ABSTRACT
Introduction
Persistent debilitating fatigue is a frequent complaint in patients with systemic autoimmune rheumatic diseases (SARDs). Fatigue is, however, frequently overlooked in the clinic, and patients who successfully achieve remission of their disease, often still have a lowered quality of life due to its persistence. How similar is this fatigue to Myalgic encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS), what is this fatigue associated with, and what tools/approaches (if any), have resulted in the improvement of fatigue in these patients is poorly defined.
Areas covered
Similarities between the pathophysiology of ME/CFS, systemic sclerosis (SSc) and primary systemic vasculitides (PSV) are discussed, followed by an in-depth review of the prevalence and correlates of fatigue in these diseases. The authors reviewed literature from MEDLINE, APA PsycInfo, Embase, and CINAHL.
Expert opinion
Persistent fatigue is a prominent feature in SARDs and may not be associated with components commonly associated with disease activity and/or progression. Immune and metabolic commonalities exist between ME/CFS, SSc, and PSVs – suggesting that common pathways inherent to the diseases and fatigue may be present. We suggest that patients with features of ME/CFS need to be identified by treating physicians, as they may require alternative approaches to therapy to improve their quality of life.
1. Introduction
Immune dysregulation (or autoimmunity) is common and affects nearly 10% of the adults [Citation1,Citation2]. In many affected patients, disease may be organ/or tissue specific (e.g. Autoimmune thyroid disease or type 1 diabetes). Autoimmunity can also affect multiple organs (systemic autoimmune diseases (SAIDs)), resulting in disease-specific morbidities and/or mortality [Citation1,Citation2]. Systemic autoimmune rheumatic diseases (SARDs) such as systemic sclerosis (SSc, or scleroderma) and ANCA-associated vasculitis (AAV) are rare with annual incidences estimated at 1.4 (1.1–1.9) per 100.000 for SSc [Citation3] and 3–5 cases per 100,000 for AAV [Citation4,Citation5]. SSc is characterized by systemic immune dysregulation, vasculopathy, and/or fibrosis [Citation6]. In contrast, AAV is associated with the formation of pathogenic anti-neutrophil cytoplasmic antibodies (ANCA) which directly promote vascular inflammation and subsequent loss of organ function [Citation7,Citation8]. The mechanisms promoting the formation of ANCA are multifactorial, and it is being recognized that patients with SSc and severe vascular complications also generate ANCA [Citation9,Citation10] – suggesting a potential overlap in both diseases.
Many patients with SARDs, including AAV and SSc, are treated with immunosuppressive therapies. In spite of this, these patients continue to suffer from general symptoms, such as fatigue, with associated loss of income, sleep disturbances, and mental health disorders [Citation11]. Fatigue in several SARDs (such as rheumatoid arthritis (RA) and systemic lupus (SLE)) is common ranging between 50% and 80% and is often linked to pain and depression. Fatigue does not improve in >50% of RA patients who achieve disease remission [Citation12,Citation13]. In SLE fatigue is correlated with obesity, depression, sleep disturbances, and pain. Like RA, fatigue appears to persist in many patients with SLE with quiescent disease [Citation14–16]. Indeed, a recent review by Dey et al. highlights both the similarities and differences between RA and SLE as they relate to fatigue, the mechanism(s) underpinning the pathogenesis of fatigue and its management [Citation17]. However, there is a range of severity in patient-reported symptoms of fatigue in patients with RA and SLE, with some having severe fatigue associated with other symptoms such as cognitive dysfunction and post-exertional malaise. These symptoms are common in myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) and are less established in most SARDs (in general) but particularly in SSc and AAV.
2. Myalgic encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS)
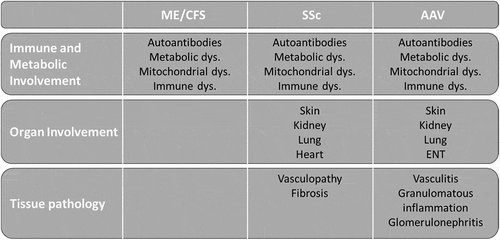
Fatigue is a common symptom and is estimated to affect approximately 50% of the people at any given time [Citation18,Citation19]. However, ME/CFS in the general population is uncommon and its prevalence is estimated to be < 3% [Citation18,Citation20]. The etiology of ME/CFS is multifactorial and is thought to stem from immune dysregulation, metabolic abnormalities associated with certain genetic predispositions [Citation18,Citation21]. The diagnosis of ME/CFS is primarily based on patient-reported symptoms and clinical manifestations, with no definitive physical biomarkers or laboratory tests [Citation22]. ME/CFS remains to be one of the largest challenges faced by numerous patients [Citation18,Citation20,Citation23] with associated loss of income, sleep disturbance, and concomitant mental health complications. The connection between inflammatory disease and ME/CFS is an emerging area of interest [Citation24–26]. Close attention has been brought to ME/CFS-like syndromes – particularly as many patients infected with SARS-CoV-2 [Citation27], subsequently developed long COVID-19 syndrome. This development has re-invigorated research into this area with nearly 100 million US dollars in research investments recently [Citation28]. In this review, we aim to link ME/CFS with the dysregulated mechanisms in AAV and SSc to potentially identify overlapping pathways in their pathogenesis (). We also aim to critically summarize the clinical data describing ME/CFS in these patient groups.
2.1. Symptoms and diagnosis of ME/CFS
Although nearly 65 years have elapsed since its first description [Citation29], identifying ME/CFS is inconsistently done among clinicians and its pathogenesis remains elusive [Citation30–32]. The primary symptom in most patients with ME/CFS is severe relentless fatigue with non-refreshing sleep and post-exertional malaise, associated with cognitive dysfunction, neurological manifestations, orthostatic intolerance, and autonomic impairment [Citation20,Citation22,Citation23]. There are nearly 20 consensus research and clinical definitions for ME/CFS, each having different selection and exclusion criteria [Citation20] which has been a large contributing factor to the skepticism of ME/CFS by clinicians, and the apparent lack of understanding felt by patients [Citation20,Citation30–32]. The Canadian Consensus Criteria (CCC) or the Canadian working definition for ME/CFS, published in 2003 are the most frequently used classification criteria in establishing research case definitions [Citation33] – as they tend to capture more severely affected patients [Citation34]. The most accepted diagnostic research criteria are derived from the 2015 Institute of Medicine (IOM), which are summarized below ().
Table 1. IOM 2015 criteria for the diagnosis of ME/CFS [Citation122].
2.2. Pathophysiology of ME/CFS
The etiology of ME/CFS is unknown. There are several potential mechanisms that may be contributing to its pathogenesis – namely dysregulated immune responses [Citation21] associated with metabolic dysregulation and neurological dysfunction. These mechanisms are associated with multiple genetic predispositions and reactivation of various viral infections, which may be associated with further potentiating this disease. In this section, we will discuss the parallels between the pathogenesis of ME/CFS with AAV and SSc.
2.2.1. Immune system dysregulation
Immune system irregularities in ME/CFS are diverse and tend to fluctuate over the course of disease. It is clear, however, that in patients with ME/CFS, AAV, and SSc, immunoregulatory pathways are attenuated, and inflammatory ones are promoted. For instance, in ME/CFS the balance between T-helper cells type 1 (Th1) and 2 (Th2), is dominated by the latter, resulting in a stronger humoral to cell-mediated immune profile [Citation35,Citation36]. Abnormal T cell polarization (TH2) has been implicated as a driver of fibrosis in SSc [Citation37], a similar pattern is evident in generalized granulomatosis with polyangiitis (GPA) and eosinophilic granulomatosis with polyangiitis (EGPA) vasculitis patients [Citation38,Citation39]. Further to this, regulatory T cells (Tregs) and antigen-specific CD8 + T cells are hyporesponsive in ME/CFS, AAV, and SSc [Citation37,Citation38].
Other important impaired immunoregulatory cells in ME/CFS, AAV, and SSc include natural killer (NK) cells. In particular, NK cell cytotoxicity is significantly reduced in ME/CFS patients [Citation40–44] – suggested the NK cells may be activated but are incapable of eliminating the target [Citation41]. Like other leukocytes, NK cell functions are regulated by the balance of activating and inhibitor receptors [Citation45]. Hence, increases in KIR3DL1, an NK cell inhibitory receptor which has been observed in ME/CFS [Citation43], may promote NK cell functional attenuation. Further to this, a reduction in NK cell cytotoxicity has been observed in both SSc and AAV [Citation46], with a reduction in the expression of various activating receptors including, NKG2D suggested to be associated with SSc [Citation47,Citation48]. Antiviral defense mechanisms may also attenuate NK cell functions [Citation49,Citation50]. Hence, increased ribonuclease L/2-5A synthetase (RNase L) activity and RNase L proteolysis may further promote this as suggested in patients with ME/CFS [Citation49,Citation50].
Perturbations within the B cell compartment are also evident in ME/CFS, with changes in IgG subclasses and increased frequencies of detectable autoantibodies. Lower levels of IgG1, IgG3, and IgG4 subclasses have been found [Citation51–53]. In contrast, IgG1 is elevated in many patients with AAV and SSc, with preferentially higher levels of IgG3 and IgG4 in SSc and AAV, respectively [Citation54–57]. During treatment of AAV and/or SSc many patients, develop low IgG and/or IgG subclass levels [Citation58,Citation59]. Numerous autoantibodies have been identified in ME/CFS patients, which include antibodies against various adrenergic and muscarinic cholinergic receptors. These antibodies are associated with increased muscle pain and weakness [Citation60–63]. Antinuclear antibodies (ANA) [Citation64] and anti-phospholipid (APL, such as anti-cardiolipin (aCL)) antibodies are more frequently found in patients with ME/CFS [Citation65,Citation66]. In SSc, the frequency of ANA is as high as 85 to 99%, though rates of antiphospilipid and ACA antibodies are less significant [Citation9]. Adrenergic and muscarinic cholinergic receptor antibodies have also been associated with fibrosis, vascular dysfunction, and gastrointestinal dysmotility in SSc [Citation67]. Interestingly up to 5% of SSc patients are positive for AAV-specific autoantibodies, and those patients positive for anti-neutrophil cytoplasmic antibodies (ANCA) and anti-topoisomerase antibodies (ATA) are at increased risk for developing AAV [Citation9]. Reports of ANA, APL, and ACL in AAV are lower – although ANA autoantibodies have been associated with more severe renal involvement in patients with AAV [Citation68]. Intriguingly, MPO-ANCA is more commonly present in patients with SSc and vascular complications [Citation8–10] suggesting a possible link between MPO-ANCA and mechanisms promoting SSc and ME/CFS.
Neutrophil activation by ANCA, drives microvascular injury in AAV patients, through the production of reactive oxygen species (ROS) and the generation of neutrophil extracellular traps (NETs) leading to cell death by NETosis [Citation7]. Excessive NETosis has also been shown to be associated with early fibrotic progression in SSc patients, as well as those who have chronic severe vascular complications [Citation8,Citation69]. In contrast, neutrophil function appears to be reduced in ME/CFS with associated neutrophil apoptosis [Citation70]. Contrary to AAV and SSc, increased NETosis has not been established in patients with ME/CFS – although it would be suspected as NETosis is a driver of autoantibody formation, and inflammatory cytokines (IL8 and TGFβ) associated with ME/CFS [Citation64–66].
ME/CFS often follows an infection, as ‘flu-like illness’ are frequent anteceding events prior to the onset of symptoms often reported by ME/CFS patients. This observation has led to a multitude of studies investigating whether a pathogen may be the trigger ME/CFS [Citation71–74]. Further to this, patients with post-acute viral syndromes, such as those described in patients infected with SARS-CoV-2, may fulfill the IOM diagnostic criteria for ME/CFS [Citation75], but often these patients have additional symptoms, which can distinguish them from idiopathic ME/CFS patients [Citation76]. There is no consistent evidence that ME/CFS results from one specific (viral) infection and many patients have no evidence of an infection as a potential trigger of ME/CFS, suggesting that infection may not be the cause of ME/CFS but may be involved in the perpetuation of the disease [Citation18,Citation74].
2.2.2. Metabolic dysregulation
An individual’s cellular chemistry is responsible for the regulation of cell-to-cell communication, inflammation, and metabolism. Metabolites detected in the circulation (such as in plasma or the serum) reflect the current functional state of an individual as affected by factors such as age, physical and emotional stress and diet [Citation77]. Naviaux et al., studying the plasma of ME/CFS patients, described 20 out of 63 dysregulated metabolic pathways, consistent with a hypometabolic state, similar to dauer [Citation77]. The dauer state of nematode worms involves a massive slowdown of metabolism, a stress resistant stage, preserving their lives when faced with starvation, overcrowding, and toxic environments [Citation78]. In particular, sphingolipids, glycosphingolipids, and phospholipids were decreased and correlated with disease severity, and all these metabolic abnormalities could be influenced by the availability of nicotinamide adenine dinucleotide phosphate (NADPH), an essential electron donor that provides reducing power for anabolic reactions and redox balance within the cellular compartments [Citation77].
