ABSTRACT
Introduction
Generalized pustular psoriasis (GPP) is a rare cutaneous and systemic inflammatory disease which is characterized by flares of widespread painful pustulation of the skin, often associated with fever and elevated inflammatory markers. Although it has historically been regarded as a severe variant of plaque psoriasis, genetic and immunological developments over the past decade have revealed that it is a distinct auto-inflammatory entity, in which over-activity of the interleukin-36 signaling pathway is fundamental. Treatments targeting the IL-36 pathway are under investigation, and in 2022 spesolimab, a monoclonal antibody against the IL-36 receptor, was licensed for treating flares of GPP
Areas covered
In this review I discuss the epidemiology, clinical features, genetics, patho-mechanisms, and current treatment options for GPP. I describe the results of clinical trials that led to the licensing of spesolimab for flares of GPP.
Expert opinion
The marked efficacy of spesolimab in GPP opens a new era of highly effective, scientifically-rational, and evidence-based treatment for this orphan disease, and has implications for other diseases in which interleukin 36 signaling is involved.
1. Introduction to GPP
Psoriasis is the name given to a diverse group of inflammatory diseases affecting the skin, encompassing plaque psoriasis (also called psoriasis vulgaris) which is common, and characterized by red scaly plaques, and pustular psoriasis which is rare and defined by the presence of sterile, subcorneal neutrophilic pustules (). The main types of pustular psoriasis are palmo-plantar pustulosis (PPP), a chronic disease in which the lesions are limited to the palms and soles, and generalized pustular psoriasis (GPP), sometimes known as von Zumbusch GPP, an acute severe systemic disease in which pustules affect the skin more generally. In the past, it was sometimes considered that plaque psoriasis, PPP, and GPP were variants of the same disease process, but recent genetic and immunological developments support the view that they are separate diseases [Citation1].
Figure 1. Typical appearance of generalized pustular psoriasis, with areas of edema, erythema, scaling and multiple pustules coalescing into lakes of pus.
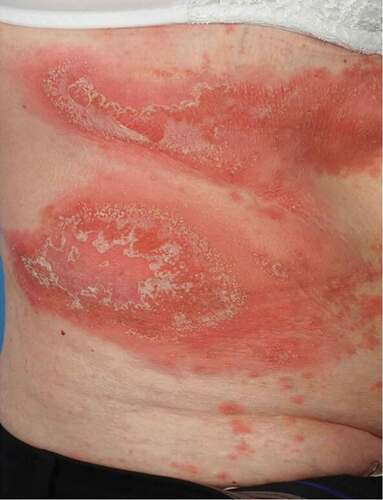
The annual prevalence of GPP is between 0.18 and 1.53 cases per 100,000, depending on the case definition applied, and with considerable regional variability [Citation2,Citation3]. The natural history is characterized by relapses and remissions, and it typically manifests with acute flares of widespread erythema, edema, and painful skin pustules that may coalesce in areas to form lakes of pus. The flares, which are often associated with systemic features such as fever, blood neutrophilia, and elevated inflammatory markers (e.g. CRP) are easily mistaken for systemic infection. Flares of GPP are sometimes spontaneous, but they can also be triggered, for instance by pregnancy, infection, psychological stress, or the withdrawal of corticosteroids [Citation4]. GPP flares frequently require hospitalization and sometimes blood pressure or ventilatory support [Citation4,Citation5], with an in-hospital mortality rate of 4.2% and 5.3% in recent large Japanese and Brazilian cohorts of GPP patients respectively [Citation6,Citation7]. GPP appears to be more common amongst patients who also have plaque psoriasis: 1% to 3% of the general adult population have plaque psoriasis [Citation8], whereas 43% to 54% of patients with GPP also have plaque psoriasis [Citation9,Citation10]. European consensus diagnostic criteria define GPP as the presence of primary, sterile, macroscopically-visible pustules not limited to the palms and soles, and excluding cases where pustules are restricted to psoriatic plaques (an entity designated ‘psoriasis with pustules’ and considered an inflammatory phase of plaque psoriasis) [Citation11]. GPP is sub-classified into either a relapsing or persistent pattern, and also based on the presence or absence of concomitant plaque psoriasis and of systemic inflammation [Citation11]. Japanese diagnostic criteria overlap in most respects, but consider systemic symptoms and histological confirmation to be primary parameters [Citation12].
A central role of the interleukin (IL)-36 inflammatory pathway in the pathogenesis of GPP was first recognized over a decade ago, based initially on genetic studies in familial cases of the disease [Citation13,Citation14]. IL-36 cytokines play a key role in epithelial innate immunity and are encoded on chromosome 2, along with other members of IL-1 superfamily of which they are a part. The IL-36 family comprises 3 pro-inflammatory agonists (IL-36α, β, and γ) and an antagonist (IL-36 receptor antagonist) at the IL-36 receptor (a heterodimer of IL-36 R and the IL-1 receptor accessory protein). Signaling through IL-36 R leads to activation of NFkB and mitogen activated protein kinases. IL-36 R ligands are over-expressed in lesional GPP skin, along with other pro-inflammatory cytokines [Citation15,Citation16]. In a significant minority of patients with GPP, this results from loss-of-function mutations in IL36RN (which encodes the IL-36 receptor antagonist), leading to a feed-forward loop of unopposed IL-36 signaling and a downstream inflammatory cascade that includes IL-17C, IL-17A/F, IL-23, TNFα, IL-1, type 1 interferon, and neutrophil chemokines (e.g. CXCL1, CXCL2, and CXCL8) [Citation1,Citation17,Citation18] (). On this basis, GPP is considered an auto-inflammatory disease. IL36RN mutations have been reported in about 24% of GPP patients, and are more prevalent in those with an earlier age at onset, and those with more severe disease [Citation9]. IL36RN mutations are not associated with plaque psoriasis [Citation19] and are seen less often in patients with GPP who also have plaque psoriasis [Citation20]. Mutations in other genes have been identified less frequently in GPP, notably in AP1S3 in 10.8% of cases [Citation9], and at the level of individual case reports in CARD14 [Citation21], SERPINA3 [Citation22] and myeloperoxidase [Citation23]. AP1S3 encodes a subunit of a protein involved autophagosome stability in keratinocytes, and its disruption causes upregulation of IL-1 signaling and overexpression of IL-36 α, further supporting the central role of IL-36 in GPP [Citation24].
