ABSTRACT
Introduction
Drug-free remission (DFR) and its maintenance have been defined as the most desirable outcome for rheumatoid arthritis (RA) patients. DFR is linked to resolution of arthritis-related symptoms and restoration of normal functioning. However, there is currently no consensus if an optimal strategy, upon the initiation of treatment to the proper drugs withdrawal, is enough to induce it, or whether it is a predetermined condition related to patients’ intrinsic characteristics.
Areas covered
This review focuses on two key concepts around DFR. First, we analyze patients’ intrinsic factors that may increase the chance of DFR, regardless of therapeutic choices. Second, we discuss on the evidence that it can be induced thanks to adequate, extrinsic disease management. Finally, we provide a glimpse into consequences of drugs discontinuation.
Expert opinion
The early initiation of DMARD and the subsequent strict monitoring and drug adjustments are of primary importance to allow patients to achieve DFR, irrespective of initial treatment strategy. Once remission is obtained and maintained, it is possible to gradually taper and discontinue drugs with no dramatic consequences on the disease course. Among those who stop medication, ACPA-negative patients more often maintain the remission. Thus, DFR might depend on a combination of intrinsic and extrinsic factors.
1. Introduction
A large proportion of rheumatoid arthritis (RA) patients are nowadays able to gain and maintain a stable control of the disease thanks to an early diagnosis, followed by a prompt initiation of disease-modifying anti-rheumatic drugs (DMARDs) with subsequent controlled monitoring. As a result, increasing numbers of patients are able to taper and discontinue DMARDs, while maintaining clinical remission, to arrive at a state of drug-free remission (DFR) [Citation1,Citation2]. An evolving concept is the chance to maintain DFR for more than 12 months (sustained DFR – SDFR), a condition associated with the resolution of pain, fatigue, and the normalization of physical functioning, which provides a glimpse into a possible ‘curable’ nature of the disease – that is, at the moment, the best proxy for cure in RA patients [Citation3].
Notably, a recent systematic literature review reported a rate of DFR up to 24% and of SDFR up to 18% in the setting of modern early arthritis cohorts [Citation4].
International guidelines currently recommend tapering DMARDs, once sustained remission is obtained, as long-term use of conventional synthetic (cs)-, biologic (b)- and targeted synthetic (ts)- DMARDs when patients are in remission no longer outweighs benefits of the treatment. In addition, adverse events (i.e. infections, malignancies) as well as high costs could result in a burden for patients and society. However, guidelines are less explicit about the question whether or not DMARDs should be tapered till zero, possibly because of uncertainty whether DFR can be obtained and maintained [Citation5].
Moreover, the pathophysiological mechanisms underlying disease resolution in RA are not known. A recent review proposed two possible (overlapping) scenarios: the first is that the whole RA population is similar at the time of the diagnosis, thus potentially all the patients are suitable to gain the DFR state thanks to a proper intervention. From a biological perspective, this implies the regaining of the immuno-tolerance that is initially lost during RA development, thanks to appropriate treatment. The second scenario is that patients able to achieve drug-free sustained remission are intrinsically different from those who are not, therefore DMARDs suspension could be attempted only in a subgroup of the whole RA population [Citation6].
Purpose of this review is to provide insights into the opportunity to increase the chance to achieve DFR, either from the identification of patients who are more likely to attempt DMARD discontinuation, or through early and intensive treatment strategies.
2. Patient-related predictors of DFR: do they support predestination or inducibility?
Few studies have primarily investigated which genetic, environmental, clinical, serological, imaging and histopathological factors may influence the chance to reach DFR [Citation6].
Many anthropometric characteristics such as age, sex, and body-mass index have been described to be not associated with a higher chance to obtain DFR [Citation4]. Nonetheless, genetic background is a factor that could influence the intrinsic probability that a patient is able to reach DFR. Shared-epitope (SE) is an important and well-known risk factor for RA development, and also is described as risk factor for not achieving DFR [Citation7]. Intriguingly, that association disappears when corrected for the presence of anti-citrullinated protein antibodies (ACPAs), suggesting a role in hampering the DFR via autoantibodies only [Citation8]. Recently, SE was described to be a risk factor for glycosylation of ACPAs’ variable domain (Fab). Glycosylation of ACPAs increases toward disease onset; interestingly, patients who later achieved DFR had lower levels of glycosylation at the time of RA onset [Citation9]. Moreover, a genetic-variant of the interleukin-2 receptor, involved in regulatory T-cells function and immune-tolerance, appears to be associated with SDFR, but only in ACPA-negative patients [Citation10].
With regard to smoking, which is known to be the most important environmental risk factor for RA, especially in the ACPA-positive subset, accordingly it appears to be linked to a lower chance for obtaining DFR as well [Citation8].
More robust data are currently available for serological status. The absence of autoantibodies, such as rheumatoid factor (RF) and ACPAs, appears to be the most important predictor of DFR. Of note, more recent studies have highlighted that (S)DFR appears to be independently driven only by the presence of ACPAs, irrespective of the coexistence of other autoantibodies [Citation11]. Accordingly, the drug-free condition is reached in about 40% of ACPA-negative patients and only 10–15% of ACPA-positive patients [Citation12]. Thus, these findings support that ACPA-positive and negative RA could be distinct disease subsets in regard to the chance for achieving DFR [Citation13].