Mitochondria function as the energy producers of the cell, providing power for biochemical reactions. Studies investigating the role of mitochondrial structure and function in ME/CFS are inconsistent with differences in diagnostic criteria and analysis methods, making comparison difficult [Citation79]. Features that have been highlighted include increased oxidative (disturbance in the balance between the production of reactive oxygen species (ROS) and antioxidant defenses) and nitrosative (disturbance in the balance of nitric acid (NO) and antioxidant defenses) stress that results in increased fatty acid, DNA, and protein damage [Citation80,Citation81]. Functionally, this is associated with a decreased mitochondrial membrane potential and increased aerobic glycolysis in circulating cells from patients with ME/CFS [Citation82–84]. Mitochondrial-derived metabolites have also been associated with ME/CFS. In particular, circulating co-enzyme Q10 (CoQ10), an essential cofactor that promotes mitochondrial ATP production in the electron transport chain has repeatedly been shown to be reduced in ME/CFS patients [Citation85,Citation86]. CoQ10 also acts as a scavenger of ROS protecting cells from oxidative damage [Citation86]. Elevated ROS levels are also associated with endothelial cell injury and vascular complications [Citation87], common to both SSc and AAV.
Mitochondria have recently been suggested to play an essential role in inflammatory mechanisms. Indeed, they are required for inflammatory cytokine production via the inflammasome (e.g. IL1 and IL33) [Citation88]. Functional mitochondria are also essential for NK cell and Treg functions, and in the differentiation of various lymphocytes [Citation89,Citation90]. Hence, it is not surprising that mitochondrial dysregulation has been linked to various SARDs, including SLE, SSc, and AAV [Citation89,Citation91,Citation92].
In SSc, an imbalance between oxidant and anti-oxidant states is classically observed, leading to increased inflammation (NLRP3, M2 macrophage polarization), immune dysregulation (increased plasmacyte differentiation and Th17/Treg imbalance) and chronic fibroblast activation through increased production of pro-inflammatory cytokines (IL1β and TGF-β) [Citation67]. In AAV, an altered redox state characterized by an increased oxidation markers and decreased anti-oxidants is present [Citation93–95]. These mechanisms may be linked to impaired immune tolerance in AAV, such as impaired Treg function, via aberrant activation of the mTOR pathway and subsequent high levels of intracellular ROS [Citation91].
2.2.3. Central nervous system, cardiovascular, and musculoskeletal abnormalities
Individuals with ME/CFS often display symptoms associated with central nervous system abnormalities, including reduced cognitive function, increased pain syndromes such as fibromyalgia, and/or postural orthostatic tachycardia syndrome (POTS). Neuroendocrine studies into hypothalamic-pituitary-adrenal (HPA) function, and cortisol levels and serotonin transmission have, provided reproducible findings, suggesting physiological changes to stress response in ME/CFS [Citation18,Citation20,Citation23]. ME/CFS patients appear to have a reduced HPA axis function resulting in adrenal insufficiency, which negatively affects immunological and neuronal homeostasis [Citation96–98], individuals who suffer from adrenal insufficiency (e.g. Addison’s disease) resulting in low cortisol levels, display ME/CFS-like symptoms such as fatigue, arthralgia, myalgia, sleep disturbance, and mood disorders [Citation23]. Glucocorticoid use is common in SARDS and is associated with adrenal suppression [Citation99,Citation100]. Low cortisol levels in SSc have been linked to symptoms of pain and depression and increased disease activity [Citation101]. Adrenal fatigue is synonymous with POTS, and this condition is present in around 16% of individuals with autoimmune diseases [Citation102].
2.2.4. Genetic factors associated with ME/CFS
The most distinct genetic feature related to the development of ME/CFS, is the profound gender bias toward females, who are four times more likely to develop ME/CFS than their male counterparts [Citation103,Citation104]. Numerous genes involved in immune and mitochondrial function are located on the X chromosome, when more than one copy is present, as is typical in women; one copy is usually deactivated [Citation105]. Studies looking at X chromosome inactivation have, however, shown that up to 23% of X-linked genes escape deactivation, leading to over activation. One such gene is TLR7, a single-stranded RNA (ssRNA) sensor, essential to innate and B cell defense from RNA viruses [Citation106]. Over activation of TLR7 can lead to activity against endogenous ssRNA, leading to autoimmunity, and has recently been implicated in increased risk of developing SLE [Citation106].
Familial heritability of ME/CFS has been investigated in a few studies, including twin studies. Family members of patients with ME/CFS have been shown to be at increased risk for developing the syndrome themselves [Citation103,Citation107]. In twins, there is higher concordance for the development of ME/CFS in monozygotes than dizygotes [Citation108–110]. Human leukocyte antigens (HLA) are familiarly inherited, and two HLAs HLA-C*07:04 or HLA-DQB1*03:03, have shown some association with the development of ME/CFS [Citation111]. These findings are, however, difficult to compare, due to study design, patient selection, phenotypic heterogeneity, and environmental factors. Interestingly, HLA-DQB1 alleles appear to be associated with nearly all SSc-specific autoantibodies [Citation112], and in AAV, HLA-DQ alleles have been shown to be relevant in MPO-ANCA associated AAV, whereas HLA-DPB1 alleles have been shown to be associated with both PR3- and MPO-ANCA associated vasculitis [Citation113–117].
GWAS studies looking at the UK Biobank, identified one gene variant (rs7337312) [Citation104], that is supported by observations in metabolic studies in ME/CFS [Citation77,Citation104], this variant results in lower amounts of SLC25A15 mRNA and therefore higher amounts of ornithine in blood. SSc disease presentation with calcinosis and joint pain has been associated with elevated levels of ornithine, though extensive skin changes are associated with lower levels [Citation118].
2.3. Management and treatment strategies in ME/CFS
The uncertainty that surrounds the underlying causes and diagnosis of ME/CFS has resulted in unclear approaches guiding specific treatments. A review of the pharmacological treatment options available to ME/CFS patients [Citation119] found that no drug therapies have universal application. Eight medications did, however, show moderate effectivity, five of which led to the improvement of cognitive dysfunction (Lisdeamfetamine dimesylate, Rintatolimod, Moclobemide, Hydrocoritisone, Acteyl-L-carnitine) and three of which led to the improvement of fatigue (Acteyl-L-carnitine, Intravenous immunoglobulin, Rituximab) [Citation119]. Although many of these medications result in improved mitochondrial and immune functions [Citation120,Citation121], none of the studies reached significance – which may reflect the heterogeneous nature of patients with ME/CFS enrolled in these studies and lack of a biomarker that can identify specific patient groups. Hence, many of these medications are not generally utilized to treat ME/CFS.
The lack of drug interventions has resulted in implementing non-pharmacological management strategies for patients with ME/CFS. Energy conservation is an important feature in ME/CFS management as patients who overexert and surpass their energy stores have subsequent ‘crashes’ resulting in multiple days in bed trying to recover. This feature is captured in the most recent classification criteria for ME/CFS [Citation33,Citation122], under post-exertional malaise. Exercise is only advised in patients who are coping with their current daily activities, in severely ill patients, that act of taking a shower may be all the exercise they can handle [Citation20]. Patients, who have learned how to pace their activity and take breaks to conserve their energy, have been shown to reduce their overall fatigue severity [Citation20,Citation123]. Improved sleep habits and pacing mental activities may also aid in symptoms of fatigue and cognitive dysfunction [Citation20]. Pain, cardiovascular, and gastrointestinal symptoms should be addressed on a symptomatic basis. Patient support is key in ME/CFS, validation of their symptoms and monitoring of their mental health is important in establishing patients who are able to successfully cope with their diagnosis [Citation20].
3. Methods for literature review
Fatigue plagues the vast majority of SARD patients, having a profound effect on their mental and physical quality of life and there is substantial overlap in the mechanisms promoting ME/CFS, SSc, and PSV (). To determine the prevalence of severe fatigue and ME/CFS in SSc and PSV, we conducted a critical review of the published literature.
3.1. Search strategy and selection criteria
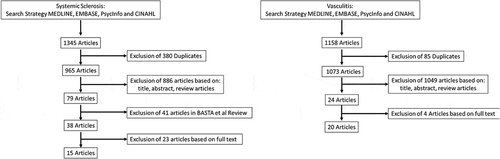
Electronic databases; Ovid, MEDLINE, APA PsycInfo, Embase, and CINAHL were searched from inception to September 2021. The exact search strategies and MESH terms are listed in the Supplement.
3.2. Inclusion and exclusion criteria
Inclusion criteria comprised: (a) cross-sectional and observational studies; (b) studies examining fatigue and factors associated with fatigue in either SSc or PSV. Data from random controlled trials and feasibility studies were included as data on fatigue interventions in these populations is limited and inclusion of these studies is informative. Exclusion criteria comprised: (a) studies that were case-reports, abstracts, expert opinion, or reviews; (b) studies that did not use published/appropriate and replicable measures to assess fatigue; (c) studies that were included in the Basta et al. [Citation124] review. Where multiple publications came from the same group of researchers and same patient group, data were retained for uniquely assessed fatigue variables. That is, if two studies represented the same patient group and one examined fatigue and quality of life and the other fatigue and education, data from both papers were included. No studies were pooled.
3.3. Screening, data extraction, and quality assessment
Two researchers (CvE, MO) independently assessed studies for eligibility and quality for inclusion in the SSc review, and two (CvE, JWCT), eligibility and quality for the PSV review (). Information from each eligible study was tabulated which include: year of publication, study design, patient characteristics, and fatigue assessment tools. Any disagreements were resolved through discussion with the third author (either MO or JWCT respectively). In total 15 articles were selected for SSc and 20 for PSV (, ).
Table 2. Summary of papers describing fatigue prevalence, correlates and interventions in systemic sclerosis.
Table 3. Summary of papers describing fatigue prevalence, correlates and interventions in primary systemic vasculitis.
3.4. Data synthesis
Due to the heterogeneity of the included studies and the broad nature of the review aim, quantitative analysis of the results was regarded unsuitable. Therefore, a descriptive synthesis was conducted. Studies were sorted based on (a) ways of measuring fatigue, (b) comparing fatigue with QoL and (c) possible explanations of the fatigue.
4. Fatigue in systemic sclerosis
Fatigue in SSc has been previously described by several studies – though the frequency of post-exertional malaise, sleep disturbances, and cognitive impairment has not [Citation124–128]. Many of the tools that have been used to assess fatigue in patients with SARDs (MFI, CFS, SF36), are validated for use in ME/CFS clinical trials [Citation129]. We have found that the MFI, SF36 and FACIT-F scores, correlated with each other in eSSc patients, as did cognitive failure (The Cognitive Failures Questionnaire (CFQ) [Citation130]) and sleep disturbances (Pittsburgh Sleep Quality Index (PSQI) [Citation131]) (van Eeden, Cohen Tervaert & Osman, unpublished). We postulate that if patients are assessed by one or more of these fatigue tools, as well as measures of cognitive failure, sleep disturbances and/or fatigability, that these patients could be further assessed for the presence of ME/CFS using the Canadian consensus and/or IOM diagnostic criteria.
A systematic review by Basta et al. [Citation124], in 2018, found that in SSc, fatigue was one of the most prevalent symptoms, having a profound impact on patient’s personal, social, and work quality of life. They also found that pharmacological treatments, particularly aimed at treating SSc were ineffective, but that encouraging data was beginning to emerge concerning exercise and alternative medicine, both of which have been shown to reduce fatigue in SSc patients. We conducted a review of the literature and found that 15 new studies had been conducted since the Basta et al. review [Citation124], with regard to fatigue in SSc. We have summarized our results below, looking at not only the prevalence and severity of fatigue and its association with disease factors in SSc but also correlations with sleep disorders, cognitive failure, and post-exertional malaise.
4.1. Prevalence and severity of fatigue in SSc
All papers describing the prevalence of fatigue in SSc used a different fatigue measure. Kesikburun et al., investigated fatigue using the Fatigue Severity scale (FSS), in 33 SSc patients (63.6% lSSc) and 34 healthy controls, and found that in their cohort 63% of patient’s experienced severe fatigue (>5 point score) [Citation132]. The only study to assess fatigue in early diffuse SSc patients (edSSc) (<3 yrs.) (n = 326) was Peytrignet et al., who found that 35.8% of the patients reported ‘quite a bit or very much fatigued’ (questionnaire item) using the Functional Assessment of Chronic Illness Therapy – Fatigue (FACIT-F) [Citation133]. Using the FSS, Cetin et al., found that 48% of SSc patients with dSSc had severe fatigue (FSS total ≥53) [Citation134]. Yakut et al., compared 35 healthy volunteers to 35 SSc patients and found that cognitive, physical, and psychosocial Fatigue Impact Scale (FIS) fatigue scores, were significantly higher in SSc patients when compared to healthy controls [Citation135].
