unlikely scenario that further fatalities may tragically occur due to OIT, even when undertaken by experienced physicians.
Optimal age for starting OIT. Some studies of peanut-OIT have reported a trend toward more successful outcomes in younger children. Two studies have reported a higher rate of sustained unresponsiveness in peanut-allergic children under 4 years than has been seen in studies in older children [Citation65,Citation66]. However, it is difficult to draw conclusions due to low numbers of children under age 24 months in both studies, and lack of head-to-head comparison with older participants. In the POISED study (which included 22 adults), there were no differences in safety or efficacy outcomes between adults and children after 2 years, although as the authors note, ‘the study was not designed to formally test the differences in these age groups.’ [Citation2] Commencing OIT earlier may be advantageous in terms of reducing the risk of potential taste aversion, thus increasing compliance and longer-term success. Studies comparing outcomes in both younger and older children within the same trial are required to address this evidence gap.
Table 4. Table outlining the perceived strengths, weaknesses, opportunities and threats (SWOT) of OIT
Much of these uncertainties result from existing knowledge gaps relating to longer-term outcomes of OIT ().
Box 1. Uncertainties in longer term outcomes of OIT
Importantly, there is a need to recognize that much of the benefit from OIT may not result from ‘immune’ desensitization, where the individual is able to ‘tolerate’ a higher dose, but from ‘psychological’ desensitization. We have previously described how the impact of food allergy on HRQL can be understood and addressed through a similar model to that underpinning cognitive behavioral therapy[Citation1]. The experience of OIT may positively impact on HRQL through:
The experience of a controlled reaction under medical supervision, which can reset the patient and carer’s perception of severity [Citation56–58].
The reality of a daily exposure to the food an individual is allergic to will, in most, psychologically desensitize the fear they have over having an allergic reaction, and thus alter their perception of the likelihood of life-threatening accidental reactions[Citation1].
Where patients undergoing OIT have reactions, the experience of self-managing these reactions (with or without parental and/or medical supervision) will boost their confidence in self-efficacy, i.e. their perceived capabilities or knowledge to manage a situation – resulting in an increased resilience when dealing with the challenges associated with food allergy[Citation67]. This benefit may well occur even in those patients who withdraw from OIT, although published data are currently absent and one concern (though lacking evidence) is that non-completion of OIT could increase IgE-sensitization and possibly increase the risk of more severe reactions at future accidental exposures).
All these factors may mean that patients with multiple food allergies can benefit from OIT to just one of their allergens – something which has not as yet been evaluated to our knowledge. They also explain the great paradox of OIT: that despite a higher rate of allergic reactions when someone is undergoing OIT (compared to allergen avoidance), there is clear and increasing demand for OIT from patients and their families.
8. The future of OIT
In considering the future landscapes of OIT provision, we have utilized a PESTLE framework to focus on the external environment into which OIT has been introduced.
Political context: Political interventions are increasingly common in the recognition and prioritization of health conditions, and remain central to facilitating outcomes that individuals themselves cannot achieve. In terms of food allergy, political intervention and lobbying has been instrumental in achieving legislative changes which might reduce the risk of accidental reactions e.g. in food labeling, allergen declaration by catering establishments, management of food-allergic children in schools etc. Recent examples include ‘Natasha’s Law’ in the UK (October 2021), which mandated comprehensive allergen labeling of prepacked foods from catering outlets (known as prepacked foods for direct sales, PPDS); [Citation68] and the Food Allergy Safety, Treatment, Education & Research (FASTER) Act in the USA, [Citation69] which amended FALCPA to include mandatory disclosure of sesame as an ingredient in prepacked foods, and facilitated data collection to improve the study of food allergy in the USA. Other legislative changes have facilitated the introduction of ‘spare’ adrenaline auto-injectors into schools [Citation70,Citation71]. With respect to OIT, it is likely that the views of patient representative organizations were an important factor in the decision by NICE to approve Palforzia for use in the UK health system[Citation28].