Along with them, data about the association between clinical features at baseline and the chance of DFR appear controversial. An insidious onset and a longer duration of symptoms before rheumatological referral are found to be inversely correlated with the chance of DFR [Citation8,Citation14]. Similarly, higher values of disease activity score (DAS) at baseline are associated with a lower chance to reach the DMARD-free remission state [Citation4]. Research on the British Early Rheumatoid Arthritis Study also observed that higher number of swollen joints resulted in a lower chance to obtain the DFR [Citation8]. Moreover, the same analysis showed an inversely correlation between functional disability at the onset (expressed by Health Assessment Questionnaire – HAQ values) and DMARD-free remission. According to the intuitive concept that the higher inflammation at baseline, the lower chance for reaching DFR, also higher levels of C-reactive protein (CRP) were described to be predictive in both univariate and multivariate analysis in the Leiden Early Arthritis cohort [Citation8]. Finally, the fulfillment of Boolean remission criteria at the time of bDMARD discontinuation was found to be predictive of remission maintenance after 1 year from drug withdrawal. However, this data was not further replicated for csDMARDs free-remission yet [Citation15].
Imaging variables at baseline are less consistent in predicting successful drug discontinuation. While no association was found between the presence of erosions and DFR, higher Sharp/van der Heijde scores at diagnosis were predictive for a lower chance to maintain remission after drug withdrawal in the Leiden Early Arthritis Clinic inception cohort [Citation8]. The presence of erosive changes at baseline was rather shown to be inversely correlated with DFR when detected by magnetic resonance imaging (MRI) [Citation6]. Moreover, in line with the findings that the higher (clinical and biochemical) inflammatory burden, the lower chance to discontinue drugs successfully, also MRI osteitis and synovitis at baseline (and at the moment of drugs withdrawal) might be negative predictive factors for DMARD-free remission [Citation16]. Whilst no studies included ultrasound (US) features as predictors of DFR, Baker and colleagues did evaluate US at the baseline. The authors excluded patients with power-doppler positivity from DMARDs suspension, thus hampering the evaluation of the possible correlation with DFR. On the other hand, grayscale synovitis and erosions were included and did not correlate with higher recurrence of flares. Interestingly, synovial hypertrophy assessed by US was suggested to be associated with a higher rate of flares in patients with psoriatic arthritis upon drugs discontinuation [Citation17].
From a biologic perspective, preliminary data indicate that pro-resolving mechanisms, e.g. anti-inflammatory mediators, might potentially operate in the rheumatoid synovium at one point in the disease course. Moreover, histopathological studies showed a higher deposition of collagen in the synovial membrane of clinical and US-inactive joints, thus suggesting a pro-fibrotic process within the remission [Citation18]. However, no studies have yet investigated the histopathological patterns of the synovial tissue under DFR.
In conclusion, available studies cannot yet help us to predict which patients could attempt drugs de-escalation; however, increasing evidence of lower DFR-rates in ACPA-positive subjects might support the ‘predestination’ model.
3. Early, intensive, and strategic treatment of RA as a prerequisite for DFR
Thanks to early diagnosis, prompt initiation of therapy, and treat-to-target (T2T) approach, RA has become a controllable disease in most patients, insofar as a disease-modifying therapy is continued to be administrated and adjusted in accordance with the disease activity indexes [Citation19]. This last concept had become an increasing matter of debate among the experts, and interest began to grow in considering DMARD-free remission as one of the best possible outcomes, strongly related to the aforementioned treatment strategies [Citation20,Citation21]. Accordingly, data from the Leiden Early Arthritis inception cohort have recently revealed an increasing chance to achieve DFR over the last decade in patients who were treated in an early and strategic manner [Citation3,Citation12].
This observation supports a specific treatment strategy to induce DFR as ‘extrinsic’ factor, irrespective of patients’ characteristics. As a consequence, these strategies might potentially modify the disease course in the whole RA population.
3.1. Window of opportunity
International guidelines emphasize the importance to start DMARD therapy as soon as the diagnosis is made [Citation5]. The concept of the ‘window of opportunity’ refers to the time span after symptom onset in which the disease progression is more susceptible to be modified and patients are more likely to obtain remission as well as anatomical and functional ability preservation. In the FINnish Rheumatoid Arthritis Combination therapy (FIN-RACo) trial, a delay of more than 4 months in initiation of therapy was the only predictor of not achieving remission among 195 RA patients who started a single disease-modifying drug [Citation22]. Furthermore, a very early referral and subsequent intervention with a DMARD demonstrated to be of primary importance to reach the major outcomes in a large Italian multicenter study [Citation23]. Collectively, data from clinical trials and observational cohorts nowadays indicate a 12-weeks-time as the optimal period in which treatment should be initiated in order to achieve better mid- and long-term outcomes [Citation2,Citation23,Citation24].
Few studies have evaluated DFR as a disease outcome related to early referral and treatment. van der Linden and colleagues examined the association between delay in assessment by a rheumatologist and the consequent later disease-modifying therapy start, with the rate of joint destruction and the probability of achieving DFR in a real-life setting of 1674 patients with early arthritis from the Leiden Early Arthritis inception cohort [Citation14]. Authors found that a delay in rheumatological referral (≥12 weeks) was associated not only with a higher radiographic progression over 6 years, but also with a minor chance of obtaining DFR as compared to a shorter delay in assessment. This relationship was also analyzed in a systematic literature review and metanalysis of 18 randomized controlled trials (RCT) available performed by van Nies et al., which concluded that prolonged symptoms duration before DMARD treatment initiation was independently associated with a lower chance on achieving DFR [Citation24]. With respect to the drug-free sustained remission as the main outcome, the same author subsequently performed an analysis on data from the Leiden Early Arthritis Clinic and the French Evaluation et Suivi de POlyarthrites Indifférenciées Récentes (ESPOIR) cohort. The study confirmed the presence of inverse correlation between symptom duration before disease-modifying therapy initiation and drug-free sustained remission and, interestingly, showed a non-linear association between these variables, with a decreasing of the log-hazard on DMARD-free sustained remission after a certain symptom duration. Further analysis on the Leiden Early Arthritis Clinic cohort also showed a higher chance for patients to reach sustained DMARD-free remission if referred to a rheumatologist within 6 weeks, compared to a period of 7–12 weeks [Citation25]. This confirms de facto the existence of a restricted period in which RA is more susceptible to treatment, that is supposed to be the window of opportunity [Citation26].