Studies comparing the level of fatigue between the SSc subtypes, have contradictory findings. Two studies found no difference between SSc subtypes; Murphy et al., investigated fatigue (Patient-Reported Outcomes Measurement Information System (PROMIS-29)) in 267 SSc patients, with scores for limited SSc (58.4 ± 10.4) and diffuse SSc (57.5 ± 10.4) being similar (51.06% of patients had lSSc) [Citation136]. Santiago et al., investigated fatigue (FACIT-F) in 142 SSc patients and found that disease subtype did not correlate with the level of fatigue, 75.5% of the cohort had lSSc [Citation137]. Sierakowska et al., on the other hand found fatigue (VAS) in dSSc 47.33 (27.30) to be significantly higher than in lcSSc 38.56 (30.20) (49.4% of the patients had lcSSc) [Citation138].
Three studies compared fatigue in SSc to fatigue in healthy controls, all found fatigue in SSc to be significantly higher than controls. Mean FSS scores where 4.8 ± 1.8 and 1.3 ± 0.5, and mean FIS scores; 45 (26–66) vs 20 (17–24.3) and 74.62 ± 31.54 vs 11.05 ± 10.20, respectively, for SSc vs healthy controls [Citation132,Citation135,Citation139]. Two studies compared the severity and frequency of fatigue in SSc and RA, both found limited difference between the two groups; Sierakowska et al. reported a mean VAS score of (46.6 ± 29.5) in SSc 140 patients, 59.2% lSSc) compared, to (52.9 ± 22) in RA (277 patients) [Citation140] and Gok et al. found FSS scores to be 4.7 ± 1.8 and 4.4 ± 1.9, for SSc and RA, respectively [Citation141].
4.2. Fatigue associations with disease factors, disability, and quality of life
Investigating dyspnea and pulmonary function markers; Kesikburun et al. found that dyspnea correlated with fatigue severity in SSc, no correlation was observed for pulmonary function measurements; HRCT (high resolution computed tomography), FVC (forced vital capacity), MVV (maximum voluntary ventilation), and PASP (Pulmonary arterial systolic pressure) [Citation132]. Yakut et al. also found dyspnea, as well as age correlated with fatigue [Citation135]. They also found some pulmonary function markers (diffusing capacity (DLCO), maximal inspiratory pressure (MIP), and maximal expiratory pressure (MEP)) correlated with fatigue and others (FVC, forced expiratory volume in 1 s (FEV1) or Peak Expiratory Flow (PEF)) did not [Citation135].
Peytrignet et al. investigated various clinical measures in 326 edSSc patients, they found fatigue (FACIT-F) correlated with corticoid steroid use, organ involvement (renal, cardiac, and pulmonary), skin thickening, elevated ESR and CRP and lower hemoglobin [Citation133]. No correlation was seen for age or autoantibodies (anti-topoisomerase, anti-ScL70, anti-RNA polymerase III, and anti-centromere) [Citation133]. Sierakowska et al. found that age and disease duration correlated with fatigue (VAS) in both SSc and RA patients [Citation140], conversely however, Yakut et al. found disease duration did not correlate with fatigue in SSc patients (FIS) [Citation135]. Utilizing the FACIT-F, Santiago et al. found that in 142 SSc patients fatigue correlated with disease severity (VAS) and disease activity European Scleroderma Trials and Research group (EUSTAR) [Citation137].
Multiple papers highlight the association of decreased quality of life, disability, and reduced physical function as correlates of fatigue in SSc. Quality of life (QoL) measured by the EuroQOL five dimensions questionnaire (EQ-5D) was found to be correlated with fatigue in studies by both Santiago et al. and Murphy et al. [Citation136,Citation137]. QoL as measured by the Scleroderma Health Assessment Questionnaire (SHAQ), also correlated with fatigue in two studies [Citation133,Citation135]. Additionally, Sierakowska et al. found that in dSSc patients, fatigue was correlated with QoL as measured by the Systemic Sclerosis Quality of Life Scale (SScQoL) [Citation138]. Finally, Murphy et al. found that fatigue severity at baseline was not predicative of reduced quality of life, during long-term follow-up [Citation142].
Three studies identified an association between worsening disability scores as measured by the Health Assessment Questionnaire Disability Index (HAQ-DI) and fatigue in SSc patients [Citation133,Citation135,Citation141]. Gok et al., Peytrignet et al., and Murphy et al., all found fatigue to correlate with the reduced physical function as measured by the SF-36 PCS and PROMIS-29 tools [Citation133,Citation136,Citation141]. Two studies also found an association between reduced social participation (PROMIS-29) and fatigue [Citation137,Citation142].
4.3. Fatigue as related to cognitive failure, sleep disorders, and fatigability
No studies investigated cognitive failure directly, but mental function as measured by the SF-36 mental component summary score (MCS) was shown to be associated with fatigue in SSc patients [Citation133,Citation135,Citation141,Citation143]. Gok et al., also showed SF-36 MCS values to be similar between SSc (58.3 ± 21.5) and RA (56.7 ± 22.6) patients [Citation141]. March et al. found that depression correlated with fatigue in SSc patients, and that cognitive failure as measured by the SF-36 MCS (61.3 ± 19.1), strongly correlated with depression [Citation143].
Only one study looked at sleep and its association with fatigue; Horsley-Silva et al. found that in 287 SSc patients (lSSc 64%), patients with poor sleep quality as measured by the Pittsburgh Sleep Quality Index (PSQI) had higher mean fatigue (FSS) scores (44.6) than those with average sleep quality (33.15) [Citation144]. Poor sleep quality was also associated with increased gastroesophageal reflux disease (GERD), and moderate-to-severe GERD correlated with fatigue [Citation144].
Reduced muscle strength and fatigability were investigated in three studies. Justo et al. evaluated muscle performance in 33 women with SSc [Citation139]. Using isokinetic measurements, they observed loss of muscle strength and endurance, resulting in reduced fatigue tolerance in the SSc patients when compared to healthy controls. Most isokinetic measurements correlated with reduced physical function including lower handgrip strength (16 (12–20) and 31 (27.8–37.5) in SSc and healthy controls, respectively) and increased disability (HAQ-DI), but were not shown to correlate with the FIS (45 (26–66)) and FACIT (29.5 (21.8–33.8)) fatigue scores [Citation139]. Yakut et al. evaluated quadriceps and handgrip (16.47 (±3.77)) muscle strength in 35 SSc patients and found it to be reduced when compared to healthy controls. SSc patients also had increased fatigability as measured by the 6 minute walking test (6MWT) (MWT distance 395.08 ± 75.00) [Citation135]. In contrast to Justo et al., muscle strength and fatigability did correlate with FIS fatigue scores (74.62 (±31.54)), as well as both the physical (39.60 (±18.38)) and mental (42.57 (±15.20)) components of the SF-36 [Citation135].
4.4. Exercise and education in fatigue management
Two studies investigated the role exercise may play in improving fatigue in SSc patients; particularly they compared structured supervised exercise to unsupervised home exercise. Cetin et al. conducted a randomized trial to investigate the effects of a Tai Chi exercise program on patients with dSSc. After 10 weeks, the control (home exercise group n = 14) showed no improvement in either fatigue impact (FIS) or severity (FSS), in the Tai Chi group (n = 14); however, both significantly improved [Citation134]. In addition, Tai Chi was superior to home exercise in the improvement of sleep quality and trunk lateral endurance [Citation134].
Yakut et al. carried out a randomized trial investigating a supervised structured exercise program (n = 20) (breathing exercises, resistance training, and aerobic training twice a week for 12 weeks under the supervision of a physiotherapist) to a home exercise group (n = 20) (introduction to course with physiotherapist, but no supervision over the 12 weeks) [Citation145]. After 12 weeks, there was a significant reduction in fatigue (FIS), disability (HAQ-DI and SHAQ), the physical component of the SF-36 and 6MWT walking distance in both groups. As in the Cetin et al. study, improvements found the supervised exercise plan was superior to the home exercise plan [Citation145].
Sierakowska et al., assessed level of education and educational needs as factors in SSc patient fatigue. They found that fatigue in 140 SSc patients correlated with their educational needs using the Polish version of the Educational Needs Assessment Tool (pol-ENAT), conversely in RA patients fatigue did not correlate with education needs [Citation140]. Additionally, they found that patients with a lower level of education had higher levels of fatigue [Citation146].
4.5. Summary
Fatigue levels in SSc patients are higher than in the general population. Between this review and the review by Basta et al., the prevalence of fatigue in SSc had a range between 33% and 89%. This variation is likely a reflection of the number of different tools used to assess fatigue in these patients since 10 different scales/measures of fatigue were used, additionally, in most instances the definition of ‘fatigue’ is not clearly stipulated. The severity of fatigue in SSc patients is higher than in healthy controls and is comparable to the levels of fatigue seen in RA patients.
Findings on association of SSc subtype and disease duration are inconclusive and may not be a factor in the development of fatigue in SSc patients. Few studies looked at SSc disease factors; though dyspnea, lung, and gastrointestinal involvement were highlighted as factors associated with fatigue in SSc (). All studies that looked at QoL in SSc patients found that fatigue was associated with reduced QoL resulting in reduced physical and social function, which often leads to increased work disability ().
Figure 3. Factors associated with fatigue in SSc and AAV. Factors were weighted by how many times they were found to be associated with fatigue within each respective review.
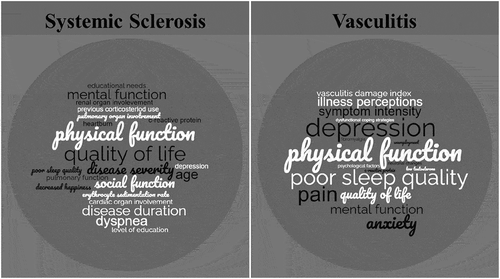
Few studies looked at symptoms that are associated with ME/CFS. Only the SF-36 MCS was used to assess mental function and although not a direct measure of cognitive failure, the reduced function is indicative of decline, and was found to be associated with fatigue in all studies that investigated it. Poor sleep quality was rarely assessed, but one study in this review [Citation144], and two in the Basta et al. review [Citation147,Citation148], found sleep quality to be poor in SSc patients, correlating with increased fatigue levels. Fatigability as measured by the 6MWT was shown to be high in SSc patients in multiple studies, though contrasting results dispute its influence on the development of fatigue.
All studies investigating exercise as a management tool for fatigue in SSc found it to be of significant benefit. Our review did not review any additional studies on pharmacological, complementary, or alternative medicines and their effect on fatigue in SSc.
An author in each review found an association between higher fatigue levels and less than post-secondary education in SSc patients [Citation140,Citation149]. Intriguingly, Sierakowska et al. found that patients with higher levels of fatigue had greater educational needs and would likely benefit from patient education [Citation140].
5. Fatigue in primary systemic vasculitis
Primary systemic vasculitis (PSV) is a group of heterogeneous diseases that result in vascular inflammation [Citation150]. They are classified based on the size of affected vessels, e.g. large-vessel vasculitis (LVV) such as Takayasu arteritis or giant cell arteritis (GCA), medium-vessel vasculitis such as polyarteritis nodosa (PAN) and small-vessel vasculitis (SVV) such as antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis (AAV), IgA vasculitis, and/or cryoglobulin associated vasculitis [Citation150,Citation151]. AAV can be subclassified further in granulomatosis with polyangiitis (GPA, formerly known as Wegener’s granulomatosis), microscopic polyangiitis (MPA), eosinophilic GPA (eGPA, formerly known as Churg-Strauss vasculitis) and renal-limited vasculitis (RLV) [Citation152]. AAV is further defined by ANCA serotype, PR3-ANCA associated vasculitis and MPO-ANCA associated vasculitis [Citation152].
Although the mortality rate in PSV has precipitously declined since the implementation of corticosteroids and other immunosuppressive therapies [Citation153,Citation154], they continue to have a huge toll on patient-related QoL [Citation155–158]. In a study by Hoffman et al. 60/100 patients with GPA, 80% of these patients experienced significant loss of energy and had to adjust daily life accordingly, 43% of patients also suffered depression related to their disease [Citation159]. This was further demonstrated by Boomsma et al., who found many patients continued to have decreased energy and a large number (33%) of patients reported depression [Citation160].
In spite of the frequency of these symptoms affecting patients with PSV, only few studies have explored and described these symptoms, which largely may stem from a lack of validated tools investigating these problems. To address this limitation, the Outcome Measures in Rheumatology (OMERACT) Vasculitis Working Group has been developing patient-reported outcome tools for AAV and PSV in order to improve assessment [Citation161]. To better understand the role of chronic fatigue, we reviewed the current knowledge regarding chronic fatigue in PSV (and its subtypes) with a focus on its prevalence and QoL sequelae.
