1. Introduction
Tezepelumab is the sixth biologic approved for severe asthma. Interestingly, the five already approved demonstrated clinical improvement in chronic rhinosinusitis with nasal polyps (CRSwNP), and by extension, in Chronic rhinosinusitis (CRS). What would be the benefit of adding a sixth in this rapidly changing landscape? Actually, the mechanism of action looks essential for determining the potential benefits of these drugs. Mechanistic overlap between these monoclonal antibodies (mAbs) can explain some of the overlapping reported evidence. Indeed, by targeting the inflammatory cascade upstream of the others, tezepelumab would at least in theory provide substantial additional benefits.
CRS and asthma are chronic inflammatory disorders that frequently coexist. Nose and bronchial epithelium have the same embryological origin and also share common molecular and cellular pathophysiology, leading to the concept of ‘unified airway disease’ [Citation1]. There is a clear correlation between possibilities of achieving clinical control both of the upper and the lower airways in patients with coexisting CRS and asthma, and these patients are more difficult to treat [Citation2]. More than 80% of the patients with CRSwNP exhibit type 2 (T2) inflammation, characterized by eosinophilic tissue infiltration. Indeed, severe CRSwNP is typically characterized by T2 inflammation.
Surgical intervention remains a mainstay of therapy for CRSwNP. Surgical treatment has a reported recurrence rate highly varying from 40% to 79%, depending on multiple factors including presence of type 2 inflammation. Tissue eosinophilic infiltration and the presence of galectin-10, the constitutive protein of Charcot Leyden crystals are other known prognostic factors for recurrence after surgery [Citation3]. Thus, patients who have recurrence despite surgery may benefit from a biologic agent. As such, biologics, which target T2 inflammation, represent promising therapeutic options for patients with severe CRSwNP.
The type 2 inflammatory pattern is characterized by eosinophils, dendritic cells (DCs), innate lymphoid cells type 2 (ILC2), mast cells, and T-helper 2 (Th2) cells. Eosinophils are found in up to 80% of surgically removed nasal polyps. Increased amounts of IL-4, IL-5, and IL-13 can be found in surgical tissue resection of nasal polyp. TSLP is increased in the epithelium from the upper airways in the polyps and, more interestingly, also in the non-polyp areas [Citation4]. TSLP expression was uniquely restricted to basal cells, especially in eosinophilic CRSwNP .
Figure 1. Inflammatory T2 pathway and mechanisms of action of Tezepelumab during chronic rhinosinusitis (CRS) and during chronic rhinosinusitis with nasal polyps (CRSwNP).
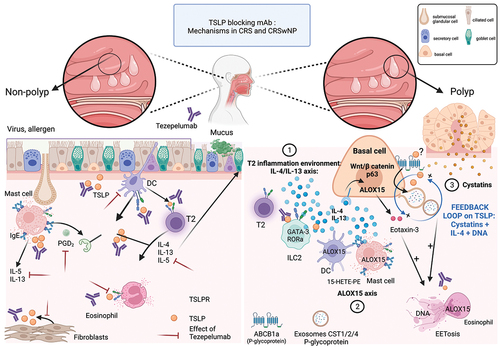
Table 1. Indirect evidence for TEZEPELUMAB efficacy in patients with CRSwNP.
There is increasing evidence of the heterogeneity of inflammation during CRS. Although type 2 inflammation is the hallmark of most CRSwNP, a non-type 2 endotype with predominant neutrophilic inflammation has also been described, also known as type 1 and type 3 endotypes. Non T2 CRSwNP/CRS are less associated with asthma and are more prevalent in Asian population. Inflammatory mechanisms are poorly understood. In non-T2 inflammation, IL-8 promotes neutrophils recruitment. Type 1 inflammation is characterized by a Th1 polarization with high levels of IFN-γ and TGFβ, whereas type 3 is more likely to be driven by Th17 pathway, through IL-17-A.
T2-polyps have been shown to be mainly composed of monotonous p63-expressing basal cells associated to a loss of the other classical epithelial subtypes notably secretory and ciliated cells [Citation5]. Single cell RNA sequencing of NP highlighted an impairment in the differentiation ability of basal cells that accumulate at this early ontogenic state (‘basal cell hyperplasia’). Basal cells in eosinophilic CRSwNP showed the highest expression of proinflammatory genes, especially those linked to type 2 inflammation, including the nitric oxide synthase (NOS2), that may be linked to high exhaled nitric oxide (FeNO) levels, TSLP, IL-4/13-induced genes, chemokines such as eotaxin-3, CLC (Charcot-Leyden crystal), and protease inhibitors such as cystatins. Eotaxin 3 is a potent chemoattractant for eosinophils through the binding to the cell surface chemokine receptor CCR3.
