ABSTRACT
Introduction
Type 2 targeting biologics have reached the market first for asthma and since 2019 also for CRSwNP. As clear guidelines and predictors for optimal biological choice are missing, patients are sometimes required to switch biologic therapy in order to find the optimal treatment result. In this paper, we evaluate reasons for switching biologics and the treatment effects after each sequential switch.
Materials and methods
Ninety-four patients who switched from one biologic to another for their treatment of CRSwNP and asthma were evaluated.
Results
Twenty patients experienced satisfactory control of CRSwNP, but insufficient control of severe asthma. Fifty-one patients experienced satisfactory control of severe asthma, but insufficient control of CRSwNP/EOM. Twenty-eight patients experienced insufficient control of both upper and lower airways. Thirteen patients had to switch because of side effects. Furthermore, two cases are described to clarify clinical decision-making.
Discussion
For abovementioned patients, a multidisciplinary approach is mandatory to find the best suitable biologic. It seems ineffective to switch to a second anti-IL5 treatment if the first one is not successful. Most patients that failed omalizumab and/or an anti-IL-5 treatment are well controlled on dupilumab. Therefore, we suggest to use dupilumab as first choice when switching biologic agents.
1. Introduction
Chronic Rhinosinusitis (CRS) with Nasal Polyps (CRSwNP) is an inflammation of the nose and sinuses that in the Western world usually has a type 2 inflammatory (T2) profile [Citation1,Citation2]. More than half of the patients with CRSwNP also have (late onset) asthma, conjointly also dubbed T2 airway disease.
CRSwNP can be treated with appropriate medical treatment, like nasal corticosteroids, saline rinsing, and an occasional course of oral corticosteroids. Patients who still experience low quality of life despite optimal medical treatment might need (functional) endoscopic sinus surgery [Citation3]. In a minority of the patients the combination of appropriate medical therapy and surgery results in insufficient control of the symptoms. These patients often have co-morbid (late onset) asthma, non-steroidal anti-inflammatory drugs (NSAID)-exacerbated respiratory disease N-ERD and other manifestations of systemic type 2 driven inflammation, such as eosinophilic otitis media (EOM). Patients with severe CRSwNP and asthma are at increased risk for a decreased quality of life, fixed airway obstruction, repetitive surgery, and hospitalization, despite receiving local or systemic corticosteroid treatment.
In the last two decades, type 2-targeting biologics reached the market first for asthma (omalizumab 2003-FDA/2005-EMA, mepolizumab 2015, reslizumab 2016, benralizumab 2017, dupilumab 2018) [Citation4] and since 2019 also became available for CRSwNP (dupilumab 2019, omalizumab 2020, mepolizumab 2020). Biologics have been shown to be effective in reducing the burden of the disease in asthma and CRSwNP [Citation5,Citation6] both in randomized controlled trials and in real-life experience [Citation7–12]. Although most patients with severe CRSwNP and/or asthma have a type 2 endotype, the underlying mechanisms driving this inflammation may vary drastically across patients. This problem has been underscored by the variation in biological therapy response.
Biomarkers, such as blood eosinophils, total serum IgE, periostin, and fractional exhaled nitric oxide (FeNO), are associated with type 2 inflammation and can be integrated to endotype patients together with coexisting disease conditions. This might be helpful to predict the optimal biologic choice. In a proposal by Pavord et al., the blood eosinophil count, regular OCS use, and presence of comorbid CRSwNP are the predictive variables that have been shown consistently to relate to treatment efficacy and allow differentiation of biologics [Citation13]. In addition, an indirect treatment comparison from Peters et al. [Citation14] stated that in patients with CRSwNP and asthma, dupilumab seems to significantly improve nasal congestion scores compared to omalizumab. Lastly, a retrospective analysis from Bavaro et al. [Citation15] suggested that AERD patients who failed to get sufficient control of upper and lower airways with anti-IL-5/IL-5 Rα therapy improved during subsequent dupilumab treatment in terms of reduced asthma exacerbations and smell. Since few head-to-head studies have been conducted regarding this subject and clear guidelines and predictors for optimal biologic choice are missing, patients are sometimes required to switch biologic therapy in order to find the optimal drug for their specific condition(s). As mentioned, there is scarce data on patients switching biological therapy. Therefore, patients with CRSwNP and asthma treated with biologics for type 2 airway disease who switched biologic agents were evaluated. Switching is defined as sequential or simultaneous use of >1 biologic agent. We concentrated on the reasons for switching and the treatment effects after each sequential switch.