5.1. Prevalence and severity of fatigue in PSV
One of the initial studies investigating the prevalence of fatigue in vasculitis was by Basu et al. 2010 [Citation162]. Using the Chalder Fatigue Scale (CFS), they found that 38.4% of AAV patients (n = 90, 66.7% GPA) had moderate-to-severe fatigue compared to 26.1% of healthy controls (n = 781) [Citation162]. In a later study, they found that 74.8% of AAV patients (n = 410, 64.6% GPA) had high levels of fatigue (dichotomized at the general population mean, i.e. upper 50% of the general population) [Citation163]. Macfarlane et al. also utilized the CFS and found that 69.9% of AVV patients (n = 166) reported high levels of fatigue (dichotomized at the general population mean) [Citation164]. In 55 GPA patients, Hajj-Ali et al. 76.4% experienced chronic fatigue (VAS-fatigue) [Citation165]. Grayson et al. and Mayor & Reidy et al. utilized the general fatigue domain of the MFI in PSV patients and reported fatigue levels of 76% (score ≥13) (n = 692, 49.9% GPA) and 91.2% (n = 249, 40.2%), respectively [Citation166,Citation167].
Fatigue severity in vasculitis patients was shown to be significantly higher than in healthy controls. Can-Sadicki et al., found that the mean Multidimensional Assessment of Fatigue (MAF) score in patients with active Behcet’s disease (BD) (26.8 ± 9.7) was significantly higher than those with inactive disease (20.4 ± 9.4) and healthy controls (19.2 (7.3–44.2) [Citation168]. A similar study by Ilhan et al. found that the mean MAF score in BD (n = 123) was 20.6 ± 14.7, compared to 12.8 ± 9.9 in healthy controls (n = 71) [Citation136]. McClean et al. investigated fatigue (MFI) in 48 AAV patients, and found their fatigue levels to be significantly higher (13 (8–16)) than in the healthy controls (5.5 (4–8)) [Citation169].
Multiple studies looked at fatigue levels between the different vasculitis subgroups. Basu et al. 2010 found fatigue (CFS) varied between subtypes, with MPA patients reporting higher rates, than GPA and eGPA patients [Citation162]. In a later study, the MPO-ANCA serotype was found to be a contributing factor to increased fatigue [Citation163]. Similarly, Koutanji et al., using the VAS-fatigue scale found that levels of fatigue were significantly different between disease groups, GPA patients had much lower scores (35.32 ± 23.31), than eGPA (60.57 ± 29.70) and MPA (70.0 ± 36.74) patients [Citation170]. In contrast to these findings, Rimland et al., McClean et al. and Tomasson et al., reported no significant difference with regard to vasculitis subtype and fatigue [Citation169,Citation171,Citation172].
5.2. Disease factors associated with fatigue in vasculitis
Four studies looked at age and disease duration as correlates of fatigue in vasculitis patients; none found any association [Citation163,Citation165,Citation168,Citation173]. Basu et al. 2010 using the SF-8 [Citation162], and Rimland et al. Ilhan et al., and Basu et al. 2014 using the SF-36, found that fatigue was associated with reduced physical function in vasculitis patients [Citation172–174]. The association of C-reactive protein (CRP) levels and fatigue was investigated; Rimland et al. (PSV, n = 112) and Basu et al., 2010 (AAV, n = 90) found fatigue was not associated with CRP levels [Citation162,Citation172]. Conversely, Tuin et al. (AAV, n = 70) and Basu et al. 2013 (AAV, n = 410) found that CRP did correlate with fatigue [Citation163,Citation175].
Organ involvement was mentioned in a few studies; McClean et al. found that in 48 AAV patients, fatigue (MFI) was not correlated with lung or renal involvement, or cardiovascular fitness [Citation169]. Similarly, Ilhan et al. and Can Sandicki et al. also found no correlation with organ involvement and fatigue (MAF) [Citation168,Citation173]. Contrary to these findings, Basu et al., found associations between fatigue (CFS) and renal [Citation162], and chest involvement [Citation163]. Basu et al., also carried out two studies investigating neural correlates and MRI changes in GPA patients, fatigued patients showed greater structural connectivity in the fornix and cingulum nerve bundles when compared to healthy controls [Citation176], and involvement of the striato-thalamo-frontal structures of the brain when compared to non-fatigued GPA and healthy controls [Citation177]. Similar cingulate cortex connectivity changes have also been observed in ME/CFS patients [Citation178].
Disease activity and illness perception were investigated in multiple studies. Disease activity as measured by the Behcet’s Disease current activity form (BDCAF), and/or the Birmingham vasculitis activity score (BVAS), were shown to correlate with increased fatigue by Tuin et al., McClean et al., and Can Sandicki et al. [Citation168,Citation169,Citation175]. In contrast, Hajj-Ali et al., and Basu et al. 2013, found no correlation between fatigue and BVAS [Citation163,Citation165]. Rimland et al., found that fatigue as measured by the MFI, correlated with, disease activity (PtGlobal), illness perception (BIPQ), and vascular FDG-PET activity, in 112 PSV patients [Citation172]. Fatigue levels were not shown to change significantly between active and remission state of disease [Citation172]. Grayson et al. 2013 found that in 692 PSV patients, fatigue (MFI) was correlated with illness perception as measured by the Illness Perception Questionnaire (IPQ-R) [Citation166]. The authors later investigated fatigue in these patients at both the onset of disease and at relapse. They found that at onset fatigue did not correlate with medical risks factors (items including; ‘diet or eating habits,’ ‘ageing,’ ‘alcohol,’ ‘smoking,’ and ‘accident or injury.’), infectious or hereditary factors or altered immunity, whereas at relapse, medication factors (items including; ‘change in my medications’ and ‘stopped taking my medications.’) correlated with fatigue [Citation179].
Four papers investigated the association of pain with increased fatigue. Mayor and Reidy, found that in 249 PSV patients, fatigue (MFI) was associated with pain (number of days in last month) [Citation167]. Koutanji et al. found an association between VAS-fatigue, VAS-pain, and SF-36 vitality scores in 51 PSV patients [Citation170]. Two of the studies investigated pain in terms of fibromyalgia, Hajj-Ali et al., using the London Fibromyalgia Epidemiologic Study Screening Questionnaire (LFESSQ) and the Symptom Intensity (SI) scale [Citation165] and Basu et al. 2010, using the ACR chronic widespread pain index [Citation162], both studies found fibromyalgia to be associated with fatigue in vasculitis patients.
5.3. Anxiety, depression, and quality of life as related to fatigue
Anxiety and depression were often investigated. Basu et al. found depression (Beck’s depression Inventory, BDI); to be associated with moderate/severe fatigue (CFS) in the 90 AAV patients assessed [Citation162]. In a later study, with 140 AAV patients, anxiety, depression, and dysfunctional coping strategies were again found to be associated with fatigue [Citation163]. Comparing AAV patients to healthy controls, McClean et al. showed strong correlations between fatigue (MFI) and anxiety (HADS), depression (HADS) in AAV patients [Citation169]. Two studies compared BD patients and healthy controls; both found that in BD patients, fatigue (MAF) was correlated with both anxiety and depression (HADS) [Citation168,Citation173]. These same factors correlated with fatigue in healthy controls, though their levels of fatigue were significantly lower [Citation173]. Hajj-Ali et al. also found depression (Brief Patient Health Questionnaire-9 (BPHQ-9)), to correlate with fatigue in GPA (n = 55) patients [Citation165]. Looking at fatigue in 692 PSV patients, Grayson et al. 2014 compared patients at the onset of disease and at relapse and found that psychological factors (including terms; ‘stress or worry,’ ‘my mental attitude,’ ‘family problems,’ ‘overwork,’ ‘my emotional state,’ and “my personality) were associated with fatigue at both time points [Citation179].
Quality of life was assessed in a few studies. Can Sandicki et al. found fatigue (MAF) in BD patients was correlated with QoL (BDQoL) [Citation168] and Koutanji et al. found that in 51 PSV patients, QoL (SF-36 vitality) was correlated with VAS fatigue [Citation170]. QoL scores also correlated with disability (HAQ) and symptom severity as measured by VAS [Citation170]. In a feasibility and construct validation study, Tomasson et al., investigated fatigue as defined by the PROMIS CAT instrument in 973 PSV patients, and also found fatigue to correlate with SF-36 vitality scores [Citation171].
5.4. Fatigue as related to cognitive failure, sleep disorders, and fatigability
One study directly investigated cognitive failure; Tomasson et al. found fatigue (PROMIS) to correlate with reduced cognition as measured by PROMIS, in 937 PSV patients [Citation171]. A few studies looked at mental function as measured by the SF-36 MCS, including Tomasson et al., who found that fatigue correlated with quality of life (vitality, SF-36) and the mental component score of the SF-36 (48.7 (11.7)) [Citation171]. Basu et al. 2014 and Rimland et al., also both found fatigue was strongly associated with the MCS SF-36, in 410 AAV and 112 patients, respectively [Citation172,Citation174]. In a study comparing BD patients (42.1 ± 9.5) and healthy controls (49.0 ± 8.2), Ilhan et al. found that in BD fatigue correlated with the mental component of the SF-36, and that MCS scores were significantly lower in the BD patients [Citation173]. McClean et al. compared MFI scores in AAV patients (48.4 (39.2–55.0)) and healthy controls (56.3 (54.4–58.4)) they too found that fatigue correlated with MCS and that AAV patients had significantly lower scores [Citation169].
Multiple studies investigated sleep disturbance; Koutanji et al., found that in 51 PSV patients, VAS fatigue, VAS sleep, and SF-36 vitality scores were correlated [Citation170]. Mayor and Reidy and Basu et al. 2013 found that sleep disturbances (Estimation of Sleep Problems Questionnaire (ESQ)) were associated with fatigue (MFI) in 249 PSV and 140 AAV patients, respectively [Citation167]. Using the Epworth Sleepiness Scale (ESS), Hajj-Ali et al., also found fatigue (VAS-fatigue) to correlate with sleep disturbances in GPA patients [Citation165]. McClean et al. showed strong correlations between fatigue (MFI) and sleep disturbances as measured by the PSQI, in 48 AAV patients [Citation169].
Only a single study looked at muscle endurance/fatigability, and it was found that fatigability as measured by quadriceps contraction was correlated with fatigue (MFI) scores in 48 AAV patients [Citation169].
5.5. Medication, exercise, and education in PSV patients with fatigue
Two studies reported on clinical trials involving vasculitis patients. O’Malley et al. reported on two trials RITUXVAS [Citation180] (n = 33) (compared rituximab plus two doses of Cyclophosphamide, compared with conventionally dosed pulse cyclophosphamide followed by azathioprine for maintenance therapy) and MYCYC [Citation181] (n = 117) (compared mycophenolate mofetil with pulsed cyclophosphamide followed by azathioprine for maintenance therapy) [Citation182]. The median fatigue level as measured by the vitality domain of the SF-36 improved after the first 6 months of treatment (45 (IQR 30–60), but then remained stable [Citation182]. Significant improvement was reported in 70% of patients, though fatigue remained worse than seen in the healthy controls. Longitudinally 25% of patients had deteriorating levels of fatigue and 12% had no change at all [Citation182]. Strand et al., investigated quality of life in GCA patients enrolled in the GiACTA RCT, four groups receiving either tocilizumab and prednisone or placebo and prednisone were investigated [Citation183]. Patients receiving tocilizumab (TCZ) had significant and clinically relevant improvement in their symptoms of fatigue with a mean FACIT score change of 5.30, compared to 0.09 and −0.42 in the prednisone only test groups [Citation183]. In two studies investigating the correlates of fatigue in PSV patients, the use of other immunosuppressive drugs was not shown to be associated with less fatigue [Citation165,Citation173].
Our search found a single study investigating physical activity in support of the management of fatigue in AAV. Harper et al. compared a standard care group to an intervention group who received physical activity, behavioral change support and standard care [Citation184]. Protocol adherence was less than optimal in this feasibility trial, though there was an indication that MFI scores improved more in the intervention group [Citation184].
Only one study investigated education as a determining factor in fatigue in vasculitis patients; Can-Sandikci et al. found that BD patients level of education was not correlated with fatigue as measured by the MAF [Citation168].
5.6. Summary
The prevalence of fatigue in PSV varied widely between studies, ranging from 38.4% to 91.2%, this may be a reflection of both the variation in the patient population with regard to vasculitis type and the use of multiple fatigue scoring tools. Most studies, however, show that fatigue is a common and significant factor for vasculitis patients. All studies showed that rates of fatigue are higher in PSV than in healthy controls.
Studies investigating variation in fatigue severity between vasculitis subgroups were inconclusive, as were studies looking at CRP levels, organ involvement, and disease activity, suggesting these factors may not factor in the development of fatigue in PSV patients. Age and disease duration were not found to be associated with fatigue, whereas pain and physical function were, in all studies that included these factors as a measure (). Anxiety, depression, and quality of life were shown to be associated with fatigue ().