However, there continues to be a real concern as to whether the approval of a standardized product for OIT might result in a situation where only standardized products can be used for OIT. [Citation72] As raised by Perkin, while standardized products might reduce the risk of dosing-errors, ‘the issuing of [for example] peanut flour to a patient with peanut allergy may result in the peanut being deemed an unlicensed medicinal product by regulatory organizations in some countries. Once a product such as [Palforzia] appears, such regulators will insist that a licensed product be used when it is available, thus preventing the ongoing use of peanut flour itself.’ [Citation73] Use of commercial products significantly increases the cost of OIT, impacting on cost-effectiveness: this is a major issue, given that the cost of the raw material is just ‘peanuts.’ Furthermore, there is no evidence that systemic reactions during OIT are commonly due to dosing errors. Rates of adverse reactions to Palforzia in the clinical trials are not dissimilar to those studies using noncommercial, less standardized products [Citation5,Citation72] (although it can be argued that in the research setting, there is greater diligence over dosing than might be the case with ‘real world’ OIT – something which could be mitigated against through adequate clinical supervision). For some food allergens such as cow’s milk, it is unlikely that a commercial product for OIT will offer greater standardization than supermarket-sourced fresh cow’s milk. Thus, it is likely that political process will significantly impact on the future of OIT.
Economic: Even prior to the COVID-19 pandemic, healthcare systems across the globe were subject to increasing pressures to meet rising demand amid limited resources, resulting in unsustainable healthcare spending[Citation74]. As such, cost-effectiveness is a key barrier to the implementation of OIT. The rate of fatal food anaphylaxis is fortunately very rare[Citation75]: However, this means that OIT cannot be justified on reducing the risk of fatal outcomes from food allergy. Instead, cost-effectiveness is dependent on improvements in HRQL. OIT is likely to be cost-effective if it can be undertaken for a cost of around $4000 per annum (assuming OIT reduces the risk of anaphylaxis, can achieve longer-term sustained unresponsiveness and results in improved HRQL[Citation55]. The recent NICE assessment in the UK has demonstrated that higher costs might be tolerated within a public health setting, although crucially the NICE evaluation did not include the cost of establishing OIT service provision within the health system[Citation28].
Social: The management of food allergy has remained largely unchanged for over three decades. However, allergen avoidance is not a treatment strategy. OIT provides a fundamental sea-change in management, and is likely to be socially desirable as highlighted in a recent Research Prioritization exercise[Citation76]. However, while recent media attention has increased awareness of the risks and impact of food allergy, much of this is focused on nut allergies to the detriment of those with non-nut allergies (such as to cow’s milk), [Citation77,Citation78] and whom may be at greater risk of severe, truly life-threatening reactions[Citation34]. There are already significant socioeconomic and ethnic disparities in the provision of care to those with food allergy[Citation79]. The introduction of OIT into routine clinical practice must not be allowed to further exacerbate this.
Technology: OIT has not progressed much since the first description of OIT appeared in the literature over 100 years ago[Citation12]. In many, there may not be a need to develop OIT further, due to satisfactory efficacy in at least half of individuals. However, there is a clear need to improve efficacy and minimize AEs in those who do not tolerate OIT well. This may be achieved through various strategies including the use of adjuvants such as anti-IgE monoclonals, or by combining OIT with other forms of allergen immunotherapy including the use of other routes of allergen exposure (sublingual, epicutaneous) and T-cell directed peptide-based vaccines[Citation6].
In addition, the adoption of technology and advanced tools for data analysis may improve cost-effectiveness by identifying predictors of safe and efficacious OIT, and potentially allowing remote monitoring of individuals undergoing OIT to reduce the burden on healthcare systems and minimize costs of treatment (including loss of earnings due to hospital visits).
Legal: The potential impact of legislation has already been discussed above. There are, however, important lessons to be drawn from the interaction between law and health policy. Legislation requires certainty, and thus there can be a lack of flexibility to respond and adapt to new clinical data and technologies. This has already been shown to create challenges with respect to allergen labeling and allergen risk assessment/management[Citation80]. Caution will therefore be needed in negotiating the gray zone of foodstuffs being used for OIT, to prevent more harm than good from being achieved. Existing legislation in many countries already requires fair and equitable access to all patient populations in state-funded healthcare systems; there is a risk that the rollout of OIT may adversely impact existing healthcare disparities in allergy provision [Citation1,Citation79]. Given that OIT is not without risk, and at least 2 fatalities have now been reported due to participation in OIT (one at initial food challenge[Citation81], and one during treatment [Citation7]), it is important for there to be a consensus in terms of what must be discussed during the consent procedure to ensure informed consent can be documented[Citation82].
