1. Introduction
Evidence shows that breastfeeding significantly prevents asthma as it modulates the gut microbiome [Citation1,Citation2]. Consistently, children with asthma present dysbiosis of the gut and lung microbiome, such as a qualitative and/or quantitative derangement [Citation3]. Namely, the disruption and/or change of microbiota homeostasis cause an imbalance, a shift of microbiota functional composition, and a reassessment of metabolic activities and immunomodulatory properties [Citation4]. The dysbiosis promotes the activation of inflammatory pathways and contributes to bronchial obstruction and airway hyperresponsiveness. In addition, dysbiosis and reduced microbial diversity affect the gut-lung axis, such as the bidirectional cross-talk between the digestive tract and airways. It is indeed well known as ‘the microbiota hypothesis,’ suggesting that early life exposures to certain inflammatory bacteria may influence the composition of the microbiota and consequently promote immune dysregulation in the form of hypersensitivity disorders. Recently, it has been seen that other bacteria may offer a ‘eubiotic chance’: dysbiosis may be corrected using ‘good bacteria,’ such as probiotics [Citation5]. This concept paved the way for using oral probiotics to manage allergic disorders, including asthma, by manipulating the immune system and restoring ‘eubiosis.’
2. Body
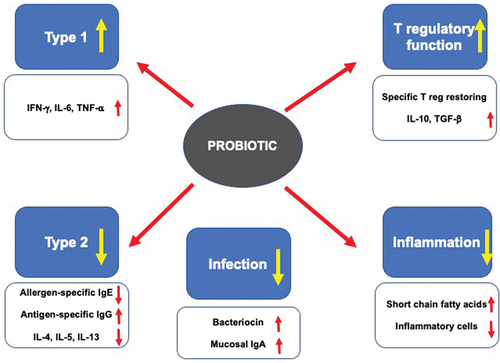
Probiotics are ‘ live microorganisms that, when administered in adequate amounts, confer a health benefit on the host’ [Citation6]. The benefits associated with probiotic supplementation depend on the multifaceted mechanisms of action [Citation7]. After administration, oral probiotics interact with the intestinal epithelial cells (IECs) and immunocompetent cells associated with the lamina propria through Toll-like receptors. This interaction stimulates the production of mediators, cytokines, and chemokines. In addition, probiotics activate regulatory T cells to release IL-10, the main regulatory and anti-inflammatory cytokine. Finally, probiotics modulate intestinal microbiota, inhibiting the growth of potentially pathogenic bacteria in the intestinal tract. Notably, long-term supplementation with some specific probiotics does not affect intestinal homeostasis.
Therefore, probiotics represent an exciting and safe natural strategy for support in asthma management. They could promote the expansion of type-1 response, downregulation of IgE production, dampening airway inflammation, and reinforce immune defense against respiratory infections, as summarized in [Citation7].
Consequently, many studies explored this issue. The outcomes, however, were conflicting, as some studies provided positive findings, whereas some trials failed to improve asthma course, as underscored by recent meta-analyses [Citation8,Citation9]. This consideration derives from the high heterogeneity of the included studies, the frequent low quality, the use of a considerable quantity of different probiotic strains, and the different strains used (strain-specificity demand).
In this regard, based on randomized controlled trials (RCT), there is evidence that some probiotics could significantly improve asthma outcomes in children. summarizes the most relevant results provided by rigorous studies. The inclusion criteria were the study’s methodology, such as randomization of enrolled patients and the presence of a control group.
Table 1. Randomized controlled studies investigating a role of probiotics in children with asthma.
