ABSTRACT
Introduction
Generalized pustular psoriasis (GPP) is a rare, severe, immune-mediated and potentially life-threatening skin disease. The rarity, differential diagnoses, relapsing nature, skin and systemic symptoms, complications and limited therapeutic approaches for this disease pose a clinical and psychological burden on patients and their families.
Areas covered
Epidemiologic data of GPP in Chinese patients, including the disease prevalence and age of disease onset, as well as epidemiologic data in global populations were reviewed. Multiple proinflammatory cytokines are involved in the disease development and clinical presentation of GPP and the interleukin (IL)-36-mediated signaling pathway play a central role. Furthermore, loss-of-function mutations in IL-36 RN (encoding the IL-36 receptor antagonist) are associated with GPP, suggesting a potential drug target for developing a disease-specific therapeutic approach. Biologic agents, including IL-36 R targeted agents, are promising treatment options, especially as existing conventional therapies are inadequate. Chinese guidelines for the diagnosis and treatment of psoriasis recommend systemic and topical treatment options for GPP and disease complications, as well as for GPP during pregnancy and juvenile GPP.
Expert Opinion
This review summarizes the epidemiology, pathogenesis, clinical characteristics, disease burden and management of patients with GPP in China, and also describes future treatment targets and related clinical trials.
1. Introduction
Generalized pustular psoriasis (GPP) is a rare, severe, potentially life-threatening immune-mediated inflammatory skin disease with extracutaneous complications that can occur during the disease course contributing to the disease burden [Citation1]. GPP has a heterogeneous presentation, typically characterized by visible erythematous and pustular lesions, often accompanied by systemic symptoms, such as chills, high fever and fatigue. Laboratory test abnormalities, such as neutrophilia, elevated C-reactive protein and general hypoproteinemia, are common [Citation1]. GPP is genetically and phenotypically distinct from plaque psoriasis [Citation2]; its rapid occurrence and recurrence of disease flares contribute to the disease burden and difficulties in its management, particularly in China. The aim of this review is to summarize the disease burden, pathogenesis, clinical characteristics and risk factors, as well as potential treatment options, novel targets and unmet needs for patients with GPP in China.
2. Epidemiology of GPP
Global epidemiologic data for GPP are limited. In studies from Japan and France, the prevalence was estimated to be 7.46 cases/million patients (1983–1989) and 1.76 cases/million patients (2004), respectively [Citation3]. A recent epidemiologic study in China based on data from the national health insurance database (2012–2016) found that the prevalence of GPP was approximately 1.4/100,000 person-years (Feng et al. The disease prevalence and burden of GPP in China: A study based on the national health insurance data between 2012–2016, unpublished data). The study in France found the highest prevalence of GPP to be in patients aged 40–59 years compared with those in other age groups [Citation3]. However, in China, the highest prevalence of GPP was seen in patients aged 0–3 and 30–39 years (Feng et al. China, unpublished data); the mean age of disease onset in Chinese patients has been reported to be 25.78–40.96 years (range <18 to >70 years)(including unpublished data from Feng et al. China) [Citation4–7]. Disease onset may be earlier in patients with a genetic predisposition; interleukin (IL)-36 RN (encoding the IL-36 receptor antagonist) biallelic mutations have been shown to be associated with a younger age of disease onset (around 16 years) than heterozygous mutations (around 23 years) or IL-36 RN without mutation (around 39 years) [Citation8]. Globally, the prevalence of GPP is generally higher in females than males (male:female ratio = 0.56:1) [Citation9], although in China the prevalence in males is higher than in females (male:female ratio range = 1.1:1–3.0:1) (including unpublished data from Feng et al. China) [Citation4–6,Citation8,Citation9]. One study in China has reported a higher prevalence in females than males (male:female ratio = 0.74:1) [Citation10]. Mortality rates for GPP vary across Asian, European and American populations (2–16%) [Citation11].
3. Pathogenesis
Abnormal activities of keratinocytes, neutrophils and monocytes contribute to GPP development and its clinical presentation [Citation2]. Activated keratinocytes release IL-36 cytokines, which promote the recruitment of immune cells to inflammatory sites through signaling pathways mediated by proinflammatory cytokines and chemokines [Citation2]. Studies have shown that gene levels of IL-1, IL-17A, IL-36, IL-22, tumor necrosis factor (TNF)-α and interferon (IFN)-γ are higher in skin biopsies of patients with GPP than normal skin, indicating that multiple cytokines are involved in the inflammatory response in skin lesions [Citation2,Citation12].
3.1. Environmental and genetic risk factors
Many environmental and genetic factors can trigger GPP in individuals predisposed to the condition. Common environmental triggers include use and subsequent withdrawal of systemic corticosteroids, pregnancy, upper respiratory tract infections, certain medications (including traditional medicine, non-steroidal anti-inflammatory drugs and vaccinations) and stress [Citation11,Citation13].