Environmental influences: The human diet is increasingly moving toward a plant-based diet: this is expected to increase exposure to allergens, whether in the form of new (novel) allergens (a danger also posed by the introduction of alternative animal-based proteins such as insects) or changes in the use of existing proteins resulting in much higher exposures than before. An example of the latter is the introduction of high-protein foods containing pea protein, which have resulted in an increase in pea protein-related allergic reactions in those not previously known to be pea-allergic. Thus, it is likely that OIT will have to respond, in due course, to changes in dietary consumption and the evolving patterns of food allergy. A clear example of this can be seen in China and elsewhere, where the introduction of cow’s milk into the diet has been associated with a significant increase in anaphylaxis presentations to hospital[Citation83].
9. Conclusion
The uptake of OIT has the potential to radically change the management of food allergy. Successful desensitization can be achieved with relative ease in around 50% of patients, with a further 20–30% likely to benefit if AEs can be managed well. However, around 1 in 5 are unlikely to tolerate OIT: these tend to be the patients with higher levels of IgE-sensitization and lower clinical reaction thresholds – but also the same individuals who have most to benefit from OIT. Persisting desensitization might be achieved through longer durations of treatment, but is unlikely to offer a cure with current protocols without ongoing maintenance dosing (although this might not need to be daily).
Given this complex balance, any decision to commence OIT must be through a shared decision making process [Citation82,Citation84,Citation85], with clear dialogue between clinicians and the food-allergic individual, and their families to support them (). When the individual concerned is a child or young person, it is essential to fully involve them in the decision process. Clinicians must ensure that patients’ goals are clearly defined at the outset, and that they have the most up-to-date information available to consider the risks and benefits, something which can be challenging to achieve in a busy clinic setting. However, this approach promotes a rigorous informed consent process, allowing families the space to clearly outline their goals and preferences, and time to come to the right decision.
Figure 4. The 3 steps of a shared-decision making process. Reproduced from Graham et al [Citation83] under the terms of the Creative Commons Attribution 4.0 International License.
![Figure 4. The 3 steps of a shared-decision making process. Reproduced from Graham et al [Citation83] under the terms of the Creative Commons Attribution 4.0 International License.](/cms/asset/0b50954c-8b52-40ba-ac38-e3f838dd8572/ierm_a_2053675_f0004_b.gif)
10. Expert opinion
Oral immunotherapy (OIT) for food allergy presents an opportunity for clinicians to actually treat food allergy, rather than just provide advice in terms of allergen avoidance and management of accidental reactions. The extensive evaluation of OIT over the past decade has paved the way for this shift toward a more dynamic, interventional approach, modifying the disease trajectory through immunomodulation. However, successful desensitization is limited to 80% and dependent upon ongoing dosing, which can be an issue due to taste aversion (common) and the potential for dose-related anaphylaxis (uncommon) even years after starting OIT [Citation1,Citation6].
The need for standardized products remains controversial [Citation72,Citation73]. There is now a single product licensed in Europe and North America for peanut (Palforzia), but OIT using peanut-containing foods can also be done at a fraction of the cost. For other allergens, OIT can only be done using non-pharma grade products (i.e. food), but for some foods e.g. cow’s milk pharma-grade products for OIT are unlikely to confer a useful level of standardization compared to fresh milk. More important is the need for standardized protocols and to avoid complex food matrices which can increase the unpredictability of absorption and potentially increase the risk of severe events. At least one fatality has been reported to OIT (using a baked matrix containing cow’s milk), in a 9 year old child in whom OIT was being undertaken using a ‘milk-ladder’ approach[Citation7]. Our own experience and perspective is that using baked foods for OIT can result in more unpredictable reactions, although this seems to be less of an issue in preschool-aged children [Citation30,Citation31]. The trend toward using baked foods as part of a ‘food ladder’ for OIT in older children – particularly without the rigorous medical supervision used for standard OIT – is therefore concerning, as this is wrongly perceived as a low-risk intervention.
Around 1 in 5 food-allergic patients are unlikely to tolerate OIT: these are often ‘more allergic’ patients who have most to benefit from treatment[Citation6]. Research is needed to develop personalized protocols for this group, perhaps using adjuvant therapies or using immunotherapy products administered via non-oral routes, to improve safety and outcomes.