2. Material and methods
2.1. Ethics statement
The study conformed to the Declaration of Helsinki. Assessment of the institutional Medical Ethical Review Committee of the PolyREG registry deemed it not to be subject to the Dutch Medical Research Involving Human Subjects Act (MREC ID: W21_030#21.034). All patients consented to data-collection and use in line with the GDPR in all three hospitals (Amsterdam, Gemelli, and Wiesbaden).
2.2. Patients
Patients treated with a biologic for CRSwNP and asthma in Amsterdam UMC, location AMC, Amsterdam, The Netherlands, Fondazione Policlinico Universitario A.Gemelli IRCCS, Rome, Italy and Center for Rhinology and Allergy, Wiesbaden, Germany, and seen in the combined airway clinic for at least 1 year were evaluated. The patients that sequentially or simultaneously used more than one biologic were selected, all of whom were discussed within a multidisciplinary team of otorhinolaryngologists and pulmonologists before switching (and if needed other specialties).
Insufficient treatment effect was determined (decided) within the team in collaboration with the treated patient. Insufficient CRSwNP-control under biologic treatment was decided when the patient had severe symptoms of CRS including anosmia, and a bilateral NPS score of ≥1 (on both sides), and either continued need for OCS, or need for surgery.
Insufficient asthma-control for biologics in asthma was defined when chronic OCS use persisted, or exacerbation rate was not decreased <1/y or if there was an airflow limitation by a FEV1 <80% predicted or asthma symptoms measured by ACQ > 1.5 or ACT < 20 [Citation9].
For all patients, baseline characteristics were registered (see ).
Table 1. Baseline characteristics.
2.3. Reasons for switching to another biologic (sequential or simultaneous use)
The reason for switching to another biologic was evaluated. Reasons for switching were categorized into:
Biologic satisfactory for CRSwNP but insufficient control asthma
Biologic satisfactory for asthma but insufficient CRSwNP-control
Biologic treatment results in insufficient CRSwNP-control and insufficient asthma control
Side effect of biologic severe enough to switch to another biologic
3. Results
3.1. Patients
In total around 2000 (Amsterdam 800, Rome 400, Wiesbaden 850) patients were evaluated.
Ninety-four patients (Amsterdam 39, Rome 18, Wiesbaden 37) either switched from one biologic to another (n = 89), or finally received two biologics (n = 5).
shows the baseline characteristics of the 94 patients in the three centers.
3.2. Reasons for using more than one biologic (sequential or simultaneous use)
We recognized four reasons to switch from one biologic to another (more than one reason can apply to one patient). One group experienced satisfactory control of symptoms and signs of CRSwNP, but insufficient control of severe asthma (n = 20; n = 15 for the first switch, 5 for the second switch). Vice versa, a second group experienced satisfactory control of severe asthma, but insufficient control of CRSwNP/EOM (n = 51; n = 43 for the first switch, 8 for the second switch). A third group experienced insufficient control of both upper and lower airways (n = 28; n = 23 for the first switch, 5 for the second switch). Finally, a fourth group had to switch because of side effects that could not be sufficiently reduced or controlled in another way (n = 13; n = 11 for the first switch, 2 for the second switch).
3.3. Order of choice and nature of given biologic
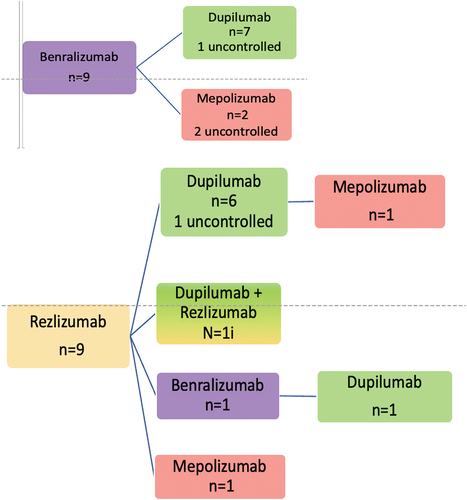
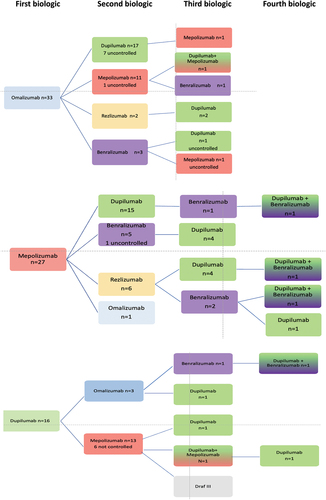
As can be seen in , most patients received omalizumab as first choice, followed by mepolizumab, usually because of their asthma. Seventy-three patients (of 94) eventually achieved control, 48 at the second choice biologic (usually dupilumab), but 6 patients only achieved control at the fourth choice. Seven patients received a combination of two biologics before they achieved control, one of whom could switch back to dupilumab only after less than a year of double treatment. The reasons for double treatment were persistent hyper-eosinophilia during dupilumab treatment (n = 3), hyper-eosinophilic syndrome HES (>5) when treated with dupilumab and insufficient control of CRSwNP when treated with anti-IL5 treatment only (n = 2), or insufficient control of asthma while on treatment with dupilumab only and insufficient control of CRSwNP when on anti-IL5 treatment only (n = 1), side effects during dupilumab (arthritis), insufficient control of CRSwNP with anti-IL5, and addition of low-frequency dupilumab (arthritis did not improve when stopping dupilumab).
