ABSTRACT
Introduction
Patients with common variable immunodeficiency (CVID) have a high frequency of inflammatory complications like autoimmune cytopenias, interstitial lung disease and enteropathy. These patients have poor prognosis and effective, timely, and safe treatment of inflammatory complications in CVID is essential, but guidelines and consensus on therapy are often lacking.
Areas covered
This review will focus on current medical treatment of inflammatory complications in CVID and point out some future perspectives based on literature indexed in PubMed. There are a number of good observational studies and case reports on treatment of specific complications, but randomized controlled trials are scarce.
Expert opinion
In clinical practice, the most urgent issues that need to be addressed are the preferred treatment of GLILD, enteropathy and liver disease. Treating the underlying immune dysregulation and immune exhaustion in CVID is an alternative approach that potentially could alleviate these and other organ-specific inflammatory complications. Therapies of potential interest and wider use in CVID include mTOR-inhibitors like sirolimus, JAK-inhibitors like tofacitinib, the monoclonal IL-12/23 antibody ustekinumab, the anti-BAFF antibody belimumab and abatacept. For all inflammatory complications, there is a need for prospective therapeutic trials, preferably randomized controlled trials, and multi-center collaborations with larger cohorts of patients will be essential.
1. Introduction
Common variable immunodeficiency disorder (CVID) is the most common symptomatic inborn error of immunity (IEI) among adults with an estimated prevalence of 1:25000 [Citation1]. Patients are clinically characterized by increased susceptibility to infections particularly in the airways, but importantly 20–70% of patients also have autoimmune or inflammatory complications like immune thrombocytopenia, interstitial lung disease or enteropathy [Citation2–6]. There are different immunological definitions of CVID, but all include low levels of IgG, IgA (or IgM), poor response to immunization, and the exclusion of other apparent causes of hypogammaglobulinemia, including combined immunodeficiencies and malignancies [Citation7,Citation8]. Genetically, a monogenic cause of CVID is found only in 10–20% of patients whereas many patients with a previous CVID diagnosis have been reclassified after finding a specific genetic variation, most notably CTLA4 haploinsufficiency [Citation9].
The clinical presentation of autoimmune and inflammatory complications in CVID is well described elsewhere and will only briefly be touched upon here [Citation2–6]. This review will focus on current medical treatment of these inflammatory complications and point out some future perspectives.
CVID patients with inflammatory complications have a poor prognosis and effective, timely, and safe treatment of these complications is essential [Citation10–12]. Treating immunodeficient patients with immunosuppressants for autoimmune and inflammatory complications carries a risk of inflicting secondary infections, but so do inflammatory organ damage. Indeed, the treatment of the immune dysregulation could through reduction of inflammation lead to less and not more infections. The identification of relevant biomarkers for the different conditions is central to evaluating this treatment ().
Table 1. Biomarkers associated with specific inflammatory complications and/or complications phenotype in CVID.
CVID patients with end-stage lung or liver disease are candidates for organ transplant. Reported results are mixed, but more modern immunosuppressive regimes may have a favorable effect on survival [Citation13–18]. Likewise, there are mixed results of hematopoietic stem cell transplantation (HSCT) which would be the ultimate treatment of the underlying immune dysregulation in CVID, but an option in selected cases only [Citation19,Citation20].
The summary and review of a rare disorder like CVID rely to a considerable degree on observational studies and case reports as larger randomized studies are mostly lacking. The clinical evidence for specific treatment of the individual patient is therefore not optimal and further compromised by the variable nature of CVID. As we do not know the immunopathogenic mechanism behind most CVID cases, there could be a rationale for trying out the more specific therapies used in monogenic CVID-like disease.
2. Targeting common features of inflammatory complications
Despite the variable presentation and immunopathogenic mechanisms in CVID, there are some frequent features of the autoimmune and inflammatory complications. Patients can be characterized by activation of B cells and T cells, and granulomas and lymphoproliferation or a combination thereof may appear in almost any organ [Citation2,Citation21–26]. Targeting these features can therefore be particularly relevant for patients with multi-organ disease.
2.1. B-cell activation
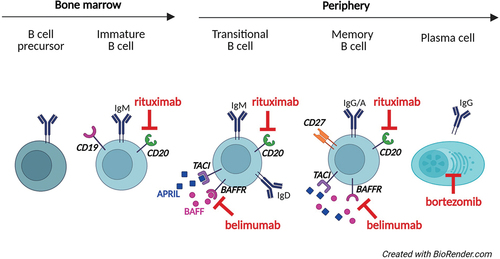
CVID is fundamentally caused by a maturation defect in B cells leading to low levels of immunoglobulins, but paradoxically many patients have activated B cells contributing to inflammatory complications. Patients can have increased frequency of transitional and CD21low B cells that are pro-inflammatory B-cell subsets with an increased capacity for antigen presentation [Citation27–30]. B cells can therefore be a target for therapy in suspected B-cell-driven disease (). There are several drugs that target cells of the B-cell lineage, first and foremost the anti-CD20 antibody rituximab, which is widely used, but anti-BAFF antibody in the form of belimumab could be an alternative or even a supplement to rituximab [Citation31–33]. Bortezomib is a proteasome inhibitor that primarily targets plasma cells and is used in hematological malignancy like multiple myeloma, but might also play a role in groups of patients with CVID [Citation34].
2.2. T-cell activation
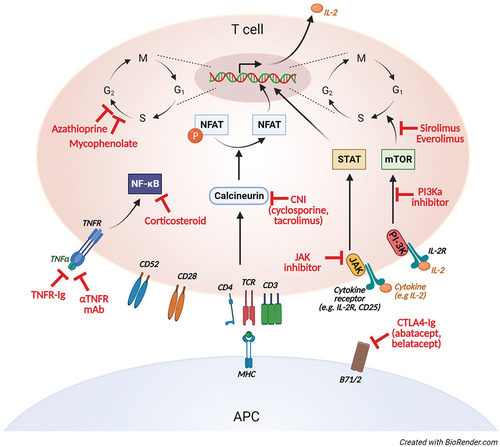
Several inflammatory complications in CVID are characterized by activation and infiltration of T cells in afflicted tissue. T cells in CVID have been targeted with immunosuppressants widely used also in immunocompetent patients, including calcineurin inhibitors like cyclosporine and tacrolimus, anti-metabolites like azathioprine and mycophenolate, corticosteroids, and also the mTOR inhibitor sirolimus () [Citation33,Citation35–37]. There seems to be no preferred choice of agent. Corticosteroids are often the first-line treatment, perhaps more due to its broad anti-inflammatory profile and flexible administration rather than the effect on T cells. Corticosteroids inhibit synthesis and secretion of inflammatory cytokines like IL-1, IL-2, IL-6, IL-8, and TNF involved in activation of a range of immune cells, and more specifically T-cell lymphopenia partially through induction of apoptosis. The T-cell costimulation modulator abatacept mimics the effect of CTLA4 and could be effective also in patients with genetically normal expression of CTLA4 [Citation38]. Moreover, targeting antigen-presenting cells like B cells and macrophages will also attenuate T-cell activation.
2.3. Granulomas
Granulomas are frequent in CVID and while the formation remains an enigma, the main clinical question is often whether to treat them or not. There is a clear case for treating symptomatic granulomas but often the granulomas are incidental findings with uncertain clinical consequences. Choosing to treat, a number of therapeutics have been reported effective in CVID, as summarized in a systematic review by van Stigt and colleagues [Citation25]. Rituximab, anti-TNF but also the combination of corticosteroids and immunoglobulins seem to have the highest rate of remission and are reported frequently [Citation21,Citation33,Citation39–42]. Importantly, these epithelioid granulomas must not be confused with the nodular hyperplasia seen in many patients with CVID-associated liver disease.