Arachidonic acid 15-lipoxygenase (ALOX15) encodes the enzyme 15-lipoxygenase (15-LO), which can oxidize polyunsaturated fatty acids and also promote the production of proteins such as eotaxin-3 and eoxins in a IL-13 dependent manner, which are associated with CRS and asthma [Citation6,Citation7].
In the polyp tissue, the top genes expressed are related to cystatins proteins family, CST1, 2, & 4 [Citation8]. CST1&2 are cysteine protease inhibitors that are supposed to play a protective role against allergen, viral, and bacterial proteases. They are among the most highly overexpressed proteins in both nasal polyp epithelium and mucus-derived exosomes in CRSwNP and are correlated with CRSwNP disease severity [Citation8]. CST1 expression can be induced by TSLP and can also stimulate TSLP expression in the presence of IL-4 and double-stranded DNA, thereby forming a positive feedback loop. P-glycoprotein (permeability glycoprotein, P-gp, encoded by ABCB1a gene) is an ATP-dependent efflux pump that is capable of regulating cytokine transport and is expressed in the nasal and can also be transferred through exosomes [Citation9]. One hypothesis is that the structure of TSLP enables the alarmin to act as a P-gp pump substrates.
2. Tezepelumab for managing CRS with or without NP
The use of biologics in severe CRSwNP is now based on high levels of clinical evidence, making them the treatment of choice in severe CRS. Thus, the collaboration between Ear-Nose-Throat (ENT) specialists and pulmonologists is increasingly necessary to manage type-2 inflammatory diseases. To date, there is no data in the literature regarding biologic switching in CRSwNP, when a first option has not been effective enough; this comes in contrast with what happens in the management of severe asthma where switches are now commonly admitted and performed. When there is no associated asthma, the ENT must consider the clinical and biological characteristics of the patient to choose a second option. In the case of moderate to severe asthma associated with CRS comorbidity, collaboration between clinical disciplines is critical to enhance multidisciplinary approach (pulmonologist, allergy specialist dermatologist, pediatrician, gastroenterologist, ideally a pathologist and ENT physician) to define the most adequate therapeutic strategy, especially for the choice of biologic, that will be mainly driven by asthma phenotype. If no asthma disorder is present, ENT specialist will initiate the biologic. But the difficulty lies in cases of dissociated response, when asthma is well controlled, but rhinosinusitis is not. The ENT specialist could propose recurrent sinonasal surgeries to remove the polyps or a switch of biologics with the risk of altering the control of asthma. In a network meta-analysis that aimed to compare the efficacy of biologics on a composite outcome (7 criteria), dupilumab was the only one that achieved an improvement of all the items compared to other biologics (i.e. omalizumab, mepolizumab, benralizumab [Citation10]).
In case of CRSwNP control failure with dupilumab, while asthma is controlled, the clinical challenge will then be to achieve outcomes qualitatively and quantitatively within the range of those achieved by dupilumab. Whether non-eosinophilic patients with CRSwNP will also improve with tezepelumab as shown in asthma will be of great interest.
Recent cases in the literature report the combination of biologics in severe asthma, either to reach a better control of asthma in poor controlled severe asthma patients or insufficiently controlled associated comorbidities such as CRSwNP. The only randomized controlled clinical trial that evaluated the safety and efficacy of combination of monoclonal antibodies during severe asthma did not highlighted an increase of side effects nor efficacy in the combination mAbs arm (i.e. anti-IL-33 itekepimab and dupilumab. The safety and efficacy of such therapeutic strategies (i.e. switching and combination of biologics) need to be address in randomized clinical trials.
3. Indirect evidence of Tezepelumab efficacy from asthma clinical trials ()
Tezepelumab is currently FDA approved for the treatment of moderate-to-severe asthma. In the phase 2b PATHWAY and phase 3 NAVIGATOR studies, it has been shown to reduce exacerbation rates independently of baseline blood eosinophil count and improved the level of asthma control, quality of life, and even lung function [Citation11,Citation13]. Next to that, there is some indirect evidence from clinical trials that it could also be effective in the treatment of chronic rhinosinusitis with nasal polyposis.
First, in the phase 2 placebo-controlled study CASCADE, Tezepelumab showed a reduction in the number of airway submucosal eosinophils by 85%, even in patients with a low submucosal eosinophil counts at baseline, suggesting that tezepelumab may act through other unknown mechanisms, expected in blocking an upstream epithelial cytokine. However, no impact was shown on other subtypes of inflammatory cells hypothetically due to a « less severe » profile of patients included in comparison with other clinical trials [Citation12].