Figure 2. Immuno-pathogenesis of generalized pustular psoriasis. Self-amplification of IL-36 and IL-17C in the absence of functional IL-36Ra leads to very high levels of CXCL8 (IL-8) and other CXCL chemokines that produce massive neutrophil influx. CXCL, C-X-C motif chemokine ligand; GPP, generalized pustular psoriasis; IL, interleukin; R, receptor. (adapted from [Citation1]).
![Figure 2. Immuno-pathogenesis of generalized pustular psoriasis. Self-amplification of IL-36 and IL-17C in the absence of functional IL-36Ra leads to very high levels of CXCL8 (IL-8) and other CXCL chemokines that produce massive neutrophil influx. CXCL, C-X-C motif chemokine ligand; GPP, generalized pustular psoriasis; IL, interleukin; R, receptor. (adapted from [Citation1]).](/cms/asset/e25c47d4-d24d-4bda-a8f5-156ddd6dc500/ierm_a_2195165_f0002_oc.jpg)
2. Overview of available drug options in GPP
Patients with a flare of GPP need urgent access to clinicians experienced in the diagnosis and management of acute inflammatory skin disease for a comprehensive assessment of the extent and severity of the disease, co-morbidities and complications. When extensive or associated with significant systemic involvement, treatment as a hospital inpatient is often needed for symptom control and supportive care, including emollients and often topical corticosteroids. Unless the flare is mild or of short duration, systemic treatment is also usually required. As the evidence base for the efficacy and safety of therapy in GPP is limited to case reports and small open-label studies, treatments that have a license for severe plaque psoriasis have often been used, including acitretin, methotrexate, ciclosporin and targeted biologic treatments [Citation25]. The use of the systemic retinoid acitretin (and the closely-related etretinate) has been reported in small retrospective case series in GPP and subsequently widely adopted globally [Citation7,Citation26]. Although not quickly or reliably effective, the fact that it is not immuno-suppressive, as most other available options are, is useful in a situation in which systemic infection can be difficult to exclude. Ciclosporin is generally more effective and quicker to act, making it a useful choice when the speed of onset is important, but it is not ideal for maintenance treatment because of the risk of cumulative toxicity.
Various of the biologics that are licensed in severe plaque psoriasis have been studied in small open label trials in GPP (). There is a long history of the use of infliximab for GPP flares and more recently other TNFα inhibitors, particularly adalimumab and certolizumab pegol. IL-17A inhibitors (secukinumab, ixekizumab) and IL-23 inhibitors (guselkumab, risankizumab, ustekinumab) have also been studied in open-label studies of up to 12 patients, which appear to show efficacy, and on this basis several biologics are licensed for use in GPP in Japan [Citation26]. The IL-17 receptor antagonist brodalumab is also licensed in GPP in Taiwan and Thailand based on improvement in symptoms in 10 of 12 patients with GPP in an open-label Japanese study [Citation31]. There are also individual case reports of GPP responding to IL-1 inhibition with anakinra [Citation38], canakinumab and gevokizumab [Citation25]. It is difficult to select between these treatments based on the published data, because of the small size of the studies, and variation in study design, for instance diagnostic criteria, severity scores applied, and the timing of endpoints.
Table 1. Evidence for efficacy of biologic treatments targeting TNF?, IL-17, IL-23 in generalized pustular psoriasis.
There are no international guidelines for the pharmacological treatment of GPP; guidelines published by the Japanese Dermatological Association in 2018 made no strong recommendations for treatment (level A), recommended infliximab or adalimumab (level B) and advised that other oral and biologic treatments could be considered but that the evidence was insufficient [Citation12]. There is retrospective evidence suggesting that in-hospital mortality is lower in patients treated with biologic therapies compared with conventional oral agents, and higher in those treated with corticosteroids alone [Citation6].
The unmet need for safe and effective treatment for treating GPP is substantial. Prior to the introduction of spesolimab, there were no specific treatments licensed for GPP in Europe or U.S.A. There is a scarcity of well-conducted clinical trials, with sufficient statistical power, use of placebo or comparator arms, and appropriate severity scores and response criteria. In a recent survey of 29 North American dermatologists experienced in treating GPP, 67% thought that treatment did not adequately prevent new flares, 72% thought treatment too slow to work, and 83% reported that patients had residual symptoms between flares, despite using the full range of systemic and biologic therapies [Citation39]. The majority of patients consider that their condition is not well controlled, and the emotional and physical impact of the disease is considerable [Citation40,Citation41]. The economic costs to individuals affected and to healthcare systems are high, and greater than for plaque psoriasis, with inpatient treatment a major cost driving factor [Citation10].
