1. Introduction
Autoimmune diseases, such as systemic sclerosis (SSc), likely arise from a convergence of host genetic and environmental factors. While a number of environmental factors have been implicated in the pathogenesis of SSc (e.g. exposure to silica, organic solvents, various infectious agents, diet), many gaps in knowledge exist, particularly the mechanistic intricacies that link environment and genetics to the development of disease.
The gut microbiome may represent an important mediator of SSc pathogenesis as the membership and function of the human gut microbiome are exquisitely sensitive to dietary and environmental changes [Citation1]. In animal models of other autoimmune diseases, such as inflammatory bowel disease (IBD), disease does not develop or is substantially mitigated under germ-free conditions [Citation2]. In SSc, the gut microbiota exhibits alterations in bacterial diversity, overall composition and abundance compared with healthy individuals [Citation3]. Emerging research also suggests that these alterations arise relatively early in the disease course [Citation4]. The present article provides a selective review and commentary on the role of the gut microbiota in SSc. In addition to reviewing the evidence to support a microbial basis for SSc, this article aims to provide a conceptual framework for the future study of microbiome research in SSc with a focus on developing microbiome-based interventions to treat and potentially prevent the progression of this disease.
2. Role of the gut microbiome in SSc
Early research on the role of the gut microbiome in SSc demonstrated distinct differences in the composition and abundance of specific bacterial genera [Citation5]. For example, in a study evaluating colonic lavage specimens from the cecum and sigmoid areas, we demonstrated that patients with SSc had distinct microbial community differences compared with unaffected, age- and sex-matched healthy controls [Citation5]. Specifically, patients with SSc had decreased abundance of genera typically deemed commensal (e.g. Faecalibacterium and Clostridium) [Citation5], as well as increased abundance of genera typically deemed pathobiont (e.g. Fusobacterium, Ruminococcus, and Akkermansia) [Citation5]. Subsequent studies have affirmed enrichment of the pathobionts, Fusobacterium, Ruminococcus, Akkermansia, in patients with SSc compared with unaffected controls [Citation6–9]. Interestingly, increased abundance of Ruminococcus gnavus is associated with increased disease activity in Crohn’s disease, a condition that similar to SSc, possesses both inflammatory and fibrosing pathological features [Citation10]. illustrates specific microbial community differences between patients with SSc and controls across different geographic cohorts.
Table 1. Differentially abundant bacterial genera in patients with SSc compared with unaffected controls across distinct geographic cohorts.*
Studies have also demonstrated a consistent link between dysbiosis and SSc-gastrointestinal (GI) symptoms in SSc [Citation5]. For example, in a cross-sectional analysis, increased abundance of the pathobiont, Fusobacterium, was associated with increased severity of GI symptoms [Citation5], as measured by the UCLA GIT 2.0. In IBD, Fusobacterium is considered a marker of disease activity that may also predict future disease course. In a longitudinal study of patients with SSc, decreased abundance of the commensal species, Bacteroides fragilis, was associated with worsening of SSc-GI symptoms over time, even after adjustment for disease duration and disease subtype (e.g. limited versus diffuse cutaneous disease) [Citation11]. During this study, which included microbial and symptom assessment every 3 months, the abundance and presence of specific genera did not significantly change over the course of 1 year. While this was a small study, future studies aiming to characterize changes in the gut microbiota of patients with SSc may need longer follow up periods.
3. Early alterations in gut microbiota in SSc
Bacterial overgrowth is a recognized feature of patients with long-standing SSc, historically thought to arise due to intestinal stasis. Slowing of intestinal motility facilitates the local proliferation of coliform bacteria. However, while intestinal motor complexes affect the diversity and abundance of the microbiota, new research has confirmed that this relationship is not unidirectional [Citation12]. In other words, gut microbiota can influence motor patterns. Studies in germ-free animals demonstrate disruptions not only in motor patterns, but also in enteric neural and muscular morphology and function [Citation12]. Colonization of germ-free rats with commensal species, such as Lactobacillus acidophilus and Bifidobacterium bifidum, reverses intestinal transit delays. Various bacterial components and products may affect motility, including short-chain fatty acids and bile salts [Citation12].
Deciphering the mechanisms by which gut bacteria and their metabolites influence gut motility is central to understanding the role these bacteria play in the pathogenesis of SSc. It is now evident that gut dysbiosis occurs early in the SSc disease course, prior to overt signs of intestinal stasis. For example, in a study of fecal specimens from patients with SSc in Sweden, patients with recent onset SSc (Median disease duration of 2 years), exhibited increased abundance of several genera deemed pathobiont (e.g. Desulfovibrio, Ruminococcus) and decreased abundance of several genera deemed commensal (e.g. Faecalibacterium) compared with age- and gender-matched controls [Citation4]. Very few patients in this cohort had any clinical signs of SSc-related lower GI tract involvement. These findings suggest that alterations in gut microbiota may proceed the development of clinically overt dysmotility in SSc.