Lactobacillus rhamnosus (Lacticaseibacillus rhamnosus) GG slightly increased the symptom-free days and significantly reduced the new sensitizations [Citation10]. Lactobacillus gasseriA5 supplementation improved lung function and asthma control, reduced asthma and rhinitis symptom scores, and diminished the production of TNF-α, IFN-γ, IL-12, and IL-13 [Citation11]. Oral Lactobacillus reuteri (Limosilactobacillus reuteri) significantly decreased the fractional exhaled nitric oxide (FeNO) values and IL-2 levels and significantly increased IL-10 levels, dampening type-2 inflammation [Citation12]. Supplementing the Bifidobacteria mixture containing Bifidobacterium longum BB536, Bifidobacterium infantis M-63, and Bifidobacterium breve M-16 V significantly reduced respiratory symptoms and improved respiratory symptoms QoL, whereas the placebo group experienced worsened symptoms and QoL [Citation13]. Finally, a multi-strain synbiotic containing Lactobacillus casei, Lactobacillus rhamnosus, Streptococcus thermophilus, Bifidobacterium breve, Lactobacillus acidophilus, Bifidobacterium infantis, Lactobacillus bulgaricus (Lactobacillus delbrueckii), and fructooligosaccharides, reduced the rate of viral respiratory infections and, consistently, the use of bronchodilators [Citation14]. The clinical relevance of these outcomes concerned the ability of this synbiotic to prevent acute respiratory infections, a significant cause of asthma exacerbations. In other words, preventing upper airways viral infections represents a valuable goal in managing children with asthma. Another study with the same synbiotic compound showed a significant reduction in outpatient visits for asthma-related problems [Citation15]. Unfortunately, this study failed to demonstrate the possible prevention of asthma attacks and hospitalizations due to asthma exacerbations. This negative outcome remarks the importance of the study design and methodology used to test probiotics.
Very recently, Bifidobacterium infantis 35,624 has been positively tested in asthmatic adults [Citation16]. This study provided interesting results, as evidenced that this probiotic significantly improved asthma control in the subgroup of patients with initial uncontrolled asthma. In particular, after eight weeks, all treated patients had well-controlled asthma. Furthermore, this outcome was associated with improved lung function. Interestingly, the B. infantis 35,624 reduced a subset of T regulatory cells with T helper 2-like function (CRTH2+ Treg cells). Consistently, the percentage of these Treg cells significantly correlated with asthma control test (ACT) scores. Therefore, this RCT demonstrated the link between asthma improvement (both concerning the control and lung function) and immunological changes provided by a probiotic supplementation.
The PRObiotics in Pediatric Asthma Management (PROPAM) study explored the efficacy of a probiotic mixture containing Ligilactobacillus Salivarius LS01 (DSM 22775) and Bifidobacterium Breve B632 (DSM 24706) in 500 children with asthma or wheezing [Citation17]. This probiotic mixture significantly reduced the number of asthmatic exacerbations (OR = 3.17). In addition, children with two exacerbations were less than a third in the active group (OR = 3.65). Further post hoc analyses confirmed the efficacy in selected sub-groups, such as preschoolers, schoolchildren, and allergic subjects [Citation18–20].
These outcomes may have relevant consequences in clinical practice. For example, asthma exacerbations, mainly if associated with hospitalization, implicate a relevant burden on the healthcare system, worsen lung function, and negatively affect children and their families. In other words, such a dramatic reduction of respiratory crises provided by an RCT represents a relevant result. In addition, the number of previous asthma exacerbations predicts further asthma exacerbations [Citation21]. As a result, preventing asthma exacerbation per se constitutes a relevant target [Citation22].
It might be speculated that this probiotic mixture could modulate the immune response reducing type 2 inflammation and restoring type 1 immunity. However, further confirmatory studies should investigate this issue. In addition, this study had some limitations, including the report of only per protocol data, 80% of the included subjects had intermittent asthma, and 40% did not even use asthma medications at baseline. Also, compliance was poorly monitored, and asthma triggers needed to be adequately identified, but the study was conducted in a primary care setting.