The central role of the IL-36 signaling pathway suggests that targeting IL-36 R may provide an effective treatment strategy for GPP. Moreover, rare individuals with deleterious mutations in IL-36 R have been identified, and have no recognizable phenotype, nor do they appear to be at risk of opportunistic infections (including varicella, candida or tuberculosis), suggesting that IL-36 R inhibition may be well tolerated [Citation42]. Two monoclonal antibodies directed against IL-36 R are in clinical development; spesolimab and imsidolimab. Results of an open label study of imsidolimab in 8 patients with GPP have been presented (NCT03619902), suggesting efficacy in this small study, and this drug is now in a phase 3 clinical trial (NCT05352893) [Citation43]. The subject of this review is spesolimab, which has recently received approval for the treatment of flares of GPP in U.S.A and Japan, and is recommended for approval in the EU.
3. Introduction to spesolimab
Spesolimab is a humanized IgG1 monoclonal antibody (molecular weight 146kDa) that binds specifically to IL-36 R with high affinity and inhibits signaling by IL-36 agonists [Citation44]. After a single intravenous infusion of 900 mg spesolimab, the Cmax (95%CI) in healthy volunteers and patients with GPP is modeled at 238 (218, 256) mcg/ml and the terminal half-life 25.5 (24.4, 26.3) days, in the absence of anti-drug antibodies (prescribing leaflet). Plasma levels are lower in subjects with higher body weight. Pharmacokinetics do not appear to be affected by age, gender or race and, as a monoclonal antibody, spesolimab is not expected to undergo hepatic or renal elimination.
4. Clinical efficacy of spesolimab
Data concerning spesolimab in GPP are derived from 2 trials: a small proof-of-concept open-label study [Citation45], and a subsequent larger pivotal phase II randomized, placebo-controlled trial [Citation46,Citation47].
4.1. Phase 1 trial of spesolimab in GPP
The first evidence for the efficacy and safety of targeting the IL-36 pathway in GPP came from an initial phase I proof-of-concept study which treated seven patients with GPP from five countries (France, Malaysia, Republic of Korea, Taiwan and Tunisia) in 2017 (NCT02978690) [Citation45]. Patients were between the ages of 22 and 58 years, and had a documented history of GPP, with evidence of prior systemic inflammation. Two patients carried homozygous loss-of-function IL36RN mutations and a further patient was heterozygous for a CARD14 mutation and also homozygous for an intronic IL36RN mutation. The activity of the disease was measured with a newly developed score, the Generalized Pustular Psoriasis Physician Global Assessment (GPPGA), which has subsequently been validated [Citation48]. The investigator scored the severity of 3 key cutaneous features of GPP (pustulation, erythema and scaling), each on a 5 point scale ranging from 0 (clear skin) to 4 (severe). A total GPPGA (from 0 to 4) was then derived from the mean of the 3 individual component values. Patients were eligible for enrollment if the baseline total GPPGA was 3 or more, and the GPPGA pustulation sub-score was 2 or more, with at least 10% body surface affected. Each patient was treated with a single open-label intravenous infusion of spesolimab 10 mg/Kg body weight, and then monitored for 20 weeks. Patients responded rapidly irrespective of their IL36RN mutation status: 5 of 7 patients achieved clear or almost clear skin (GPPGA 0 or 1) within 1 week of the spesolimab infusion, and all patients had achieved this response by week 4. Pustules completely cleared within 48 hours in three patients, and within 1 week in five patients. RNA sequencing of skin biopsies revealed down regulation within 1 week of signatures of IL-36 signaling (e.g. IL36A, IL36G), Th1 and Th17 signaling (e.g. IL12B, IL23A), innate immunity (e.g. TNFa and IL6) and neutrophil recruitment (e.g. CXCL8) [Citation16]. Markers of systemic inflammation also improved, with CRP and neutrophil counts returning to normal within 2 weeks [Citation45] and marked down regulation of serum biomarkers, including CXCL1, IL-8, and IL-17A [Citation16]. No severe or serious drug-related adverse events were seen [Citation45].