Next, considering the « allergic response pathway» which may maintain a high level of Type 2 inflammation in some patients with CRS, interesting insights have been brought by the CATNIP study [Citation14]. The objective was to assess the impact of combination treatment with tezepelumab and subcutaneous cat allergen immunotherapy (SCIT) on responses to Nasal Allergen Challenge (NAC). At week 52, the association of tezepelumab and SCIT was the most effective in reducing the NAC induced total nasal symptom scores (TNSS). More interestingly, one year after treatment cessation, a persistent clinical tolerance was observed that could be explained by a downregulation of a gene network related to type 2 inflammation, especially a reduced expression of a mast cell gene signature in nasal brushings.
From a more clinical perspective, in NAVIGATOR, tezepelumab impressively improved nasal symptoms (SNOT-22 score) in patients with comorbid nasal polyps [Citation15]; these patients had a greater response in terms of asthma as well.
4. Safety
Additional data from both real-life and NCT studies are needed to address the question of the long-term safety of tezepelumab. The DESTINATION study (NCT03706079) has shown that tezepelumab is well tolerated in patients with severe asthma over 2 years. Indeed, the roles of TSLP in different type of cancers highlighted that TSLP can have pleiotropic effects and has both pro-tumoral and anti-tumoral effects, depending on the type of tumor. TSLP can also be protective regarding host defense against helminth or in S. aureus infections. However, the incidences of benign and malignant neoplasms and severe infections were similar in the tezepelumab and placebo groups in different NCT studies (NAVIGATOR, CASCADE, and PATHWAY studies). No hypersensitivity reactions have been reported. In the long-term DESTINATION study, significantly higher incidence of cardiac serious adverse events has been reported in patients receiving tezepelumab compared to placebo, without an increase of cardiovascular deaths.
5. Expert opinion
CRS is a heterogeneous disease divided into the two phenotypes: CRS with and without nasal polyps. Eosinophilic inflammation is present in 85% of CRSwNP. However, during Chronic Rhinosinusitis without nasal polyps (CRSsNP), inflammation is highly heterogeneous, with 5%, 45%, and 15% of type 1, type 2, and type 3 inflammation, respectively, classified on nasal protein expression of IFN-γ, eosinophil, and IL-17-A. Multiple biologics are efficient for treating either lower and upper airways. The challenge is now to treat both sides and even all the manifestations falling within the T2 spectrum. Targeting T2 inflammation in CRSwNP, and especially TSLP, may reverse structural changes such as nasal polyps through the ‘reprogramming’ of structural cells and their interaction with the environment. TSLP is produced by the epithelia and is found in the airways, skin, and eye mucosa, sustaining the hypothesis of a universal molecular pathway to transduce a warning signal and supporting, beyond the concept of the unified airways, the idea that one biologic in a patient should target all the spectrum of T2 inflammation expressing in different organs. The ongoing study WAYPOINT (NCT04851964) might confirm those findings of tezepelumab for the treatment of CRSwNP.
Tezepelumab is actually considered as an available drug to cover the unmet need in T2 and low T2 severe asthma and acts upstream the inflammation cascade. Data are needed to evaluate its efficacy in non T2 CRS/CRSwNP.
Abbreviations
| ACQ-6 | = | Asthma Control Questionnaire |
| AAER | = | Annualized Asthma Exacerbation Rate |
| Arachidonic acid 15-lipoxygenase | = | ALOX15, 15-lipoxygenase |
| ATP | = | adenosine triphosphate |
| CD | = | Cluster of differentiation |
| CRS | = | Chronic Rhinosinusitis |
| CRSwNP | = | Chronic Rhinosinusitis with nasal polyps |
| CRSsNP | = | Chronic Rhinosinusitis without nasal polyps |
| DC | = | dendritic cells |
| FeNO | = | Fractional exhaled nitric oxide |
| MC | = | mast cells |
| IgE | = | immunoglobulin |
| ILC | = | innate lymphoid cell |
| IL | = | interleukin |
| IL-R | = | interleukin-receptor |
| mAb | = | monoclonal antibodies |
| Nitric oxide synthase | = | NOS |
| NP | = | nasal polyp |
| RORα | = | RAR-related orphan receptor alpha. |
| T2 | = | Type 2 |
| TSLP | = | thymic stromal lymphopoietin |
| TSPLR | = | TSLP receptor |
Declaration of interest
A Bourdin reports grants, personal fees, non-financial support, and/or other from AstraZeneca and Boehringer Ingelheim; Astra Zeneca, GSK, Novartis, Sanofi Regeneron, Boehringer Ingelheim and Chiesi. C Jeremy reports funding’s by GlaxoSmithKline, Astra Zeneca, Sanofi. L Crampette reports speaker fees, non-financial support and/other from Medtronic, Novartis, Zambon, ALK, AstraZeneca, Sanofi Genzyme. A Engi reports personal fees, non-financial support from Sanofi, Astra Zeneca The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Additional information
Funding
References
- Passalacqua G, Ciprandi G, Canonica GW. United airways disease: therapeutic aspects. Thorax. 2000;55(2):S26–27.