2.4. Lymphoproliferative infiltrates
Lymphoproliferative infiltrates are also frequent findings raising the question of whether to treat or not, with the ominous threat being the development of lymphoma, even if most patients do not. Lymphomas are reported in 4–8% of patients in studies from different centers, while some form of lymphoid hyperplasia is found in approximately 20% [Citation12,Citation43–48]. It is however, not known whether treating benign lymphoid infiltrates will prevent a transformation to malignant disease. Infiltrates may be symptomatic with mass-effect, perhaps most critically in infiltration of the CNS [Citation49,Citation50]. Corticosteroids are often reported as the treatment of choice for lymphoproliferative infiltrates, but there are also reports on the use of rituximab [Citation50].
3. Treatment of specific inflammatory complications
3.1. Immune driven cytopenias
Immune-driven cytopenias like autoimmune hemolytic anemia (AIH) and immune thrombocytopenic purpura (ITP) are common in CVID [Citation2,Citation10,Citation51]. The immunopathogenic mechanisms likely involve autoantibodies, but also autoreactive T cells and macrophages in the spleen [Citation42,Citation52]. Corticosteroids have been first-line treatment and intravenous immunoglobulins (IVIG) adjunctive treatment for both immunocompetent and immunodeficient patients for many years and several regimens exist () () [Citation53–56]. Splenectomy has been an important alternative for treatment of immune cytopenias in CVID when refractory to corticosteroids and/or IVIG, and while the frequency of splenectomies in general is decreasing, it remains an option. The procedure seems reasonably safe with surgical complications around 6–7% and effective in about 80% of cases [Citation57].
Figure 3. Established (bold), reported and potential (italics) treatment for autoimmune and inflammatory complications of CVID. Scientific evidence for established treatment is variable, with no randomized controlled trials performed.
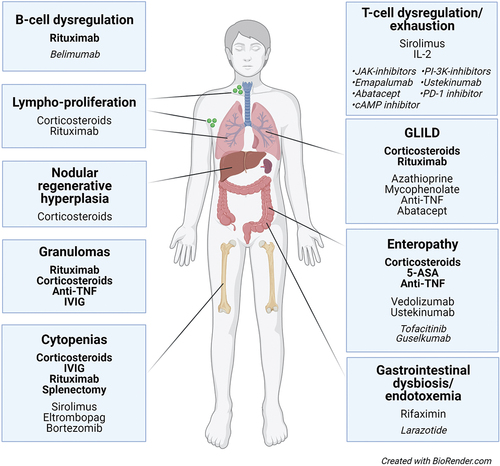
Table 2. First-line treatment of autoimmune cytopenias in CVID.
The main reason for the now limited use of splenectomy is the success of rituximab in treating immune-driven cytopenias both in immunocompetent and immunodeficient patients. In a combined French-American study of CVID patients with ITP or autoimmune hemolytic anemia by Gobert and colleagues, there was an initial response in 85% of patients and a sustained response in 60% of patients [Citation31]. Rituximab has several potential side effects, including opportunistic infections and hypogammaglobulinemia [Citation58,Citation59]. Theoretically, rituximab can impair any preserved immunoglobulin production in CVID-patients leading to increased need of immunoglobulin substitution, but there are no clinical reports of this potential side effect. Severe infections have been observed in CVID patients with autoimmune cytopenias treated with rituximab, but it is unclear whether this occurs more frequently than in other patients.Ig substitution therapy should however, be optimized [Citation31].
There are also other medical alternatives as shown by several case reports. Autoreactive T cells may play a pathogenic role in ITP and are therefore potential therapeutic targets. In a patient with CVID, lymphadenopathy and splenomegaly who developed thrombocytopenia, Deenick and colleagues found an expansion of CD21low B cells, but also increased activation of the mTOR signaling pathway in CD4+ T cells [Citation36]. The patient was subsequently treated with the mTOR inhibitor sirolimus (aka rapamycin) with clear effect on thrombocyte levels and generalized lymphadenopathy.
Thrombopoietin-receptor agonists have a clear place in treatment of chronic ITP in patients with no known IEI, as impaired megakaryopoiesis seems to be an important pathogenic mechanism [Citation60]. There is one case-report on the successful use of eltrombopag in a CVID patient who had poor response to prednisone and IVIG [Citation61]. The use of thrombopoietin-receptor agonists is associated with elevated liver function tests and myelofibrosis, but perhaps most importantly there is a substantial risk of relapse after discontinuation.
Bortezomib is a potent proteasome-inhibitor predominantly targeting plasma cells [Citation62]. The main role of bortezomib is in treating multiple myeloma, but there are several reports on experimental treatment of autoantibody-mediated disease [Citation63,Citation64]. In a patient with CVID and Evans syndrome with poor response to corticosteroids, splenectomy, mycophenolate mofetil, and rituximab, the administration of bortezomib had sustained effect on both hemoglobin and thrombocyte levels [Citation34].
After the stabilization of a CVID patient with immune cytopenia, there is a risk of relapse regardless previous therapy, and some patients seem particularly vulnerable with repeated relapses despite effective initial treatment. These patients will usually be on immunoglobulin treatment and as intravenous, in contrast to subcutaneous, immunoglobulins are part of the initial treatment, regular treatment with IVIG could theoretically reduce the risk of relapse. However, in a study by Scheuerlein and colleagues on adult CVID patients with autoimmune thrombocytopenia, there was no apparent difference in risk of relapse between patients on IVIG and subcutaneous treatment [Citation65]. The IgG through levels were, however, significantly lower in patients who had ITP.
3.2. Enteropathy
Gastrointestinal symptoms are frequent in CVID, but cannot all be related to a histologically verified enteropathy, and conversely not all patients with histologic enteropathy have gastrointestinal symptoms [Citation66]. There is no consensus on the indication for treatment of CVID enteropathy, but symptomatic patients with histologically verified enteropathy should be considered for immunosuppressive treatment after the exclusion of any infectious cause to enteropathy. Notably, Norovirus has been frequently found in some CVID cohorts but its role as a true pathogen has been debated [Citation66,Citation67].
Patients can present with lymphoid inflammatory infiltrates of predominantly T cells sometimes resembling graft versus host disease (GVHD) and/or granulomas both in the upper and lower gastrointestinal tract [Citation66–68]. In contrast, inflammatory bowel disease (IBD)-like lesions are rarely seen, but there is a lack of plasma cells mirroring the overall B-cell maturation defect in CVID. Patients can have villous atrophy resembling celiac disease, but transcriptional features in biopsies in CVID are distinctly different from celiac disease, and there is currently no rationale for the use of a gluten free diet in CVID [Citation66,Citation69].
There are several case reports and case series on the treatment of enteropathy in CVID, but no larger specific observational studies or clinical trials. An IBD-approach is therefore often applied starting with corticosteroids either in the form of budesonide 3–9 mg/day or low dose prednisone<10 mg/day combined with 5-aminosalicylates, and adding on anti-TNF in the form of infliximab 5 mg/kg/q 6 weeks or adalimumab 40 mg/q 2 weeks (maintenance doses) when needed, in particular with the presence of granulomas [Citation25,Citation70–72]. The integrin antibody vedolizumab inhibits T-cell migration to the gut and is part of the regular regimen for IBD, primarily in steroid-refractory disease. Vedolizumab has also shown effect in graft-versus-host-disease (GVHD) and there are several case reports on the successful use of vedolizumab in CVID enteropathy [Citation73,Citation74]. The monoclonal antibody ustekinumab targeting IL-12 and IL-23 is also part of the first-line therapy for IBD and there is one case report of a CVID-patient with Crohn-like features who was successfully treated with ustekinumab (90 mg/q 8 weeks maintenance dose) after failing to improve on corticosteroids and anti-TNF treatment [Citation75]. Guselkumab is a novel inhibitor of IL-23 that potentially also could be of use in CVID. The JAK-inhibitor tofacitinib (10 mg/day maintenance dose) is approved for treatment of ulcerative colitis and could be an alternative that so far has not been reported in CVID enteropathy [Citation76].