There is a strong genetic association between GPP and mutations in three key genes: IL-36 RN, CARD14 (encodes keratinocyte nuclear factor κB adaptor protein) and AP1S3 (encodes a subunit of the adaptor protein-1 complex) [Citation1]. Over 33% of patients with GPP carry a variant in these three genes [Citation8]. IL-36 RN mutations are more frequent in European and Asian populations than CARD14 and AP1S3, suggesting that IL-36 RN plays a more impactful role in GPP [Citation8,Citation14]. A study also found that Chinese patients with GPP with biallelic mutations in IL-36 RN tend to have earlier disease onset than patients with heterozygous mutation or without IL-36 RN mutation (26.83 years versus 33.27 and 38.03 years, respectively) [Citation15]. p.Arg10ArgX1 is the most frequently reported IL-36RN variant in Chinese patients [Citation16]. Interestingly, the frequencies of all IL-36RN mutations and mutation in p.Arg10ArgX1 showed genetic heterogeneity in Chinese patients, between those with GPP alone and GPP with psoriasis vulgaris (PV) (70.59% versus 37.78%, odds ratio = 3.95; P = .02; 70.59% versus 26.67%, odds ratio = 6.60; P = 2.0 × 10−3), which are different from those in European and African populations that p.Arg10ArgX1 was not found in those two populations. In addition, the most common mutations in the IL-36RN, p.Ser113Leu and p.Leu27Pro, in European population and African population, respectively, were not found in the Chinese population [Citation16–18]. CARD14 gain-of-function mutations are also associated with GPP in Chinese and Japanese populations, with the p.Asp176His variant contributing to an increased disease risk [Citation19]. Although the frequency of CARD14 mutation with p.Asp176His is low in East Asian populations, it was shown to be 5.6-fold and 6.2-fold higher in patients with GPP than in individuals without GPP in China and Japan, respectively [Citation19]. The 17q25.3 locus of the CARD14 p.Arg820Trp variant has shown a strong association with GPP in Chinese patients [Citation20]. Furthermore, CARD14 shows genetic heterogeneity between GPP alone and GPP with PV, with CARD14 mutation only presenting in patients with GPP and PV [Citation15]. Interestingly, a study found that the CARD14 mutation was observed in Chinese patients with GPP, not in European patients [Citation14]. AP1S3 mutations have not been identified in Chinese patients with GPP [Citation15], whereas a German study found that 10% of German patients with IL-36RN mutations also carry AP1S3 mutations [Citation8] (). The locus 5q33.1 has also shown a strong association with GPP in Chinese patients; two genes (TNIP1 and ANXA6) located in this region may play a role in predisposing individuals to GPP [Citation20]. Moreover, mutations in IL-36RN were found in Tunisian, European and Japanese populations who had a family history of GPP; mutations in CARD14 were also found in Japanese patients with GPP who had a family history, which suggests that mutations in IL-36RN and CARD14 underlie familial GPP [Citation21,Citation22].
Table 1. Genetic risk factors in Chinese patients with GPP.
3.2. Role of IL-36 signaling pathway in GPP
Among the several cytokines identified in the pathogenesis of GPP, IL-36 plays the most central role. IL-36 is a member of the IL-1 family of inflammatory cytokines and comprises three isoforms: IL-36α, β and γ. Both gene and protein expression levels of IL-36α and γ are elevated in skin biopsies of patients with GPP, and gene expression levels of IL-36α and γ in patients with GPP are significantly higher than in PV [Citation12]. Studies on primary human keratinocytes suggest that overexpression of IL-36 increases chemokine levels (e.g. IL-8) and immune cell infiltration, hence contributing to the development of GPP [Citation12,Citation23]. The IL-36 receptor is expressed in keratinocytes, monocytes and dendritic cells; binding of IL-36 isoforms to the IL-36 receptor initiates the signaling pathway that stimulates proinflammatory cytokine and chemokine production. This leads to immune cell recruitment and keratinocyte proliferation, resulting in the characteristic inflammatory responses seen in GPP [Citation2,Citation23,Citation24]. An anti-inflammatory cytokine, the IL-36 receptor antagonist (IL-36RA), also binds to the IL-36 receptor, reducing the possibility of IL-36 isoforms binding, consequently suppressing downstream inflammatory activities of the IL-36 signaling pathway [Citation23]. IL-36RA deficiency (termed deficiency of interleukin-36 receptor antagonist), caused by mutations in IL-36RN, contributes to dysregulation of the IL-36 signaling pathway, promoting further keratinocyte chemokine expression, epidermal neutrophil infiltration and pustule formation, as well as inducing systemic inflammatory responses. Dysregulated IL-36 signaling and the subsequent aberrant inflammatory cascade in GPP form a positive feedback loop and IL-36RN deficiency is proposed to be a key driver (shown in ) [Citation23–25].
Figure 1. IL-36 signaling pathway in normal skin and in skin with GPP.
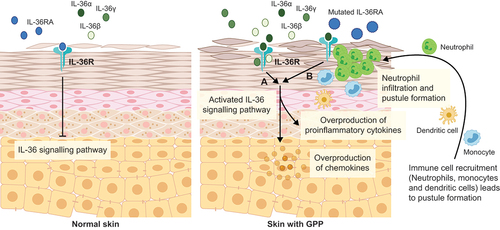
4. Clinical characteristics and disease assessment
The European Rare and Severe Psoriasis Expert Network (ERASPEN) defines GPP as primary, sterile, macroscopically visible pustules on non-acral skin that can occur with/without systemic inflammation and with/without PV, and can relapse (>1 episode) or persist (>3 months) [Citation26]. Five types of GPP have been reported in the literature to date. 1) Acute GPP (von Zumbusch type) [Citation13]. 2) GPP of pregnancy, which most commonly arises in the second to third trimester of pregnancy and rapidly resolves after delivery [Citation13]. 3) Annular GPP [Citation27]. 4) Juvenile and infantile GPP [Citation27]. 5) Localized GPP, which is characterized by pustular lesions occurring in and around psoriasis plaques [Citation11,Citation27]. The Chinese guidelines use the same categorization for these five GPP types [Citation28]. The systemic and skin symptoms reported in Chinese patients are similar to those in Japanese patients; moreover, muscle pain is common and the sterile pustules can appear on patients’ palms and feet, as well as around nails and nailfold in patients [Citation28].
Based on literature and our clinical experience to date, the differential diagnoses of GPP include acute generalized exanthematous pustulosis, superficial candidiasis, subcorneal pustular dermatosis, Stevens-Johnson syndrome – a severe mucocutaneous disease characterized by severe purulent conjunctivitis, severe stomatitis with extensive mucosal necrosis, and purpuric macule, and is caused by drug exposure or infections; with a pustular eruption as an unusual form of the disease [Citation29,Citation30], amicrobial pustulosis of the folds, pemphigus foliaceous, IgA pemphigus subcorneal pustular dermatosis type, transient neonatal pustular melanosis, acropustulosis of infancy and SAPHO syndrome (synovitis, acne, pustulosis, hyperostosis, osteitis), adding to the challenge of correctly diagnosing GPP [Citation1,Citation31–34]. Moreover, diagnostic criteria for GPP vary between research entities. ERASPEN criteria stipulate that a diagnosis of GPP can be confirmed only upon disease relapse >1 episode or persistence >3 months [Citation26]. However, the Japanese Dermatological Association (JDA) guidelines state that a definitive diagnosis of GPP can be made if patients show the following four features: 1) systemic symptoms, including fever and fatigue; 2) systemic or extensive flush accompanied by multiple sterile pustules that can sometimes merge as lakes of pus; 3) neutrophilic subcorneal pustules histopathologically characterized by Kogoj’s spongiform pustules; and 4) the above clinical and histological features occur repeatedly [Citation35]. GPP should be suspected in patients with the second and third features [Citation35].