Multiple studies investigated symptoms regularly associated with ME/CFS. Five studies investigated sleep disturbances as a correlate of fatigue in PSV, and all found an association. Although only one study explored cognitive failure, multiple studies looked at mental function (SF-36 MCS), all these studies found impaired mental function to be associated with fatigue in PSV patients. Only one study looked at fatigability, and it was found to be associated with fatigue.
In contrast to the number of studies investigating treatment strategies in SSc, very few are available for PSV. Three pharmacological trials were conducted investigating the use of tocilizimab, rituximab and cyclophosphamide, there was significant improvement in fatigue scores for most patients, however fatigue levels were not comparable to healthy volunteers. Also, in a subset of patients showed no improvement all.
One study investigating exercise and behavioral changes as a treatment option for fatigued AAV patients, suggested there may be some improvement [Citation184]. A later study (published after database search) explored the experience of the patients following this intervention, and found they could mitigate their symptoms and side-effects, with self-management [Citation185]. Additionally, a recent study by Astley et al. found that a home-based exercise program improved functionality and physical fatigue scores in patients with Childhood-onset Takayasu Arteritis [Citation186]. One study investigated patient education level, and in contrast to what was seen in SSc patients, level of education did not correlate with perceived fatigue.
6. Conclusion
ME/CFS, Systemic Sclerosis, and Vasculitides are unique diseases with vastly different presentations. However, their pathogenesis shares a multitude of inflammatory, immune, and metabolic perturbations. In particular, mitochondrial dysregulation, Th2 polarization, low IgG levels and reduced NK cell cytotoxicity have been highlighted in all three groups – suggesting that in patients with ME/CFS and SSc or vasculitis, common mechanisms may be present. Patients with these diseases also present with high levels of persistent, debilitating fatigue.
To our knowledge, this is the first review investigating fatigue in PSV. Our review of both SSc and PSV, highlights the burden of fatigue in both patient groups. The average prevalence of fatigue in PSV (71%) was higher than that reported in SSc (59%). Factors correlated with fatigue were better highlighted in the PSV group, with physical function, depression, pain, and poor sleep quality, having the strongest association. In SSc, quality of life and physical function had the strongest association. Disease-specific factors such as disease severity, disease activity, and organ involvement had little to no association with fatigue. These findings help elucidate why disease-specific pharmacological interventions have had mixed success in improving SARD patient fatigue. Although these approaches are being disputed in ME/CFS, our review highlighted that in SARDS, exercise, and behavioral changes may result in improved fatigue, sleep, anxiety, and depression symptoms.
The strength of this review is that it covered an extensive literature search, which was conducted using a replicable methodology, with abstract screening and data extraction being carried out by independent researchers in order to reduce bias. However, there are limitations to this study. This review included only English language articles and smaller sample size studies were included. There is a need for more research looking into the determinants of fatigue in SARDs patients, in larger cohorts. This study may be influenced by publication bias. The vast heterogeneity between patient groups and fatigue assessment tools precluded a meta-analysis, and it was thus not possible to formally assess the extent of publication bias. The assessment tools had distinct standards, so the integrated results in our article, may only relatively reveal the true value Future studies looking at fatigue in SARDs should reach consensus on which patients and assessment tools to employ, to allow for quantitative analysis. Cross-sectional, prospective studies, feasibility, and randomized control trials were included, although the majority of studies were cross-sectional. Therefore, there is a limit to the conclusions that can be drawn about cause and effect. Many of the disease-related factors examined in this review (such as pain, depression, sleep disorders, and disease severity) are self-reported, and thus patient bias cannot be excluded. The majority of measures have, however, been validated.
The mechanisms leading to life altering fatigue in SARDs may not be fully addressed by treating disease-specific parameters, and physicians managing these diseases should be familiar with the tools utilized for diagnosing ME/CFS in order to better identify these patients, as other strategies may be important in their management. In particular, physicians should affirm fatigued patients reduced quality of life, and make concerted efforts, in informing patients of possible management strategies.
7. Expert opinion
There are numerous classifiable inflammatory diseases, and many others that are not classifiable. Their development appears to be multifactorial, and poorly understood. Persistent, debilitating fatigue is frequent in most SARDs, and is generally under-recognized. The notion that fatigue in SARDs can be fully explained by the pathology of the disease from which they suffer, may be short sighted as inflammatory manifestations associated with these diseases may improve with immunomodulation, but fatigue symptoms do not. Importantly, fatigue may not be associated with common outcomes utilized for assessing disease activity and/or progression – suggesting that it may be an independent manifestation of these diseases which is not captured using traditional biomarkers associated with disease activity.
We brought attention to the immune and metabolic similarities between ME/CFS, SSc, and PSV. Importantly, mitochondria are known to regulate activation, differentiation, and survival of immune cells. Currently, the use of low-dose Naltrexone hydrochloride (NTX) is being studied in PSV (NCT03482479). More research is needed, specifically concerning the use of approved drugs, to improve mitochondrial and immune cell function in these patients. For instance, Ursodeoxycholic acid (UDCA), a treatment for primary biliary cirrhosis (PBC), has been shown to modulate mitochondrial transmembrane potential, respiration and reduced ROS production [Citation182]. UDCA has also been shown to beneficially affect mitochondrial function in Alzheimer’s [Citation183] and Parkinson’s disease [Citation184]. On the other hand, NTX used to treat opioid and alcohol use disorders, has been shown to improve NK cell cytotoxicity [Citation185] and reduce microglia activation [Citation186]. Low dose NTX has been shown to have beneficial effects on the symptoms of chronic fatigue and chronic pain in ME/CFS and fibromyalgia patients [Citation186,Citation187].
This review highlights the significant burden of persistent fatigue in both SSc and PSV patients, as well as the strong association of this fatigue with factors associated with quality of life, rather than disease associated factors. In particular, the role of patient education, behavioral adjustment and exercise as potential management strategies is highlighted. Education and behavioral therapy have shown success in improving patient outcomes, studies in RA [Citation187], SLE [Citation188], and SSc [Citation189] and multiple trials are currently evaluating these strategies in the management of SARDs [Citation190,Citation191] and ME/CFS (NCT01765725). The effect of exercise should not be neglected. Exercise has been shown to be of benefit in the management of fatigue in RA [Citation192], SLE [Citation193] as well as SSc and PSV. Exercise alone has been shown to improve both mitochondrial function and biogenesis [Citation194], as well as NK cell function [Citation195].
In spite of studies describing fatigue in many SARDs, research is still seriously lacking as only a few studies assessed fatigue in these diseases as the sole outcome. Furthermore, the lack of consensus on the use of objective fatigue measurements using subjective questionnaires and biomarkers in both SSc and PSV has limited the generalizability of some studies. The field of research into ME/CFS has undergone rapid expansion in recent years, particularly following the identification of post-COVID-19 syndrome (long COVID). There is much overlapped in the symptoms, immune, and metabolic profiles of long COVID and those of ME/CFS patients [Citation75,Citation196], just as we have highlighted in SARDs. Many long COVID patients fulfill the diagnostic criteria for ME/CFS [Citation76,Citation197]. We have identified that patients with early SSc and AAV patients with fatigue fulfill the diagnostic criteria for ME/CFS. Although these patients cannot be diagnosed as having ME/CFS, the criteria surrounding the diagnosis of ME/CFS have been very well researched. Using ME/CFS criteria as a standardized approach to identifying persistent fatigue in SARDs may help streamline research, including the identification of potential biomarkers as well as improved treatment strategies.
In the next few years, the recent increase in research interest and funding opportunities for ME/CFS, will likely lead to exciting new developments and improvements in the understanding of the development of fatigue in these patients. Due to the overlap in symptoms, immune, and metabolic profiles of ME/CFS and SARDs patients with fatigue, results from these studies may inform future directions, beneficial to all patients who fulfill the criteria for ME/CFS, independent of the underlying disease.
Finally, it is of great importance for physicians treating patients suffering from SARDs recognize and acknowledge that fatigue is a prominent, life-altering feature in these diseases. This fatigue may not improve with disease remission, and the mental and physical toll that these symptoms may have on patients are poorly appreciated. Treating physicians should be encouraged to educate themselves for methods utilized to identify patients suffering from persistent fatigue, and some of the non-pharmacological management strategies that may be required. As studies continue to unlock the mechanisms underpinning these symptoms, treating physicians will play fundamental roles in educating and empowering their patients in dealing with the challenges associated with fatigue.
Article highlights
Immune and metabolic similarities exist between Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS) patients, Systemic Sclerosis (SSc), and primary small vessel vasculitis (PSV).
Persistent fatigue is a prominent feature in patients with SARDs, and does not appear to be fully related to disease activity or progression.
Studies specifically looking at immune and metabolic dysregulation, and its association with fatigue in SARDs are lacking
Until fatigue in SARDs is fully understood, physicians should educate patients on possible management strategies, such as education and exercise.
With fatigue in SARDs having potential overlap with ME/CFS, there is a need to recognize and acknowledge the impact of persistent fatigue on SARDs patients’ quality of life.
Declaration of interest
J Cohen Tervaert and M Osman’s research is supported by unrestricted grants from the University of Alberta (Canada), Dutch Kidney Foundation, the University Hospital Foundation and Kaye Grants, Scleroderma Canada, and Boehringer Ingelheim. Additionally, M Osman is funded by the Arthritis Society (STAR career development award) and Scleroderma Canada. J Cohen Tervaert received speaker honoraria from Pfizer and Medexus. M Osman received speaker honoraria from Boehringer Ingelheim. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Supplemental Material
Download MS Word (19 KB)Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/1744666X.2022.2116002
Additional information
Funding
References
- Cooper GS, Stroehla BC. The epidemiology of autoimmune diseases. Autoimmun Rev. 2003 May;2(3):119–125.
- Ramos-Casals M, Brito-Zeron P, Kostov B, et al. Google-driven search for big data in autoimmune geoepidemiology: analysis of 394,827 patients with systemic autoimmune diseases. Autoimmun Rev. 2015 Aug;14(8):670–679.
- Bairkdar M, Rossides M, Westerlind H, et al. Incidence and prevalence of systemic sclerosis globally: a comprehensive systematic review and meta-analysis. Rheumatology (Oxford). 2021 Jul 1;60(7):3121–3133.
- Berti A, Dejaco C. Update on the epidemiology, risk factors, and outcomes of systemic vasculitides. Best Pract Res Clin Rheumatol. 2018 Apr;32(2):271–294.
- Hellmich B, Lamprecht P, Spearpoint P, et al. New insights into the epidemiology of ANCA-associated vasculitides in Germany: results from a claims data study. Rheumatology (Oxford). 2021 Oct 2;60(10):4868–4873.
- Derk CT, Jimenez SA. Systemic sclerosis: current views of its pathogenesis. Autoimmun Rev. 2003 Jun;2(4):181–191.
- Kitching AR, Anders HJ, Basu N, et al. ANCA-associated vasculitis. Nat Rev Dis Primers. 2020 Aug 27;6(1):71.
- Osman MS, Tervaert JWC. Anti-neutrophil cytoplasmic antibodies (ANCA) as disease activity biomarkers in a “personalized medicine approach” in ANCA-associated vasculitis. Curr Rheumatol Rep. 2019 Dec 26; 21(12):76.
- Mehra S, Walker J, Patterson K, et al. Autoantibodies in systemic sclerosis. Autoimmun Rev. 2013 Jan;12(3):340–354.
- Osman M, Redmond D, Tervaert JWC. Comment on: ANCA in systemic sclerosis, when vasculitis overlaps with vasculopathy: a devastating combination of pathologies. Rheumatology (Oxford). 2021 Nov 3; 60(11):e407–e9.
- AARDA. Profound, debilitating fatigue found to be a major issue for autoimmune disease patients in new national survey. 2015. [cited 2021 Aug]. Available from: https://www.aarda.org/fatigue-survey-results-released/
- Druce KL, Bhattacharya Y, Jones GT, et al. Most patients who reach disease remission following anti-TNF therapy continue to report fatigue: results from the British society for rheumatology biologics register for rheumatoid arthritis. Rheumatology (Oxford). 2016 Oct;55(10):1786–1790.
- Pollard LC, Choy EH, Gonzalez J, et al. Fatigue in rheumatoid arthritis reflects pain, not disease activity. Rheumatology (Oxford). 2006 Jul;45(7):885–889.
- Tench CM, McCurdie I, White PD, et al. The prevalence and associations of fatigue in systemic lupus erythematosus. Rheumatology (Oxford). 2000 Nov;39(11):1249–1254.
- Wang B, Gladman DD, Urowitz MB. Fatigue in lupus is not correlated with disease activity. J Rheumatol. 1998 May;25(5):892–895.
- Ahn GE, Ramsey-Goldman R. Fatigue in systemic lupus erythematosus. Int J Clin Rheumtol. 2012 Apr 1; 7(2):217–227.