4.2. Phase II trial of spesolimab (Effisayil 1) in GPP
The results of the small open-label study informed the design of the first randomized, placebo-controlled trial to be reported in patients with GPP (NCT03782792) [Citation46]. Fifty-three patients from 52 centers in 12 countries were enrolled to this 12 week trial, with a 5 year open label extension for responders at week 12 (39 patients). GPP was diagnosed according to ERASPEN criteria irrespective of IL36RN mutation status [Citation11]. Inclusion criteria included age between 18 and 75 years, and an acute flare of GPP at the time of randomization (defined by a GPPGA score of 3 or more, GPPGA pustulation sub-score of 2 or more and at least 5% body surface area affected). Exclusion criteria included an immediately life-threatening flare of GPP, plaque psoriasis without pustules, or with pustules restricted to psoriatic plaques (‘psoriasis with pustules’). Conventional systemic immunosuppressants or biologics were discontinued, with an appropriate washout period for the biologics prior to randomization. Subjects were randomized 2 to 1 to receive a single intravenous infusion of spesolimab 900 mg or placebo on day 1. The primary endpoint (GPPGA pustulation subscore of 0 at week 1) was achieved by 19/35 (54%) randomized to spesolimab and 1/18 (6%) assigned placebo (P < 0.001); the key secondary endpoint (GPPGA score of 0 or 1 at week 1) was reached in 15/35 (43%) of the spesolimab arm and 2/18 (11%) in placebo (p = 0.02) [Citation47]. At the primary endpoint at week 1, subjects from either arm of the study could receive an open-label spesolimab 900 mg intravenous infusion without breaking the original blinding, if they had not responded sufficiently (GPPGA≥2 and GPPGA pustulation sub-score≥2). Twelve of 35 (34%) patients originally randomized to spesolimab received a second infusion, whereas this was required by 15 of the 18 (83%) patients who had been assigned placebo; hence there was no significant placebo arm beyond the first week of the trial. Of the 15 patients randomized to placebo who received an open-label infusion of spesolimab at week 1, 11 (73%) had achieved GPPGA pustulation subscore of 1 at week 2 (7 days after the infusion) and 8 (53%) had GPPGA total score of 0 or 1. One further dose of spesolimab was permitted for patients whose disease recurred having previously responded, and over the course of the trial 50 patients had at least one spesolimab infusion combining both treatment arms. Of the patients originally randomized to spesolimab, 21 patients (60%) had a GPPGA pustulation sub-score of 0 and GPPGA total score of 0 or 1 at week 12. Peripheral neutrophil counts returned to the normal range over the first week in the spesolimab arm in those in whom they had been elevated, and the CRP returned to normal in 2 weeks. Patient-reported outcomes (e.g. pain on visual analogue scale, Dermatology Life Quality Index) improved substantially over the first 1 to 4 weeks and remained low until the end of the trial.
4.3. Safety of spesolimab in GPP
During the first week of the Effisayil 1 trial, adverse events were seen in 66% of the spesolimab group and 56% in the placebo group with a higher rate of infection (17.1% vs 5.6%). Over the course of the trial, infections were reported in 47% of patients who had received at least one dose of spesolimab, although there were no patterns in terms of the nature of the organism or site affected.
Two cases of drug reaction with eosinophilia and systemic symptoms (DRESS) were reported in patients who had received spesolimab. DRESS is a severe cutaneous adverse drug reaction, with an onset usually delayed by 2 to 6 weeks after exposure to the culprit drug, a prolonged course, and is most frequently triggered by a limited range of drugs, especially allopurinol, anticonvulsants, and antibiotics [Citation49]. It is unexpected to see two cases of DRESS in this small trial, as it is a rare condition and is not usually triggered by biologic agents. Moreover, the diagnosis of DRESS is challenging, with diagnostic features that may overlap with other cutaneous diseases, for instance the fever, rash with pustules, edema and neutrophilia seen in GPP [Citation4,Citation49]. In the first of the reported cases, subsequent external expert review concluded that the features were not compatible with a diagnosis of DRESS according to standard criteria (RegiSCAR score 1) because of the time course (onset within 2 days of spesolimab exposure and resolution within 10 days), there were other potential triggers, and symptoms were compatible with a flare of GPP. External review of the second case arrived at a diagnosis of ‘possible DRESS’ (RegiSCAR score 3); however, the patient had received the antibiotic spiramycin prior to symptoms, and the reaction recurred in the same pattern some months later on re-challenge with spiramycin. In summary, there is doubt as to the link between spesolimab and DRESS although it is listed as a warning in the prescribing information.
Anti-drug antibodies were detected in 23 of 50 patients who had received at least one dose of spesolimab after a median of 2.3 weeks, and in 12 of these the maximum titer was greater than 4,000 and they were neutralizing. Titers below 4,000 did not appear to affect the pharmacokinetics of spesolimab. In some patients with titers above 4,000, plasma concentrations of spesolimab were lower but as yet there are few data concerning the clinical effect on safety or efficacy (https://www.ema.europa.eu/en/documents/rmp-summary/spevigo-epar-risk-management-plan_en.pdf).
5. Post-marketing surveillance
As spesolimab only received marketing authorization for GPP in late 2022, at the time of writing there are no post-marketing safety and tolerability data available. Spesolimab is also under investigation in several other inflammatory conditions in which IL-36 signaling may be important, including palmoplantar pustulosis, hidradenitis suppurativa, atopic dermatitis and ulcerative colitis. Safety data for intravenous spesolimab at various doses has been published from clinical trials in PPP and ulcerative colitis [Citation50,Citation51].
In a phase IIa placebo-controlled controlled trial in PPP (NCT03135548), 59 patients were randomized 1:1:1 to receive spesolimab 900 mg intravenous infusion, 300 mg intravenous infusion or placebo every four weeks over 16 weeks, with follow up for a further 16 weeks [Citation50]. In this population, the infusions were well tolerated, the adverse event profile was similar between active arms and placebo, with no relevant treatment-emergent safety signals and no increased risk of infection.