However, as diverse clinical phenotypes of SSc exist, a single microbial community profile for all patients with SSc is unlikely. For example, while SSc patients from different geographic cohorts exhibit shared patterns of reduced microbial diversity and decreased relative abundance of Firmicutes [Citation6], a recent study demonstrated differences in microbial composition between SSc patients with interstitial lung disease (ILD) compared with those without ILD in two independent cohorts [Citation4]. This study [Citation4] and another smaller study [Citation13] also reported differences in microbial composition between patients with and without small intestinal bacterial overgrowth (SIBO). In a multivariable analysis controlling for cohort, disease duration, and the presence of SIBO, ILD remained significantly associated with microbial composition (as measured by beta diversity) in the aforementioned study [Citation4]. Whether these microbiota alterations contribute to ILD pathogenesis in SSc is unclear; nevertheless, these findings provide compelling evidence that distinct microbial community profiles are associated with specific SSc disease manifestations and warrant future study.
4. Microbiome-based interventions for SSc
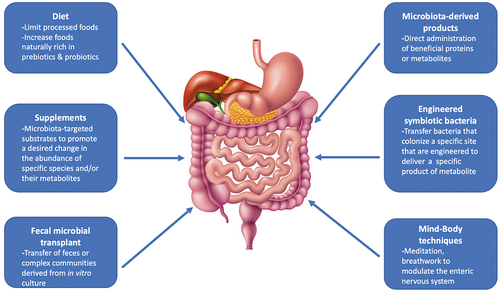
As our understanding of the gut microbiome in SSc evolves, modifying the composition of the microbiota may play a future role in the treatment of SSc. Below are some examples of microbiome-based interventions that may benefit patients with SSc ().
4.1. Prebiotics
Undigestible oligosaccharides and polysaccharides, or prebiotics, promote the proliferation and/or metabolic activity of bacteria in a manner deemed beneficial to the host. In irritable bowel syndrome (IBS), a condition with overlapping symptoms of SSc-GI dysfunction, treatment with a short-chain inulin-type fructan for 6 weeks reduced IBS symptoms and improved quality of life compared with placebo [Citation14]. Prebiotics, such as inulin, occur naturally in foods, including asparagus, chicory root and Jerusalem artichokes. Future studies are needed to determine whether prebiotic supplementation can modify the gut microbiota and improve symptoms in SSc.
4.2. Probiotics
Probiotics are live or attenuated microorganisms that may alter the composition of gut microbial communities and may also benefit the host. While probiotics have been extensively studied in IBD and IBS, only two small randomized controlled trials (RCTs) have evaluated the effects of probiotics on GI symptoms in SSc [Citation15,Citation16]. Both studies failed to detect a treatment effect. However, the probiotic supplement used in these studies were comprised of Bifidobacterium and Lactobacillus species. Prior studies have demonstrated that patients with SSc have increased abundance of these genera compared with healthy controls [Citation5–8]. Thus, further research is required to understand the optimal strain of probiotics for SSc. In addition, dietary supplements in the US do not undergo regulatory review by the Food and Drug Administration prior to introduction to the market; whether the supplement contains viable organisms at the time of purchase is generally unknown. Fermented (e.g. sauerkraut, tempeh, Kombucha) and cultured (e.g. yogurt, kephir, cheese) food products naturally contain various probiotics and may represent a more effective and cost-conscious alternative to probiotic supplements.
4.3. Diet
Various dietary modifications can profoundly affect the composition of the gut microbiome. For example, transition to a plant-based diet or a diet devoid of gluten has immediate effects on gut microbial communities. A diet low in fermentable oligosaccharides, disaccharides, and monosaccharides and polyols (FODMAPs) is commonly recommended to patients with SSc to reduce symptoms, such as gas and bloating. While this intervention has not been studied in RCTs, a recent small study found that patients who adhered to a low FODMAP diet had no appreciable alterations in gut microbial composition, nor GI symptoms, compared with patients who did not adhere to a low FODMAP diet [Citation17]. There is also some concern that patients consuming a low-FODMAP diet may experience reductions in beneficial commensal microbes, as well as micronutrient deficiencies, particularly if the low FODMAP diet is consumed long-term. It is important to remember that elimination of high FODMAP foods is only the first step in this process; selective and sequential re-introduction of foods containing individual FODMAPs is critical for achieving dietary diversification.
5. Future directions
While strategies such as fecal microbial transplant [Citation18] and broad-spectrum antibiotic therapies [Citation19] aim to reestablish intestinal homeostasis, the sustainability of these approaches is limited due to the stability and resilience of the preexisting host microbiota. More personalized interventions are needed that replete beneficial microbes and eliminate pathobiont microbes, specific to the SSc disease state, without collateral damage to the rest of the gut microbiome. Finally, recognizing alterations in the gut microbiome in early SSc may lead to the discovery of therapies aimed at averting the development of complications of SSc, including ILD.