- Dey M, Parodis I, Nikiphorou E. Fatigue in systemic lupus erythamatosus and rheumatoid arthritis: a comparison of mechanisms, measures and management. J Clin Med. 2021;10(16):3566.
- Afari N, Buchwald D. Chronic fatigue syndrome: a review. Am J Psychiatry. 2003 Feb;160(2):221–236.
- Pawlikowska T, Chalder T, Hirsch SR, et al. Population based study of fatigue and psychological distress. BMJ. 1994 Mar 19;308(6931):763–766.
- Bested AC, Marshall LM. Review of myalgic encephalomyelitis/chronic fatigue syndrome: an evidence-based approach to diagnosis and management by clinicians. Rev Environ Health. 2015;30(4):223–249.
- Komaroff AL. Advances in understanding the pathophysiology of chronic fatigue syndrome. JAMA. 2019 Aug 13; 322(6):499–500.
- Bateman L, Bested AC, Bonilla HF, et al. Myalgic encephalomyelitis/chronic fatigue syndrome: essentials of diagnosis and management. Mayo Clin Proc. 2021 Nov;96(11):2861–2878.
- Cortes Rivera M, Mastronardi C, Silva-Aldana CT, et al. Myalgic encephalomyelitis/chronic fatigue syndrome: a comprehensive review. Diagnostics (Basel). 2019 Aug 7;9(3). 10.3390/diagnostics9030091.
- Louati K, Berenbaum F. Fatigue in chronic inflammation - a link to pain pathways. Arthritis Res Ther. 2015 Oct 5;17(1):254.
- Montoya JG, Holmes TH, Anderson JN, et al. Cytokine signature associated with disease severity in chronic fatigue syndrome patients. Proc Natl Acad Sci U S A. 2017 Aug 22;114(34):E7150–E8.
- Davies K, Dures E, Ng WF. Fatigue in inflammatory rheumatic diseases: current knowledge and areas for future research. Nat Rev Rheumatol. 2021 Nov;17(11):651–664.
- Komaroff AL, Bateman L. Will COVID-19 lead to myalgic encephalomyelitis/chronic fatigue syndrome? Front Med (Lausanne). 2020;7:606824.
- Congress.Gov. H.R.2754 - COVID–19 long haulers act. 2021. [cited 2022 Jun]. Available from: https://www.congress.gov/bill/117th-congress/house-bill/2754?r=5&s=1
- NoListedAuthors. A new clinical entity? Lancet. 1956 May 26;270(6926):789–790.
- Hewlett S, Cockshott Z, Byron M, et al. Patients’ perceptions of fatigue in rheumatoid arthritis: overwhelming, uncontrollable, ignored. Arthritis Rheum. 2005 Oct 15;53(5):697–702.
- Sapra A, Bhandari P. Chronic fatigue syndrome. Treasure Island (FL): StatPearls; 2021.
- Deale A, Wessely S. Patients’ perceptions of medical care in chronic fatigue syndrome. Soc Sci Med. 2001 Jun;52(12):1859–1864.
- Carruthers BM, Jain AK, De Meirleir KL, et al. Myalgic encephalomyelitis/chronic fatigue syndrome: clinical working case definition, diagnostic and treatment protocols. J Chron Fatigue Syndr. 2003;11(1):7–115.
- Jason LA, McManimen S, Sunnquist M, et al. Examining the institute of medicine’s recommendations regarding chronic fatigue syndrome: clinical versus research criteria. J Neurol Psychol. 2015(Suppl 2) .
- Torres-Harding S, Sorenson M, Jason LA, et al. Evidence for T-helper 2 shift and association with illness parameters in chronic fatigue syndrome (CFS). Bull IACFS ME. 2008 Fall;16(3):19–33.
- Skowera A, Cleare A, Blair D, et al. High levels of type 2 cytokine-producing cells in chronic fatigue syndrome. Clin Exp Immunol. 2004 Feb;135(2):294–302.
- O’Reilly S, Hugle T, van Laar JM. T cells in systemic sclerosis: a reappraisal. Rheumatology (Oxford). 2012 Sep;51(9):1540–1549.
- Abdulahad WH, Lamprecht P, Kallenberg CG. T-helper cells as new players in ANCA-associated vasculitides. Arthritis Res Ther. 2011 Aug 23; 13(4):236.
- Wilde B, Thewissen M, Damoiseaux J, et al. T cells in ANCA-associated vasculitis: what can we learn from lesional versus circulating T cells? Arthritis Res Ther. 2010;12(1):204.
- Fletcher MA, Zeng XR, Maher K, et al. Biomarkers in chronic fatigue syndrome: evaluation of natural killer cell function and dipeptidyl peptidase IV/CD26. Plos One. 2010 May 25;5(5):e10817.
- Brenu EW, van Driel ML, Staines DR, et al. Immunological abnormalities as potential biomarkers in chronic fatigue syndrome/myalgic encephalomyelitis. J Transl Med. 2011 May 28;9(1):81.
- Eaton-Fitch N, du Preez S, Cabanas H, et al. A systematic review of natural killer cells profile and cytotoxic function in myalgic encephalomyelitis/chronic fatigue syndrome. Syst Rev. 2019 Nov 14;8(1):279.
- Brenu EW, Hardcastle SL, Atkinson GM, et al. Natural killer cells in patients with severe chronic fatigue syndrome. Auto Immun Highlights. 2013 Dec;4(3):69–80.
- Strayer D, Scott V, Carter S. Low NK cell activity in chronic fatigue syndrome (CFS) and relationship to symptom severity. J Clin Cell Immunol. 2015;6:1–9.
- van Eeden C, Khan L, Osman MS, et al. Natural killer cell dysfunction and its role in COVID-19. Int J Mol Sci. 2020;21(17):6351.
- Fuchs S, Scheffschick A, Gunnarsson I, et al. Natural killer cells in anti-neutrophil cytoplasmic antibody-associated vasculitis - A review of the literature. Front Immunol. 2021;12:796640.
- Benyamine A, Magalon J, Sabatier F, et al. Natural killer cells exhibit a peculiar phenotypic profile in systemic sclerosis and are potent inducers of endothelial microparticles release. Front Immunol. 2018;9:1665.
- Puxeddu I, Bongiorni F, Chimenti D, et al. Cell surface expression of activating receptors and co-receptors on peripheral blood NK cells in systemic autoimmune diseases. Scand J Rheumatol. 2012 Aug;41(4):298–304.
- Nijs J, De Meirleir K. Impairments of the 2-5A synthetase/RNase L pathway in chronic fatigue syndrome. Vivo. 2005 Nov-Dec;19:1013–1021.
- Suhadolnik RJ, Peterson DL, O’Brien K, et al. Biochemical evidence for a novel low molecular weight 2-5A-dependent RNase L in chronic fatigue syndrome. J Interferon Cytokine Res. 1997 Jul;17(7):377–385.
- Guenther S, Loebel M, Mooslechner AA, et al. Frequent IgG subclass and mannose binding lectin deficiency in patients with chronic fatigue syndrome. Hum Immunol. 2015 Oct;76(10):729–735.
- Peterson PK, Shepard J, Macres M, et al. A controlled trial of intravenous immunoglobulin G in chronic fatigue syndrome. Am J Med. 1990 Nov;89(5):554–560.
- Wakefield D, Lloyd A, Brockman A. Immunoglobulin subclass abnormalities in patients with chronic fatigue syndrome. Pediatr Infect Dis J. 1990 Aug;9(Supplement):S50–3.
- Brouwer E, Tervaert JW, Horst G, et al. Predominance of IgG1 and IgG4 subclasses of anti-neutrophil cytoplasmic autoantibodies (ANCA) in patients with Wegener’s granulomatosis and clinically related disorders. Clin Exp Immunol. 1991 Mar;83(3):379–386.
- Holland M, Hewins P, Goodall M, et al. Anti-neutrophil cytoplasm antibody IgG subclasses in Wegener’s granulomatosis: a possible pathogenic role for the IgG4 subclass. Clin Exp Immunol. 2004 Oct;138(1):183–192.
- Ma Y, Chen L, Xu Y, et al. Clinical and pathological features of patients with antineutrophil cytoplasmic antibody-associated vasculitides concomitant with IgG4-related disease. Int J Rheum Dis. 2019 Dec;22(12):2143–2150.
- Zhang H, Li P, Wu D, et al. Serum IgG subclasses in autoimmune diseases. Medicine (Baltimore). 2015 Jan;94(2):e387.
- Barahona-Correa JE, De la Hoz A, Lopez MJ, et al. Infections and systemic sclerosis: an emerging challenge. Rev Colome Reumatol. 2020;27:62–84.
- Tieu J, Smith RM, Gopaluni S, et al. Rituximab associated hypogammaglobulinemia in autoimmune disease. Front Immunol. 2021;12:671503.
- Loebel M, Grabowski P, Heidecke H, et al. Antibodies to beta adrenergic and muscarinic cholinergic receptors in patients with chronic fatigue syndrome. Brain Behav Immun. 2016 Feb;52:32–39.
- Yamamoto S, Ouchi Y, Nakatsuka D, et al. Reduction of [11C](+)3-MPB binding in brain of chronic fatigue syndrome with serum autoantibody against muscarinic cholinergic receptor. Plos One. 2012;7(12):e51515.
- Tanaka S, Kuratsune H, Hidaka Y, et al. Autoantibodies against muscarinic cholinergic receptor in chronic fatigue syndrome. Int J Mol Med. 2003 Aug;12(2):225–230.
- Scheibenbogen C, Loebel M, Freitag H, et al. Immunoadsorption to remove ss2 adrenergic receptor antibodies in chronic fatigue syndrome CFS/ME. Plos One. 2018;13(3):e0193672.
- Sotzny F, Blanco J, Capelli E, et al. Myalgic encephalomyelitis/chronic fatigue syndrome - Evidence for an autoimmune disease. Autoimmun Rev. 2018 Jun;17(6):601–609.
- Hokama Y, Campora CE, Hara C, et al. Anticardiolipin antibodies in the sera of patients with diagnosed chronic fatigue syndrome. J Clin Lab Anal. 2009;23(4):210–212.
- Hokama Y, Empey-Campora C, Hara C, et al. Acute phase phospholipids related to the cardiolipin of mitochondria in the sera of patients with chronic fatigue syndrome (CFS), chronic Ciguatera fish poisoning (CCFP), and other diseases attributed to chemicals, Gulf War, and marine toxins. J Clin Lab Anal. 2008;22(2):99–105.
- Cabral-Marques O, Riemekasten G. Functional autoantibodies targeting G protein-coupled receptors in rheumatic diseases. Nat Rev Rheumatol. 2017 Nov;13(11):648–656.
- Zhao X, Wen Q, Qiu Y, et al. Clinical and pathological characteristics of ANA/anti-dsDNA positive patients with antineutrophil cytoplasmic autoantibody-associated vasculitis. Rheumatol Int. 2021 Feb;41(2):455–462.
- Didier K, Giusti D, Le Jan S, et al. Neutrophil extracellular traps generation relates with early stage and vascular complications in systemic sclerosis. J Clin Med. 2020 Jul 7;9(7):2136.
- Brenu EW, Staines DR, Baskurt OK, et al. Immune and hemorheological changes in chronic fatigue syndrome. J Transl Med. 2010 Jan 11;8(1):1.
- Ablashi DV. Viral studies of chronic fatigue syndrome. Clin Infect Dis. 1994 Jan;18 Suppl 1(Supplement_1):S130–3.
- Hickie I, Davenport T, Wakefield D, et al. Post-infective and chronic fatigue syndromes precipitated by viral and non-viral pathogens: prospective cohort study. BMJ. 2006 Sep 16;333(7568):575.
- Rasa S, Nora-Krukle Z, Henning N, et al. Chronic viral infections in myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS). J Transl Med. 2018 Oct 1;16(1):268.
- O’Neal AJ, Hanson MR. The enterovirus theory of disease etiology in myalgic encephalomyelitis/chronic fatigue syndrome: a critical review. Front Med (Lausanne). 2021;8:688486.
- Komaroff AL, Lipkin WI. Insights from myalgic encephalomyelitis/chronic fatigue syndrome may help unravel the pathogenesis of postacute COVID-19 syndrome. Trends Mol Med. 2021 Sep;27(9):895–906.
- Davis HE, Assaf GS, McCorkell L, et al. Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. EClin Med. 2021 Aug;38:101019.
- Naviaux RK, Naviaux JC, Li K, et al. Metabolic features of chronic fatigue syndrome. Proc Natl Acad Sci U S A. 2016 Sep 13;113(37):E5472–80.
- Fielenbach N, Antebi AC. C. elegans dauer formation and the molecular basis of plasticity. Genes Dev. 2008 Aug 15; 22(16):2149–2165.