3.2. Treat-to-target strategy
Current experts’ recommendations stress on the need for T2T approach in order to obtain better outcomes [Citation19]. The treatment strategy of tight control (TC) for rheumatoid arthritis (TICORA) study analyzed the differences between a sustained, tight-controlled, DAS-driven approach compared with clinical routine outpatient care of RA patients. The study first demonstrated the advantages of an intensive management in terms of improvement of disease activity as well as better long-term outcomes such as radiographic progression, physical function and quality of life [Citation27]. These findings were subsequently replicated by other studies, which confirmed the beneficial and long-lasting effect of DMARDs in inducing remission when initiated early in the course of the disease and administrated according to an intensive and strict protocol, compared to routine care treatment [Citation28,Citation29].
Insights into the concept of DFR as an outcome of T2T and TC-based strategies were provided by a post-hoc analysis of the Behandel Strategie en treatment strategies (BeSt) study, originally designed for the assessment of the advantage of T2T and TC strategies in reaching better radiographic and functional outcomes in early RA. Over the first 10 years of the study, the BeSt trial demonstrated that early-targeted treatments, irrespective of initial treatment strategy, and subsequent tight monitoring of disease activity, improved maintenance of remission after discontinuation of DMARDs [Citation30].
In the treatment in the Rotterdam Early Arthritis Cohort (tREACH) study, early RA patients were randomized to receive an initial triple DMARD therapy or methotrexate (MTX) monotherapy. Similarly, this trial showed the beneficial effect of an early T2T-based treatment in order to reach remission and maintain it after drug tapering, regardless of the initial treatment strategy [Citation31].
As in controlled clinical trials, T2T approach has been shown to be useful in achieving the DFR also in real-life settings. In the Dutch RhEumatoid Arthritis Monitoring (DREAM) remission induction cohort, patients with early RA started DMARDs aiming at disease activity score 28 (DAS28)-remission (<2.6), followed by tapering and discontinuation of therapy when remission was sustained (>6 months.) After a follow-up of 5 years, the study demonstrated that more than 36% of patients achieved SDFR [Citation13].
Further confirmation is given by van der Woude and colleagues over a 5-years follow-up among patients treated according to a DAS-steered or a non-DAS-steered approach. The author found a similar rate of SDFR among the two groups with more general favorable outcomes in the DAS-steered cohort. Noteworthy, the DAS-driven approach increased the chance to achieve SDFR in ACPA-positive but not in ACPA-negative patients [Citation7]. The latter finding was also observed in a recent study performed by Burgers et al., which showed a tendency to reach DFR thanks to intensive DAS-steered treatment in ACPA-positive RA patients only [Citation32].
In the Induction therapy with MTX and Prednisone in Rheumatoid Or Very Early arthritic Disease (IMPROVED) study, DAS-steered early remission appeared to be predictive for SDFR in an RA population [Citation33]. More recently, Verstappen and colleagues confirmed that a strong DAS decline within the first 4 months of treatment was associated with a higher probability of SDFR development, but only in ACPA-negative patients [Citation34].
With respect to initial therapy, as described before, the BeSt and the tREACH trials showed a similar rate of DFR among patients treated with different initial treatment strategies [Citation30,Citation31]. On the other hand, the Productivity and Remission in a randomIZed controlled trial of etanercept (ETA) in Early rheumatoid arthritis (PRIZE) displayed a predictive role of the initial combination of ETA plus MTX in induction and maintenance of DFR compared to MTX alone [Citation35]. Similarly, in A Very Early Rehabilitation Trial (AVERT) patients initially treated with abatacept (ABA) plus MTX more often reached and sustained DFR compared to those treated with MTX alone [Citation36]. At last, in the U-Act-Early randomized clinical trial, newly diagnosed RA patients were randomized in 3 treatment arms (group 1: tocilizumab (TCZ) monotherapy; group 2: TCZ + MTX; group 3: MTX monotherapy). When evaluating the rate of SDFR as a secondary endpoint, this trial showed a significantly favorable effect of the two TCZ arms compared to the MTX monotherapy [Citation37].
Collectively, DFR is more probable if DMARDs are initiated promptly, especially in ACPA-positive subjects, if remission is achieved early in the disease course and if treatment is steered at the target. Together these might imply that DFR is an ‘inducible’ condition, achievable by improving our management strategies.
4. Strategies for DMARD withdrawal
Alongside the improvement of the induction treatment strategies, the concept of a correct DMARD discontinuation strategy arises once the disease remission is obtained and maintained over time. Notably, the European Alliance of Associations of Rheumatology (EULAR) guidelines suggest a clinical- and economical-based sequence in drugs discontinuation, i.e. stopping glucocorticoids first (even if in a low dosage), followed by tapering and withdrawing bDMARDs and, ultimately, csDMARDs [Citation5]. Similarly, the American College of Rheumatology (ACR) also integrates the possibility to withdraw disease-modifying drugs once the remission is achieved and sustained [Citation38]. Noteworthy, no randomized controlled trial has yet compared different strategies in DMARD-tapering aiming to obtain DFR as primary endpoint.