In a phase II randomized controlled trial in ulcerative colitis (NCT03482635) with a 12 week treatment period and further 12 week follow up, 98 patients were randomized 1:1:1:1 to a single intravenous infusion of spesolimab 300 mg, spesolimab 450 mg every 4 weeks, spesolimab 1,200 mg every 4 weeks, or placebo (NCT013482635). Approximately half of the patients continued to take systemic corticosteroids and 10% remained on azathoprine. A second phase IIa randomized placebo-controlled trial in ulcerative colitis (NCT03123120) studied spesolimab as add-on therapy to a TNF inhibitor (NCT03123120). Twenty-two patients were randomized 2:1 to receive intravenous infusions of spesolimab 1,200 mg at weeks 0, 4 and 8 or placebo over a 12 week treatment phase with a further 24 weeks of follow-up. These trials failed to meet their efficacy endpoint, but the results of safety analyses were combined with an additional 8 subjects from an open label study in ulcerative colitis of intravenous spesolimab 1,200 mg at weeks 0, 4 and 8 (NCT03100864), to derive a safety analysis set of 127 subjects (spesolimab 97, placebo 30) [Citation51]. In these 3 studies, the overall frequency of adverse events was similar between spesolimab and placebo treated patients, they were mostly of mild to moderate severity and no deaths were reported. The were no cases of DRESS, and no increase in serious or opportunistic infections, despite concomitant treatment with corticosteroids or TNF inhibitors, and the generally higher doses of spesolimab than in the trials in GPP.
6. Regulatory affairs
Spesolimab (brand name Spevigo ®) was approved by the Federal Drug Agency in the United States of America in September 2022, by the Pharmaceuticals and Medical Devices Agency in Japan for the treatment of GPP flares in adults (https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/761244s000lbl.pdf) and is marketed by Boehringer Ingelheim International GmbH. In October 2022 the Committee for Medicinal Products for Human Use adopted a positive opinion, granting conditional marketing authorization in the EU for spesolimab for the treatment of flares in adult patients with GPP as monotherapy (https://www.ema.europa.eu/en/medicines/human/summaries-opinion/spevigo). The approved dosage for GPP is a single spesolimab 900 mg intravenous infusion, which may be repeated if the flare symptoms persist one week after the first infusion.
7. Conclusion
GPP is a rare, orphan disease characterized by severe flares of cutaneous pustulation often with associated systemic inflammation. Although it sometimes occurs in patients who also have plaque psoriasis, it is distinct from plaque psoriasis in its presentation, histology, immunology and genetics. The discovery IL36RN mutations in a significant number of individuals affected by GPP (and not seen in plaque psoriasis) has opened up a new target for treatment of GPP, which has previously been limited to largely off-label use of therapies approved for plaque psoriasis. Early encouraging results were seen in an open label proof-of-concept study in seven individuals with GPP treated with a single intravenous infusion with spesolimab, all of whom responded rapidly, with a clearance of skin manifestations, and resolution of systemic inflammation (Bachelez 2019).
The Effisayil 1 trial of spesolimab against placebo was groundbreaking, in being the largest clinical trial to be carried out in GPP (53 subjects) and the only trial in GPP to date with a placebo arm or, indeed any comparator arm. It achieved the primary outcome of a clearance of pustules at day 8 after of a single infusion (54% spesolimab, 6% placebo), and secondary efficacy outcome measures, markers of systemic inflammation and patient reported outcomes similarly improved. The fact that all but 3 subjects in the placebo arm required open label spesolimab (12 out of 35 in active arm) is another reflection of the marked efficacy of spesolimab, but unfortunately means that there was effectively no placebo arm for the remaining 11 weeks of the trial. Responses to spesolimab were seen in patients with or without an IL36RN mutation. Overall, the tolerability of the treatment was acceptable given the severity of the disease, with a similar rate of adverse events in both arms of the trial in the placebo-controlled phase, and no adverse events leading to treatment discontinuation. There is a reassuring lack of safety signals in clinical trials of spesolimab in PPP and ulcerative colitis.
8. Expert opinion
IL-36 inhibition is an important new development in GPP and potentially other neutrophilic diseases, with spesolimab the first drug in this class to be approved. It represents a move away from treatments inherited from the field of plaque psoriasis, that inhibit TNFα, IL-17 and IL-23, toward a more appropriately targeted approach. It is difficult to compare outcomes achieved by spesolimab with these other biologics, because the evidence base for their efficacy is so limited in GPP. Significant challenges in clinical trial design in GPP arise from the rarity of the disease, it’s episodic nature, and the need for a short placebo-controlled phase for ethical reasons due to disease severity. In the Effisayil 1 placebo-controlled trial of spesolimab, the primary endpoint was set at day 8, which seems clinically appropriate, and contrasts with an endpoint at 12 to 16 weeks traditionally adopted in clinical trials in plaque psoriasis, and in the small open-label studies of biologics in GPP ().
There is also a need to agree and validate core outcome measures and suitable clinical trial endpoints for GPP that are uniformly adopted in clinical trials. GPPGA and its sub-scores utilized in Effisayil 1 proved to be effective outcome measures and have been validated, but only measure the cutaneous component of the disease. A head to head trial between spesolimab and one of the biologics approved for GPP in Japan would be informative, if the most effective treatment comparator can be agreed. Despite these caveats, based on the published data currently available, responses to spesolimab seem to be more complete than with other biologics, and the speed of onset appears to be more rapid (), which is important because of the severity of the disease. Spesolimab has now become the standard of care for treatment for GPP flares, given the scientific rationale and evidence for its efficacy, and that, in Europe and U.S.A at least, it is the only treatment licensed for this indication.