The clinical outcomes, provided by these mentioned studies concerning the use of probiotics in asthma, may depend on the relevant changes in the immune response. Even though there a very few mechanistic studies, the probiotic effect on regulatory cells could be determinant in shifting the immune response from a type 2 endotype to type 1 endotype [Citation23]. A reduction of type 2 immunity results in reduced expression of pro-inflammatory type 2 cytokines. As a result, a reduction in eosinophilic inflammation can occur [Citation24]. At the same time, a restoration of regulatory function may result in a type 1 polarization that enhances immune defense against infection [Citation25]. Physiological type 1 immunity reduces respiratory infections both in number and severity. Thus, supplementation with probiotics can on the one hand reduce type 2 inflammation and on the other reduce infections. The latter point makes sure that asthma exacerbations resulting from a respiratory infection are reduced [Citation26].
Moreover, future research should investigate the possible influence of genetics, and also epigenetics, on the response to probiotic supplementation. Probably, genetic factors could significantly affect the immunological changes consequent to probiotics. It could be envisaged, similarly to pharmacogenetics, a probiotic-genetics that could identify responders to specific strains to an ideal tailoring of treatments.
In conclusion, we underline a practical consideration. It is undoubtedly wrong and misleading to think that all probiotics can positively affect patients with asthma. In clinical practice, if probiotic supplementation is to be used, one must choose those products that have documented evidence of efficacy and safety in patients with asthma. Furthermore, it should always be kept in mind that probiotics are not drugs and should always be used as an add-on treatment to complement standard asthma therapy. Therefore, the choice of a possible probiotic supplementation in patients with asthma should be restricted to products with documented evidence. In addition, there is a need to obtain robust and convincing data from studies exploring the mechanistic aspects, mainly concerning the immunologic effects. Moreover, further studies should identify the specific probiotic for each individual, considering all those factors that may interact with probiotic metabolism and personal response (diet, immunity, concomitant treatments, comorbidities, stress). This way, we could have a personalized choice of the ideal probiotic for every asthma patient.
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
One peer reviewer declares receiving royalties from UptoDate on probiotics for allergy treatment and prevention. Peer reviewers on this manuscript have no other relevant financial relationships or otherwise to disclose.
Additional information
Funding
References
- Davis EC, Castagna VP, Sela DA, et al. Gut microbiome and breast-feeding: implications for early immune development. J Allergy Clin Immunol. 2022;150(3):523–534.
- Rosas-Salazar C, Shilts MH, Tang -Z-Z, et al. Exclusive breast-feeding, the early-life microbiome and immune response, and common childhood respiratory illnesses. J Allergy Clin Immunol. 2022;150(3):612–621.
- Hufnagl K, Pali-Schoell I, Roth-Walter F, et al. Dysbiosis of the gut and lung microbiome has a role in asthma. Sem Immunopathol. 2020;42(1):75–93.
- Weiss GA, Hennet T. Mechanisms and consequences of intestinal dysbiosis. Cell Mol Life Sci. 2017;74(16):2959–2977.
- Huang J, Zhang J, Wang X, et al. Effect of probiotics on respiratory tract allergic disease and gut microbiota. Front Nutr. 2022;9:821900.
- Hill C, Guarner F, Reid G, et al. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol. 2014;11(8):506–514.
- Ciprandi G, Tosca MA. Probiotics in children with asthma. Children (Basel). 2022;9(7):978.
- Wei X, Jiang P, Liu J, et al. Association between probiotic supplementation and asthma incidence in infants: a meta-analysis of randomized controlled trials. J Asthma. 2020;57(2):167–178.
- Uwaezuoke SN, Ayuk AC, Eze JN, et al. Postnatal probiotic supplementation can prevent and optimize treatment of childhood asthma and atopic disorders: a systematic review of randomized controlled trials. Front Pediatr. 2022;10:956141.
- Rose MA, Stieglitz F, Koksal A, et al. Efficacy of probiotic Lactobacillus GG on allergic sensitization and asthma in infants at risk. Clin Exp Allergy. 2010;40(9):1398–1405.