Most of currently available studies focus on bDMARD discontinuation. Importantly, very little studies address complete DMARD discontinuation (both conventional and biologic), and algorithms which could help clinicians to successfully taper drugs, as well as the correct order to do it, are yet to be defined.
The first clinical trial about D-penicillamine withdrawal displayed a high rate of arthritis flare: about 80% of patients who discontinued the drug. Of importance, the reintroduction of the former dose of penicillamine was successful in controlling relapses [Citation39]. A recent prospective study provided further information about conventional DMARDs discontinuation. Forty-four patients with established RA in remission (DAS28-CRP <2.4) completely stopped DMARD therapy at once. Twenty-one of them (48%) were able to maintain DFR over a 6-month follow-up period [Citation40].
Most studies about bDMARD tapering focus on tapering or spacing of biologic agents with continuation of csDMARDs. In addition, they do not provide indications about the chance of reaching DFR according to different biologic drugs-discontinuation strategies [Citation1].
A pivotal controlled trial performed by Quinn et al. in 2005 and its 8-years follow-up analysis showed the ability of an infliximab (IFX)-plus-MTX-based induction regimen to attain faster improvement of disease activity, MRI-detected inflammation and quality of life compared to MTX alone. Moreover, after one year of treatment, patients were able to maintain remission after IFX discontinuation, and one patient was able to reach DFR. Though, the study was not designed to detect the percentage of DFR as an outcome of drug tapering [Citation41,Citation42].
A sub-analysis of the BeSt trial provided further insights into DMARD tapering strategies. According to the treatment protocol, if the DAS<2.4 for more than 6 months, medication was tapered. After 2 years all medication could be stopped if DAS<1.6 for more than 6 months. Order of tapering was glucocorticoids, IFX, and thereafter csDMARDs. Consequently, DFR was achieved and maintained in 8–18% of patients for at least one year [Citation43].
In the three-phase PRIZE trial, patients with early RA who achieved DAS28-remission (<2.6) over 52 weeks of combined therapy with ETA (50 mg) injections + oral MTX (10–25 mg) weekly (phase 1 of the study), were double-blinded randomized to receive: 1) ETA (25 mg) injection + oral MTX; 2) placebo injections + oral MTX; 3) placebo injections + oral placebo. They were then followed for another 39 weeks (phase 2 of the study). At week 39, patients able to maintain at least the low disease activity (LDA) state (DAS28 ≤ 3.2) had the study drugs withdrawn and then followed through week 65 (phase 3 of the study). Regarding discontinuation strategy, ETA and placebo injections were stopped abruptly; MTX and placebo capsules were tapered over 2 to 4 weeks. Despite DFR was not a study outcome, the trial showed that, at week 52, patients in the combination-therapy group maintained remission significantly longer after induction compared to the patients in the MTX-alone group or the placebo group; this suggests that adopting a gradual tapering strategy in biological drug discontinuation could be more beneficial instead of abrupt interruption. Of note, no data about disease flares rate during the drug-free phase are available [Citation44].
Further information on DMARD tapering strategies were recently provided by the Tapering strategies in Rheumatoid Arthritis (TARA) trial. Patients with established RA in stable control of the disease over a 6-months period (DAS ≤2.4 and swollen joint count ≤ 1) under both csDMARD and TNF-inhibitors (TNFi) therapy were randomized into tapering the csDMARD in the first year followed by tapering the TNFi in the second year, or vice versa. The rate of flares was not influenced by tapering strategy adopted, thus suggesting a possible preferential choice in tapering TNFi first due to financial arguments [Citation45].
Although not conclusive, currently available data suggest that gradual tapering results in low flare rates compared to abrupt cessation. This suggest that DFR could be more easily reached by improving disease management, thus strengthening the ‘inducibility’ hypothesis.
5. Consequences of DMARD withdrawal
Risk of disease flares is the most important consequence of drugs discontinuation. Because of different study designs as well as definition of flares, the rate of disease relapses during or after drugs discontinuation may vary widely between clinical trials and observational cohorts. Despite of that, currently available data seem to indicate that risk of flares is time-dependent, as most of them occur during drugs de-escalation and reach the lowest percentage after one year from drugs suspension. In particular, a recent systematic literature review reports flare rates of 41.8–75% during tapering, 10.4–11.8% during the first year after achieving the DMARD-free status (so called ‘early flares’) and 0.3–3.5% after the first year (‘late flares’) [Citation4].
Importantly, it is now fairly described that the majority of patients who experience a flare are able to recapture remission after restarting the same DMARD [Citation45]. Reassuringly, a recent trial reports that up to 92–100% of patients can regain at least the LDA state after drug reintroduction [Citation46].
Another important issue is whether complete DMARDs suspension could lead to radiographic progression. A literature meta-analysis performed by Henaux et al. revealed a higher risk of structural progression after complete withdrawal of bDMARDs, while not present after tapering them [Citation47]. More recently, the Aiming for Remission in Rheumatoid Arthritis (ARCTIC) Rewind trial showed that tapering of csDMARDs may lead to more radiographic progression compared to tapering of TNFi [Citation48]. On the contrary, no differences in anatomic damage were seen in two different tapering strategies in the aforementioned TARA study [Citation45].
Patient-reported outcomes also showed to benefit from a drug-free state, especially when sustained. In particular, arthritis-related symptoms as pain, fatigue and morning stiffness are significantly lower during SDFR compared to diagnosis, and patients are able to regain their functional ability as well [Citation3].
Last but not of less importance, tapering and discontinuing DMARDs could help to save on healthcare costs. The Dose REduction Strategy of Subcutaneous TNF inhibitors (DRESS) study showed a significant cost-saving related to TNFi injections spacing [Citation49]. The data was furtherly confirmed by the Spacing of TNF-Blocker Injections in Rheumatoid Arthritis (STRASS) trial [Citation50]. Intriguingly, the two-year cost-utility analysis of the TARA trial, which, compared to the formers, evaluated also the burden of presenteeism-related costs, found no differences between the two aforementioned tapering strategies [Citation51].