Another factor underlying the difficulty in managing GPP is the lack of sufficient and validated scoring systems for disease and treatment assessment. Four scoring systems have been reported to date: 1) the Generalized Pustular Psoriasis Area and Severity Index (GPPASI), adapted from the Psoriasis Area Severity Index (PASI) for PV, which evaluates skin components (erythema, scaling and pustules) and percentage of the body surface area affected; 2) the Generalized Pustular Psoriasis Physician Global Assessment (GPPGA), adapted from the Physician Global Assessment (PGA) for psoriasis, which evaluates the same skin components as GPPASI; 3) the JDA-severity index (JDA-SI), which evaluates both skin components (overall erythema, erythema with pustules and edema) and systemic symptoms (pyrexia, white blood cell count, C-reactive protein and serum albumin) [Citation36]; and 4) the Dermatology Life Quality Index (DLQI), a questionnaire that assesses the impact of skin diseases and their treatment in patients with different dermatological conditions [Citation37], has been used to evaluate the impact of GPP on patients’ quality of life (QoL) and treatment outcomes [Citation11,Citation38]. The JDA-SI is considered the most practical and standard scoring system for GPP in terms of clinical utility [Citation36].
GPP poses a significant clinical burden on patients and can be life threatening in some cases. It is associated with extracutaneous complications that can occur at any stage during the disease course, including sepsis, renal, respiratory and heart failure [Citation1,Citation11]. Moreover, the relapsing pattern and uncertainty of GPP flares throughout patients’ lives is a major burden to patients and their families [Citation9], resulting in substantial impact on patients’ QoL, even during the quiescent phase of the disease [Citation9,Citation11]. Moreover, patients with GPP experience a number of comorbidities (including hypertension, peptic ulcer disease, osteoporosis, psoriasis arthritis and insomnia) and substantial emotional stress during the course of the disease or when the symptoms are under control [Citation39,Citation40]. In addition, a variety of prodromes, such as nausea, high fever, joint pain and skin symptoms, in patients with GPP were observed in our clinical practice, could also add discomfort to patients. Also, disease burden of patients with GPP is heavier than patients with plaque psoriasis, in terms of inpatient visits, use of medications, duration of hospitalization as well as medical costs [Citation39,Citation41].
5. Management of GPP
5.1. Global management of GPP
Available management guidelines generally recommend conventional psoriasis medications (e.g. cyclosporine, acitretin, methotrexate [MTX]) and biologic agents as first-line therapies for GPP [Citation35,Citation42]; however, no conventional drugs have been approved specifically for GPP [Citation1,Citation2,Citation35]. Although cyclosporine and MTX are used for GPP treatment, cyclosporine may only be considered for short-term treatment due to its poor safety and tolerability profile, while MTX should be considered with caution for patients with comorbidities (e.g. diabetes mellitus, renal or hepatic injury) [Citation43]. In addition, due to the potential teratogenic effects of acitretin and MTX on the fetus, both agents are contraindicated in pregnant women. MTX is also contraindicated in lactating women, and contraception should be used for 3 months after discontinuing MTX or until at least 3 years after discontinuing acitretin [Citation2,Citation28,Citation35]. Furthermore, real-world data have shown that the median drug survival (the duration from drug initiation to discontinuation to measure therapeutic success) of biologic agents for GPP is longer than that for cyclosporine and MTX (infliximab: 13 months versus cyclosporine: 7 months and MTX: 4 months, respectively). This real-world study used the Cox regression model to adjust variables of age, gender, PsA, and the number of previous systemic antipsoriatic treatments that might affect drug survival. In addition, percentages of patients who discontinued the conventional therapies and biologic agents, as well as the reasons for drug discontinuation were presented in this study; adverse events and ineffectiveness were the common reasons for treatment discontinuation, other reasons such as patient’s request also contributed to drug discontinuation, however, this study did not discuss how the variables of self-discontinuation of patients and adverse events were controlled for calculating drug survival [Citation43].
Some biologic agents have been approved for treating GPP in Japan, including TNF-α inhibitors (adalimumab and infliximab), IL-17/IL-17 R inhibitors (secukinumab, brodalumab and ixekizumab) and IL-23 inhibitors (risankizumab and guselkumab) [Citation35,Citation44–46]; brodalumab has also been approved for GPP in Thailand [Citation47]. It is important to note that these agents were approved based on case reports and single-arm studies with small sample sizes, as the rarity and relapsing pattern of GPP make it challenging for investigators to initiate randomized controlled trials (RCTs) [Citation2]. Currently, there are a number of case reports that continue to support the use of biologic agents in patients with GPP, including combination therapy with infliximab and low-dose acitretin in adult patients [Citation48–50]. Furthermore, no approved biologic agents in the USA [Citation51], Europe [Citation2] or China specifically target GPP, to act fast and effectively prevent disease flares. There is therefore an unmet need for treatments that can act rapidly and have an acceptable safety profile.