Figure 1. Switching order. Name of biologic (and surgery) and number of patients. Patients are controlled in last step unless stated otherwise.
Patients were treated for a mean period of 15 months (s.d. 15 months) with the first biologic and a mean of 16 months (s.d. 12 months) with the second biologic before a switch was considered.
3.4. Order in which biologics were given
The order in which a biologic was given depended on the choice made by the physician but also the availability of the biologic. For asthma, it has been possible to prescribe biologics for a long time. In this analysis, patients are included from 2014 (omalizumab), 2016 (mepolizumab), 2018 (reslizumab and benralizumab), and 2019 (dupilumab) onwards, respectively, for the primary indication of asthma. For the primary indication of CRSwNP from 2019 (dupilumab), 2020 (omalizumab), and 2021 on (mepolizumab), respectively.
3.5. Omalizumab as first choice
Of the 33 patients starting on omalizumab, 17 switched to dupilumab, 11 to mepolizumab, 2 to reslizumab and 3 to benralizumab, as the second biologic. At the time of this evaluation (December 2022), 23 patients had satisfactory control of their upper and lower airway disease with their second biologic of which 12 received dupilumab, and 9 mepolizumab. Reasons for switching to the second biologic were satisfactory control of symptoms and signs of asthma, but insufficient control of signs and symptoms of CRSwNP/EOM (n = 16), satisfactory control of symptoms and signs of CRSwNP but insufficient control of signs and symptoms of lower airway disease (n = 9), insufficient control of both upper and lower airways (n = 6), and side effects (n = 2).
3.6. Mepolizumab as first choice
Of the 27 patients starting on mepolizumab, 15 switched to dupilumab because of insufficient control of their CRSwNP, with satisfactory control of upper and lower airways in 14 patients. Eleven switched first to either benralizumab or reslizumab, of which eight only reached control after switching to dupilumab. The others usually ended up with a combination of anti-IL5 treatment and dupilumab. Reasons for switching were a satisfactory control of symptoms and signs of asthma but insufficient control of signs and symptoms of CRSwNP/EOM (n = 17), or a satisfactory control of symptoms and signs of CRSwNP but insufficient control of signs and symptoms of severe asthma (n = 3), or insufficient control for both upper and lower airways (n = 8).
3.7. Dupilumab as first choice
Of the 16 patients receiving dupilumab as first choice, 13 switched to mepolizumab and 3 to omalizumab; 9 patients because of (perceived) side effects, 2 with insufficient control of both upper and lower airways, 3 for insufficient control of the CRSwNP and also 3 for insufficient control the asthma (more than one reason may apply). Five patients had hyper-eosinophilia that could not be controlled with lengthening of the dose interval or short-term systemic corticosteroid treatment. Before start of dupilumab treatment, the eosinophil levels of these patients were 0.50, 1.43, 0.94, 3.57, and 0.05 × 109 cells per liter, respectively. The latter patient was using 10 mg of prednisolone daily to control asthma at the time. Thus, in general, baseline eosinophil levels of patients who developed hyper-eosinophilia were high. Treatment duration with dupilumab in these patients ranged from 3 to 24 months. All patients except one showed no clinical manifestations of hyper-eosinophilic syndrome or related end organ damage. One patient developed disabling plantar fasciitis in the left foot. Any association with dupilumab was ruled out by the rheumatologist. Despite that, the multidisciplinary team (otorhinolaryngologist, pulmonologist, and rheumatologist) decided to discontinue dupilumab treatment. For the rest of the patients, dupilumab treatment was stopped after shared decision-making between the otorhinolaryngologist and the patient. Of the 13 patients switching to mepolizumab, 9 remained uncontrolled, 2 switched back to dupilumab, and one eventually ended up undergoing Draf III surgery.
3.8. Benralizumab as first choice
Of the nine patients starting on benralizumab, seven switched to dupilumab of which six had sufficient control for both upper and lower airway disease and two switched to mepolizumab without reaching sufficient control.