6. Conclusion
A non-neglectable number of patients with RA are nowadays able to maintain (sustained) remission after drugs discontinuation. The early diagnosis and prompt initiation of DMARD, the strict monitoring and the DAS-steered intensification of immunosuppression are still the cornerstones for RA management, ultimately aiming to achieve drug-free sustained remission. This probably happens irrespective of initial drug(s) choice. This supports the concept that the whole RA population could at some point obtain DFR thanks to clinicians’ intervention. However, queries regarding the existence of an optimal algorithm for treatment start and tapering remain open and need to be elucidated in further studies.
Along with these inducible, patient-independent parameters, ACPA-negativity appears to be the most important ‘intrinsic’ predictor for achieving DFR. Since only a small number of ACPA-positive patients reach DMARD-free remission, then the ‘predestination’ hypothesis could be rather suggested. Future research should focus on tailored approach among different subgroups of RA patients.
7. Expert opinion
Many studies define the (S)DFR as the best possible achievable condition for RA patients. Being able to successfully stop immunosuppressive drugs could be beneficial for many reasons: 1) to reduce the load of drug’s adverse effects, especially in terms of infections, once the remission is obtained and the damage-benefit ratio of the use of DMARDs is no longer clear; 2) to reduce the load of costs on the health system, especially for biological or targeted-synthetic drugs; 3) to improve patient-reported outcomes; 4) to be able to distinguish between inflammation suppression or ‘true cure’ of RA [Citation1]. Although the rate of DFR varies according to different variables, increasing available data should reassure the clinicians to attempt drug discontinuation once the remission is reached and sustained, in selected patients and in a balanced way.
Once the diagnosis is made, being able to identify RA patients who will be more susceptible to achieve DFR is still extremely challenging. As long as we consider the sustained remission after drug discontinuation the most desirable disease outcome, the existence of ‘intrinsic’ patients’ characteristics which are independent from the clinicians’ intervention may lead to question if RA is a ‘curable’ disease in every patient or only in a lucky subgroup of them. The most robust current available data identify ACPA-positivity as important predictive factors for not achieving DFR [Citation8], as SDFR is obtained only in about 10–15% of seropositive patients [Citation12]. Thus, the detection of ‘extrinsic’ treatment strategies which can increase the chance for SDFR in the ACPA positive and negative subgroups is inherently different and need to be studied separately in further research.
Advantages over the last decades in the diagnosis and treatment of RA were shown to have a favorable impact also into the chance to achieve DFR [Citation4].
The early diagnosis and prompt initiation of treatment with DMARD within a ‘window of opportunity’ of approximately 12 weeks from the disease onset are, among others, of great importance to allow patients to achieve a drug-free sustained remission [Citation23]. Similarly, the T2T strategy is now demonstrated to be useful for that purpose [Citation30,Citation31]. The recent TREAT Early Arthralgia to Reverse or Limit Impending Exacerbation to Rheumatoid arthritis (TREAT EARLIER) trial showed that initiation of MTX during the pre-arthritic stage could be able to reduce disease burden. In this trial, patients with clinically suspect arthralgia treated with MTX displayed a similar rate of RA occurrence compared to non-treated group; however, after arthritis development, the treated-group demonstrated better disease outcomes, such as less pain and less morning stiffness [Citation52]. This might open interesting scenarios among the opportunity to improve the ability to obtain DFR, if therapy is commenced before the clinical onset of arthritis.
Along with the prompt initiation of treatment when the diagnosis is made, also the prompt response to treatment seems to be associated with a major chance to achieve sustained remission after drug discontinuation. Accordingly, the DAS response during the very early phase of the disease course upon DMARD initiation could be a useful parameter to predict which patients can obtain SDFR [Citation33], especially for ACPA-negative patients [Citation34].
Although many studies have shown a favorable effect related to the use of combinations of drugs or second-line therapies ab initio in patients with recent-onset RA in terms of obtaining an early remission [Citation53], if a preferential and/or a sequential choice of one or more medications (both cs- and b/ts-DMARDs) has a different impact on the opportunity to gain DFR is still controversial. It was shown that early start of treatment is predictive for DFR independently of initial treatment [Citation30,Citation31]. On the contrary, few trials focused on biological therapies as initial therapy, and therefore hampers comparison with initial conventional therapy. According to them, DFR could be reached more easily through the use of specific biological drugs. This might suggest the presence of specific pathways that should be preferentially inhibited to achieve this outcome [Citation35–37]. Nevertheless, it should be noted that patients initially treated with biological DMARDs were able to reach a sustained remission earlier compared to those treated with MTX alone. Thus, one remains uncertain whether the increased DFR is truly driven by the use of biologic drug, or by the earlier remission.
Nowadays, more data are available for the detection of a proper initial and ongoing strategy in drug-escalation aimed to induce DFR. Instead, the same cannot be said for the medications tapering phase, and better algorithms are yet to be defined. Nevertheless, one could suggest preferring a gradual tapering strategy than an abrupt discontinuation, and to follow a clinical and financial-driven schedule, as also recommended by EULAR and ACR guidelines.