The systematic reviews analyzed case reports and case series of the conventional psoriatic treatments and biologic agents used in patients with GPP. These analyses found that both the conventional treatments (acitretin, MTX and cyclosporine) and biologic agents (such as infliximab, secukinumab, brodalumab and guselkumab) have shown favorable clinical outcomes in patients with GPP, although the endpoints used in each study to evaluate the efficacy of the medications varied. Biologic agents had more favorable safety profiles than conventional treatments, due to their specific mechanisms of action [Citation52,Citation53]. Studies have reported a few adverse events (AEs) in patients who used infliximab, including headache, diarrhea, rash, pharyngitis, rhinitis, cough, upper respiratory infection and urinary tract infection [Citation52]. Nasopharyngitis, urticaria, diabetes mellitus and arthralgia were the most frequently reported AEs in 12 Japanese patients with GPP who used secukinumab in a Phase III, open-label, multicenter study [Citation54]. Nasopharyngitis was the most common AE that occurred in 12 Japanese patients with GPP who used brodalumab [Citation38]. Nasopharyngitis, pruritus and hypoalbuminemia were the most common AEs in 10 Japanese patients with GPP who used adalimumab [Citation55]. Although the AEs reported in patients who used secukinumab, brodalumab and adalimumab in each clinical trial varied, the safety outcomes of those three biologic agents were favorable. Also, the efficacy of individual biologic agent (including infliximab, adalimumab, etanercept, ustekinumab and brodalumab) was comparable, reflected by the number of patients showing clinical improvement, with 58.1%, 85.7% or 66.7% of patients who used biologic agents targeting TNF-α, IL-12/IL-23 or IL-17, achieving a complete response with a mean treatment duration of 9.3 months, 20.9 months and 12.7 months, respectively [Citation27].
5.2. Management of GPP in China
Cyclosporine, acitretin and MTX are most frequently used for treating GPP in China [Citation56], although only acitretin has been approved for pustular psoriasis [Citation28]. Although cyclosporine A, acitretin and MTX have generally favorable safety profiles, a meta-analysis of Chinese studies in GPPshowed that the rates of AEs induced by cyclosporine A, acitretin and MTX were 59.04%, 80.45% and 58.97%, respectively. The most common AEs associated with cyclosporine A were arterial hypertension and nephrotoxicity. AEs associated with acitretin were dry skin, itch, dyslipidaemia and changes in hepatic function; those associated with MTX included myelosuppression and changes in hepatic function [Citation56].
No biologic agents are currently approved for GPP in China, although TNF-α inhibitors (etanercept, infliximab and adalimumab), IL-17 inhibitors (secukinumab) and IL-12/23 inhibitors (ustekinumab) have been approved for moderate and severe plaque psoriasis with favorable safety reported; the most recent approval was the IL-23 inhibitor, guselkumab [Citation57,Citation58]. Two case studies from China have reported the efficacy of adalimumab and secukinumab in patients with GPP [Citation59,Citation60]. Moreover, short-term use of infliximab showed efficacy in a child aged 8 years with juvenile GPP [Citation61]. In addition, a wide variety of traditional Chinese herbal medicine therapies characterized by the use of herbal formulae that consist of at least two medicinal herbs, have been investigated in GPP, including combination therapies of those herbal formulae and Western medicine (i.e. cyclosporine, acitretin or MTX), with some combinations showing superior efficacy to Western medicine alone in patients with GPP [Citation62,Citation63]. Also, based on our clinical experience in China, systemic therapies with composite medicines that consist of several medicinal herbs, such as Natural indigo composite capsule, and Xiaoyin granules or tablet; topical therapies such as Qingpeng cream; wet wrap therapy or smear therapy with herbal formulae have shown efficacy in patients.
The Chinese guidelines recommend several systemic, topical and physical therapies for GPP [Citation28]. Systemic therapies include cyclosporine, acitretin, MTX and biologic agents as first-line therapies for adult patients with GPP. Tripterygium wilfordii, dapsone and mycophenolate mofetil are also recommended for GPP. Glucocorticoids are not recommended as systemic treatments for GPP generally and are only considered for severe and life-threatening or uncontrollable disease, or if other medications are contraindicated. The mainstay of topical therapy is to sustain skin hygiene and protect the damaged skin; calamine can be applied to the undamaged pustule, because calamine can protect the pustules, reduce inflammation and swelling of the lesion, as well as reduce irritation; a bland emollient can be applied after the damaged pustule has recovered [Citation28]. Physical therapy using narrow-band ultraviolet B (NB-UVB) or psoralen and ultraviolet A (PUVA) has shown efficacy in Chinese patients with GPP [Citation64]; a Chinese herbal formulae bath combined with a Chinese herbal medicine topical therapy have also shown efficacy [Citation62,Citation64]. Complications of GPP must also be considered; physicians should closely monitor patients’ vital signs, fluid, electrolyte balance and infection status during treatment. The impact of GPP on infants should be considered when managing GPP in pregnancy; glucocorticoids can be considered when appropriate. Cyclosporine should be used with caution due to safety concerns during pregnancy [Citation28]; TNF-α inhibitors may be efficacious in this setting [Citation65]. There is limited evidence on safe, effective treatments for children with GPP; cyclosporine, acitretin, MTX and TNF-α inhibitors (e.g. adalimumab) [Citation65] may be considered. Intravenous immunoglobulin can be considered for infants; cyclosporine is preferred in children aged >1 year, and is superior to acitretin and MTX in terms of safety. Acitretin should be used with caution (shown in ) [Citation28]. Moreover, for patients with familial GPP, we recommend the patients to pay attention to the disease progress and receive therapy as soon as possible; we also recommend other family members to do a genetic screening for GPP, once they are aware of the family history of GPP.
Table 2. Summary of therapies recommended by global guidelines for management and treatment for GPP and clinical experience in China for GPP, based on disease conditiona.
Table 3. Summary of therapies for adult patients with GPP in China, based on therapy categorya.
5.3. Treatment for annular GPP and localized GPP
Annular GPP and localized GPP are milder than the other three subtypes of GPP and, therefore, easier to treat. Topical therapies are the mainstay approach based on the literature [Citation42] and clinical experience in China. Combination systemic and physical therapy may also be used if treatment response is inadequate [Citation64]. Etanercept monotherapy and a combination of etanercept and cyclosporine, respectively, have shown efficacy in managing GPP symptoms in Caucasian patients [Citation66,Citation67].
5.4. Prognosis
For patients with early disease onset of pustular psoriasis and slow disease progression (slow disease progress: no marked changes in the standardized clinical outcome measures, such as GPPASI, GPPGA and BSA, are observed in patients, suggesting that no symptoms of the acute phase of the disease occur in patients with GPP), prognosis is promising [Citation68]. Prognosis tends to be poor in: patients who experience rapid disease progression, particularly those with comorbid plaque psoriasis, with inadequate treatment response or those with extracutaneous complications who are receiving treatment. For elderly patients with uncontrolled GPP, complications (e.g. heart failure and respiratory tract infection) can affect prognosis. The prognosis for children with GPP can be promising if MTX or systemic hormones, such as prednisone, are avoided. Patients with subacute annular GPP or impetigo herpetiformis with a clear etiology generally have a good prognosis [Citation69].