The barriers to widespread implementation of OIT are significant but not insurmountable: OIT is a resource-intensive treatment and dose-related allergic reactions including anaphylaxis are common. There are increasing pressures on healthcare systems to meet rising demand amid limited resources, even prior to the COVID-19 pandemic. Deaths from food allergy are fortunately a very rare event, but as a result, OIT is extremely unlikely to reduce food allergy mortality. Cost-effectiveness is therefore dependent on improvements in quality of life – and data relating to these outcomes are currently very limited. This is a key evidence gap which needs to be addressed, if a strong economic case for OIT is to be made.
However, OIT is likely to be cost-effective in most patients, particularly if noncommercial products can be used. There may be a trade-off: in those patients with lower reaction thresholds, a commercial product may be needed to provide the very low doses of allergen needed for initial updosing, until patients reach a level of desensitization where they can be switched to foods containing the relevant allergen. However, such a protocol limits the potential for commercial profit, and thus reduces the incentive for commercial funding to develop the field further. This risk could be addressed through enhanced noncommercial funding of multicenter, collaborative studies.
Over the next 5 years, we envisage that OIT will become far more mainstream as a therapeutic option in food allergy, but the healthcare community needs to proactively address the issues of accessibility and equity, so that it does not exacerbate existing disparities in healthcare provision for food-allergic individuals. An urgent international consensus is needed to facilitate the analysis of real-world safety and efficacy data, allowing the development of personalized protocols to improve outcomes for patients while minimizing AEs. Although the current delivery of OIT may not yet be ideal, the concept and outcomes of treatment are well worth further exploration, investment and development.
Article highlights
OIT can achieve desensitization in 50-80% of food-allergic individuals, but there are concerns as to longer-term efficacy. Adverse events are common including anaphylaxis, and at least one fatality has been reported.
The need for standardized products (as opposed to standardized protocols using foods) is controversial.
Cost-effectiveness is dependent on improved quality of life, but evidence for this is currently lacking, and is a key evidence gap.
OIT is very likely to become a routine therapeutic option in food allergy, but strategies are needed to address issues of accessibility and equity, so OIT dones not further increase existing disparities in healthcare delivery for food-allergic individuals.
International consensus is needed to agree the reporting of safety and efficacy data, to aid comparisons between studies and facilitate the development of personalized protocols to improve safety outcomes.
Abbreviations
AE Adverse Events CoFAR Consortium for Food Allergy Research DBPCFC Double-blind, placebo-controlled food challenge EAACI European Academy of Allergy and Clinical Immunology FALCPA Food Allergen Labelling and Consumer Protection Act FDA Food and Drug Administration GMP Good Manufacturing practice HRQL Health-related quality of life ICER Institute for Clinical and Economic Review NICE National Institute for Health and Care Excellence NNT Number needed to treat OFC Oral Food challenge OIT Oral immunotherapy PESTLE Political, Economic, Social, Technology, Legal, Environmental PROMs Patient-reported outcome measures SWOTStrengths, Weaknesses, Opportunities, Threats
Declaration of interest
P Turner reports grants from the UK Medical Research Council, Food Standards Agency, Jon Moulton Charity Trust and Imperial/NIHR BRC; personal fees from UK Food Standards Agency, Aimmune Therapeutics, Allergenis, Aquestive Therapeutics, outside the submitted work; other research support (data access) from DBV Technologies. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Duca B, Patel N, Turner PJ. GRADE-ing the benefit/risk equation in food immunotherapy. Curr Allergy Asthma Rep. 2019;19(6):30.
- Chinthrajah RS, Purington N, Andorf S, et al. Sustained outcomes in oral immunotherapy for peanut allergy (POISED study): a large, randomised, double-blind, placebo-controlled, phase 2 study. Lancet. 2019;394(10207):1437–1449.
- FDA approves first drug for treatment of peanut allergy for children. Cited 2020 Jan 31. Available from: https://www.fda.gov/news-events/press-announcements/fda-approves-first-drug-treatment-peanut-allergy-children Cited 2021 Dec 24
- Committee for Medicinal Products for Human Use (CHMP), European medicines agency. Cited 2020 Oct 15. Available from: https://www.ema.europa.eu/en/documents/assessment-report/palforzia-epar-public-assessment-report_en.pdf Cited 2021 Dec 24
- Wasserman RL, Hague AR, Pence DM, et al. Real-world experience with peanut oral immunotherapy: lessons learned from 270 patients. J Allergy Clin Immunol Pract. 2019;7(2):418–426.e4.