3.9. Reslizumab as first choice
Of the nine patients starting on reslizumab, eight switched to dupilumab (one via benralizumab) because of insufficient control of the CRSwNP (n = 5) or insufficient control of asthma (n = 3) (of which one had insufficient control of both CRSwNP and severe asthma). All but one now have sufficient control of both upper and lower airways.
3.10. Reasons for double treatment
Seven patients received treatment with a combination of two biologics. Five because of hyper-eosinophilia (2 with signs of HES) with insufficient control of CRSwNP with anti-IL5. One switched because control of upper and lower airways disease could not be achieved with a single biologic and one patient had arthritis with an unclear relation to dupilumab, but insufficient control of CRSwNP without dupilumab.
3.11. Predicting the need to switch, best first choice
This selection of patients that needed to switch to another biologic is not the best population to evaluate differences in response due to the small size and heterogeneity of the cohort. Nevertheless, we could compare the patients that eventually ended up being controlled by anti-IL5 treatment to those being controlled with dupilumab or a combination of two biologics (for omalizumab there were insufficient patients). It was not possible to find biomarkers that predicted the response.
4. Case descriptions of considerations in clinical decision making
4.1. Case 1
A 62-year-old male with symptoms of CRSwNP since 2016. He had a functional endoscopic sinus surgery (FESS) in 2018. Since 2018, he also suffered from a late onset eosinophilic asthma.
The patient presented to the Amsterdam UMC in 2019 with uncontrolled CRSwNP and late onset eosinophilic asthma. In the past year, he had had two serious exacerbations of asthma treated with prednisolone 30 mg daily for 2 weeks and had received a depo-medrol injection, with a partial effect on his symptoms. He complained of nasal blockage, loss of the sense of smell and pressure on the ears, mainly the right ear (AD) with reduced hearing of AD.
His asthma seriously hampered his functioning: he could not perform his work as a luggage carrier at the airport anymore.
At nasal endoscopy, we saw grade 3 polyps on both sides and thick eosinophilic mucus. The patient had a grommet in the AD, and the left ear (AS) showed a normal aspect of the eardrum. CT scan showed total opacification of the sinuses. Spirometry results show FVC = 4,29 L(113,44%); FEV1 = 2,63 L(87,86%); FEV1%M = 61%. Biomarkers tested showed exhaled NO 58ppb (increased), blood eosinophils 0.87 × 109/L (increased) and a total IgE of 55 kU/L.
Because of the dominant lower airway problems, the patient was started on reslizumab 250 mg, intravenously once per month. In the months after the start of therapy, his lower airway symptoms significantly improved: he could go back to work again and his asthma seemed well controlled. At the 3-month follow-up visit spirometry showed the following results: FVC = 4,70 L(124,93%); FEV1 = 3,39 L(113,99%); FEV1%M = 72% and FeNO 44ppb (slightly increased), blood eosinophils 0.05 × 109/L.
However, the effects on the upper airways were moderate: his sense of smell was variably bad and he still had symptoms of Eustachian tube dysfunction. Nasal endoscopy showed grade 1 left-sided NPS and grade 3 right-sided NPS, otoscopic findings were comparable to baseline.
Treatment was continued and his asthma control was excellent; however, CRSwNP symptoms remained bothersome. After 2 years of treatment with reslizumab, his asthma control remained excellent, but the upper airways were deteriorating with increasing symptoms: thick sticky mucus, no smell, and pressure on ears AD>AS. At the clinic visit, we saw grade 2 left NPS and grade 3 right NPS at nasal endoscopy, with bilateral thick clear mucus. Otoscopy showed an otitis media with effusion of the AD (grommet had extruded). After discussion with the team, we decided to switch to dupilumab 300 mg/2 wk. At the first visit, 1 month after start of the treatment, the patient reported a significant improvement of the upper airway problems: his sense of smell was coming back and he had less pressure on the ears. His asthma was still excellently controlled. Nasal endoscopy showed grade 0 left NPS (no polyps) and grade 1 right NPS. Otoscopy showed AD as retracted tympanic membrane, with an aerated middle ear. AS was normal.