5.5. Future treatment targets
No GPP-specific therapies are currently available worldwide. Targeted therapy, such as IL-36R inhibition, is a novel and specific approach. Spesolimab is a humanized monoclonal antibody that binds IL-36R with high affinity to inhibit the IL-36 signaling pathway and downstream inflammatory activities. A phase I study involving seven patients with GPP flares who received a single dose of spesolimab showed that all patients achieved a GPPGA score of 0 or 1 (clear or almost clear skin) by Week 4, with five patients achieving this by Week 1. Mean improvements from baseline in GPPASI score of all seven patients were 59.0% at Week 1, 73.2% at Week 2 and 79.8% at Week 4. Three, five and six patients achieved complete pustule clearance within 48 hours, 1 week and 2 weeks after treatment, respectively; improvements were maintained through 20 weeks [Citation70]. A phase II, double-blind, placebo-controlled trial, Effisayil 1, is the first RCT conducted in patients with GPP and showed that a single dose of spesolimab rapidly cleared pustules by Week 1 in patients presenting with flare, with improvements maintained throughout the 12-week study period and with an acceptable safety profile [Citation71]. A phase IIb, double-blind, placebo-controlled RCT, Effisayil 2, is investigating the efficacy and safety of three different doses of spesolimab in preventing GPP flares in patients with GPP (NCT04399837). Another humanized monoclonal antibody, imsidolimab (IL-36R antagonist), is under investigation for GPP (NCT03619902).
In China, off-label use of biologic agents for GPP has been reported in some case studies; currently available biologic agents are considered effective options when patients are refractory to conventional systemic treatments [Citation65].
6. Impact of COVID-19 on GPP
Two case reports have shown that infection with SARS-CoV-2 caused development of GPP in patients, including a 12-year-old patient with plaque psoriasis [Citation72,Citation73]. In addition, one patient without existing skin diseases developed GPP due to the Oxford‐AstraZeneca vaccine [Citation74], and one patient with psoriasis developed GPP after receiving the CoronoVac vaccine [Citation75]. The Pfizer-BioNTech vaccine was reported to exacerbate the AGPP condition in an adult patient [Citation76]. Moreover, studies have reported the management of patients with plaque psoriasis who receive biologic agents during COVID-19 pandemic [Citation77–79]. There is a literature gap on the impact of psoriatic treatments on COVID-19 or vaccination in patients with GPP.
7. Conclusion
The rarity and clinical characteristics of GPP make its research, diagnosis and treatment challenging. Conventional medications and biologic agents are used for patients with GPP in China, while no effective GPP-specific treatments are available. The proinflammatory cytokines involved in GPP pathogenesis suggest potential drug targets for developing specific treatments for GPP in the future.
8. Expert opinion
Our review summarized the disease burden, pathogenesis, clinical characteristics and risk factors, and the scoring systems that are currently being used for disease and treatment assessment, as well as potential treatment options and novel drug targets for GPP. By summarizing the most up-to-date information about GPP globally and in China, we can improve awareness of this rare and debilitating disease among Chinese health care professionals (HCPs). This review captures the latest treatment strategies and the most recent data from studies of currently available biologic agents in GPP, as well as describing the unmet needs of GPP. We also encourage the HCP readership to develop a systematic perspective on GPP treatment to allow them to effectively implement these treatment strategies in their clinical practice.
Currently, there are discrepancies in diagnosis criteria and recognizing the differential diagnoses of GPP. There is also an insufficient disease severity scoring system, hence the precise diagnosis of GPP and appropriate management for individual patient remains a challenge, which needs to be improved and standardized in clinical practice. Notably, there are no GPP-specific treatments that have been approved to date, which is a gap in clinical practice worldwide. Therefore, a GPP-specific treatment with an acceptable safety profile is a major unmet clinical need. Inhibition of IL-36R is a novel and specific approach, based upon our emerging understanding of GPP pathogenesis, which needs to be further investigated in the future. An IL-36R inhibitor, spesolimab, is currently in clinical development and has shown promising outcomes in patients with GPP in phase I and phase II studies.
Biologic agents are considered as effective treatment options for GPP, especially when patients are unresponsive to conventional systemic treatments. However, evidence-based treatment options are lacking; head-to-head studies that compare biologic agents and conventional systemic treatments are necessary to expand the evidence base on the efficacy and safety of biologic agents. The currently approved biologic agents are based on case reports and single-arm studies with small sample sizes, and it is challenging for investigators to initiate randomized controlled trials due to the rarity and relapsing nature of the disease, which are hurdles preventing research in this field from making progress.
From our perspective, biologic agents, especially drugs targeting the IL-36 pathway, are the leading candidates in this field and may become the main treatment option for GPP in the future; however, the cost of biologic agents will need to be taken into consideration, as the high cost of these agents might still limit their use in many patients. Moreover, increasing patients’ understanding of their disease may also help improve treatment outcomes and their quality of life in the long term.
In China, the Chinese guidelines on the diagnosis and treatment for GPP will become the standard for guiding HCPs on diagnosis and selecting treatments for patients in the following 5 or 10 years. In terms of how the field will have progressed in 5 years from when this review was written, we believe that there will be growing global attention on the burden of GPP disease as understanding of the disease pathogenesis and management increases following the emergence of more basic science and clinical study data.
During the COVID-19 pandemic, several case reports have reported that COVID-19 or certain COVID-19 vaccines can cause the development of GPP in patients with or without psoriasis. There is a literature gap in the impact of conventional psoriatic treatments or biologic agents on patients with GPP during the pandemic.
Article highlights
Generalised pustular psoriasis (GPP) is a rare, severe and potentially life-threatening immune-mediated skin disease.
The skin and systemic symptoms, relapsing pattern and extracutaneous complications associated with GPP pose a clinical burden in patients.
Conventional medications and biologic agents are used for treating GPP with limited study evidence.