- Vazquez-Ortiz M, Turner PJ. Improving the safety of oral immunotherapy for food allergy. Pediatr Allergy Immunol. 2016;27(2):117–125.
- Ontario mother warns of allergy therapy risks after daughter’s death. Available from: https://www.ctvnews.ca/health/it-seems-unbelievable-ont-mother-warns-of-allergy-therapy-risks-after-daughter-s-death-1.5716985 Cited 2021 December 24
- Wüthrich B. History of food allergy. Chem Immunol Allergy. 2014;100:109–119.
- Maimonides M. Treatise on asthma. Muntner S, editor. Philadelphia: Lippincott; 1963. p. 2.
- Directive 2000/13/EC of The European Parliament and of The Council. Available from: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=CONSLEG:2000L0013:20090120:EN:PDF Cited 2021 Dec 24
- US Food and Drug Administration. Food Allergen Labeling and Consumer Protection Act of 2004 (FALCPA). Available from: https://www.fda.gov/food/food-allergensgluten-free-guidance-documents-regulatory-information/food-allergen-labeling-and-consumer-protection-act-2004-falcpa Cited 2021 December 24)
- Schofield AT. A case of egg poisoning. Lancet. 1908;171:716.
- Patriarca G, Schiavino D, Nucera E, et al. Food allergy in children: results of a standardized protocol for oral desensitization. Hepatogastroenterology. 1998;45(19):52–58.
- Morisset M, Moneret-Vautrin DA, Guenard L, et al. Oral desensitization in children with milk and egg allergies obtains recovery in a significant proportion of cases. A randomized study in 60 children with cow’s milk allergy and 90 children with egg allergy. Eur Ann Allergy Clin Immunol. 2007;39(1):12–19.
- Nelson HS, Lahr J, Rule R, et al. Treatment of anaphylactic sensitivity to peanuts by immunotherapy with injections of aqueous peanut extract. J Allergy Clin Immunol. 1997;99(6 Pt 1):744–751.
- Martorell A, Alonso E, Echeverría L, et al.; Expert panel selected from members of the Spanish Society of Pediatric Allergology, Asthma and Clinical Immunology (SEICAP) and the Spanish Society of Allergology and Clinical Immunology (SEAIC). Oral immunotherapy for food allergy: a Spanish guideline. immunotherapy egg and milk spanish guide (ITEMS guide). Part I: cow milk and egg oral immunotherapy: introduction, methodology, rationale, current state, indications, contraindications, and oral immunotherapy build-up phase. J Investig Allergol Clin Immunol. 2017;27(4):225–237.
- Ebisawa M, Ito K, Fujisawa T. Committee for Japanese pediatric guideline for food allergy, The Japanese Society of Pediatric Allergy and Clinical Immunology; Japanese Society of Allergology. Japanese guidelines for food allergy 2020. Allergol Int. 2020;69(3):370–386.
- Muraro A, Werfel T, Hoffmann-Sommergruber K, et al.; EAACI Food Allergy and Anaphylaxis Guidelines Group. EAACI food allergy and anaphylaxis guidelines: diagnosis and management of food allergy. Allergy. 2014;69(8):1008–1025.
- Pajno GB, Fernandez-Rivas M, Arasi S, et al.; EAACI Allergen Immunotherapy Guidelines Group. EAACI guidelines on allergen immunotherapy: IgE-mediated food allergy. Allergy. 2018;73(4):799–815.
- Bégin P, Chan ES, Kim H, et al. CSACI guidelines for the ethical, evidence-based and patient-oriented clinical practice of oral immunotherapy in IgE-mediated food allergy. Allergy Asthma Clin Immunol. 2020;16:20.
- Calvani M, Giorgio V, Miceli Sopo S. Specific oral tolerance induction for food. A systematic review. Eur Ann Allergy Clin Immunol. 2010;42(1):11–19.
- Nurmatov U, Venderbosch I, Devereux G, et al. Allergen-specific oral immunotherapy for peanut allergy. Cochrane Database Syst Rev. 2012;9:CD009014. DOI:https://doi.org/10.1002/14651858.CD009014.pub2
- Yeung JP, Kloda LA, McDevitt J, et al. Oral immunotherapy for milk allergy. Cochrane Database Syst Rev. 2012;11(11):CD009542.