GPP is heterogeneous in both genotype and phenotype, with imperfect genotype-phenotype correlation. At present, the genetic basis of the disease is known in less than half of affected patients. Attempts to further the understanding of the genetic architecture of the disease, for instance by the International Rare And Severe Psoriasis Expert Network (IRASPEN https://www.dermregister.com/iraspen.html) may explain variations in the natural history of the disease and allow for more effective treatment selection. For instance, it is interesting that both IL36RN mutated and non-mutated subjects responded to spesolimab in the Effisayil 1 trial, but it might be expected that the speed and duration of response may differ when a sufficiently large number of GPP patients have been treated.
More needs to be known about how best to use spesolimab in clinical practice. It is clear that one or two intravenous infusions is a highly effective treatment for the rapid control of flares of the disease. In view of the development of anti-drug antibodies in a significant number of subjects in spesolimab trials, the effect that these may have on the pharmacokinetics of the drug and clinical efficacy on re-treatment needs to be established. Patients who have had a flare of GPP are understandably fearful of recurrence, and treatment strategies to prevent future flares would also be very helpful. In this respect, the results of a recently completed trial of subcutaneous spesolimab in flare prevention (NCT04399837) will be important, and could lead to a label extension of spesolimab as an ongoing maintenance treatment to prevent flares [Citation52].
At this early stage in the introduction of spesolimab, which at the time of writing has only recently been licensed for GPP flares, there are inevitably many gaps in the evidence which hopefully will be filled as clinical experience develops, and as data from open label extensions of clinical trials and from treatment registries become available. The most acute need is for safety data in larger cohorts and in a real world population. While data from safety studies in PPP and ulcerative colitis are reassuring, the infections seen more frequently in the treatment arm of the trial of spesolimab in GPP raise a concern. This is particularly the case because it can be difficult to exclude systemic infection in individuals when they present with a flare of GPP, with widespread pustulation, fever and often very high inflammatory markers.
As IL-36 signaling may contribute to inflammation in a variety of epithelial tissues and disease states, IL-36 R blockade has potential beyond pustular psoriasis, including in inflammatory bowel disease, chronic obstructive pulmonary disease, rheumatoid arthritis, hidradenitis suppurativa, and spesolimab is in clinical trials in several of these indications [Citation44,Citation53]. However, its role in treating plaque psoriasis is perhaps most relevant. Whilst there is a wide range of very effective treatments available for the treatment of plaque psoriasis (including TNF inhibitors, IL-17 inhibitors and IL-23 inhibitors), a scientific case can be made for the potential efficacy of IL-36 R blockade also [Citation42]. As a significant proportion of patients with GPP also have plaque psoriasis, it is important to establish whether spesolimab will treat both diseases in these individuals, whether treatment will need to be added to spesolimab, or whether a TNFα inhibitor, IL-17 inhibitor or IL-23 inhibitor will be used in preference to spesolimab in this situation.
Article highlights
GPP is a rare severe auto-inflammatory disease clinically and genetically distinct from common plaque psoriasis
overactivity of IL-36 signaling is fundamental to the immuno-pathogenesis of GPP, sometimes due to inactivating germline mutations in the anti-inflammatory receptor antagonist encoded by IL36RN
Inhibition of IL-36 signaling with the monoclonal antibody against the IL-36 receptor spesolimab is rapidly effective in controlling flares of GPP and is well tolerated, leading it to be the first treatment to be licensed for this indication in U.S.A, Europe, and Japan in 2022.
Declaration of interest
AD Burden has undertaken paid consultancy work for research, lecturing or advisory boards for Abbvie, Almirall, Boehringer Ingelheim, Bristol Meyer Squibbs, Celgene, Janssen, Leo Pharma, Novartis, and UCB. The author has no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
One peer reviewer declares: research for Amgen, Argenx, Anaptysbio, Bristol-Myer-Squibb, Pfizer, and Regeneron. One peer reviewer declares: employee of Janssen and have Janssen/JnJ stock. Peer reviewers on this manuscript have no other relevant financial relationships or otherwise to disclose.
Boehringer Ingelheim provided a scientific accuracy review at the request of the journal editor.
Additional information
Funding
References
- Bachelez H, Barker J, Burden AD, et al. Generalized pustular psoriasis is a disease distinct from psoriasis vulgaris: evidence and expert opinion. Exp Rev Clin Immunol. 2022;18:1033–1047.
- Löfvendahl S, Norlin JM, Schmitt-Egenolf M. Prevalence and incidence of generalized pustular psoriasis in Sweden – a population-based register study. Br J Dermatol. 2022;186:970–976.
- Zheng M, Jullien D, Eyerich K. The prevalence and disease characteristics of generalized pustular psoriasis. Am J Clin Dermatol. 2022;23:5–12.
- Choon SE, Lai NM, Mohammad NA, et al. Clinical profile, morbidity and outcome of adult-onset generalized pustular psoriasis: analysis of 102 cases seen in a tertiary hospital in Johor, Malaysia. Int J Dermal. 2014;53:676–684.
- Hanna ML, Singer D, Bender SD, et al. Characteristics of hospitalizations and emergency department visits due to generalized pustular psoriasis in the United States. Curr Med Res Opin. 2021;37:1697–1703.
- Miyachi H, Konishi T, Kumazawa R, et al. Treatments and outcomes of generalized pustular psoriasis: a cohort of 1516 patients in a nationwide inpatient database in Japan. J Am Acad Dermatol. 2022;86:1266–1274.