An unmet need exists for GPP-specific treatments that can rapidly resolve the symptoms of GPP flares and prevent recurrence, with acceptable safety.
Recent epidemiologic data for GPP in China, as well as the prevalence of gene mutations that are associated with GPP in Chinese patients, with mutations in IL-36RN being more prevalent in the Chinese population than European population.
Conventional systemic psoriatic treatments such as cyclosporine, acitretin and methotrexate are most frequently used for treating GPP in China. However, no safe and effective therapies are currently approved for GPP.
The recommended management strategies and treatment options for GPP from recent Chinese guidelines and from clinical experience in China, including systemic, topical and physical therapies.
IL-36RN mutation may be a pathogenetic driver for GPP, which makes it a potential treatment target. Our review discussed the ongoing clinical trials targeting IL-36R in patients with GPP.
Declaration of interest
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
One peer reviewer declares: advisory boards for AbbVie, Boehringer Mannheim, Celgene, Janssen Cilag, Lilly, Pfizer, MSD, Mundipharma, Novartis, Amgen, Leo, Sanofi, UCB; speaker board for Abbvie, Boehringer Mannheim Celgene, Janssen Cilag, Leo, MSD, Novartis, Pfizer; clinical studies for AbbVie, Amgen, Boehringer Mannheim, Celgene, Galderma, GSK, Janssen Cilag, Leo, Novartis, Pfizer, Regeneron, Sanofi. One peer reviewer declares: advisor to Eli Lilly, Dermira, Amgen, AbbVie, Incyte Corp, Galderma, UCB, Sun Pharma, Aslan pharm, AnaptysBio, Castle Bioscience, CorEvitas Registry (formerly Corrona); paid consultant/advisor for Eli Lilly, Amgen, Arcutis, AbbVie; investigator in an IL-36 antagonist trial. Peer reviewers on this manuscript have no other relevant financial relationships or otherwise to disclose.
Acknowledgments
Shaowei Duan (Boehringer Ingelheim) provided medical input for developing the manuscript. Editorial support was provided by Xu Hu, PhD and Tim Stentiford, BSc (Hons), PgDip (SciComm), CMPP (Nucleus Global, Shanghai), and funded by Boehringer Ingelheim.
Additional information
Funding
References
- Bachelez H. pustular psoriasis: the dawn of a new era. Acta Derm Venereol. 2020;100:adv00034.
- Gooderham MJ, Van Voorhees AS, Lebwohl MG. An update on generalized pustular psoriasis. Expert Rev Clin Immunol. 2019;15:907–919.
- Augey F, Renaudier P, Nicolas JF. Generalized pustular psoriasis (Zumbusch): a French epidemiological survey. Eur J Dermatol. 2006;16:669–673.
- Feng Q, Guo J, Zhang Q, et al. Evaluation of prevalence and disease burden of generalized pustular psoriasis in China: based on data (2012-2016) from the national health insurance database. unpublished. 2021
- Yu S, Luo Z. clinical analysis of 120 patients with generalized pustular psoriasis. Zhongguo Mafeng Pifubing Zazhi. 2019;5:260–5,75.
- Li Y, Gan X, Xiao R, et al. Clinical analysis of 32 adult patients with acute generalized pustular psoriasis. Linchuang Pifuke Zazhi. 2004;6:351–352.
- Niu R, Lu G, Zhang HE. Clinical analysis of 92 patients with generalized pustular psoriasis. Zhongguo Pifu-Xingbingxue Zazhi. 2012;2:129–131.
- Mössner R, Wilsmann-Theis D, Oji V, et al. The genetic basis for most patients with pustular skin disease remains elusive. Br J Dermatol. 2018;178:740–748.
- Kharawala S, Golembesky AK, Bohn RL, et al. The clinical, humanistic, and economic burden of generalized pustular psoriasis: a structured review. Expert Rev Clin Immunol. 2020;16:239–252.
- Luo Y. Clinical analysis of 130 patients with generalized pustular psoriasis [Master thesis]. China Medical University; 2017.
- Choon SE, Lai NM, Mohammad NA, et al. Clinical profile, morbidity, and outcome of adult-onset generalized pustular psoriasis: analysis of 102 cases seen in a tertiary hospital in Johor, Malaysia. Int J Dermatol. 2014;53:676–684.
- Johnston A, Xing X, Wolterink L, et al. IL-1 and IL-36 are dominant cytokines in generalized pustular psoriasis. J Allergy Clin Immunol. 2017;140:109–120.
- Benjegerdes KE, Hyde K, Kivelevitch D, et al. Pustular psoriasis: pathophysiology and current treatment perspectives. Psoriasis (Auckl). 2016;6:131–144.
- Twelves S, Mostafa A, Dand N, et al. Clinical and genetic differences between pustular psoriasis subtypes. J Allergy Clin Immunol. 2019;143:1021–1026.
- Li L. Association of gene IL36RN, CARD14 and AP1S with generalized pustular psoriasis in Chinese Han population. China: Jinan University; 2018.
- Li X, Chen M, Fu X, et al. Mutation analysis of the IL36RN gene in Chinese patients with generalized pustular psoriasis with/without psoriasis vulgaris. J Dermatol Sci. 2014;76:132–138.
- Li M, Han J, Lu Z, et al. Prevalent and rare mutations in IL-36RN gene in Chinese patients with generalized pustular psoriasis and psoriasis vulgaris. J Invest Dermatol. 2013;133:2637–2639.
- Setta-Kaffetzi N, Navarini AA, Patel VM, et al. Rare pathogenic variants in IL36RN underlie a spectrum of psoriasis-associated pustular phenotypes. J Invest Dermatol. 2013;133:1366–1369.
- Berki DM, Liu L, Choon SE, et al. Activating CARD14 mutations are associated with generalized pustular psoriasis but rarely account for familial recurrence in psoriasis vulgaris. J Invest Dermatol. 2015;135:2964–2970.
- Zhang Z, Ma Y, Zhang Z, et al. Identification of two loci associated with generalized pustular psoriasis. J Invest Dermatol. 2015;135:2132–2134.
- Farooq M, Nakai H, Fujimoto A, et al. Mutation analysis of the IL36RN gene in 14 Japanese patients with generalized pustular psoriasis. Hum Mutat. 2013;34:176–183.