After 6 months of treatment, his asthma control remained excellent without any work impairment and without exacerbations. In addition, he was able to exercise vigorously. Also, his upper airway symptoms strongly improved with improved sense of smell, but infrequent mild pressure on AD persisted. Nasal endoscopy showed minimal polyps on the right side (NPS grade 1) and none on the left (NPS grade 0). Sniffin’s Sticks-12 (olfactory) identification test (SSIT-12: 0–12; 0–6 anosmia, 7–10 hyposmia, 11–12 normosmia) was 6, indicating borderline anosmia. Spirometry results showed FVC = 5,11 L(137,71%); FEV1 = 3,59 L(123,10%); FEV1%M = 70%. Otoscopy showed an unaltered retracted tympanic membrane AD and no abnormalities in AS. We decided to reduce the dose of dupilumab to 300 mg/4 wks. At a recent visit one year after the start of treatment with dupilumab and now 6 months on a dose of 300 mg/4 weeks, he has excellent control of both upper and lower airways. His only remaining complaint is infrequent aural pressure in AD, without conductive hearing loss. Nasal endoscopy showed grade 0 NPS on both sides, and SSIT remained 6. Otoscopy showed a retracted eardrum on the right side, AS normal. The patient indicated that he could perceive all odors during the olfaction identification test, which he was unable to do previously, but he continued to have trouble identifying them. We advised him on smell training. In the end, we decided to further lengthen the dupilumab dosing interval to 300 mg/6 weeks. The patient is continuing on this dose and has well-controlled CRSwNP and asthma.
4.2. Case 2
The second case is a 57-year-old woman who received long-term oral prednisolone, latest dose 10 mg per day, because of type 2 late onset asthma, CRSwNP and EOM. She had had FESS twice, most recently in 2015. In 2017, she started treatment with mepolizumab, on which she could reduce the prednisolone to 5 mg every other day. Her asthma improved significantly, she slept much better and had less exercise intolerance. However, the CRSwNP and EOM did not improve, but actually deteriorated when the prednisolone was reduced. Because of the bilateral grade 3 polyps, a revision sinus surgery was performed in 2018. Stopping the prednisolone treatment was not possible and she had a few pulmonary exacerbations because of which the prednisolone was increased again. FeNO showed a result of 52ppb, during treatment with mepolizumab 100 mg/4 weeks subcutaneously and 5 mg of prednisolone. Mid 2019, she switched biologic treatment to reslizumab because of the impossibility to stop prednisolone. At that time, the FeNO was 52ppb, while under mepolizumab treatment and 5 mg of prednisolone. Mid 2020 she was seen again, with no sense of smell, bad hearing, grade 3 NPS polyps bilaterally and otitis media with effusion (OME) in both ears. At the end of 2020, the patient started dupilumab 300 mg/2 wk sc. Within 1 month of treatment, she experienced significant improvement of both upper and lower airways, and at 6 months, her nasal polyps had totally disappeared, but OME remained bilaterally. Also, it was not possible to reduce the prednisolone to less than 5 mg daily and she kept having significant blood eosinophilia and increasing lower airway symptoms. A new analysis of vasculitis syndromes was performed which was negative. Because of prednisolone dependency and high blood eosinophils (>2 × 109/L) it was decided to switch the patient to benralizumab. With benralizumab the lower airways improved, however, within 6 months she had grade 3 NPS bilaterally and an EOM with a middle ear polyp on one side. Her hearing was significantly impaired. It was decided to combine dupilumab with benralizumab in June 2022. With this combination of two biologics, upper and lower airways improved and became sufficiently controlled. Prednisolone could finally be phased out and stopped altogether. On nasal endoscopy, polyps proved to have disappeared bilaterally, without edema and eosinophilic secretions, and otoscopy showed aerated middle ears without granulation of the tympanic membrane. Unfortunately, until now, the sense of smell has not returned.
5. Discussion
This paper describes a proportionally very small group of patients that has combined issues of upper and lower type 2 inflammatory airway disease and does not do well on the first biologic treatment given. We must emphasize that this is a small minority of less than 5% of the patients treated with a biologic in our centers. Most patients do well on the monotherapy of first choice.
The very small group described above either achieves insufficient control of upper and/or lower airways/ears (usually when treated with anti-IL5), or significant side-effects/hyper-eosinophilia (usually when treated with dupilumab).
For these patients, a careful multidisciplinary approach is mandatory to find the most suitable biologic, or, seldomly, decide upon a combination of biologics.
Although direct comparative trials are missing, a systematic review and network meta-analysis ranked dupilumab highest in the most relevant outcomes, such as symptoms, nasal polyp score, and olfaction [Citation16]. In a real-life setting, dupilumab has been shown to be effective in the large majority of patients fulfilling the EPOS2020 criteria [Citation7,Citation8]. For severe asthma, on the other hand, the comparative efficacy and safety of the biologics currently approved are less clear. A Bayesian network meta-analysis on effect parameters as exacerbation rates, prebronchodilator FEV1, the Asthma Control Questionnaire, and serious adverse events in individuals with eosinophilic asthma of eight randomized clinical trials (n = 6461) showed minimal differences in the efficacy and safety of mepolizumab, benralizumab, and dupilumab [Citation17]. An algorithm on the assessment and treatment in asthma was developed by integrating clinical characteristics, biomarkers, and coexisting conditions in order to choose the appropriate initial biologic therapy.