3.3. Liver disease
Liver disease is reported to affect 10–80% of CVID patients, but notably the definition of liver disease and selection of patients vary in different reports [Citation2,Citation10,Citation77–81]. Histologically, CVID associated liver disease is typically present in the form of nodular regenerative hyperplasia (NRH), but epithelioid granulomas and features resembling autoimmune hepatitis are also seen [Citation2,Citation10,Citation77–81]. These presentations may be found together and sometimes progress to end-stage liver disease where liver transplantation is the only therapeutic option [Citation15–17,Citation82]. NRH is a porto-sinusoidal vascular disorder associated with a range of chronic diseases including rheumatoid arthritis, HIV-infection and is also seen in a post-liver transplant setting [Citation83,Citation84]. In CVID, NRH is associated with an intrasinusoidal inflammatory infiltrate of CD8+ T cells and in a case report, Sousa eSilva and colleagues treated a patient with CVID and NRH with budesonide 9 mg/day with improvement of liver enzyme alterations [Citation81,Citation85]. There is no established indication nor therapy for NRH in CVID, but based on histopathological findings, treatment affecting T cells like corticosteroids could be effective. Notably, few B cells are found infiltrating the liver in NRH in CVID, even if there is an association to raised levels of circulating CD21low B cells in patients with inflammatory complications in general [Citation29,Citation86].
Corticosteroids have also been reported used in CVID patients with autoimmune hepatitis like disease, but with varying results [Citation78]. Granulomatous lesions can be seen also in the liver in CVID, and corticosteroids and anti-TNF treatment have indeed been found effective in single cases [Citation40,Citation87].
Liver disease is a severe complication of CVID with poor prognosis, and there is an urgent need for systematically exploring the effect of budesonide or more potent immune modulators in patients with inflammatory histological features.
3.4. Interstitial lung disease
Interstitial lung disease, often referred to as granulomatous lymphocytic interstitial lung disease (GLILD), is another severe inflammatory complication of CVID, but in contrast to liver disease there has lately been a more active approach to treatment [Citation88]. Infections can cause a similar radiographic appearance, and lymphoma is an important differential diagnosis that needs to be carefully considered before starting treatment [Citation89]. In a position paper from the UK, the indication for treatment was the presence of radiologic features and falling pulmonary function tests with or without symptoms in the form of dyspnea and cough [Citation89]. Corticosteroids seem to be the preferred first line treatment at many centers with doses ranging 0.5–1.0 mg/kg prednisolone, but with tapering of the dose there is a clear risk of relapse [Citation89]. In a retrospective study of patients receiving high-dose corticosteroids (>0.3 mg/kg/day), Smits and colleagues found significant improvement of lung function and CT scans with a sustained remission in 42% of patients [Citation90]. Rituximab (typically 1000–2000 mg q 6 months) either alone or in combination with azathioprine (1–2 mg/kg/day), mycophenolate (500–2000 mg/day) or anti-TNF has shown to be effective in several retrospective and observational studies [Citation24,Citation33,Citation41,Citation91]. Again, the presence of pulmonary granulomas would suggest a favorable effect of anti-TNF, but this has not been systematically evaluated. Immunosuppressive treatment of GLILD has a clear effect on pathologic CT-scores while improvement in pulmonary function tests are more modest and any symptomatic change remains unreported [Citation33]. There are also case reports with favorable effect of anti-metabolites, anti-TNF and abatacept alone or in combination with corticosteroids [Citation38,Citation92,Citation93]. There are no clear consensus or guidelines regarding the length of treatment and data on the clinical development after discontinuation of treatment is lacking.
In a systematic review by Lamers and colleagues published in April 2021 which included 42 articles and 233 patients, they found widely varied responses to treatment and concluded that well-controlled evidence was lacking [Citation94]. They called for standardized data collection and uniform treatment regimens to better compare studies and effect.
4. Modulating immune dysregulation and systemic inflammation
Patients with CVID can present with frequent infections and a number of organ-specific inflammatory complications that become the natural focus of clinical attention. The underlying systemic inflammation and immune dysregulation in CVID are more elusive, but might provide an important perspective on treatment of the inflammatory complications in CVID. This systemic perspective implies weight on systemic biomarkers for therapeutic considerations and evaluation, and not only organ function and pathology. GLILD has for instance been described as the pulmonary manifestation of a systemic lymphoproliferative disease, and we have previously shown how treating GLILD-patient with rituximab not only led to the resolution of pulmonary changes, but also diminished lymph node hyperplasia [Citation83]. Targeting immune dysregulation could potentially also restore some of the immunodeficiency in these patients.
4.1. B -cell dysregulation
CVID patients have a maturation defect in their B cells and at the same time many patients have an expansion of the inflammatory and autoreactive CD21low B cells [Citation95,Citation96]. Interestingly, CVID patients have elevated levels of the B-cell activating factor BAFF, first shown by Cunningham-Rundles group in 2007 and again in a study first authored by Paul Maglione [Citation24,Citation97]. In this latter study, there were particularly high levels in patients with progressive GLILD. Mutation of the TACI gene was also associated to high levels of BAFF possibly through a loss of binding of BAFF to the TACI receptor. High levels of BAFF irrespective of cause might contribute to the expansion of the CD21low B cells through the non-canonical NF kappa B pathway as suggested also by Maglione’s group [Citation98]. BAFF is central in other immune-driven diseases for much the same reason as in CVID, and can be blocked by the monoclonal antibody belimumab. Belimumab has, in contrast to rituximab, shown effect in treatment of systemic lupus erythematosus (SLE), and could modulate B-cell dysregulation in CVID patients with GLILD, TACI-mutation or any other cause of high levels of BAFF [Citation99]. Importantly, there are significant functional differences between belimumab and rituximab and a potential for synergistic effects if combined [Citation100]. There are so far no reports on the use of belimumab in CVID.
4.2. T-cell dysregulation
The T cells are also dysregulated in CVID, typically with an inverted CD4/CD8 ratio, a high proportion of effector-memory cells and a Th1 dominance, in particular in patients with an inflammatory phenotype [Citation23,Citation86,Citation101–103]. Unger and colleagues showed a skewed Th1 phenotype in both peripheral blood and lymph nodes [Citation23]. Interestingly, there was an inverse relation to not only Th2 but also Th-17-cells. Th1-driven inflammation is of relevance for several immune driven diseases, like multiple sclerosis and diabetes mellitus type 1, and can be modulated intra- or extracellularly [Citation104,Citation105]. Tbet is the defining Th1 transcription factor but cannot so far be targeted directly [Citation106]. However, there is an increasing number of JAK-inhibitors, some targeting specific JAKs, which can modulate different STATs, including STAT1 and STAT4 that induces Tbet [Citation107,Citation108]. JAK-inhibitors like tofacitinib (JAK1/2/3) and ruxolitinib (JAK1/2) have been reported effective in other inborn errors of immunity, notably in STAT1 and STAT3 gain-of-function mutations, but there are no reports of use in CVID [Citation109–111].
The PI-3-kinase – mTOR pathway is upregulated in Th1-cells and treatment with everolimus/sirolimus or a PI-3K inhibitor like idelalisib or leniolisib might reduce the burden of the Th1-driven inflammation [Citation112]. As previously mentioned, sirolimus has been reported effective in ITP and granulomatous disease in CVID, and for inflammatory complications of LRBA- and CTLA4-insufficiency [Citation113–118]. Interferon-gamma is the signal cytokine of Th1-inflammation and can be targeted by the monoclonal antibody emapalumab [Citation119]. Emapalumab is approved for treatment of HLH, but has been associated with a high risk of infections in that patient group. Interleukin-12 is another Th1-associated cytokine and can be targeted with ustekinumab that already is widely used for immune driven diseases, and that has a more favorable safety profile [Citation120,Citation121].
We and others have shown that CVID-patients are characterized by not only dysregulation of Th1 cells, but also by low levels of regulatory T cells (TReg) [Citation122–124]. This is a phenomenon known from several inflammatory diseases, and can as such be related to the inflammatory process per se as well as an innate feature of CVID. Expansion of the TReg compartment has been suggested as an alternative strategy for treatment of autoimmune diseases in general with both ex and in vivo methods applied [Citation125,Citation126]. The most promising approach seems to be the use of sirolimus or low dose IL-2 that also will be discussed later.