6. Conclusion
Accumulating evidence supports the hypothesis that dysbiosis plays a role in the pathogenesis of SSc. The incorporation of functional analyses of the microbiome, using metagenomic, transcriptomic, proteomic and metabolomic analyses, may reveal new mechanistic insights and transform our understanding of how the gut microbiota shapes immune function in SSc. The identification of specific microbial populations or species may furthermore lead to the development of highly specific targeted therapies.
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Additional information
Funding
References
- Ahn J, Hayes RB. Environmental influences on the human microbiome and implications for noncommunicable disease. Annu Rev Public Health. 2021;42:277–292.
- Hernández-Chirlaque C, Aranda CJ, Ocón B, et al. Germ-free and antibiotic-treated mice are highly susceptible to epithelial injury in DSS colitis. J Crohns Colitis. 2016;10:1324–1335.
- Volkmann ER, Hoffmann-Vold AM. Gastrointestinal tract microbiota modifications in systemic sclerosis. Eur J Rheumatol. 2020;7:S228–36.
- Andréasson K, Lee SM, Lagishetty V, et al. Disease features and gastrointestinal microbial composition in patients with systemic sclerosis from two independent cohorts. ACR Open Rheumatol. 2022;4:417–425.
- Volkmann ER, Chang YL, Barroso N, et al. Association of systemic sclerosis with a unique colonic microbial consortium. Arthritis Rheumatol. 2016;68:1483–1492.
- Volkmann ER, Hoffmann-Vold AM, Chang YL, et al. Systemic sclerosis is associated with specific alterations in gastrointestinal microbiota in two independent cohorts. BMJ Open Gastroenterol. 2017;4:e000134.
- Andréasson K, Alrawi Z, Persson A, et al. Intestinal dysbiosis is common in systemic sclerosis and associated with gastrointestinal and extraintestinal features of disease. Arthritis Res Ther. 2016;18:278.
- Patrone V, Puglisi E, Cardinali M, et al. Gut microbiota profile in systemic sclerosis patients with and without clinical evidence of gastrointestinal involvement. Sci Rep. 2017;7:14874.
- Bellocchi C, Fernández-Ochoa Á, Montanelli G, et al. Microbial and metabolic multi-omic correlations in systemic sclerosis patients. Ann N Y Acad Sci. 2018;1421:97–109.
- Henke MT, Kenny DJ, Cassilly CD, et al. Ruminococcus gnavus, a member of the human gut microbiome associated with Crohn’s disease, produces an inflammatory polysaccharide. Proc Natl Acad Sci U S A. 2019;116:12672–12677.
- Volkmann ER, Hoffmann-Vold A-M, Chang Y-L, et al. Longitudinal characterisation of the gastrointestinal tract microbiome in systemic sclerosis. EMJ Rheumatology. 2020;5. 110–118.
- Waclawiková B, Codutti A, Alim K, et al. Gut microbiota-motility interregulation: insights from in vivo, ex vivo and in silico studies. Gut Microbes. 2022;14:1997296.
- Levin D, De Palma G, Zou H, et al. Fecal microbiome differs between patients with systemic sclerosis with and without small intestinal bacterial overgrowth. J Scleroderma Relat Disord. 2021;202(6):290–298
- Silk DB, Davis A, Vulevic J, et al. Clinical trial: the effects of a trans-galactooligosaccharide prebiotic on faecal microbiota and symptoms in irritable bowel syndrome. Aliment Pharmacol Ther. 2009;29:8–508.
- Low AHL, Teng GG, Pettersson S, et al. A double-blind randomized placebo-controlled trial of probiotics in systemic sclerosis associated gastrointestinal disease. Semin Arthritis Rheum. 2019;49:411–419.
- Marighela TF, Arismendi MI, Marvulle V, et al. Effect of probiotics on gastrointestinal symptoms and immune parameters in systemic sclerosis: a randomized placebo-controlled trial. Rheumatology (Oxford). 2019;58:1985–1990.
- Howlett N, Lee S, Lagishetty V, et al. A low FODMAP diet is not associated with decreased GI symptoms or changes in GI microbial composition in patients with systemic sclerosis [Abstract]. Arthritis Rheumatol. 2020;72(Suppl 10).
- Fretheim H, Chung BK, Didriksen H, et al. Fecal microbiota transplantation in systemic sclerosis: a double-blind, placebo-controlled randomized pilot trial. PLoS One. 2020;15:e0232739.
- Polkowska-Pruszyńska B, Gerkowicz A, Rawicz-Pruszyński K, et al. Gut microbiome in systemic sclerosis: a potential therapeutic target. Postepy Dermatol Alergol. 2022;39:101–109.