In a recent paper, Pavord et al. suggested to start with dupilumab when patients with severe asthma have CRSwNP and eosinophils are between 150 and 1500 [Citation13]. In patients with very high eosinophilia (>1500) an evaluation of a potential vasculitis (EGPA) has to be performed. In patients with very low eosinophils, FeNO (>25 ppb) can be helpful to decide which biologic will be the best treatment [Citation13].
However, very few of the currently clinically applicable biomarkers seem to be able to predict what is the best choice in a certain patient. Also, in this paper, we were not able to find such predictive biomarkers.
A number of things can be learned from this paper. In general, it seems ineffective to switch to a second anti-IL5 treatment if the first one is not successful. Only two patients out of 15 patients that switched from one anti-IL5 treatment to another got controlled. Most patients that failed omalizumab and/or an anti-IL-5 treatment are well controlled on dupilumab, and we therefore suggest to use dupilumab as first choice when switching biologic agents. A few patients needed treatment with two biologics for different reasons. In our experience so far, this could be done safely. The limited literature on double biologic treatment in asthma also showed safety [Citation18], but is hampered by short duration of follow-up and the mostly retrospective nature of the studies. To the best of our knowledge, no papers have been published on the topic of simultaneous biologic therapy in CRSwNP. When switching to dupilumab from an anti-IL5 treatment, it is advised to start directly with the dupilumab and use the window of low eosinophils created by the anti-IL5 treatment to reduce the chance of having issues with hyper-eosinophilia.
An intrinsic weakness of this paper is that the introduction of biologics has not been simultaneous, both for the indications of severe asthma and CRSwNP, and for the different agents within these indications. Therefore, experience in asthma has been longest with omalizumab, then with anti-IL5 treatment, and only very recently experience could be obtained with dupilumab. For CRSwNP, almost the opposite has occurred, with first the introduction of dupilumab, then omalizumab and only recently mepolizumab (the other two anti-IL5 agents are not yet registered for treatment of CRSwNP).
Although a significant percentage of the patients discussed in this paper participated in prospective real-life registries, some patients already started with biologic treatment before these registries came about. In those patients, detailed clinical data were often not available.
Also, the registries of the three centers participating in this analysis are not identical. As a consequence, some data were not available for all patients, in particular hampering the analysis of biomarkers.
For now, biologics are a paradigm shift for the group of patients with type 2 inflammation-induced diseases in the upper and lower airways and middle ears. The international guidelines from EAACI, EPOS, and GINA propose recommendations for selecting the most effective biological for a certain patient, including clinical characteristics, biomarkers, and co-morbidities. We have learned from this analysis that in a very small group, a combination of two biologics may be necessary to achieve optimal control of disease.
6. Expert opinion
Biological treatment of patients with severe CRSwNP and asthma failing to achieve control with the first biologic warrants a multi-disciplinary evaluation. Choosing the right biologic for these patients can be challenging and at the moment it is often a matter of trial and error. When switching from anti-IL5 to dupilumab the dupilumab treatment can best be started directly after the anti-IL5 treatment to prevent hyper-eosinophilia.
The majority of the patients with CRSwNP and/or asthma that are treated with a biologic do well. However, choosing the best biologic treatment for a certain patients can be a challenge. Direct comparisons of biologics are missing in both CRSwNP and in asthma.
For a long time, asthma treatment was centered around the reduction of eosinophils. In CRSwNP the good effect of dupilumab and the hallmark study of Tanya Laidlaw showing complete depletion of tissue eosinophils in CRSwNP by dexpramipexole without any effect on symptoms and signs of CRSwNP pointed to the potentially more limited role of eosinophils. Recent Bayesian network meta-analysis show comparable results for all evaluated biologics in severe eosinophilic asthma [Citation16] and superiority of dupilumab over the other two available biologics in the treatment of severe CRSwNP [Citation13].
For this reason, some authors advise to choose dupilumab whenever there is comorbidity of CRSwNP in patients with severe asthma and blood eosinophils between 150 and 1500 [Citation12]. Whether this advice can be generalized to all patients or that certain subgroups do equally well or even better on anti-IL5 or anti-IgE treatment has to be determined. In this group of patients that were not controlled with their first choice, half of the patients were ultimately controlled on dupilumab.
Choices have been influenced by the appearance of biologics on the market, the preferences of health care professionals and insurance companies.