4.3. T-cell exhaustion and anergy
T cells in CVID are not only activated, they are also exhausted, as indicated in a study from our group where patients with GLILD had high levels of soluble CD25, IFN-gamma and TNF, but also of the T-cell exhaustion marker soluble TIM3 [Citation127]. Exhausted T cells have low proliferative capacity, a consistent feature of CVID that has been known for decades, and with some studies presenting therapeutic measures to enhance the proliferative potential of T cells. Our group showed in the late 90ties that the low proliferation of T cells in CVID could be partially reversed by inhibitors of cyclic AMP in vitro [Citation128]. Interleukin-2 (IL-2) is a strong inducer of T-cell growth and proliferation. Cunningham-Rundles and her colleagues tried giving CVID-patients low dose interleukin-2 and showed not only that T cells from patients improved their proliferative capacity, but that there also was an increase in immunoglobulin levels [Citation129].
The stimulation of exhausted or suppressed T cells is central to the effect of checkpoint inhibitors in cancer treatment, and could present an alternative strategy for immune reconstitution in IEI, with potential side effects as important concerns. Perreau and colleagues have shown that functional impairment of CD4+ T cells from CVID patients is restricted to the bacteria-specific T cells [Citation130]. These bacteria-specific T cells expressed high levels of programmed cell death protein 1 (PD1), and by blocking the PD-1 axis in vitro the proliferative response improved. The initiation of IVIG-therapy also improved T-cell proliferation and at the same time reduced the expression of PD-1.
Interestingly, the high expression of PD1 was associated with endotoxemia. The authors speculate that bacterial translocation from the gut could be the source of this endotoxemia and ultimately driving the functional impairment of T cells. This opens up for a very different approach to treatment of immune dysregulation in CVID, focusing on the composition and functional properties of gut microbiota in CVID.
4.4. Gastrointestinal dysbiosis as a cause of immune dysregulation and inflammation
Gut microbiota has earned an important place in biomedical research with associations to disease beyond the gastrointestinal tract, and others and we have shown a potential role for gut microbiota also in the pathogenesis of CVID [Citation131–135]. In our cohort, CVID patients with inflammatory complications had a gastrointestinal dysbiosis compared to CVID patients with infection only and healthy controls, and interestingly, this dysbiosis was related not only to endotoxemia but also to high levels of the inflammatory T-cell marker soluble CD25. Modulating the disturbed CVID microbiota could therefore be a way to modulate also the systemic inflammation in these patients. In a randomized controlled study with rifaximin, we did show a change in the composition of the gut microbiota in the treated patients, but not in the levels of the inflammatory markers [Citation136]. There were important limitations to the study, but the concept might still be relevant.
An important factor in gut microbiotas` relevance for CVID might be the loss of mucosal integrity. Downregulation of tight junctions renders a leaky gut with increased potential for endotoxemia, and fortifying the mucosal integrity can therefore be a potential approach. The synthetic amino acid peptide larazotide has been reported as a regulator of intestinal tight junction and has shown promising results in small studies in celiac disease and IBD, but there are so far no reports in CVID [Citation137,Citation138].
5. Conclusion
There are a number of good observational studies and case reports on treatment of inflammatory complications in CVID, but randomized controlled trials are scarce. Immune cytopenias can be treated with corticosteroids with IVIG as potential supplement, but rituximab has shown good overall response and is well tolerated. There are few systematic studies on treatment of enteropathy but an IBD-approach with corticosteroids, 5-aminosalicylates and anti-TNF is often used, and there are several case reports on the effect of vedolizumab. Liver disease can present with NRH, granulomas and/or hepatitis and there are reports of effect of corticosteroids and anti-TNF. Corticosteroids is considered first-line treatment of interstitial lung disease, but there is observational support for effect of rituximab either alone or in combination with anti-metabolites. T cells are central in inflammatory complications of CVID and there are reports on the successful use of the mTOR-inhibitor sirolimus in treatment of immune cytopenia and granulomatous disease. There is a wide range of novel immunomodulatory drugs with potential benefit in CVID that should be systematically evaluated in prospective trials.
6. Expert opinion
Patients with CVID have a high frequency of inflammatory complications like autoimmune cytopenias, interstitial lung disease, and enteropathy. These patients have poor prognosis and effective, timely, and safe treatment of inflammatory complications in CVID is essential, but guidelines and consensus on therapy are often lacking. In clinical practice, the most urgent issues that need to be addressed are the preferred treatment of:
−GLILD
−Enteropathy
−Liver disease
Activation of B cells and T cells, granulomas and lymphoproliferative infiltrates are frequent features of inflammatory complications, and targeting these features can therefore be particularly relevant for patients with multi-organ disease. Rituximab is routinely used in the treatment of immune cytopenias and GLILD, but the anti-BAFF antibody belimumab could be an alternative or supplement to rituximab in B-cell driven disease () [Citation24,Citation31–33]. There are numerous reports on immunosuppressants with effect on T cells in CVID. The mTOR-inhibitor sirolimus is particularly promising as it modulates the Th1-response seen in CVID, and also is reported to stimulate and expand the TReg population () [Citation36,Citation37,Citation125,Citation126]. The monoclonal antibody ustekinumab targets the Th1-associated cytokine IL-12 (and the Th17-associated cytokine IL-23), is approved for the treatment of ulcerative colitis and has been reported effective in CVID enteropathy () [Citation75]. The JAK-inhibitor tofacitinib (JAK1/2/3) also targets T cells, is used in treatment of inflammatory diseases like ulcerative colitis and reported safe and effective in patients with STAT1 or STAT3 gain-of-function disease [Citation110]. Tofacitinib could therefore be of particular interest in treatment of CVID enteropathy. Abatacept mimics the effect of CTLA4, have found an important role in the treatment of CTLA4-haploinsufficiency, but could be of interest also in CVID-patients with normal CTLA4-expression, perhaps particularly in treatment of lymphoproliferative infiltrates [Citation38].
Treating the underlying immune dysregulation and immune exhaustion in CVID could potentially alleviate the organ-specific inflammatory complications while also restoring some of the immunodeficiency. There is no clear strategy for doing this, but finding a way to safely stimulate the immune system is one option, modulating the microbiota could be another. Low-dose IL-2 have been shown to improve both T-cell proliferation and immunoglobulin levels in CVID, while reported also to expand the TReg compartment [Citation126,Citation129]. Check-point inhibitors can be effective stimulators of exhausted T cells, and blocking PD-1 on T cells from CVID patients in vitro has improved proliferation [Citation130]. There will, however, be important concerns about side effects of check-point inhibitors used in vivo. Functional impairment of T cells is associated with endotoxemia, and bacterial translocation from the gut could be driving T-cell activation and exhaustion. CVID-patients are characterized by microbial dysbiosis of the gut that can be modulated with antibiotics like rifaximin, and loss of mucosal integrity can be targeted with regulators of intestinal tight-junctions [Citation131,Citation136–138].
For all inflammatory complications of CVID, there is a need for prospective therapeutic trials, preferably randomized controlled trials, to find what is really effective and safe. At the same time, we have to appreciate the variable nature of CVID and the need for a personalized approach. This is a conflict not easily resolved, but multi-center collaborations with larger cohorts of patients will be essential. The eGLILD-net project sponsored by the European Respiratory Society in association with the European Society for Immunodeficiencies is a good example of a strategic initiative that can contribute to these collaborations [Citation139]. More such initiatives could bring the treatment of inflammatory complications of CVID forward.
Article highlights
Patients with CVID have a high frequency of inflammatory complications and guidelines and consensus on treatment are lacking
There is an urgent need for better treatment of interstitial lung disease, enteropathy, and liver disease
Belimumab, sirolimus, JAK-inhibitors, and ustekinumab are drugs of particular interest in treatment of inflammatory complications in CVID
Multi-center prospective therapeutic trials will be essential in future clinical research
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Acknowledgments
The author wants to thank Dr Pål Aukrust and Dr Ingvild Nordøy for valuable advice on the manuscript. Sincere thanks also to senior researcher Kari Otterdal for preparing the illustrations.