- Takeichi T, Kobayashi A, Ogawa E, et al. Autosomal dominant familial generalized pustular psoriasis caused by a CARD14 mutation. Br J Dermatol. 2017;177:e133–e5.
- Marrakchi S, Guigue P, Renshaw BR, et al. Interleukin-36-receptor antagonist deficiency and generalized pustular psoriasis. N Engl J Med. 2011;365:620–628.
- Furue K, Yamamura K, Tsuji G, et al. Highlighting interleukin-36 signalling in plaque psoriasis and pustular psoriasis. Acta Derm Venereol. 2018;98:5–13.
- Sugiura K, Takemoto A, Yamaguchi M, et al. The majority of generalized pustular psoriasis without psoriasis vulgaris is caused by deficiency of interleukin-36 receptor antagonist. J Invest Dermatol. 2013;133:2514–2521.
- Navarini AA, Burden AD, Capon F, et al. European consensus statement on phenotypes of pustular psoriasis. J Eur Acad Dermatol Venereol. 2017;31:1792–1799.
- Boehner A, Navarini AA, Eyerich K. Generalized pustular psoriasis - a model disease for specific targeted immunotherapy, systematic review. Exp Dermatol. 2018;27:1067–1077.
- Expert Committee of Psoriasis SoD, Chinese Medical Association. Guidelines of diagnosis and treatment for psoriasis in China (2018 complete edition). Zhonghuo Pifuke Zazhi. 2018;10:667–710.
- Reichert-Penetrat S, Barbaud A, Antunes A, et al. An unusual form of Stevens-Johnson syndrome with subcorneal pustules associated with mycoplasma pneumoniae infection. Pediatr Dermatol. 2000;17:202–204.
- Harr T, French LE. Toxic epidermal necrolysis and Stevens-Johnson syndrome. Orphanet J Rare Dis. 2010;5:39.
- Ly K, Beck KM, Smith MP, et al. Diagnosis and screening of patients with generalized pustular psoriasis. Psoriasis (Auckl). 2019;9:37–42.
- Naik HB, Cowen EW. Autoinflammatory pustular neutrophilic diseases. Dermatol Clin. 2013;31:405–425.
- Varman KM, Namias N, Schulman CI, et al. Acute generalized pustular psoriasis, von Zumbusch type, treated in the burn unit. A review of clinical features and new therapeutics. Burns. 2014;40:e35–9.
- Romiti R, Hirayama A, Arnone M, et al. Generalized pustular psoriasis (von Zumbusch). An Bras Dermatol. 2022;97:63–74.
- Fujita H, Terui T, Hayama K, et al. Japanese guidelines for the management and treatment of generalized pustular psoriasis: the new pathogenesis and treatment of GPP. J Dermatol. 2018;45:1235–1270.
- Neuhauser R, Eyerich K, Boehner A. Generalized pustular psoriasis-Dawn of a new era in targeted immunotherapy. Exp Dermatol. 2020;29:1088–1096.
- Finlay AY, Khan GK. Dermatology Life Quality Index (DLQI)–a simple practical measure for routine clinical use. Clin Exp Dermatol. 1994;19:210–216.
- Yamasaki K, Nakagawa H, Kubo Y, et al. Efficacy and safety of brodalumab in patients with generalized pustular psoriasis and psoriatic erythroderma: results from a 52-week, open-label study. Br J Dermatol. 2017;176:741–751.
- Morita A, Kotowsky N, Gao R, et al. Patient characteristics and burden of disease in Japanese patients with generalized pustular psoriasis: results from the medical data vision claims database. J Dermatol. 2021;48:1463–1473.
- Reisner DV, Johnsson FD, Kotowsky N, et al. Impact of generalized pustular psoriasis from the perspective of people living with the condition: results of an online survey. Am J Clin Dermatol. 2022;23:65–71.
- Okubo Y, Kotowsky N, Gao R, et al. Clinical characteristics and health-care resource utilization in patients with generalized pustular psoriasis using real-world evidence from the Japanese medical data center database. J Dermatol. 2021;48:1675–1687.
- Robinson A, Van Voorhees AS, Hsu S, et al. Treatment of pustular psoriasis: from the medical board of the national psoriasis foundation. J Am Acad Dermatol. 2012;67:279–288.
- Kromer C, Loewe E, Schaarschmidt ML, et al. Drug survival in the treatment of generalized pustular psoriasis: a retrospective multicenter study. Dermatol Ther. 2021;34:e14814.
- Secukinumab (Genetical recombination) approval review report. Pharmaceuticals and Medical Devices Agency; 2015 [cited 2021 Sept 22]. Available from: https://www.pmda.go.jp/files/000216877.pdf
- Risankizumab (Genetical recombination) approval review report. Pharmaceutical Evaluation Division, Pharmaceutical Safety and Environmental Health Bureau, Ministry of Health, Labour and Welfare; 2019 [cited 2021 Sept 22]. Available from: https://www.pmda.go.jp/files/000239461.pdf
- Guselkumab (Genetical recombination) approval review report. Pharmaceutical Evaluation Division, Pharmaceutical Safety and Environmental Health Bureau, Ministry of Health, Labour and Welfare; 2018 [cited 2021 Sept 22]. Available from: https://www.pmda.go.jp/files/000234741.pdf
- Kirin KH. Lumicef® (Brodalumab) prescribing information. Thailand: Kyowa Hakko Kirin (Thailand) Co., Ltd. Bangkok. 2018.
- Zhu H, Song P, Du D, et al. Successful treatment of a 3-year-old girl with generalized pustular psoriasis using secukinumab monotherapy. Pediatr Dermatol. 2021;38:1366–1367.
- Hansel K, Marietti R, Tramontana M, et al. Childhood generalized pustular psoriasis: successful long-term treatment with Adalimumab. Dermatol Ther. 2020;33:e13294.
- Kołt-Kamińska M, Żychowska M, Reich A. Infliximab in combination with low-dose acitretin in generalized pustular psoriasis: a report of two cases and review of the literature. Biologics. 2021;15:317–327.