- Chen Y-S, Jan R-L, Lin Y-L, et al. Randomized placebo-controlled trial of Lactobacillus on asthmatic children with allergic rhinitis. Ped Pulmonol. 2010;45(11):1111–1120.
- Miraglia Del Giudice M, Maiello N, Decimo F, et al. Airways allergic inflammation and L. reuterii treatment in asthmatic children. J Biol Regul Homeost Agents. 2012;26(1 Suppl):S35–S40.
- Miraglia Del Giudice M, Indolfi C, Capasso M, et al. Bifidobacterium mixture (B longum BB536, B infantis M-63, B breve M-16V) treatment in children with seasonal allergic rhinitis and intermittent asthma. It J Ped. 2017;43:25
- Ahanchian H, Jafari SA, Ansari E, et al. A multi-strain synbiotic may reduce viral respiratory infections in asthmatic children: a randomized controlled trial. Electr Physicians. 2016;8(9):2833–2839.
- Hassanzad M, Mostashari KM, Ghaffaripour H, et al. Synbiotics and treatment of asthma: a double-blinded, randomized, placebo-controlled clinical trial. GMJ. 2019;8:e1350.
- Sangkanjanavanich S, Pradubpongsa P, Mitthamsiri W, et al. Bifidobacterium infantis 35624 efficacy in patients with uncontrolled asthma: a randomized placebo-controlled trial. Ann Allergy Asthma Immunol. 2022;129(6):790–792.
- Drago L, Cioffi L, Giuliano M, et al. The PRObiotics in Pediatric Asthma Management (PROPAM) study in the primary care setting: a randomized, controlled, double-blind trial with Ligilactobacillus Salivarius LS01 (DSM 22775) and Bifidobacterium Breve B632 (DSM 24706). J Immunol Res. 2022;2022:3837418.
- Ciprandi G, Cioffi L, Giuliano M, et al. The PRObiotics in Pediatric Asthma Management (PROPAM) study: a post-hoc analysis in preschoolers. Ped Pulmonol. 2022;57(5):1355–1357.
- Drago L, Cioffi L, Giuliano M, et al. A post hoc analysis on the effects of a probiotic mixture on asthma exacerbations frequency in schoolchildren. Eur Resp J Open Res. 2022;8:00020–2022
- Ciprandi G, Schiavetti I, Cioffi L, et al. The PRObiotics in Pediatric Asthma Management (PROPAM) study: a post-hoc analysis in allergic children. Ann Allergy Asthma Immunol. 2022;129(1):111–113.
- Ciprandi G, Cioffi L, Schiavetti I, et al. Factors associated with wheezing recurrence in clinical practice. J Asthma. in press.
- Murray CS, Jackson DJ, Teague WG. Prevention and outpatient treatment of asthma exacerbations in children. J Allergy Clin Immunol Pract. 2021;9(7):2567–2576.
- Wang C, Bai J, Chen X, et al. Gut microbiome-based strategies for host health and disease. Crit Rev Food Sci Nutr. 2023;1–16. in press. DOI:10.1080/10408398.2023.2176464
- Ghadimi D, Fölster-Holst R, de Vrese M, et al. Effects of probiotic bacteria and their genomic DNA on TH1/TH2-cytokine production by peripheral blood mononuclear cells (PBMCs) of healthy and allergic subjects. Immunobiology. 2008;213(8):677–692.
- Abbasi-Dokht T, Sadrifar S, Forouzandeh S, et al. Multistrain probiotics supplement alleviates asthma symptoms via increasing treg cells population: a randomized, double-blind, placebo-controlled trial. Int Arch Allergy Immunol. 2023;184(3):291–301.
- Du T, Lei A, Zhang N, et al. The beneficial role of probiotic lactobacillus in respiratory diseases. Front Immunol. 2022;13:908010.