Additional information
Funding
References
- Cunningham-Rundles C. Common variable immune deficiency: dissection of the variable. Immunol Rev. 2019 Jan;287(1):145–161.
- Ho HE, Cunningham-Rundles C. Non-infectious complications of common variable immunodeficiency: updated clinical spectrum, sequelae, and insights to pathogenesis. Front Immunol. 2020;11:149.
- Fischer A, Provot J, Jais JP, et al. Autoimmune and inflammatory manifestations occur frequently in patients with primary immunodeficiencies. J Allergy Clin Immunol. 2017 Nov;140(5):1388–1393.e8.
- Gathmann B, Mahlaoui N, Gérard L, et al. Clinical picture and treatment of 2212 patients with common variable immunodeficiency. J Allergy Clin Immunol. 2014 Jul;134(1):116–126.
- Cabañero-Navalon MD, Garcia-Bustos V, Nuñez-Beltran M, et al. Current clinical spectrum of common variable immunodeficiency in Spain: the multicentric nationwide GTEM-SEMI-CVID registry. Front Immunol. 2022;13:1033666.
- Westh L, Mogensen TH, Dalgaard LS, et al. Identification and characterization of a nationwide danish adult common variable immunodeficiency cohort. Scand J Immunol. 2017 Jun;85(6):450–461.
- Bonilla FA, Barlan I, Chapel H, et al. International Consensus Document (ICON): common variable immunodeficiency disorders. J Allergy Clin Immunol Pract. 2016 Jan;4(1):38–59.
- Seidel MG, Kindle G, Gathmann B, et al. The European Society for Immunodeficiencies (ESID) Registry Working Definitions for the Clinical Diagnosis of Inborn Errors of Immunity. J Allergy Clin Immunol Pract. 2019 Jul;7(6):1763–1770.
- de Valles-Ibáñez G, Esteve-Solé A, Piquer M, et al. Evaluating the genetics of common variable immunodeficiency: monogenetic model and beyond. Front Immunol. 2018;9:636.
- Chapel H, Lucas M, Lee M, et al. Common variable immunodeficiency disorders: division into distinct clinical phenotypes. Blood. 2008;112(2): 277–286.
- Bates CA, Ellison MC, Lynch DA, et al. Granulomatous-lymphocytic lung disease shortens survival in common variable immunodeficiency. J Allergy Clin Immunol. 2004 Aug;114(2):415–421.
- Resnick ES, Moshier EL, Godbold JH, et al. Morbidity and mortality in common variable immune deficiency over 4 decades. Blood. 2012 Feb 16;119(7):1650–1657. DOI:10.1182/blood-2011-09-377945
- Hill AT, Thompson RA, Wallwork J, et al. Heart lung transplantation in a patient with end stage lung disease due to common variable immunodeficiency. Thorax. 1998 Jul;53(7):622–623.
- Burton CM, Milman N, Andersen CB, et al. Common variable immune deficiency and lung transplantation. Scand J Infect Dis. 2007;39(4):362–367.
- Jørgensen SF, Macpherson ME, Bjøro K, et al. Liver transplantation in patients with primary antibody deficiency. J Allergy Clin Immunol. 2017 May;139(5):1708–1710.e2.
- Azzu V, Elias JE, Duckworth A, et al. Liver transplantation in adults with liver disease due to common variable immunodeficiency leads to early recurrent disease and poor outcome. Liver Transpl. 2018 Feb;24(2):171–181.
- Ramlaul N, Ooi J, Jeffrey GP, et al. Liver transplantation in adults with liver disease due to common variable immunodeficiency leads to early recurrent disease and poor outcome. Liver Transpl. 2018 Nov;24(11):1622–1626.
- Andersen IM, Reims HM, Grzyb K, et al. Long-term survival after liver transplantation in patients with common variable immunodeficiency. Liver Transpl. 2023 Mar 20. Online ahead of print.
- Wehr C, Gennery AR, Lindemans C, et al. Multicenter experience in hematopoietic stem cell transplantation for serious complications of common variable immunodeficiency. J Allergy Clin Immunol. 2015 Apr;135(4):988–997.e6.
- Albert MH, Sirait T, Eikema DJ, et al. Hematopoietic stem cell transplantation for adolescents and adults with inborn errors of immunity: an EBMT IEWP study. Blood. 2022 Oct 6;140(14):1635–1649.
- Boursiquot JN, Gérard L, Malphettes M, et al. Granulomatous disease in CVID: retrospective analysis of clinical characteristics and treatment efficacy in a cohort of 59 patients. J Clin Immunol. 2013 Jan;33(1):84–95.
- Maglione PJ, Ko HM, Beasley MB, et al. Tertiary lymphoid neogenesis is a component of pulmonary lymphoid hyperplasia in patients with common variable immunodeficiency [Original]. J Allergy Clin Immunol. 2014 Feb;133(2):535–542.
- Unger S, Seidl M, van Schouwenburg P, et al. The T(H)1 phenotype of follicular helper T cells indicates an IFN-γ-associated immune dysregulation in patients with CD21low common variable immunodeficiency. J Allergy Clin Immunol. 2018 Feb;141(2):730–740.
- Maglione PJ, Gyimesi G, Cols M, et al. BAFF-driven B cell hyperplasia underlies lung disease in common variable immunodeficiency. JCI Insight. 2019 Mar 7;4(5). DOI:10.1172/jci.insight.122728
- van Stigt AC, Dik WA, Kamphuis LSJ, et al. What works when treating granulomatous disease in genetically undefined CVID? A systematic review. Front Immunol. 2020;11:606389.
- Berbers RM, van der Wal MM, van Montfrans JM, et al. Chronically activated t-cells retain their inflammatory properties in common variable immunodeficiency. J Clin Immunol. 2021 Oct;41(7):1621–1632.
- Warnatz K, Denz A, Drager R, et al. Severe deficiency of switched memory B cells (Cd27(+)igm(-)IgD(-)) in subgroups of patients with common variable immunodeficiency: a new approach to classify a heterogeneous disease. Blood. 2002 Mar 1;99(5):1544–1551.
- Berglund LJ, Wong SW, Fulcher DA. B-cell maturation defects in common variable immunodeficiency and association with clinical features. Pathology. 2008 Apr;40(3):288–294.
- Wehr C, Kivioja T, Schmitt C, et al. The EUROclass trial: defining subgroups in common variable immunodeficiency. Blood. 2008 Jan 1;111(1):77–85. DOI:10.1182/blood-2007-06-091744
- Reincke ME, Payne KJ, Harder I, et al. The antigen presenting potential of CD21(low) B Cells. Front Immunol. 2020;11:535784.
- Gobert D, Bussel JB, Cunningham-Rundles C, et al. Efficacy and safety of rituximab in common variable immunodeficiency-associated immune cytopenias: a retrospective multicentre study on 33 patients. Br J Haematol. 2011 Nov;155(4):498–508.
- Ng J, Wright K, Alvarez M, et al. Rituximab monotherapy for common variable immune deficiency-associated granulomatous-lymphocytic interstitial lung disease [Case reports]. Chest. 2019 May;155(5):e117–121.
- Verbsky JW, Hintermeyer MK, Simpson PM, et al. Rituximab and antimetabolite treatment of granulomatous and lymphocytic interstitial lung disease in common variable immunodeficiency. J Allergy Clin Immunol. 2021 Feb;147(2):704–712.e17.
- Knight T, Ravindranath Y, Callaghan MU. Successful treatment of an adolescent male with severe refractory evans syndrome using bortezomib-based therapy. J Pediatr Hematol Oncol. 2020 Mar;42(2):e110–113.
- Sigmon L, Greene K, Hansen JJ. IV cyclosporine to treat refractory CVID enteropathy. Scand J Gastroenterol. 2012 Nov;47(11):1396–1397.