For asthma, omalizumab has been available for the treatment of allergic asthma since 2005. The first anti-IL5 treatment was approved in 2015 (mepolizumab) and others followed. Dupilumab was only approved in 2019. For CRSwNP, the order of availability is almost reversed with dupilumab being the first approved biologic in 2019, omalizumab in 2020 and mepolizumab in 2021. For that reason and because of the fear of severe eosinophilia (as a potential side effect of dupilumab treatment) health care professionals treating severe asthma heavily relied on anti-IL5 treatment. Indeed, dupilumab seems to be the biologic with most adverse events, mainly hyper-eosinophilia which can be difficult to treat in a minority of patients. This can be a reason to switch to or combine with an anti-IL5 treatment.
The available data until now seem to point to the advice to start with dupilumab in patients with severe asthma and eosinophils between 150 and 1500. However, further relevant biomarkers for optimal precision medicine are lacking and in the near future, we anticipate there will be a small, but difficult group who need to switch biologics or even combine them.
Studies evaluating precision medicine and relevant biomarkers are needed to improve our care that is now too often based on trial and error.
In our opinion, the evaluation of biologic efficacy should cover both the upper and lower airways. It is, for example, not sufficient to be satisfied when a patient has attained completely controlled asthma, but still has no sense of smell and a completely blocked nose. Nor can we accept insufficiently controlled asthma when the upper airways are doing well. In such cases, a multidisciplinary approach is advised to estimate the chances of a successful switch. As described, patient numbers will be small, but it seems that switching can be performed safely if under close monitoring. Again, the evaluation following such a switch should cover both the upper and lower airways. In the rare case that a patient cannot achieve disease control in both areas with a single biologic, double treatment could be considered. In our opinion, this is an important reason for referral to a tertiary care center with a wide experience in biological treatment.
Article highlights
Since clear guidelines and predictors for optimal biological choice are missing, patients are sometimes required to switch biologic therapy in order to find the optimal drug for their specific condition
Evaluation of biological treatment should include upper and lower airways
Biological treatment of patients with severe CRSwNP and asthma failing to achieve control with the first biological warrants a multi-disciplinary evaluation
When disease control in both upper- and lower airways cannot be achieved during treatment with a single biologic, treatment with two biologics combined could be considered
When switching from anti-IL5 treatment to dupilumab, dupilumab can best be started directly after the anti-IL5 treatment to prevent hyper-eosinophilia.
Declaration of interest
R van der Lans has acted as a consultant and/or advisory board member for GSK. W Fokkens is an advisory board member of Sanofi, GSK, and Dianosic. S Reitsma has acted as a consultant and/or advisory board member for Sanofi, GSK, and Novartis. The department of Otorhinolaryngology and Head/Neck Surgery of the Amsterdam UMC has received research funding from Sanofi, GSK, and Novartis. L Klimek has received research grants from Allergy Therapeutics/Bencard, Great Britain/Germany; ALK-Abelló, Denmark; Allergopharma, Germany; ASIT Biotech, Belgium; AstraZeneca, Sweden, Bionorica, Germany; Biomay, Austria, Boehringer Ingelheim, Germany, Circassia, U.S.A; Stallergenes, France; Cytos, Switzerland; Curalogic, Denmark; HAL, Netherlands; Hartington, Spain; Lofarma, Italy; Viatris/Mylan, U.S.A; Novartis, Switzerland, Leti, Spain; ROXALL, Germany; GlaxoSmithKline (GSK), Great Britain; Sanofi, France and/or has served on the speaker’s bureau or was consulting for the above mentioned pharmaceutical companies.
L Klimek is the current President of German Society of Allergology AeDA, Vice-President of the European Academy for Allergy and Clinical Immunology (EAACI), Vice-President of German Academy for Allergy and Environmental Medicine and Editor-in-Chief of AllergoJournal and AllergoJournal International. EDC reports lecture fees and participations in experts board meeting of GSK, Novartis, Sanofi, Astrazeneca. M Bonini has received speaker and advisory board fees from AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmith Kline, Menarini, and Sanofi. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
R van der Lans, J Otten, K Dziadziulia, C Montuori, B Hilvering, E Weersink, M Bonini, J Hagemann, W Thaitrakool, E de Corso, L Klimek, S Reitsma, W Fokkens collected data for the paper. R van der Lans, J Otten, W Thaitrakool, W Fokkens analyzed the data and prepared the tables and figures S Reitsma, L Klimek, E de Corso and W Fokkens designed the analysis All authors reviewed, commented and approved the final manuscript for publication.