- Romantsik O, Bruschettini M, Tosca MA, et al. Oral and sublingual immunotherapy for egg allergy. Cochrane Database Syst Rev. 2014;11:CD010638. DOI:https://doi.org/10.1002/14651858.CD010638.pub2
- Nurmatov U, Devereux G, Worth A, et al. Effectiveness and safety of orally administered immunotherapy for food allergies: a systematic review and meta-analysis. Br J Nutr. 2014;111(1):12–22.
- Nurmatov U, Dhami S, Arasi S, et al. Allergen immunotherapy for IgE-mediated food allergy: a systematic review and meta-analysis. Allergy. 2017;72(8):1133–1147.
- de Silva D, Rodríguez Del Río P, de Jong NW, et al.; GA2LEN Food Allergy Guidelines Group. Allergen immunotherapy and/or biologicals for IgE-mediated food allergy: a systematic review and meta-analysis. Allergy. 2022. doi:https://doi.org/10.1111/all.15211.
- National Institute for Health and Care Excellence. Single technology appraisal of palforzia for treating peanut allergy. Cited 2021 Dec 23. Available from: https://www.nice.org.uk/guidance/indevelopment/gid-ta10713 Cited 2021 Dec 24
- Venter C, Meyer R, Ebisawa M, et al. Food allergen ladders: a need for standardization. Pediatr Allergy Immunol. 2022;33(1):e13714.
- Ball HB, Luyt D. Home-based cow’s milk reintroduction using a milk ladder in children less than 3 years old with IgE-mediated cow’s milk allergy. Clin Exp Allergy. 2019;49(6):911–920.
- MacMahon J, Hourihane JO, Byrne A. A virtual management approach to infant egg allergy developed in response to pandemic-imposed restrictions. Clin Exp Allergy. 2021;51(2):360–363.
- Chomyn A, Chan ES, Yeung J, et al. Canadian food ladders for dietary advancement in children with IgE-mediated allergy to milk and/or egg. Allergy Asthma Clin Immunol. 2021;17(1):83.
- Turner PJ, Gowland MH, Sharma V, et al. Increase in anaphylaxis-related hospitalizations but no increase in fatalities: an analysis of United Kingdom national anaphylaxis data, 1992-2012. J Allergy Clin Immunol. 2015;135(4):956–963.e1.
- Baseggio Conrado A, Ierodiakonou D, Gowland MH, et al. Food anaphylaxis in the United Kingdom: analysis of national data, 1998-2018. BMJ. 2021;372:n251.
- Leonard SA. Debates in allergy medicine: baked milk and egg ingestion accelerates resolution of milk and egg allergy. World Allergy Organ J. 2016;9:1.
- Dang TD, Peters RL, Allen KJ. Debates in allergy medicine: baked egg and milk do not accelerate tolerance to egg and milk. World Allergy Organ J. 2016;9:2.
- Remington BC, Westerhout J, Campbell DE, et al. Minimal impact of extensive heating of hen’s egg and cow’s milk in a food matrix on threshold dose-distribution curves. Allergy. 2017;72(11):1816–1819.
- BSACI and The Canadian Society of Allergy and Clinical Immunology joint statement on oral immunotherapy. Available from: https://www.bsaci.org/bsaci-and-the-canadian-society-of-allergy-and-clinical-immunology-joint-statement-on-oral-immunotherapy/. Cited 2022 Feb 18.
- Yonkof JR, Mikhail IJ, Prince BT, et al. Delayed and severe reactions to baked egg and baked milk challenges. J Allergy Clin Immunol Pract. 2021;9(1):283–289.e2.
- Rodríguez Del Río P, Escudero C, Sánchez-García S, et al. Evaluating primary end points in peanut immunotherapy clinical trials. J Allergy Clin Immunol. 2018;143(2):494–506.
- Peanut curry death: Mohammed Zaman fails in appeal Bid. Available from: https://www.bbc.co.uk/news/uk-england-york-north-yorkshire-41926399 Cited 2021 Dec 24.
- Dua S, Ruiz-Garcia M, Bond S, et al. Effect of sleep deprivation and exercise on reaction threshold in adults with peanut allergy: a randomized controlled study. J Allergy Clin Immunol. 2019;144(6):1584–1594.e2.
- Patel N, Adelman DC, Anagnostou K, et al. Using data from food challenges to inform management of consumers with food allergy: a systematic review with individual participant data meta-analysis. J Allergy Clin Immunol. 2021;147(6):2249–2262.e7.