- Deenick EK, Morey A, Danta M, et al. Reversible suppression of lymphoproliferation and thrombocytopenia with rapamycin in a patient with common variable immunodeficiency. J Clin Immunol. 2018 Feb;38(2):159–162.
- Deyà-Martínez A, Esteve-Solé A, Vélez-Tirado N, et al. Sirolimus as an alternative treatment in patients with granulomatous-lymphocytic lung disease and humoral immunodeficiency with impaired regulatory T cells. Pediatr Allergy Immunol. 2018 Jun;29(4):425–432.
- von Spee-Mayer C, Echternach C, Agarwal P, et al. Abatacept use is associated with steroid dose reduction and improvement in fatigue and CD4-Dysregulation in CVID Patients with Interstitial Lung Disease. J Allergy Clin Immunol Pract. 2021 Feb;9(2):760–770.e10.
- Bonnet F, Morlat P, Viallard JF, et al. Pulmonary granuloma, polyarthritis and antiphospholipids in common variable immunodeficiency: resolution after IVIG and the role of immunoglobulin a. Clin Exp Rheumatol. 2005 May;23(3):428–429.
- Thatayatikom A, Thatayatikom S, White AJ. Infliximab treatment for severe granulomatous disease in common variable immunodeficiency: a case report and review of the literature. Annals of allergy, asthma & immunology: official publication of the American College of Allergy. Asthma, & Immunology. 2005 Sep;95(3):293–300.
- Chase NM, Verbsky JW, Hintermeyer MK, et al. Use of combination chemotherapy for treatment of granulomatous and lymphocytic interstitial lung disease (GLILD) in patients with common variable immunodeficiency (CVID). J Clin Immunol. 2013 Jan;33(1):30–39.
- Franxman TJ, Howe LE, Baker JR Jr. Infliximab for treatment of granulomatous disease in patients with common variable immunodeficiency. J Clin Immunol. 2014 Oct;34(7):820–827.
- Kralickova P, Milota T, Litzman J, et al. CVID-Associated tumors: czech nationwide study focused on epidemiology, immunology, and genetic background in a cohort of patients with CVID. Front Immunol. 2018;9:3135.
- Mayor PC, Eng KH, Singel KL, et al. Cancer in primary immunodeficiency diseases: cancer incidence in the United States Immune Deficiency Network Registry. J Allergy Clin Immunol. 2018 Mar;141(3):1028–1035.
- von Spee-Mayer C, Koemm V, Wehr C, et al. Evaluating laboratory criteria for combined immunodeficiency in adult patients diagnosed with common variable immunodeficiency. Clin Immunol. 2019 Jun;203:59–62.
- Yakaboski E, Fuleihan RL, Sullivan KE, et al. Lymphoproliferative Disease in CVID: a Report of Types and Frequencies from a US Patient Registry. J Clin Immunol. 2020 Apr;40(3):524–530.
- Smith T, Cunningham-Rundles C. Lymphoid malignancy in common variable immunodeficiency in a single-center cohort. Eur J Haematol. 2021 Nov;107(5):503–516.
- Oksenhendler E, Gérard L, Fieschi C, et al. Infections in 252 patients with common variable immunodeficiency. Clin Infect Dis. 2008 May 15;46(10):1547–1554.
- Nguyen JT, Green A, Wilson MR, et al. Neurologic complications of common variable immunodeficiency. J Clin Immunol. 2016 Nov;36(8):793–800.
- van de Ven A, Mader I, Wolff D, et al. Structural noninfectious manifestations of the central nervous system in common variable immunodeficiency disorders. J Allergy Clin Immunol Pract. 2020 Mar;8(3):1047–1062.e6.
- Mormile I, Punziano A, Riolo CA, et al. Common variable immunodeficiency and autoimmune diseases: a retrospective study of 95 adult patients in a single tertiary care center. Front Immunol. 2021;12:652487.
- Chaturvedi S, Arnold DM, McCrae KR. Splenectomy for immune thrombocytopenia: down but not out. Blood. 2018 Mar 15;131(11):1172–1182.
- Cunningham-Rundles C. Common variable immune deficiency: case studies. Blood. 2019 Nov 21;134(21):1787–1795.
- Neunert C, Terrell DR, Arnold DM, et al. American Society of Hematology 2019 guidelines for immune thrombocytopenia. Blood Adv. 2019 Dec 10;3(23):3829–3866.
- Jäger U, Barcellini W, Broome CM, et al. Diagnosis and treatment of autoimmune hemolytic anemia in adults: recommendations from the first international consensus meeting. Blood Rev. 2020 May;41:100648.
- Chawla S, Barman P, Tyagi R, et al. Autoimmune cytopenias in common variable immunodeficiency are a diagnostic and therapeutic conundrum: an update. Front Immunol. 2022;13:869466.
- Wong GK, Goldacker S, Winterhalter C, et al. Outcomes of splenectomy in patients with common variable immunodeficiency (CVID): a survey of 45 patients. Clin Exp Immunol. 2013 Apr;172(1):63–72.
- van Vollenhoven RF, Emery P, Bingham CO 3rd, et al. Longterm safety of patients receiving rituximab in rheumatoid arthritis clinical trials. J Rheumatol. 2010 Mar;37(3):558–567.
- Barmettler S, Ong MS, Farmer JR, et al. Association of immunoglobulin levels, infectious risk, and mortality with rituximab and hypogammaglobulinemia. JAMA Netw Open. 2018 Nov 2;1(7):e184169.
- Ghanima W, Cooper N, Rodeghiero F, et al. Thrombopoietin receptor agonists: ten years later. Haematologica. 2019 Jun;104(6):1112–1123.
- Carrabba M, Barcellini W, Fabio G. Use of thrombopoietin-receptor agonist in CVID-Associated immune thrombocytopenia. J Clin Immunol. 2016 Jul;36(5):434–436.
- Citrin R, Foster JB, Teachey DT. The role of proteasome inhibition in the treatment of malignant and non-malignant hematologic disorders. Expert Rev Hematol. 2016 Sep;9(9):873–889.
- Turnbull MT, Siegel JL, Becker TL, et al. Early bortezomib therapy for refractory Anti-NMDA receptor encephalitis. Front Neurol. 2020;11:188.
- Schwarz L, Akbari N, Prüss H, et al. Clinical characteristics, treatments, outcome, and prognostic factors of severe autoimmune encephalitis in the intensive care unit: standard treatment and the value of additional plasma cell-depleting escalation therapies for treatment-refractory patients. Eur J Neurol. 2023; 30(2): 474–489.
- Scheuerlein P, Pietsch L, Camacho-Ordonez N, et al. Is it safe to switch from intravenous immunoglobulin to subcutaneous immunoglobulin in patients with common variable immunodeficiency and autoimmune thrombocytopenia? Front Immunol. 2018;9:1656.
- Jørgensen SF, Reims HM, Frydenlund D, et al. A cross-sectional study of the prevalence of gastrointestinal symptoms and pathology in patients with common variable immunodeficiency. Am J Gastroenterol. 2016 Oct;111(10):1467–1475.
- Strohmeier V, Andrieux G, Unger S, et al. Interferon-driven immune dysregulation in common variable immunodeficiency-associated villous atrophy and norovirus infection. J Clin Immunol. 2023;43(2): 371–390 .
- van Schewick CM, Lowe DM, Burns SO, et al. Bowel histology of CVID patients reveals distinct patterns of mucosal inflammation. J Clin Immunol. 2022 Jan;42(1):46–59.
- Kaarbø M, Yang M, Hov JR, et al. Duodenal inflammation in Common variable immunodeficiency has altered transcriptional response to viruses. J Allergy Clin Immunol 2023;151(3):767–777 .
- Chua I, Standish R, Lear S, et al. Anti-tumour necrosis factor-alpha therapy for severe enteropathy in patients with common variable immunodeficiency (CVID). Clin Exp Immunol. 2007 Nov;150(2):306–311.