- Strober B, Kotowsky N, Medeiros R, et al. Unmet medical needs in the treatment and management of generalized pustular psoriasis flares: evidence from a survey of corrona registry dermatologists. Dermatol Ther (Heidelb). 2021;11:529–541.
- Zhou LL, Georgakopoulos JR, Ighani A, et al. Systemic monotherapy treatments for generalized pustular psoriasis: a systematic review. J Cutan Med Surg. 2018;22:591–601.
- Kearns DG, Chat VS, Zang PD, et al. Review of treatments for generalized pustular psoriasis. J Dermatolog Treat. 2021;32:492–494.
- Imafuku S, Honma M, Okubo Y, et al. Efficacy and safety of secukinumab in patients with generalized pustular psoriasis: a 52-week analysis from phase III open-label multicenter Japanese study. J Dermatol. 2016;43:1011–1017.
- Morita A, Yamazaki F, Matsuyama T, et al. Adalimumab treatment in Japanese patients with generalized pustular psoriasis: results of an open-label phase 3 study. J Dermatol. 2018;45:1371–1380.
- Ji S. Meta-analysis of treatment with cyclosporine, methotrexate and acitretin for patients with generalized pustular psoriasis. Taishanyixueyuan Xuebao. 2018;39:973–978.
- Chinese Society of Dermatology CDA, Dermatology and Venereology Specialized Committee of Chinese Association of Integrative Medicine, Chen A-J, Gao X-H, Gu H, et al. Chinese experts consensus on biologic therapy for psoriasis#. IntJ Demat Venerol. 2020;3:76–85.
- Guselkumab injection prescription information. [cited 2021 Sept 22]. Avaibale from: https://www.xian-janssen.com.cn/sites/default/files/PDF/te_nuo_ya_yu_chong_bi_ni_yin_zhi_ban_shuo_ming_shu__2.pdf.Janssen-CilagInternationalNV2020
- Wu Y, Chen J, Shi X, et al. A case study of one patient with acute generalized pustular psoriasis treated with adalimumab. Zhongguo Pifu-Xingbingxue Zazhi. 2018;9:1032–1035.
- He M, Zhang JA, Xin W, et al. Efficacy evaluation of 7 patients with psoriasis treated with secukinumab. Zhongguo Mafeng Pifubing Zazhi. 2020;9:537–540
- Pan J, Qiu L, Xiao T, et al. Juvenile generalized pustular psoriasis with IL36RN mutation treated with short-term infliximab. Dermatol Ther. 2016;29:164–167.
- Liu L, Zhao Y. Research progress on clinical therapies for generalized pustular psoriasis. Zhongguo Zhongyi Jizhen. 2020;7:1313–1316
- Wang Y, Yang H, Chen L, et al. Network-based modeling of herb combinations in traditional Chinese medicine. Brief Bioinform. 2021;22. DOI:10.1093/bib/bbab106.
- Fan P. The current situation of Treatment For Generalized Pustular Psoriasis. Zhongguo Liaoyang Yixue. 2010;6:524–525.
- Zhou J. Research progress on biological agents for generalized pustular psoriasis. Pifukexue Tongbao. 2020;37:472–476
- Ricotti C, Kerdel FA. Subacute annular generalized pustular psoriasis treated with etanercept and cyclosporine combination. J Drugs Dermatol. 2007;6:738–740.
- Lo Schiavo A, Brancaccio G, Puca RV, et al. Etanercept in the treatment of generalized annular pustular psoriasis. Ann Dermatol. 2012;24:233–234.
- Yuling S, Jun G, Zhizhong Z. The expert writing committee for the Chinese expert consensus on diagnosis and treatment in patients with pustular psoriasis. Chinese expert consensus on diagnosis and treatment in patients with pustular psoriasis (2022). Chin Jl of Dermatology. 2022;55:187–195.
- Jin H, Cho HH, Kim WJ, et al. Clinical features and course of generalized pustular psoriasis in Korea. J Dermatol. 2015;42:674–678.
- Bachelez H, Choon SE, Marrakchi S, et al. Inhibition of the interleukin-36 pathway for the treatment of generalized pustular psoriasis. N Engl J Med. 2019;380:981–983.
- Bachelez H, Choon SE, Marrakchi S, et al. Effisayil™ 1: a multicentre, randomised, double-blind, placebo-controlled study to evaluate the efficacy, safety and tolerability of spesolimab in patients with a generalized pustular psoriasis flare. N Engl J Med. 2021;385:2431–2440.
- Shahidi Dadras M, Diab R, Ahadi M, et al. Generalized pustular psoriasis following COVID-19. Dermatol Ther. 2021;34:e14595.
- Pala E, Melikoğlu M, Erkayman MH. Pediatric COVID-19 patient with exacerbated generalized pustular psoriasis. Rev Soc Bras Med Trop. 2021;54:e0318.
- Elamin S, Hinds F, Tolland J. De novo generalized pustular psoriasis following Oxford-AstraZeneca COVID-19 vaccine. Clin Exp Dermatol. 2022;47:153–155.
- Onsun N, Kaya G, Işık BG, et al. A generalized pustular psoriasis flare after CoronaVac COVID-19 vaccination: case report. Health Promot Perspect. 2021;11:261–262.
- Perna D, Jones J, Schadt CR. Acute generalized pustular psoriasis exacerbated by the COVID-19 vaccine. JAAD Case Rep. 2021;17:1–3.
- Talamonti M, Galluzzo M, Chiricozzi A, et al. Management of biological therapies for chronic plaque psoriasis during COVID-19 emergency in Italy. J Eur Acad Dermatol Venereol. 2020;34:e770–e2.
- Talamonti M, Galluzzo M, Chiricozzi A, et al. Characteristic of chronic plaque psoriasis patients treated with biologics in Italy during the COVID-19 Pandemic: risk analysis from the PSO-BIO-COVID observational study. Expert Opin Biol Ther. 2021;21:271–277.
- Galluzzo M, Tofani L, Bianchi L, et al. Status of a real-life cohort of patients with moderate-to-severe plaque psoriasis treated with secukinumab and considerations on the use of biological agents in the Covid-19 era. Expert Opin Biol Ther. 2020;20:829–830.