Along with the positive impact, clinicians should be aware of possible negative consequences of disease-modifying drugs discontinuation. One of the main concerns for tapering DMARDs in RA patients is the risk of disease flares. Nevertheless, data from modern arthritis cohorts suggest that more than one-third of patients with LDA or in remission may taper or stop DMARD therapy without experiencing a disease flare within the first year [Citation54]. Notably, the true severity of relapses and how this could influence patients’ and clinicians’ perspective in discontinuing the medications is yet to be studied systematically. Moreover, and even more important, current data show that almost the total amount of patients can re-catch remission or, at least, the LDA state after DMARD re-initiation, with no dramatic consequences in terms of accumulated damage [Citation45].
The query about the role of autoantibodies remains open. The presence of ACPAs appears to hamper the chance for reaching DFR. From a genetic and biologic perspective, this might support the idea that DFR is a predestinated state: seronegative patients are predestinated to achieve it. However, a large cohort study from the Netherlands showed that only ACPA-positive RA patients have increasingly achieved DFR during last decades thanks to more intensive treatment, while no improvement was observed in seronegative patients [Citation12]. This might rather suggest the inducibility model: if treatment strategies improve, perhaps seropositive patients will benefit most.
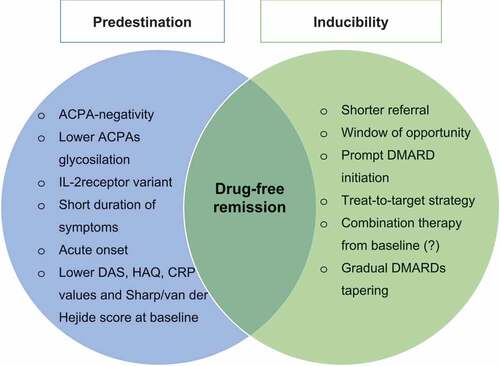
According to available findings, the likelihood to achieve DFR might therefore derive from a combination of patient- and treatment-related factors (). As a result, better characterization of both needs to be evaluated in further studies.
Article highlights
DFR has been modernly defined as the most desirable outcome for patients with RA. Despite that, the query whether DFR is due to patients’ baseline characteristics or implementation of treatment strategies is still open.
From the ‘predestination’ perspective, patients with ACPA-negative disease, lower disease activity, lower CRP values and lower radiographic severity at the baseline are believed to be more suitable to achieve DFR.
Alongside with these non-modifiable conditions, DFR is more often obtained if patients are referred to rheumatologist quicker after symptoms onset, if they promptly start DMARD within the window of opportunity and if the treatment is steered-to-target. Moreover, a gradual drugs tapering increase the chance to achieve DFR. These data support the ‘induction’ hypothesis.
Collectively, these data suggest that DFR might be both a predestinated and inducible condition.
Currently available data reassure about consequences of drugs suspensions. In case of disease flares, patients are quite often able to recapture remission or low disease activity after DMARD reintroduction.
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
One peer reviewer declares: named as an inventor on a patent by Newcastle University relating to biomarkers of drug-free remission in rheumatoid arthritis; collaboration with Genentech to test for cytokine biomarkers of remission in rheumatoid arthritis. Peer reviewers on this manuscript have no other relevant financial relationships or otherwise to disclose.
Additional information
Funding
References
- Schett G, Emery P, Tanaka Y, et al. Tapering biologic and conventional DMARD therapy in rheumatoid arthritis: current evidence and future directions. Ann Rheum Dis. 2016;75(8):1428–1437.
- Burgers LE, Raza K, van der Helm-van Mil AH. Window of opportunity in rheumatoid arthritis - definitions and supporting evidence: from old to new perspectives. RMD Open. 2019;5(1):e000870.
- Ajeganova S, van Steenbergen HW, van Nies JAB, et al. Disease-modifying antirheumatic drug-free sustained remission in rheumatoid arthritis: an increasingly achievable outcome with subsidence of disease symptoms. Ann Rheum Dis. 2016 May;75(5):867–873.
- Verstappen M, Van Mulligen E, De Jong PHP, et al., DMARD-free remission as novel treatment target in rheumatoid arthritis: a systematic literature review of achievability and sustainability. RMD Open. 2020. 6(1): e001220.
- Smolen JS, Landewé RBM, Bijlsma JWJ, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2019 update. Ann Rheum Dis. 2020;79(6):S685–99.
- Verstappen M, van der Helm-van Mil AHM. Sustained DMARD-free remission in rheumatoid arthritis – about concepts and moving towards practice. Jt Bone Spine. 2022 Nov;89(6):105418.
- van der Woude D, Visser K, Klarenbeek NB, et al. Sustained drug-free remission in rheumatoid arthritis after DAS-driven or non-DAS-driven therapy: a comparison of two cohort studies. Rheumatology (Oxford). 2012 Jun;51(6):1120–1128.
- van der Woude D, Young A, Jayakumar K, et al. Prevalence of and predictive factors for sustained disease-modifying antirheumatic drug-free remission in rheumatoid arthritis: results from two large early arthritis cohorts. Arthritis Rheum. 2009 Aug;60(8):2262–2271.
- Kissel T, Hafkenscheid L, Wesemael TJ, et al. IgG anti–citrullinated protein antibody variable domain glycosylation increases before the onset of rheumatoid arthritis and stabilizes thereafter: a cross‐sectional study encompassing ~1,500 samples. Arthritis Rheumatol. 2022 Jul 28;74(7):1147–1158.
- van Steenbergen HW, van Nies JAB, Ruyssen-Witrand A, et al. IL2RA is associated with persistence of rheumatoid arthritis. Arthritis Res Ther. 2015Sep8; 17:244. Internet
- van Wesemael Mv TJ, Knevel R, van der Helm - van Mil AHM, et al. In rheumatoid arthritis, the association between anti-modified protein antibodies and long- term outcomes is dominated by the effect of anti-citrullinated protein antibodies. Lancet Rheumatol. 2022;4:e316–e317.