- Vieira Duarte G, de Carvalho Av E, Romiti R, et al. Generalized pustular psoriasis in Brazil: a public claims database study. J Am Acad Dermatol Int. 2022;6:61–67.
- Parisi R, Iskandar IYK, Kontopantelis E, et al. Global psoriasis Atlas. National, regional, and worldwide epidemiology of psoriasis: systematic analysis and modelling study. BMJ. 2020;369:m1590.
- Twelves S, Mostafa A, Dand N, et al. Clinical and genetic differences between pustular psoriasis subtypes. J Allergy Clin Immunol. 2019;143:1021–1026.
- Löfvendahl S, Norlin JM, Schmitt-Egenolf M. Economic burden of generalized pustular psoriasis in sweden: a population-based register study. Psoriasis (Auckl). 2022;12:89–98.
- Navarini AA, Burden AD, Capon F, et al. European consensus statement on phenotypes of pustular Psoriasis. J Eur Acad Dermatol Venereol. 2017;31:1792–1799. .
- Fujita H, Terui T, Hayama K, et al. Japanese guidelines for the management and treatment of generalized pustular psoriasis: the new pathogenesis and treatment of GPP. J Dermatol. 2018;45(11):1235–1270. DOI:10.1111/1346-8138.14523. .
- Marrakchi S, Gigue P, Renshaw BR, et al. Interleukin-36-receptor antagonist deficiency and generalized pustular psoriasis. N Engl J Med. 2011;365:620–628. .
- Onoufriades A, Simpson MA, Pink AE, et al. Mutations in IL36RN/IL1F5 are associated with the severe episodic inflammatory skin disease known as Generalized Pustular Psoriasis. Am J Hum Genet. 2011;89:432–437.
- Johnston A, Xing X, Wolterink L, et al. IL-1 and IL-36 are dominant cytokines in generalized pustular psoriasis. J Allergy Clin Immunol. 2017;140:109–120.
- Baum P, Visvanathan S, Garcet S, et al. Pustular psoriasis: molecular pathways and effects of spesolimab in generalized pustular psoriasis. J Allergy Clin Immunol. 2022;149:1402–1412.
- Catapano M, Vergnano M, Romano M, et al. Interleukin-36 promotes systemic Type-I IFN responses in severe forms of psoriasis. J Invest Dermatol. 2020;140:816–826.
- Marrakchi S, Puig L. Pathophysiology of generalized pustular psoriasis. Am J Clin Dermatol. 2022;23:13–19.
- Berki D, Mahil SK, Burden AD, et al. Loss of IL36RN function does not confer susceptibility to psoriasis vulgaris. J Invest Dermatol. 2014;134(1):271–273. DOI:10.1038/jid.2013.285
- Sugiura K, Takemoto A, Yamaguchi M, et al. The majority of generalized pustular psoriasis without psoriasis vulgaris is caused by deficiency of interleukin-36 receptor antagonist. J Invest Dermatol. 2013;133:2514–2521.
- Berki DM, Liu L, Choon S-E, et al. Activating CARD14 mutations are associated with generalized pustular psoriasis but rarely account for familial recurrence in psoriasis vulgaris.J Invest Dermatol 2015;135:p. 2964–2970
- Frey S, Sticht H, Wilsmann-Theis D, et al. Rare loss-of-function mutation in SERPINA3 in generalized pustular psoriasis. J Invest Dermatol. 2020;140:1451–1455.
- Vergnano M, Mochenhaupt M, Benzian-Olsson N, et al. Loss-of-function myeloperoxidase mutations are associated with increased neutrophil counts and pustular skin disease. Am J Hum Genet. 2021;108:757.
- Mahil SK, Twelves S, Farkas K, et al. AP1S3 mutations cause skin autoinflammation by disrupting keratinocyte autophagy and up-regulating IL-36 production. J Invest Dermatol. 2016;136:2251–2259.
- Krueger J, Puig L, Thaçi D. Treatment options and goals for patients with generalized pustular psoriasis. Am J Clin Dermatol. 2022;23:51–54.
- Komine M, Morita A. Generalized pustular psoriasis: current management status and unmet medical needs in Japan. Exp Rev Clin Immunol. 2021;17:1015–1027.
- Morita A, Yamazaki F, Matsuyama T, et al. Adalimumab treatment in Japanese patients with generalized pustular psoriasis: results of an open-label phase 3 study. J Dermatol. 2018;45:1371–1380.
- Okubo Y, Umezawa Y, Sakurai S, et al. Efficacy and safety of certolizumab pegol in Japanese patients with generalized pustular psoriasis and erythrodermic psoriasis: 52 week results. Dermatol Ther. 2022;12:1397–1415.
- Esposito M, Mazzotta A, Casciello C, et al. Etanercept at different doses in the treatment of generalized pustular psoriasis: a case series. Dermatol. 2008;216:355–360.
- Viguier M, Aubin F, Delaporte E, et al. Efficacy and safety of tumor necrosis factor inhibitors in acute generalized pustular psoriasis. Arch Dermatol. 2012;148:1423–1425.
- Yamasaki K, Nakagawa H, Kubo Y, et al. Efficacy and safety of brodalumab in patients with generalized pustular psoriasis and psoriatic erythroderma: results from a 52-week, open label study. Br J Dermatol. 2017;176:741–751.
- Saeki H, Nakagawa H, Ishii T, et al. Efficacy and safety of open-label ixekizumab treatment in Japanese patients with moderate-to-severe plaque psoriasis, erythrodermic psoriasis and generalized pustular psoriasis. J Eur Acad Dermatol Venereol. 2015;29:1148–1155.