- Laidlaw TM, Mullol J, Woessner KM, et al. Chronic rhinosinusitis with nasal polyps and asthma. J Allergy Clin Immunol Pract. 2021;9(3):1133–1141. DOI:10.1016/j.jaip.2020.09.063
- Chen W, Bai Y, Kong W, et al. Predictive significance of Charcot-Leyden crystal structures for nasal polyp recurrence. Clin Transl Allergy. 2022;12(11):e12212. DOI:10.1002/clt2.12212
- Nagarkar DR, Poposki JA, Tan BK, et al. Thymic stromal lymphopoietin activity is increased in nasal polyps of patients with chronic rhinosinusitis. J Allergy Clin Immunol. 2013;132(3):593–600.e12. DOI:10.1016/j.jaci.2013.04.005
- Ordovas-Montanes J, Dwyer DF, Nyquist SK, et al. Allergic inflammatory memory in human respiratory epithelial progenitor cells. Nature. 2018;560:649–654.
- Feltenmark S, Gautam N, Brunnström A, et al. Eoxins are proinflammatory arachidonic acid metabolites produced via the 15-lipoxygenase-1 pathway in human eosinophils and mast cells. Proc Natl Acad Sci U S A. 2008;105(2):680–685. DOI:10.1073/pnas.0710127105
- Kristjansson RP, Benonisdottir S, Davidsson OB, et al. A loss-of-function variant in ALOX15 protects against nasal polyps and chronic rhinosinusitis. Nat Genet. 2019;51(2):267–276. DOI:10.1038/s41588-018-0314-6
- Nocera AL, Mueller SK, Workman AD, et al. Cystatin SN is a potent upstream initiator of epithelial-derived type 2 inflammation in chronic rhinosinusitis. J Allergy Clin Immunol. 2022;150(4):872–881. DOI:10.1016/j.jaci.2022.04.034
- Bleier BS. Regional expression of epithelial MDR1/P-glycoprotein in chronic rhinosinusitis with and without nasal polyposis. Int Forum Allergy Rhinol. 2012;2(2):122–125.
- Oykhman P, Paramo FA, Bousquet J, et al. Comparative efficacy and safety of monoclonal antibodies and aspirin desensitization for chronic rhinosinusitis with nasal polyposis: a systematic review and network meta-analysis. J Allergy Clin Immunol. 2022;149(4):1286–1295. DOI:10.1016/j.jaci.2021.09.009
- Menzies-Gow A, Corren J, Bourdin A, et al. Tezepelumab in Adults and Adolescents with Severe, Uncontrolled Asthma. N Engl J Med. 2021;384(19):1800–1809. DOI:10.1056/NEJMoa2034975.
- Diver S, Khalfaoui L, Emson C, et al. Effect of tezepelumab on airway inflammatory cells, remodelling, and hyperresponsiveness in patients with moderate-to-severe uncontrolled asthma (CASCADE): a double-blind, randomised, placebo-controlled, phase 2 trial. Lancet Respir Med. 2021;0(11):1299–1312. DOI:10.1016/S2213-2600(21)00226-5
- Corren J, Parnes JR, Wang L, et al. Tezepelumab in Adults with Uncontrolled Asthma. N Engl J Med. 2017;377:936–946.
- Corren J, Larson D, Altman MC, et al. Effects of combination treatment with tezepelumab and allergen immunotherapy on nasal responses to allergen: a randomized controlled trial. J Allergy Clin Immunol. 2023;151:192–201.
- Menzies-Gow A, Corren J, Israel E, et al. Tezepelumab efficacy in patients with severe, uncontrolled asthma and comorbid nasal polyps in NAVIGATOR. Eur Respir J. 2021;PA876. DOI:10.1183/13993003.congress-2021.PA876.