- Holden S, Maksoud R, Eaton-Fitch N, et al. A systematic review of mitochondrial abnormalities in myalgic encephalomyelitis/chronic fatigue syndrome/systemic exertion intolerance disease. J Transl Med. 2020 Jul 29;18(1):290.
- Kennedy G, Spence VA, McLaren M, et al. Oxidative stress levels are raised in chronic fatigue syndrome and are associated with clinical symptoms. Free Radic Biol Med. 2005 Sep 1;39(5):584–589.
- Morris G, Maes M. Mitochondrial dysfunctions in myalgic encephalomyelitis/chronic fatigue syndrome explained by activated immuno-inflammatory, oxidative and nitrosative stress pathways. Metab Brain Dis. 2014 Mar;29(1):19–36.
- Jastroch M, Divakaruni AS, Mookerjee S, et al. Mitochondrial proton and electron leaks. Essays Biochem. 2010;47:53–67.
- Missailidis D, Annesley SJ, Allan CY, et al. An isolated complex V inefficiency and dysregulated mitochondrial function in immortalized lymphocytes from ME/CFS patients. Int J Mol Sci. 2020 Feb 6;21(3):1074.
- Tomas C, Brown A, Strassheim V, et al. Cellular bioenergetics is impaired in patients with chronic fatigue syndrome. Plos One. 2017;12(10):e0186802.
- Castro-Marrero J, Cordero MD, Saez-Francas N, et al. Could mitochondrial dysfunction be a differentiating marker between chronic fatigue syndrome and fibromyalgia? Antioxid Redox Signal. 2013 Nov 20;19(15):1855–1860.
- Maes M, Mihaylova I, Kubera M, et al. Coenzyme Q10 deficiency in myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) is related to fatigue, autonomic and neurocognitive symptoms and is another risk factor explaining the early mortality in ME/CFS due to cardiovascular disorder. Neuro Endocrinol Lett. 2009;30(4):470–476.
- Abdulle AE, Diercks GFH, Feelisch M, et al. The role of oxidative stress in the development of systemic sclerosis related vasculopathy. Front Physiol. 2018;9:1177.
- Liu Q, Zhang D, Hu D, et al. The role of mitochondria in NLRP3 inflammasome activation. Mol Immunol. 2018 Nov;103:115–124.
- Faas MM, de Vos P. Mitochondrial function in immune cells in health and disease. Biochim Biophys Acta Mol Basis Dis. 2020 Oct 1; 1866(10):165845.
- Angajala A, Lim S, Phillips JB, et al. Diverse roles of mitochondria in immune responses: novel insights into immuno-metabolism. Front Immunol. 2018;9:1605.
- Shimojima Y, Kishida D, Ichikawa T, et al. Oxidative stress promotes instability of regulatory T cells in antineutrophil cytoplasmic antibody-associated vasculitis. Front Immunol. 2021;12:789740.
- Doridot L, Jeljeli M, Chene C, et al. Implication of oxidative stress in the pathogenesis of systemic sclerosis via inflammation, autoimmunity and fibrosis. Redox Biol. 2019 Jul;25:101122.
- Rutgers A, Heeringa P, Tervaert JW. The role of myeloperoxidase in the pathogenesis of systemic vasculitis. Clin Exp Rheumatol. 2003 Nov-Dec;21(6 Suppl 32):S55–63.
- Harper L, Nuttall SL, Martin U, et al. Adjuvant treatment of patients with antineutrophil cytoplasmic antibody-associated vasculitis with vitamins E and C reduces superoxide production by neutrophils. Rheumatology (Oxford). 2002 Mar;41(3):274–278.
- Slot MC, Theunissen R, van Paassen P, et al. Anti-oxidized low-density lipoprotein antibodies in myeloperoxidase-positive vasculitis patients preferentially recognize hypochlorite-modified low density lipoproteins. Clin Exp Immunol. 2007 Aug;149(2):257–264.
- Fuite J, Vernon SD, Broderick G. Neuroendocrine and immune network re-modeling in chronic fatigue syndrome: an exploratory analysis. Genomics. 2008 Dec;92(6):393–399.
- Torres-Harding S, Sorenson M, Jason L, et al. The associations between basal salivary cortisol and illness symptomatology in chronic fatigue syndrome. J Appl Biobehav Res. 2008 Jan 1;13(3):157–180.
- Parker AJ, Wessely S, Cleare AJ. The neuroendocrinology of chronic fatigue syndrome and fibromyalgia. Psychol Med. 2001 Nov;31(8):1331–1345.
- Spies CM, Straub RH, Cutolo M, et al. Circadian rhythms in rheumatology–a glucocorticoid perspective. Arthritis Res Ther. 2014 Nov 13;16(2):S3.
- Broersen LH, Pereira AM, Jorgensen JO, et al. Adrenal insufficiency in corticosteroids use: systematic review and meta-analysis. J Clin Endocrinol Metab. 2015 Jun;100(6):2171–2180.
- Bagnato G, Cordova F, Sciortino D, et al. Association between cortisol levels and pain threshold in systemic sclerosis and major depression. Rheumatol Int. 2018 Mar;38(3):433–441.
- Vernino S, Bourne KM, Stiles LE, et al. Postural orthostatic tachycardia syndrome (POTS): state of the science and clinical care from a 2019 national institutes of health expert consensus meeting - Part 1. Auton Neurosci. 2021 Nov;235:102828.
- Albright F, Light K, Light A, et al. Evidence for a heritable predisposition to chronic fatigue syndrome. BMC Neurol. 2011 May 27;11(1):62.
- Dibble JJ, McGrath SJ, Ponting CP. Genetic risk factors of ME/CFS: a critical review. Hum Mol Genet. 2020 Sep 30; 29(R1):R117–R24.
- Tukiainen T, Villani AC, Yen A, et al. Landscape of X chromosome inactivation across human tissues. Nature. 2017 Oct 11;550(7675):244–248.
- Laffont S, Guery JC. Deconstructing the sex bias in allergy and autoimmunity: from sex hormones and beyond. Adv Immunol. 2019;142:35–64.
- Walsh CM, Zainal NZ, Middleton SJ, et al. A family history study of chronic fatigue syndrome. Psychiatr Genet. 2001 Sep;11(3):123–128.
- Buchwald D, Herrell R, Ashton S, et al. A twin study of chronic fatigue. Psychosom Med. 2001 Nov-Dec;63(6):936–943.
- Hickie IB, Bansal AS, Kirk KM, et al. A twin study of the etiology of prolonged fatigue and immune activation. Twin Res. 2001 Apr;4(2):94–102.
- Farmer A, Scourfield J, Martin N, et al. Is disabling fatigue in childhood influenced by genes? Psychol Med. 1999 Mar;29(2):279–282.
- Lande A, Fluge O, Strand EB, et al. Human leukocyte antigen alleles associated with myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS). Sci Rep. 2020 Mar 24;10(1):5267.
- Arnett FC. HLA and autoimmunity in scleroderma (systemic sclerosis). Int Rev Immunol. 1995;12(2–4):107–128.
- Dahlqvist J, Ekman D, Sennblad B, et al. Identification and functional characterization of a novel susceptibility locus for small vessel vasculitis with MPO-ANCA. Rheumatology (Oxford). 2022;61(8): 3461–3470 .
- Merkel PA, Xie G, Monach PA, et al. Identification of functional and expression polymorphisms associated with risk for antineutrophil cytoplasmic autoantibody-associated vasculitis. Arthritis Rheumatol. 2017 May;69(5):1054–1066.
- Lyons PA, Rayner TF, Trivedi S, et al. Genetically distinct subsets within ANCA-associated vasculitis. N Engl J Med. 2012 Jul 19;367(3):214–223.
- Hilhorst M, Arndt F, Joseph Kemna M, et al. HLA-DPB1 as a risk factor for relapse in antineutrophil cytoplasmic antibody-associated vasculitis: a cohort study. Arthritis Rheumatol. 2016 Jul;68(7):1721–1730.
- Wang HY, Cui Z, Pei ZY, et al. Risk HLA class II alleles and amino acid residues in myeloperoxidase-ANCA-associated vasculitis. Kidney Int. 2019 Oct;96(4):1010–1019.
- Smolenska Z, Zabielska-Kaczorowska M, Wojteczek A, et al. Metabolic pattern of systemic sclerosis: association of changes in plasma concentrations of amino acid-related compounds with disease presentation. Front Mol Biosci. 2020;7:585161.
- Collatz A, Johnston SC, Staines DR, et al. A systematic review of drug therapies for chronic fatigue syndrome/myalgic encephalomyelitis. Clin Ther. 2016 Jun;38(6):1263–71 e9.
- Pettegrew JW, Levine J, McClure RJ. Acetyl-L-carnitine physical-chemical, metabolic, and therapeutic properties: relevance for its mode of action in Alzheimer’s disease and geriatric depression. Mol Psychiatry. 2000 Nov;5(6):616–632.
- Cooper N, Arnold DM. The effect of rituximab on humoral and cell mediated immunity and infection in the treatment of autoimmune diseases. Br J Haematol. 2010 Apr;149(1):3–13.
- Clayton EW. Beyond myalgic encephalomyelitis/chronic fatigue syndrome: an IOM report on redefining an illness. JAMA. 2015 Mar 17;313:1101–1102.
- Jason L, Benton M, Torres-Harding S, et al. The impact of energy modulation on physical functioning and fatigue severity among patients with ME/CFS. Patient Educ Couns. 2009 Nov;77(2):237–241.
- Basta F, Afeltra A, Margiotta DPE. Fatigue in systemic sclerosis: a systematic review. Clin Exp Rheumatol. 2018 Jul-Aug;36(113):150–160.
- Assassi S, Leyva AL, Mayes MD, et al., Predictors of fatigue severity in early systemic sclerosis: a prospective longitudinal study of the GENISOS cohort. Plos One. 2011;6(10):e26061.
- Sandusky SB, McGuire L, Smith MT, et al. Fatigue: an overlooked determinant of physical function in scleroderma. Rheumatology (Oxford). 2009 Feb;48(2):165–169.
- Koenig M, Joyal F, Fritzler MJ, et al. Autoantibodies and microvascular damage are independent predictive factors for the progression of Raynaud’s phenomenon to systemic sclerosis: a twenty-year prospective study of 586 patients, with validation of proposed criteria for early systemic sclerosis. Arthritis Rheum. 2008 Dec;58(12):3902–3912.
- Pokeerbux MR, Giovannelli J, Dauchet L, et al. Survival and prognosis factors in systemic sclerosis: data of a French multicenter cohort, systematic review, and meta-analysis of the literature. Arthritis Res Ther. 2019 Apr 3;21(1):86.
- Kim DY, Lee JS, Son CG. Systematic review of primary outcome measurements for chronic fatigue syndrome/myalgic encephalomyelitis (CFS/ME) in randomized controlled trials. J Clin Med. 2020;9(11): 3463.
- Broadbent DE, Cooper PF, FitzGerald P, et al. The cognitive failures questionnaire (CFQ) and its correlates. Br J Clin Psychol. 1982 Feb;21(1):1–16.
- Buysse DJ, Reynolds CFs3rd, Monk TH, et al. The Pittsburgh sleep quality index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989 May;28(2):193–213.
- Kesikburun B, Koseoglu BF, Dogan A, et al. The effects of respiratory muscle weakness and pulmonary involvement on functional status, fatigue and health related quality of life in patients with systemic sclerosis. Arch Rheumatol. 2015;30(2):116–123.
- Peytrignet S, Denton CP, Lunt M, et al. Disability, fatigue, pain and their associates in early diffuse cutaneous systemic sclerosis: the European scleroderma observational study. Rheumatology. 2018;57(2):370–381.
- Cetin SY, Calik BB, Ayan A. Investigation of the effectiveness of Tai Chi exercise program in patients with scleroderma: a randomized controlled study. Complement Ther Clin Pract. 2020;40:101181.
- Yakut H, Ozalevli S, Birlik AM. Fatigue and its relationship with disease-related factors in patients with systemic sclerosis: a cross-sectional study. Turk J Med Sci. 2021;51(2):530–539.
- Murphy SL, Kratz AL, Khanna D, et al. Fatigue and its association with social participation, functioning, and quality of life in systemic sclerosis. Arthritis Care Res. 2021;73(3):415–422.
- Santiago TL, Santos E, Duarte AC, et al. Happiness, quality of life and their determinants among people with systemic sclerosis: a structural equation modeling approach. Rheumatology (Oxford, England). 2021;60(10):4717–4727.
- Sierakowska M, Doroszkiewicz H, Sierakowska J, et al. Factors associated with quality of life in systemic sclerosis: a cross-sectional study. Qual Life Res. 2019;28(12):3347–3354.
- Justo AC, Guimaraes FS, Ferreira AS, et al. Muscle function in women with systemic sclerosis: association with fatigue and general physical function. Clin Biomech (Bristol, Avon). 2017;47:33–39.