- Saeki H, Nakagawa H, Nakajo K, et al. Efficacy and safety of ixekizumab treatment for Japanese patients with moderate to severe plaque psoriasis, erythrodermic psoriasis and generalized pustular psoriasis: results from a 52 week, open label, phase 3 study (UNCOVER-J). J Dermatol. 2017;44:355–362.
- Imafuka S, Homma M, Okubo Y, et al. Efficacy and safety of secukinumab in patients with generalized pustular psoriasis: a 52-week analysis from phase III open-label multicenter Japanese study. J Dermatol. 2016;43:1011–1017.
- Sano S, Kubo H, Morishima H, et al. Guselkumab, a human interleukin-23 monoclonal antibody in Japanese patients with generalized pustular psoriasis and erythrodermic psoriasis: efficacy and safety analyses of a 52-week, phase 3 multicenter, open-label study. J Dermatol. 2018;45:529–539.
- Yamanaka K, Okubo Y, Yasuda I, et al. Efficacy and safety of risankizumab in Japanese patients with generalized pustular psoriasis or erythrodermic psoriasis: primary analysis and 180-week follow-up results from the phase 3, multicenter IMMspire study. J Dermatol. 2023;50:195–202.
- Arakawa A, Ruzicka T, Prinz JC. Therapeutic efficacy of interleukin 12/interleukin 23 blockade in generalized pustular psoriasis regardless of IL36RN mutation status. JAMA Dermatol. 2016;152:825–828.
- Naik HB, Pichard DC, Schwartz DM, et al. Anakinra for refractory pustular psoriasis: a phase II, open-label, dose-escalation trial. J Am Acad Dermatol. 2022;87:1380–1383.
- Strober B, Kotowsky N, Medeiros R, et al. Unmet medical needs in the treatment and management of generalized pustular psoriasis flares: evidence from a survey of Corrona registry dermatologists. Dermatol Ther. 2021;11:529–541.
- Lebwohl M, Medeiros RA, Mackey RH, et al. The disease burden of generalized pustular psoriasis: real-world evidence from CorEvitas. Psoriasis Registry J Psoriasis Psoriatic Arthritis. 2022;7:71–78.
- Reisner DV, Johnsson FD, Kotowsky N, et al. Impact of generalized pustular psoriasis from the perspective of people living with the condition: results of an online survey. Am J Clin Dermatol. 2022;23:65.
- Mahil SK, Catapano M, Di Meglio P, et al. An analysis of IL-36 signature genes and individuals with IL1RL2 knockout mutations validates IL-36 as a psoriasis therapeutic target. Sci Transl Med. 2017;9:eaan2514.
- Gudjonsson JE, Randazzo B, Zhou J. Imsidolimab in the treatment of adult subjects with generalized pustular psoriasis: design of a pivotal phase 3 clinical trial and a long-term extension study. J Am Acad Dermatol. 2022;87:AB70.
- Ganesan R, Raymond EL, Mennerich D, et al. Generation and functional characterization of anti-human and anti-mouse IL36R antagonist monoclonal antibodies. MAbs. 2017;9:1143–1154.
- Bachelez H, Choon SE, Marrakchi S, et al. Inhibition of the interleukin-36 pathway for the treatment of generalized pustular psoriasis. N Engl J Med. 2019;380:981–983. .
- Choon SE, Lebwohl MG, Marrakchi S, et al. Study protocol of the global Effisayil 1 phase II, multicentre, randomized, double-blind, placebo-controlled trial of spesolimab in patients with generalized pustular psoriasis presenting with an acute flare. BMJ Open. 2021;11:e043666.
- Bachelez H, Choon SE, Marrakchi S, et al. Trial of spesolimab for generalized pustular psoriasis. N Engl J Med. 2021;385:2431–2440. .
- Burden AD, Bissonnette R, Lebwohl MG, et al. Psychometric validation of the Generalized Pustular Psoriasis Physician Global Assessment (GPPGA) and Generalized Pustular Psoriasis Area and Severity Index (GPPASI). J Eur Acad Derm Venereol. 2023 (in press). DOI:10.1111/jdv.18999.
- Kardaun SH, Sekula P, Valeyrie-Allanore L, et al. Drug reaction with eosinophilia and systemic symptoms (DRESS): an original multisystem adverse drug reaction. Results from the prospective RegiSCAR study. Br J Dermatol. 2013;169:1071–1080.
- Mrowietz U, Burden AD, Pinter A, et al. Spesolimab, an anti-interleukin-36 receptor antibody, in patients with palmoplantar pustulosis: results of a phase IIa, multicenter, double-blind, randomized, placebo-controlled pilot study. Dermatol Ther. 2021;11:571–585.
- Ferrante M, Irving PM, Selinger CP, et al. Safety and tolerability of spesolimab in patients with ulcerative colitis. Exp Opin Drug Safety. 2022;31:1–12.
- Morita A, Barker J, Choon SE, et al. Design of effisayil 2, a randomized, double-blind, placebo-controlled study of spesolimab in preventing flares in patients with generalized pustular psoriasis. Dermatol Ther. 2023;13:347–359.
- Sugiura K. Role of interleukin 36 in generalized pustular psoriasis and beyond. Dermatol Ther. 2021;12:315–328.