Additional information
Funding
References
- Hopkins C, Surda P, Walker A, et al. EPOS 4 Patients. Rhinology. 2021;0(0):1–57. DOI:10.4193/Rhin20.950
- Hellings PW, Fokkens WJ, Orlandi R, et al. The EUFOREA pocket guide for chronic rhinosinusitis. Rhinology. 2022;0(0):0–0. DOI:10.4193/Rhin22.344
- Fokkens WJ, Lund VJ, Hopkins C, et al. European position paper on rhinosinusitis and nasal polyps 2020. Rhinology. 2020;58(Suppl S29):1–464. DOI:10.4193/Rhin20.401
- Porsbjerg CM, Menzies-Gow AN, Tran TN, et al. Global variability in administrative approval prescription criteria for biologic therapy in severe asthma. J Allergy Clin Immunol Pract. 2022;10(5):1202–16.e23. DOI:10.1016/j.jaip.2021.12.027
- Agache I, Song Y, Alonso-Coello P, et al. Efficacy and safety of treatment with biologicals for severe chronic rhinosinusitis with nasal polyps: a systematic review for the EAACI guidelines. Allergy. 2021;76(8):2337–2353. DOI:10.1111/all.14809
- Agache I, Beltran J, Akdis C, et al. Efficacy and safety of treatment with biologicals (benralizumab, dupilumab, mepolizumab, omalizumab and reslizumab) for severe eosinophilic asthma. A systematic review for the EAACI Guidelines - recommendations on the use of biologicals in severe asthma. Allergy. 2020;75(5):1023–1042. DOI:10.1111/all.14221
- van der Lans RJL, Fokkens WJ, Adriaensen G, et al. Real-life observational cohort verifies high efficacy of dupilumab for chronic rhinosinusitis with nasal polyps. Allergy. 2022;77(2):670–674. DOI:10.1111/all.15134
- De Corso E, Settimi S, Montuori C, et al. Effectiveness of dupilumab in the treatment of patients with severe uncontrolled CRSwNP: a “Real-life” observational study in the first year of treatment. J Clin Med. 2022;11(10):2684. DOI:10.3390/jcm11102684
- Menzies-Gow AN, McBrien C, Unni B, et al. Real world biologic use and switch patterns in severe asthma: data from the international severe asthma registry and the US CHRONICLE study. J Asthma Allergy. 2022;15:63–78.
- Kallieri M, Zervas E, Fouka E, et al. Relight: a two-year REal-LIfe study of mepolizumab in patients with severe eosinophilic asThma in Greece: evaluating the multiple components of response. European Respiratory Journal. 2022;60:468. DOI:10.1183/13993003.congress-2022.468
- Campo P, Soto Campos G, Moreira A, et al. Real-life study in non-atopic severe asthma patients achieving disease control by omalizumab treatment. Allergy. 2021;76(6):1868–1872. DOI:10.1111/all.14668
- Renner A, Marth K, Patocka K, et al. Benralizumab rapidly improves asthma control in Austrian real-life severe eosinophilic asthmatics. Allergy. 2020;75(12):3272–3275.
- Pavord ID, Hanania NA, Corren J. Controversies in allergy: choosing a biologic for patients with severe asthma. J Allergy Clin Immunol Pract. 2022;10(2):410–419. DOI:10.1016/j.jaip.2021.12.014
- Peters AT, Han JK, Hellings P, et al. Indirect treatment comparison of biologics in chronic rhinosinusitis with nasal polyps. J Allergy Clin Immunol Pract. 2021;9(6):2461–71 e5. DOI:10.1016/j.jaip.2021.01.031
- Bavaro N, Gakpo D, Mittal A, et al. Efficacy of dupilumab in patients with aspirin-exacerbated respiratory disease and previous inadequate response to anti-IL-5 or anti-IL-5Ralpha in a real-world setting. J Allergy Clin Immunol Pract. 2021;9(7):2910–2 e1. DOI:10.1016/j.jaip.2021.02.020
- Oykhman P, Paramo FA, Bousquet J, et al. Comparative efficacy and safety of monoclonal antibodies and aspirin desensitization for chronic rhinosinusitis with nasal polyposis: a systematic review and network meta-analysis. J Allergy Clin Immunol. 2022;149(4):1286–1295. DOI:10.1016/j.jaci.2021.09.009
- Akenroye A, Lassiter G, Jackson JW, et al. Comparative efficacy of mepolizumab, benralizumab, and dupilumab in eosinophilic asthma: a Bayesian network meta-analysis. J Allergy Clin Immunol. 2022;150(5):1097–105.e12. DOI:10.1016/j.jaci.2022.05.024
- Lommatzsch M, Suhling H, Korn S, et al. Safety of combining biologics in severe asthma: asthma-related and unrelated combinations. Allergy. 2022;77(9):2839–2843. DOI:10.1111/all.15379