- Cunningham-Rundles C. How I treat common variable immune deficiency. Blood. 2010 Jul 8;116(1):7–15.
- Prieto Elordui J, Arreba Gonzalez P, Ortiz de Zarate Sagastagoitia J, et al. Infliximab as treatment of severe enteropathy in a patient with common variable immunodeficiency and cytomegalovirus infection. Gastroenterol Hepatol. 2018 Mar;41(3):163–164.
- Sifers T, Hirten R, Mehandru S, et al. Vedolizumab therapy in common variable immune deficiency associated enteropathy: a case series. Clin Immunol. 2020 Mar;212:108362.
- Johnson D, Lee G, Weber F. Vedolizumab therapy in refractory enteropathy associated with CVID. ACG Case Rep J. 2022 Jan;9(1):e00721.
- Ruiz de Morales JG, Muñoz F, Hernando M. Successful treatment of common variable immunodeficiency-associated inflammatory bowel disease with ustekinumab. J Crohns Colitis. 2017 Sep 1;11(9):1154–1155.
- Straatmijer T, van Schaik FDM, Bodelier AGL, et al. Effectiveness and safety of tofacitinib for ulcerative colitis: two-year results of the ICC Registry. Aliment Pharmacol Ther. 2023;57(1): 117–126 .
- Malamut G, Ziol M, Suarez F, et al. Nodular regenerative hyperplasia: the main liver disease in patients with primary hypogammaglobulinemia and hepatic abnormalities. J Hepatol. 2008 Jan;48(1):74–82.
- Fuss IJ, Friend J, Yang Z, et al. Nodular regenerative hyperplasia in common variable immunodeficiency. J Clin Immunol. 2013 May;33(4):748–758.
- Azzu V, Fonseca M, Duckworth A, et al. Liver disease is common in patients with common variable immunodeficiency and predicts mortality in the presence of cirrhosis or portal hypertension. J Allergy Clin Immunol Pract. 2019 Sep;7(7):2484–2486.e3.
- Crescenzi L, Pecoraro A, Fiorentino A, et al. Liver stiffness assessment by transient elastography suggests high prevalence of liver involvement in common variable immunodeficiency. Dig Liver Dis. 2019 Nov;51(11):1599–1603.
- Crotty R, Taylor MS, Farmer JR, et al. Spectrum of hepatic manifestations of common variable immunodeficiency. Am J Surg Pathol. 2020 May;44(5):617–625.
- Apostolov R, Sinclair M, Lokan J, et al. Successful liver transplantation in common variable immune deficiency with reversal of hepatopulmonary syndrome. BMJ Case Rep. 2019 Apr 3;12(4):e226095.
- De Gottardi A, Sempoux C, Berzigotti A. Porto-sinusoidal vascular disorder. J Hepatol. 2022 Oct;77(4):1124–1135.
- Kounis I, Sebagh M, Evain M, et al. Nodular regenerative hyperplasia is not a rare condition after liver transplantation: incidence, predictive factors, and impact on survival. Transplantation. 2023;107(2): 410–419 .
- Sousa eSilva R, Pereira da Silva S, Luís R, et al. Nodular regenerative hyperplasia in CVID patients: could low-dose oral glucocorticoids be part of the solution? Eur Ann Allergy Clin Immunol. 2022 Mar 18. Online ahead of print.
- Mouillot G, Carmagnat M, Gérard L, et al. B-cell and T-cell phenotypes in CVID patients correlate with the clinical phenotype of the disease. J Clin Immunol. 2010 Sep;30(5):746–755.
- Fernández-Ruiz M, Guerra-Vales JM, Francisco-Javier CF, et al. Fever of unknown origin in a patient with common variable immunodeficiency associated with multisystemic granulomatous disease. Intern Med. 2007;46(15):1197–1202.
- Bethune C, Egner W, Garcez T, et al. British Society for Immunology/United Kingdom Primary Immunodeficiency Network consensus statement on managing non-infectious complications of common variable immunodeficiency disorders. Clin Exp Immunol. 2019 Jun;196(3):328–335.
- Hurst JR, Verma N, Lowe D, et al. British Lung Foundation/United Kingdom Primary Immunodeficiency Network Consensus Statement on the Definition, Diagnosis, and Management of Granulomatous-Lymphocytic Interstitial Lung Disease in Common Variable Immunodeficiency Disorders [Review]. J Allergy Clin Immunol Pract. 2017 Jul;5(4):938–945.
- Smits B, Goldacker S, Seneviratne S, et al. The efficacy and safety of systemic corticosteroids as first line treatment for granulomatous lymphocytic interstitial lung disease. J Allergy Clin Immunol. 2022 Dec 29. Online ahead of print.
- Cereser L, De Carli R, Girometti R, et al. Efficacy of rituximab as a single-agent therapy for the treatment of granulomatous and lymphocytic interstitial lung disease in patients with common variable immunodeficiency. J Allergy Clin Immunol Pract. 2019 Mar;7(3):1055–1057.e2.
- Bucciol G, Petrone A, Putti MC. Efficacy of mycophenolate on lung disease and autoimmunity in children with immunodeficiency. Pediatr Pulmonol. 2017 Oct;52(10):E73–e76.
- Bintalib HM, Lowe DM, Mancuso G, et al. Corticosteroid-induced remission and mycophenolate maintenance therapy in granulomatous lymphocytic interstitial lung disease: long-term, longitudinal change in lung function in a single-centre cohort. ERJ Open Res. 2022 Oct;8(4):00024–2022.
- Lamers OAC, Smits BM, Leavis HL, et al. Treatment Strategies for GLILD in common variable immunodeficiency: a systematic review. Front Immunol. 2021;12:606099.
- Warnatz K, Wehr C, Dräger R, et al. Expansion of CD19(hi)CD21(lo/neg) B cells in common variable immunodeficiency (CVID) patients with autoimmune cytopenia. Immunobiology. 2002 Dec;206(5):502–513.
- Keller B, Strohmeier V, Harder I, et al. The expansion of human T-bet(high)cd21(low) B cells is T cell dependent. Sci Immunol. 2021 Oct 15;6(64):eabh0891.
- Knight AK, Radigan L, Marron T, et al. High serum levels of BAFF, APRIL, and TACI in common variable immunodeficiency [Original]. ClinImmunol. 2007 Aug;124(2):182–189.
- Matson EM, Abyazi ML, Bell KA, et al. B cell dysregulation in common variable immunodeficiency interstitial lung disease. Front Immunol. 2020;11:622114.
- Furie R, Petri M, Zamani O, et al. A phase III, randomized, placebo-controlled study of belimumab, a monoclonal antibody that inhibits B lymphocyte stimulator, in patients with systemic lupus erythematosus. Arthritis & Rheumatism. 2011 Dec;63(12):3918–3930.
- Wise LM, Stohl W. Belimumab and rituximab in systemic lupus erythematosus: a tale of two B cell-targeting agents. Front Med. 2020;7:303.
- Boileau J, Mouillot G, Gérard L, et al. Autoimmunity in common variable immunodeficiency: correlation with lymphocyte phenotype in the French DEFI study. J Autoimmun. 2011 Feb;36(1):25–32.
- Bateman EA, Ayers L, Sadler R, et al. T cell phenotypes in patients with common variable immunodeficiency disorders: associations with clinical phenotypes in comparison with other groups with recurrent infections. Clin Exp Immunol. 2012 Nov;170(2):202–211.
- Taraldsrud E, Aukrust P, Jorgensen S, et al. Patterns of constitutively phosphorylated kinases in B cells are associated with disease severity in common variable immunodeficiency. Clin Immunol. 2017 Feb;175:69–74.
- Walker LS, von Herrath M. CD4 T cell differentiation in type 1 diabetes. Clin Exp Immunol. 2016 Jan;183(1):16–29.
- Krishnarajah S, Becher BT. (H) cells and cytokines in encephalitogenic disorders. Front Immunol. 2022;13:822919.