- Turner PJ, Baumert JL, Beyer K, et al. Can we identify patients at risk of life-threatening allergic reactions to food? Allergy. 2016;71(9):1241–1255.
- Anagnostou K, Clark A, King Y, et al. Efficacy and safety of high-dose peanut oral immunotherapy with factors predicting outcome. Clin Exp Allergy. 2011;41(9):1273–1281.
- Furuta T, Tanaka K, Tagami K, et al. Exercise-induced allergic reactions on desensitization to wheat after rush oral immunotherapy. Allergy. 2020;75(6):1414–1422.
- Anagnostou K, Islam S, King Y, et al. Assessing the efficacy of oral immunotherapy for the desensitisation of peanut allergy in children (STOP II): a phase 2 randomised controlled trial. Lancet. 2014;383(9925):1297–1304.
- Chu DK, Wood RA, French S, et al. Oral immunotherapy for peanut allergy (PACE): a systematic review and meta-analysis of efficacy and safety. Lancet. 2019;393(10187):2222–2232.
- Tice JA, Guzauskas GF, Hansen RN, et al. The effectiveness and value of oral immunotherapy and viaskin peanut for peanut allergy. J Manag Care Spec Pharm. 2020;26(5):620–623.
- Burrell S, Patel N, Vazquez-Ortiz M, et al. Self-administration of Adrenaline for anaphylaxis during in-hospital food challenges improves health-related quality of life. Arch Dis Child. 2021;106(6):558–563.
- European Academy of Allergy. Clinical immunology clinical outcomes in food-AIT trials Harmonization (CO-FAITH) taskforce. Available from: https://www.eaaci.org/science/task-forces.html Cited 2021 Dec 24
- PALISADE Group of Clinical Investigators. AR101 oral immunotherapy for peanut allergy. N Engl J Med. 2018;379(21): 1991–2001.
- O’B Hourihane J, Beyer K, Abbas A, et al. Efficacy and safety of oral immunotherapy with AR101 in European children with a peanut allergy (ARTEMIS): a multicentre, double-blind, randomised, placebo-controlled phase 3 trial. Lancet Child Adolesc Health. 2020;4(10):728–739.
- Institute for Clinical and Economic Review (ICER). Oral immunotherapy and Viaskin® peanut for peanut allergy: effectiveness and value. Available from: https://icer.org/wp-content/uploads/2020/10/ICER_PeanutAllergy_Final_Report_071019.pdf Cited 2021 Dec 24
- Shaker M, Greenhawt M. Estimation of health and economic benefits of commercial peanut immunotherapy products: a cost-effectiveness analysis. JAMA Network Open. 2019;2(5):e193242.
- Patel N, Lindsley S, Vazquez-Ortiz M, et al. Significant impact of screening challenge on the improvement in health-related quality of life during oral immunotherapy (OIT). J Allergy Clin Immunol. 2020;145(2):AB135.
- DunnGalvin A, Cullinane C, Daly DA, et al. Longitudinal validity and responsiveness of the Food Allergy Quality of Life Questionnaire - Parent Form in children 0-12 years following positive and negative food challenges. Clin Exp Allergy. 2010;40:476–485.
- Soller L, Hourihane J, DunnGalvin A. The impact of oral food challenge tests on food allergy health-related quality of life. Allergy. 2014;69:1255–1257.
- Shaker MS. An economic analysis of a peanut oral immunotherapy study in children. J Allergy Clin Immunol Pract. 2017;5(6):1707–1716.
- Shaker M, Chan ES, Protudjer JLP, et al. The cost-effectiveness of preschool peanut oral immunotherapy in the real-world setting. J Allergy Clin Immunol Pract. 2021;9(7):2876–2884.e4.
- Fiocchi A, Artesani MC, Fierro V, et al. Oral immunotherapy for peanut allergy: the con argument. World Allergy Organ J. 2020;13(8):100445.
- Vazquez-Ortiz M, Alvaro-Lozano M, Alsina L, et al. Safety and predictors of adverse events during oral immunotherapy for milk allergy: severity of reaction at oral challenge, specific IgE and prick test. Clin Exp Allergy. 2013;43:92–102.
- Savilahti EM, Kuitunen M, Valori M, et al. Use of IgE and IgG4 epitope binding to predict the outcome of oral immunotherapy in cow’s milk allergy. Pediatr Allergy Immunol. 2014;25:227–235.