- Matthijssen XME, Niemantsverdriet E, Huizinga TWJ, et al., Enhanced treatment strategies and distinct disease outcomes among autoantibody-positive and -negative rheumatoid arthritis patients over 25 years: a longitudinal cohort study in the Netherlands. PLoS Med. 2020. 17(9): 1–18.
- Versteeg GA, Steunebrink LMM, Vonkeman HE, et al. Long-term disease and patient-reported outcomes of a continuous treat-to-target approach in patients with early rheumatoid arthritis in daily clinical practice. Clin Rheumatol. 2018 May;37(5):1189–1197.
- Der Linden MPM V, Le Cessie S, Raza K, et al. Long-term impact of delay in assessment of patients with early arthritis. Arthritis Rheum. 2010;62(12):3537–3546.
- Hashimoto M, Furu M, Yamamoto W, et al. Factors associated with the achievement of biological disease-modifying antirheumatic drug-free remission in rheumatoid arthritis: the ANSWER cohort study. Arthritis Res Ther. 2018 Dec 3;20(1):165.
- Ahmad HA, Baker JF, Conaghan PG, et al. Prediction of flare following remission and treatment withdrawal in early rheumatoid arthritis: post hoc analysis of a phase IIIb trial with Abatacept. Arthritis Res Ther. 2022;24(1):47.
- Araujo EG, Finzel S, Englbrecht M, et al. High incidence of disease recurrence after discontinuation of disease-modifying antirheumatic drug treatment in patients with psoriatic arthritis in remission. Ann Rheum Dis. 2015 Apr;74(4):655–660.
- Bugatti S, Sakellariou G, Luvaro T, et al. Clinical, imaging, and pathological suppression of synovitis in rheumatoid arthritis: is the disease curable? Front Med. 2018 May 15;5. 10.3389/fmed.2018.00140
- Smolen JS, Aletaha D, Bijlsma JWJ, et al. Treating rheumatoid arthritis to target: recommendations of an international task force. Ann Rheum Dis. 2010 Apr 1;69(4):631–637.
- Monti S, Montecucco C, Bugatti S, et al. Rheumatoid arthritis treatment: the earlier the better to prevent joint damage. RMD Open. 2015;1(Suppl 1):e000057.
- Scirè CA, Lunt M, Marshall T, et al. Early remission is associated with improved survival in patients with inflammatory polyarthritis: results from the Norfolk Arthritis Register. Ann Rheum Dis. 2014 Sep;73(9):1677–1682.
- Möttönen T, Hannonen P, Korpela M, et al. Delay to institution of therapy and induction of remission using single-drug or combination-disease-modifying antirheumatic drug therapy in early rheumatoid arthritis. Arthritis Rheum. 2002 Apr;46(4):894–898.
- Gremese E, Salaffi F, Bosello SL, et al. Very early rheumatoid arthritis as a predictor of remission: a multicentre real life prospective study. Ann Rheum Dis. 2013 Jun;72(6):858–862.
- Van Nies JAB, Krabben A, Schoones JW, et al. What is the evidence for the presence of a therapeutic window of opportunity in rheumatoid arthritis? A systematic literature review. Ann Rheum Dis. 2014;73(5):861–870.
- Niemantsverdriet E, Dougados M, Combe B, et al. Referring early arthritis patients within 6 weeks versus 12 weeks after symptom onset: an observational cohort study. Lancet Rheumatol. 2020 Jun;2(6):e332–8.
- Van Nies JAB, Tsonaka R, Gaujoux-Viala C, et al. Evaluating relationships between symptom duration and persistence of rheumatoid arthritis: does a window of opportunity exist? Results on the leiden early arthritis clinic and ESPOIR cohorts. Ann Rheum Dis. 2015;74(5):806–812.
- Grigor C, Capell H, Stirling A, et al. Effect of a treatment strategy of tight control for rheumatoid arthritis (the TICORA study): a single-blind randomised controlled trial. Lancet. 2004;364(9430):263–269.
- Verstappen SMM, Jacobs JWG, Van Der Veen MJ, et al. Intensive treatment with methotrexate in early rheumatoid arthritis: aiming for remission. Computer assisted management in early rheumatoid arthritis (CAMERA, an open-label strategy trial). Ann Rheum Dis. 2007;66(11):1443–1449.
- Schipper LG, Vermeer M, Kuper HH, et al. A tight control treatment strategy aiming for remission in early rheumatoid arthritis is more effective than usual care treatment in daily clinical practice: a study of two cohorts in the dutch rheumatoid arthritis monitoring registry. Ann Rheum Dis. 2012 Jun;71(6):845–850.
- Markusse IM, Akdemir G, Dirven L, et al., Long-term outcomes of patients with recent-onset rheumatoid arthritis after 10 years of tight controlled treatment: a randomized trial. Ann Intern Med. 2016. 164(8): 523–531.
- Kuijper TM, Luime JJ, de Jong PHP, et al. Tapering conventional synthetic DMARDs in patients with early arthritis in sustained remission: 2-year follow-up of the tREACH trial. Ann Rheum Dis. 2016 Dec;75(12):2119–2123.
- Burgers LE, van der Pol JA, Huizinga TWJ, et al. Does treatment strategy influence the ability to achieve and sustain DMARD-free remission in patients with RA? Results of an observational study comparing an intensified DAS-steered treatment strategy with treat to target in routine care. Arthritis Res Ther. 2019;21(1):115.
- Akdemir G, Heimans L, Bergstra SA, et al. Clinical and radiological outcomes of 5-year drug-free remission-steered treatment in patients with early arthritis: IMPROVED study. Ann Rheum Dis. 2018 Jan;77(1):111–118.