- Szabo SJ, Sullivan BM, Stemmann C, et al. Distinct effects of T-bet in TH1 lineage commitment and IFN-gamma production in CD4 and CD8 T cells. Science. 2002 Jan 11;295(5553):338–342.
- Gadina M, Gazaniga N, Vian L, et al. Small molecules to the rescue: inhibition of cytokine signaling in immune-mediated diseases. J Autoimmun. 2017 Dec;85:20–31.
- Fang D, Cui K, Cao Y, et al. Differential regulation of transcription factor T-bet induction during NK cell development and T helper-1 cell differentiation. Immunity. 2022 Apr 12;55(4):639–655.e7.
- Bloomfield M, Kanderová V, Paračková Z, et al. Utility of ruxolitinib in a child with chronic mucocutaneous candidiasis caused by a novel STAT1 gain-of-function mutation. J Clin Immunol. 2018 Jul;38(5):589–601.
- Forbes LR, Vogel TP, Cooper MA, et al. Jakinibs for the treatment of immune dysregulation in patients with gain-of-function signal transducer and activator of transcription 1 (STAT1) or STAT3 mutations. J Allergy Clin Immunol. 2018 Nov;142(5):1665–1669.
- Vargas-Hernández A, Mace EM, Zimmerman O, et al. Ruxolitinib partially reverses functional natural killer cell deficiency in patients with signal transducer and activator of transcription 1 (STAT1) gain-of-function mutations. J Allergy Clin Immunol. 2018 Jun;141(6):2142–2155.e5.
- Rao VK, Webster S, Dalm V, et al. Effective “activated PI3Kδ syndrome”-targeted therapy with the PI3Kδ inhibitor leniolisib. Blood. 2017 Nov 23;130(21):2307–2316.
- Simonson WT, Markovina S, Grossman WJ, et al. Common variable immunodeficiency with regulatory T-cell deficiency treated with rapamycin. Annals of allergy, asthma & immunology: official publication of the American College of Allergy. Asthma, & Immunology. 2009 Feb;102(2):170–171.
- Bride KL, Vincent T, Smith-Whitley K, et al. Sirolimus is effective in relapsed/refractory autoimmune cytopenias: results of a prospective multi-institutional trial. Blood. 2016 Jan 7;127(1):17–28.
- Azizi G, Abolhassani H, Yazdani R, et al. New therapeutic approach by sirolimus for enteropathy treatment in patients with LRBA deficiency. Eur Ann Allergy Clin Immunol. 2017 Sep;49(5):235–239.
- Schwab C, Gabrysch A, Olbrich P, et al. Phenotype, penetrance, and treatment of 133 cytotoxic T-lymphocyte antigen 4-insufficient subjects. J Allergy Clin Immunol. 2018 Dec;142(6):1932–1946.
- Lee AY, Huynh N, Lin MW. Granulomatous skin lesions of common variable immunodeficiency treated with sirolimus. Australas J Dermatol. 2021 Aug;62(3):434–435.
- Egg D, Rump IC, Mitsuiki N, et al. Therapeutic options for CTLA-4 insufficiency. J Allergy Clin Immunol. 2022 Feb;149(2):736–746.
- Vallurupalli M, Berliner N. Emapalumab for the treatment of relapsed/refractory hemophagocytic lymphohistiocytosis. Blood. 2019 Nov 21;134(21):1783–1786.
- Lasa JS, Olivera PA, Danese S, et al. Efficacy and safety of biologics and small molecule drugs for patients with moderate-to-severe ulcerative colitis: a systematic review and network meta-analysis. Lancet Gastroenterol Hepatol. 2022 Feb;7(2):161–170.
- Rubín de Célix C, Chaparro M, Gisbert JP. Real-world evidence of the effectiveness and safety of ustekinumab for the treatment of crohn’s disease: systematic review and meta-analysis of observational studies. J Clin Med. 2022 Jul 20;11(14):4202.
- Fevang B, Yndestad A, Sandberg WJ, et al. Low numbers of regulatory T cells in common variable immunodeficiency: association with chronic inflammation in vivo. Clin Exp Immunol. 2007 Mar;147(3):521–525.
- Genre J, Errante PR, Kokron CM, et al. Reduced frequency of CD4(+)CD25(HIGH)FOXP3(+) cells and diminished FOXP3 expression in patients with Common Variable Immunodeficiency: a link to autoimmunity? Clin Immunol. 2009 Aug;132(2):215–221.
- Yu GP, Chiang D, Song SJ, et al. Regulatory T cell dysfunction in subjects with common variable immunodeficiency complicated by autoimmune disease. Clin Immunol. 2009 May;131(2):240–253.
- Battaglia M, Stabilini A, Roncarolo MG. Rapamycin selectively expands CD4+CD25+FoxP3+ regulatory T cells. Blood. 2005 Jun 15;105(12):4743–4748.
- Pilat N, Sprent J. Treg therapies revisited: tolerance beyond deletion. Front Immunol. 2020;11:622810.
- Fraz MSA, Michelsen AE, Moe N, et al. Raised serum markers of t cell activation and exhaustion in granulomatous-lymphocytic interstitial lung disease in common variable immunodeficiency. J Clin Immunol. 2022 Jul;5:1–11.
- Aukrust P, Aandahl EM, Skålhegg BS, et al. Increased activation of protein kinase a type I contributes to the T cell deficiency in common variable immunodeficiency. J Immunol. 1999 Jan 15;162(2):1178–1185.
- Cunningham-Rundles C, Bodian C, Ochs HD, et al. Long-term low-dose IL-2 enhances immune function in common variable immunodeficiency. Clin Immunol. 2001 Aug;100(2):181–190.
- Perreau M, Vigano S, Bellanger F, et al. Exhaustion of bacteria-specific CD4 T cells and microbial translocation in common variable immunodeficiency disorders. J Exp Med. 2014 Sep 22;211(10):2033–2045.
- Jørgensen SF, Trøseid M, Kummen M, et al. Altered gut microbiota profile in common variable immunodeficiency associates with levels of lipopolysaccharide and markers of systemic immune activation. Mucosal Immunol. 2016 Nov;9(6):1455–1465.
- Shulzhenko N, Dong X, Vyshenska D, et al. CVID enteropathy is characterized by exceeding low mucosal IgA levels and interferon-driven inflammation possibly related to the presence of a pathobiont. Clin Immunol. 2018 Dec;197:139–153.
- Fiedorová K, Radvanský M, Bosák J, et al. Bacterial but not fungal gut microbiota alterations are associated with Common Variable Immunodeficiency (CVID) Phenotype. Front Immunol. 2019;10:1914.
- van Schewick CM, Nöltner C, Abel S, et al. Altered microbiota, impaired quality of life, malabsorption, infection, and inflammation in CVID patients with diarrhoea. Front Immunol. 2020;11:1654.
- Bosák J, Lexa M, Fiedorová K, et al. Patients with Common Variable Immunodeficiency (CVID) show higher gut bacterial diversity and levels of low-abundance genes than the healthy housemates. Front Immunol. 2021;12:671239.
- Jørgensen SF, Macpherson ME, Bjørnetrø T, et al. Rifaximin alters gut microbiota profile, but does not affect systemic inflammation - a randomized controlled trial in common variable immunodeficiency. Sci Rep. 2019 Jan 17;9(1):167.
- Troisi J, Venutolo G, Terracciano C, et al. The Therapeutic use of the Zonulin Inhibitor AT-1001 (Larazotide) for a Variety of Acute and Chronic Inflammatory Diseases. Curr Med Chem. 2021;28(28):5788–5807.
- Hoilat GJ, Altowairqi AK, Ayas MF, et al. Larazotide acetate for treatment of celiac disease: a systematic review and meta-analysis of randomized controlled trials. Clin Res Hepatol Gastroenterol. 2022 Jan;46(1):101782.
- Hurst JR, Warnatz K. Interstitial lung disease in primary immunodeficiency: towards a brighter future. Eur Respir J. 2020 Apr;55(4):2000089.