- Vazquez-Ortiz M, Alvaro M, Piquer M, et al. Baseline specific IgE levels are useful to predict safety of oral immunotherapy in egg allergic children. Clin Exp Allergy. 2014;44:130–141.
- Vickery BP, Berglund JP, Burk CM, et al. Early oral immunotherapy in peanut-allergic preschool children is safe and highly effective. J Allergy Clin Immunol. 2017;139(1):173–181.e8.
- Jones SM, Kim EH, Nadeau KC, et al.; Immune Tolerance Network. Efficacy and safety of oral immunotherapy in children aged 1-3 years with peanut allergy (the Immune Tolerance Network IMPACT trial): a randomised placebo-controlled study. Lancet. 2022;399(10322):359–371.
- Rasbach L, Jenkins C, Laffel L. An integrative review of self-efficacy measurement instruments in youth with type 1 diabetes. Diabetes Educ. 2015;41(1):43–58.
- UK Government. Natasha’s legacy becomes law. Cited 2019 Sept 5. Available from: https://www.gov.uk/government/news/natashas-legacy-becomes-law Cited 2021 Dec 24
- US Congress. Food Allergy Safety, Treatment, Education, and Research (FASTER) act of 2020. Available from: https://www.congress.gov/bill/116th-congress/house-bill/2117/text Cited 2021 Dec 24)
- US Congress. School access to emergency epinephrine act. Cited 2013 Sept 12. Available from: https://www.congress.gov/bill/113th-congress/senate-bill/1503 Cited 2021 Dec 24
- UK Government. The human medicines (amendment) regulations 2017. Available from: https://www.legislation.gov.uk/uksi/2017/715/made Cited 2021 Dec 24
- Wasserman RL, Hague AR, Pence DM, et al. The real world is not standardized. J Allergy Clin Immunol Pract. 2019;7(6):2098–2099.
- Perkin MR. Oral desensitization to peanuts. N Engl J Med. 2018;379(21):2074–2075.
- World Economic Forum. The Global Risks Report 2020. Available from: https://www.weforum.org/reports/the-global-risks-report-2020 Cited 2021 Dec 24
- Turner PJ, Jerschow E, Umasunthar T, et al. Fatal anaphylaxis: mortality rate and risk factors. J Allergy Clin Immunol Pract. 2017;5(5):1169–1178.
- Turner PJ, Andoh-Kesson E, Baker S, et al. Identifying key priorities for research to protect the consumer with food hypersensitivity: a UK Food Standards Agency Priority Setting Exercise. Clin Exp Allergy. 2021;51(10):1322–1330.
- Turner PJ. Persistent allergy to cow’s milk: of greater a clinical concern than other food allergies. Pediatr Allergy Immunol. 2013;24:624–626.
- Barnett J, Begen FM, Gowland MH, et al. Comparing the eating out experiences of consumers seeking to avoid different food allergens. BMC Public Health. 2018;18:1263.
- Warren CM, Turner PJ, Chinthrajah RS, et al. Advancing food allergy through epidemiology: understanding and addressing disparities in food allergy management and outcomes. J Allergy Clin Immunol Pract. 2021;9(1):110–118.
- Allen KJ, Turner PJ, Pawankar R, et al. Precautionary labelling of foods for allergen content: are we ready for a global framework? World Allergy Organ J. 2014;7(1):10.
- Pouessel G, Beaudouin E, Tanno LK, et al.; Allergy Vigilance Network®. Food-related anaphylaxis fatalities: analysis of the Allergy Vigilance Network® database. Allergy. 2019;74(6):1193–1196.
- Abrams EM, Erdle SC, Cameron SB, et al. How to incorporate oral immunotherapy into your clinical practice. Curr Allergy Asthma Rep. 2021;21(4):30.
- Baseggio Conrado A, Patel N, Turner PJ. Global patterns in anaphylaxis due to specific foods: a systematic review. J Allergy Clin Immunol. 2021;148(6):1515–1525.e3.
- Anagnostou A, Hourihane JO, Greenhawt M. The role of shared decision making in pediatric food allergy management. J Allergy Clin Immunol Pract. 2020;8(1):46–51.
- Graham F, Mack DP, Bégin P. Practical challenges in oral immunotherapy resolved through patient-centered care. Allergy Asthma Clin Immunol. 2021;17(1):31.