- Sierakowska M, Krajewska-Kutak E, Sierakowski S, et al. Comparative analysis of educational needs of patients with rheumatic diseases selected based on the polish version of the educational needs assessment tool (Pol-ENAT). Reumatologia. 2016;54:153–160.
- Gok K, Erol K, Cengiz G, et al. Comparison of level of fatigue and disease correlates in patients with rheumatoid arthritis and systemic sclerosis. Arch Rheumatol. 2018;33(3):316–321.
- Murphy SL, Whibley D, Kratz AL, et al. Fatigue predicts future reduced social participation, not reduced physical function or quality of life in people with systemic sclerosis. Journal of Scleroderma and Related Disorders. 2021;6(2):187–193.
- March C, Huscher D, Preis E, et al. Prevalence, risk factors and assessment of depressive symptoms in patients with systemic sclerosis. Arch Rheumatol. 2019;34(3):253–261.
- Horsley-Silva JL, Umar SB, Vela MF, et al. The impact of gastroesophageal reflux disease symptoms in scleroderma: effects on sleep quality. Dis Esophagus. 2019;32(5). 10.1093/dote/doy136.
- Yakut H, Ozalevli S, Aktan R, et al. Effects of supervised exercise program and home exercise program in patients with systemic sclerosis: a randomized controlled trial. Int J Rheum Dis. 2021;24(9):1200–1212.
- Sierakowska M, Krajewska-Kulak E, Sierakowski S, et al., Pain, fatigue and functional disability are associated with higher educational needs in systemic sclerosis: a cross-sectional study. Rheumatol Int. 2018;38(8):1471–1478.
- Frech T, Hays RD, Maranian P, et al. Prevalence and correlates of sleep disturbance in systemic sclerosis–results from the UCLA scleroderma quality of life study. Rheumatology (Oxford). 2011 Jul;50(7):1280–1287.
- Sariyildiz MA, Batmaz I, Budulgan M, et al. Sleep quality in patients with systemic sclerosis: relationship between the clinical variables, depressive symptoms, functional status, and the quality of life. Rheumatol Int. 2013 Aug;33(8):1973–1979.
- Levis B, Kwakkenbos L, Hudson M, et al. The association of sociodemographic and objectively-assessed disease variables with fatigue in systemic sclerosis: an analysis of 785 Canadian scleroderma research group registry patients. Clin Rheumatol. 2017 Feb;36(2):373–379.
- Jennette JC, Falk RJ, Bacon PA, et al. revised international chapel hill consensus conference nomenclature of vasculitides. Arthritis Rheum. 2012 Jan];65(1):1–11.
- Bossuyt X, Cohen Tervaert JW, Arimura Y, et al. Position paper: revised 2017 international consensus on testing of ANCAs in granulomatosis with polyangiitis and microscopic polyangiitis. Nat Rev Rheumatol. 2017 Nov;13(11):683–692.
- Hilhorst M, van Paassen P, Tervaert JW, et al. Proteinase 3-ANCA vasculitis versus Myeloperoxidase-ANCA Vasculitis. J Am Soc Nephrol. 2015 Oct;26(10):2314–2327.
- Flossmann O, Berden A, de Groot K, et al. Long-term patient survival in ANCA-associated vasculitis. Ann Rheum Dis. 2011 Mar;70(3):488–494.
- Hilhorst M, Wilde B, van Paassen P, et al. Improved outcome in anti-neutrophil cytoplasmic antibody (ANCA)-associated glomerulonephritis: a 30-year follow-up study. Nephrol Dial Transplant. 2013 Feb;28(2):373–379.
- Exley AR, Moots RM, Carruthers D, et al. Short-form 36 in vasculitis. Clin Exp Immunol. 1995;63:101.
- Herlyn K, Reinhold-Keller E, Zeidler A, et al. Health-related quality of life in primary systematic vasculitides. Arthritis Rheum. 1998;41(1):122.
- Raza K, Wilson A, Carruthers DM, et al. Impaired SF36 in patients with primary systematic necrotizing vasculitis (PSNV): influence of factors besides disease activity and damage. Arthritis Rheam. 1999;42:315.
- Gill N, Tervaert JWC, Yacyshyn E. Vasculitis patient journey: a scoping review of patient experiences with vasculitis. Clin Rheumatol. 2021 May;40(5):1697–1708.
- Hoffman GS, Drucker Y, Cotch MF, et al. Wegener’s granulomatosis: patient-reported effects of disease on health, function, and income. Arthritis Rheum. 1998 Dec;41(12):2257–2262.
- Boomsma MM, Stegeman CA, Tervaert JW. Comparison of dutch and US patients’ perceptions of the effects of Wegener’s granulomatosis on health, function, income, and interpersonal relationships: comment on the article by Hoffman et al. Arthritis Rheum. 1999 Nov;42(11):2495–2497.
- Robson JC, Dawson J, Doll H, et al. Validation of the ANCA-associated vasculitis patient-reported outcomes (AAV-PRO) questionnaire. Ann Rheum Dis. 2018 Aug;77(8):1157–1164.
- Basu N, Jones GT, Fluck N, et al. Fatigue: a principal contributor to impaired quality of life in ANCA-associated vasculitis. Rheumatology (Oxford). 2010 Jul;49(7):1383–1390.
- Basu N, McClean A, Harper L, et al. Explaining fatigue in ANCA-associated vasculitis. Rheumatology (Oxford). 2013 Sep;52(9):1680–1685.
- Macfarlane GJ, Jones GT, Swafe L, et al. Alternative population sampling frames produced important differences in estimates of association: a case-control study of vasculitis. J Clin Epidemiol. 2013 Jun;66(6):675–680.
- Hajj-Ali RA, Wilke WS, Calabrese LH, et al. Pilot study to assess the frequency of fibromyalgia, depression, and sleep disorders in patients with granulomatosis with polyangiitis (Wegener’s). Arthritis Care Res (Hoboken). 2011 Jun;63:827–833.
- Grayson PC, Amudala NA, McAlear CA, et al. Illness perceptions and fatigue in systemic vasculitis. Arthritis Care Res (Hoboken). 2013 Nov;65(11):1835–1843.
- Mayor H, Reidy J. The role of pain, perseverative cognition and goal adjustment in vasculitis-associated fatigue. J Health Psychol. 2018 Sep;23(10):1299–1308.
- Can Sandikci S, Colak S, Omma A, et al. An evaluation of depression, anxiety and fatigue in patients with Behcet’s disease. Int J Rheum Dis. 2019;22(6):974–979.
- McClean A, Morgan MD, Basu N, et al. Physical fatigue, fitness, and muscle function in patients with antineutrophil cytoplasmic antibody-associated vasculitis. Arthritis Care Res (Hoboken). 2016;68(9):1332–1339.
- Koutantji M, Harrold E, Lane SE, et al. Investigation of quality of life, mood, pain, disability, and disease status in primary systemic vasculitis. Arthritis Rheum. 2003 Dec 15;49(6):826–837.
- Tomasson G, Farrar JT, Cuthbertson D, et al. Feasibility and construct validation of the patient reported outcomes measurement information system in systemic vasculitis. J Rheumatol. 2019;46(8):928–934.
- Rimland CA, Quinn KA, Rosenblum JS, et al. Outcome measures in large vessel vasculitis: relationship between patient-, physician-, imaging-, and laboratory-based assessments. Arthritis Care Res (Hoboken). 2020;72(9):1296–1304.
- Ilhan B, Can M, Alibaz-Oner F, et al. Fatigue in patients with Behcet’s syndrome: relationship with quality of life, depression, anxiety, disability and disease activity. Int J Rheum Dis. 2016;21(12):2139–2145.
- Basu N, McClean A, Harper L, et al. The characterisation and determinants of quality of life in ANCA associated vasculitis. Ann Rheum Dis. 2014 Jan;73(1):207–211.
- Tuin J, Sanders JS, Buhl BM, et al. Androgen deficiency in male patients diagnosed with ANCA-associated vasculitis: a cause of fatigue and reduced health-related quality of life? Arthritis Res Ther. 2013;15(5):R117.
- Basu N, Murray AD, Jones GT, et al. Fatigue-related brain white matter changes in granulomatosis with polyangiitis. Rheumatology (Oxford, England). 2013;52(8):1429–1434.
- Basu N, Murray AD, Jones GT, et al. Neural correlates of fatigue in granulomatosis with polyangiitis: a functional magnetic resonance imaging study. Rheumatology (Oxford). 2014 Nov;53(11):2080–2087.
- Kim BH, Namkoong K, Kim JJ, et al. Altered resting-state functional connectivity in women with chronic fatigue syndrome. Psychiatry Res. 2015 Dec 30;234(3):292–297.
- Grayson PC, Amudala NA, McAlear CA, et al. Causal attributions about disease onset and relapse in patients with systemic vasculitis. J Rheumatol. 2014;41(5):923–930.
- Jones RB, Tervaert JW, Hauser T, et al. Rituximab versus cyclophosphamide in ANCA-associated renal vasculitis. N Engl J Med. 2010 Jul 15;363(3):211–220.
- Jones RB, Hiemstra TF, Ballarin J, et al. Mycophenolate mofetil versus cyclophosphamide for remission induction in ANCA-associated vasculitis: a randomised, non-inferiority trial. Ann Rheum Dis. 2019 Mar;78(3):399–405.
- O’Malley L, Druce KL, Chanouzas D, et al. The longitudinal course of fatigue in antineutrophil cytoplasmic antibody-associated vasculitis. J Rheumatol. 2020;47(4):572–579.
- Strand V, Dimonaco S, Collinson N, et al. Health-related quality of life in patients with giant cell arteritis treated with tocilizumab in a phase 3 randomised controlled trial. Arthritis Res Ther. 2019;21(1):64.
- Harper L, Hewitt CA, Litchfield I, et al. Management of fatigue with physical activity and behavioural change support in vasculitis: a feasibility study. Rheumatology. 2021;60(9):4130–4140.
- Litchfield I, Greenfield S, Harper L, et al. Addressing the transition to a chronic condition: exploring independent adoption of self-management by patients with ANCA-associated vasculitis. Rheumatol Adv Pract. 2021;5:rkab075.
- Astley C, Clemente G, Terreri MT, et al. Home-based exercise training in childhood-onset takayasu arteritis: a multicenter, randomized, controlled trial. Front Immunol. 2021;12:705250.
- Katz P. Causes and consequences of fatigue in rheumatoid arthritis. Curr Opin Rheumatol. 2017 May;29(3):269–276.
- Zahiri S, Jahani S, Sayadi N, et al. The effects of an educational intervention on fatigue and activities of daily living in patients with systemic lupus erythematosus [Original Article]. Nursing and Midwifery Studies. 2022Jan 1;11:24–30.
- Carandang K, Poole J, Connolly D. Fatigue and activity management education for individuals with systemic sclerosis: adaptation and feasibility study of an intervention for a rare disease. Musculoskeletal Care. 2022 Feb 6. DOI:10.1002/msc.1617
- Raunsbaek Knudsen L, Lomborg K, Ndosi M, et al. The effectiveness of e-learning in patient education delivered to patients with rheumatoid arthritis: the WebRA study-protocol for a pragmatic randomised controlled trial. BMC Rheumatol. 2021 Dec 20;5(1):57.
- Nordlund J, Henry RS, Kwakkenbos L, et al. The scleroderma patient-centered intervention network self-management (SPIN-SELF) program: protocol for a two-arm parallel partially nested randomized controlled feasibility trial with progression to full-scale trial. Trials. 2021 Nov 27;22(1):856.
- Kelley GA, Kelley KS, Callahan LF. Aerobic exercise and fatigue in rheumatoid arthritis participants: a meta-analysis using the minimal important difference approach. Arthritis Care Res (Hoboken). 2018 Dec;70(12):1735–1739.
- Wu ML, Yu KH, Tsai JC. The effectiveness of exercise in adults with systemic lupus erythematosus: a systematic review and meta-analysis to guide evidence-based practice. Worldviews Evid Based Nurs. 2017 Aug;14(4):306–315.
- Menshikova EV, Ritov VB, Fairfull L, et al. Effects of exercise on mitochondrial content and function in aging human skeletal muscle. J Gerontol A Biol Sci Med Sci. 2006 Jun;61(6):534–540.
- Llavero F, Alejo LB, Fiuza-Luces C, et al. Exercise training effects on natural killer cells: a preliminary proteomics and systems biology approach. Exerc Immunol Rev. 2021;27:125–141.
- Paul BD, Lemle MD, Komaroff AL, et al. Redox imbalance links COVID-19 and myalgic encephalomyelitis/chronic fatigue syndrome. Proc Natl Acad Sci U S A. 2021 Aug 24;118(34). DOI: 10.1073/pnas.2024358118.
- Mancini DM, Brunjes DL, Lala A, et al. Use of cardiopulmonary stress testing for patients with unexplained dyspnea post-coronavirus disease. JACC Heart Fail. 2021 Dec;9(12):927–937.