- Verstappen M, Niemantsverdriet E, Matthijssen XME, et al., Early DAS response after DMARD-start increases probability of achieving sustained DMARD-free remission in rheumatoid arthritis. Arthritis Res Ther. 2020. 22(1): 1–11.
- Nam JL, Villeneuve E, Hensor EMA, et al. A randomised controlled trial of etanercept and methotrexate to induce remission in early inflammatory arthritis: the EMPIRE trial. Ann Rheum Dis. 2014 Jun;73(6):1027–1036.
- Emery P, Burmester GR, Bykerk VP, et al. Evaluating drug-free remission with Abatacept in early rheumatoid arthritis: results from the phase 3b, multicentre, randomised, active-controlled AVERT study of 24 months, with a 12-month, double-blind treatment period. Ann Rheum Dis. 2015 Jan;74(1):19–26.
- Bijlsma JWJ, Welsing PMJ, Woodworth TG, et al. Early rheumatoid arthritis treated with tocilizumab, methotrexate, or their combination (U-Act-Early): a multicentre, randomised, double-blind, double-dummy, strategy trial. Lancet. 2016 Jul;388(10042):343–355.
- Singh JA, Saag KG, Bridges SL, et al. American college of rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Rheumatol (Hoboken, NJ). 2015 [2016 Jan];68(1):1–26.
- Ahern MJ, Hall ND, Case K, et al. D-penicillamine withdrawal in rheumatoid arthritis. Ann Rheum Dis. 1984 Apr 1;43(2):213–217.
- Baker KF, Skelton AJ, Lendrem DW, et al. Predicting drug-free remission in rheumatoid arthritis: a prospective interventional cohort study. J Autoimmun. 2019 Dec;105:102298.
- Quinn MA, Conaghan PG, O’Connor PJ, et al. Very early treatment with infliximab in addition to methotrexate in early, poor-prognosis rheumatoid arthritis reduces magnetic resonance imaging evidence of synovitis and damage, with sustained benefit after infliximab withdrawal: results from a twelve-m. Arthritis Rheum. 2005 Jan;52(1):27–35.
- Bejarano V, Conaghan PG, Quinn MA, et al. Benefits 8 years after a remission induction regime with an infliximab and methotrexate combination in early rheumatoid arthritis. Rheumatology. 2010 Oct 1;49(10):1971–1974.
- Van Den Broek M, Lems WF, Allaart CF. BeSt practice: the success of early-targeted treatment in rheumatoid arthritis. Clin Exp Rheumatol. 2012;30(4 SUPPL.73) S35–8.
- Emery P, Hammoudeh M, FitzGerald O, et al. Sustained remission with etanercept tapering in early rheumatoid arthritis. N Engl J Med. 2014 Nov 6;371(19):1781–1792.
- van Mulligen E, Weel AE, Hazes JM, et al. Tapering towards DMARD-free remission in established rheumatoid arthritis: 2-year results of the TARA trial. Ann Rheum Dis. 2020;79(9):1174–1181.
- Curtis JR, Emery P, Karis E, et al. Etanercept or methotrexate withdrawal in rheumatoid arthritis patients in sustained remission. Arthritis rheumatol. (Hoboken NJ): 2021.Vol. 73(5)759–768.
- Henaux S, Ruyssen-Witrand A, Cantagrel A, et al. Risk of losing remission, low disease activity or radiographic progression in case of bDMARD discontinuation or tapering in rheumatoid arthritis: systematic analysis of the literature and meta-analysis. Ann Rheum Dis. 2018;77(4):515–522.
- Lillegraven S, Paulshus Sundlisæter N, Aga A-B, et al. Effect of half-dose vs stable-dose conventional synthetic disease-modifying antirheumatic drugs on disease flares in patients with rheumatoid arthritis in remission: the arctic rewind randomized clinical trial. JAMA. 2021;325(17):1755–1764.
- Kievit W, van Herwaarden N, van den Hoogen FH, et al. Disease activity-guided dose optimisation of Adalimumab and etanercept is a cost-effective strategy compared with non-tapering tight control rheumatoid arthritis care: analyses of the DRESS study. Ann Rheum Dis. 2016 Nov;75(11):1939–1944.
- Vanier A, Mariette X, Tubach F, et al. Cost-effectiveness of TNF-blocker injection spacing for patients with established rheumatoid arthritis in remission: an economic evaluation from the spacing of TNF-blocker injections in rheumatoid arthritis trial. Value Heal. 2017 Apr;20(4):577–585.
- van Mulligen E, Weel AE, Kuijper TM, et al. Two-year cost effectiveness between two gradual tapering strategies in rheumatoid arthritis: cost-utility analysis of the TARA trial. Ann Rheum Dis. 2020;79(12):1550–1556.
- Krijbolder DI, Verstappen M, van Dijk BT, et al. Intervention with methotrexate in patients with arthralgia at risk of rheumatoid arthritis to reduce the development of persistent arthritis and its disease burden (TREAT EARLIER): a randomised, double-blind, placebo-controlled, proof-of-concept trial. Lancet. 2022 Jul;400(10348):283–294.
- Ajeganova S, Huizinga T. Sustained remission in rheumatoid arthritis: latest evidence and clinical considerations. Ther Adv Musculoskelet Dis. 2017 Oct 2;9(10):249–262.
- Kuijper TM, Lamers-Karnebeek FBG, Jacobs JWG, et al. Flare rate in patients with rheumatoid arthritis in low disease activity or remission when tapering or stopping synthetic or biologic DMARD: a systematic review. J Rheumatol. 2015 Nov;42(11):2012–2022.