ABSTRACT
Background
Primary Raynaud’s phenomenon (pRP) is difficult to distinguish from secondary (sRP). Although nailfold capillaroscopy (NFC) may detect early alterations, no universal criteria yet discriminate between pRP from sRP.
Objectives
To create and validate two NFC scores that could distinguish pRP from sRP and that could predict systemic sclerosis (SSc), respectively.
Methods
We performed NFC on two separate cohorts with isolated RP, and recorded number of capillaries per field, enlarged/giant capillaries, crossed/bizarre patterns, microhemorrhages, neoangiogenesis, rarefaction, edema, blood flow velocity, stasis. By multivariate regression analysis, we evaluated the adjusted prognostic role of these features in a derivation cohort of 656 patients. Results were used to construct algorithm-based prognostic scores (A and B). These scores were then tested on a confirmation cohort of 219 patients.
Results
Score A was unable to discriminate sRP from pRP (low negative predictive values with high positive predictive values for any cut-point); score B was unable to discriminate progression to SSc or a SSc-spectrum disorder (low positive predictive values with high negative predictive values for lower cut-points).
Conclusion
NFC patterns, believed as specific, showed low discriminatory power and on their own are unable to reliably discriminate sRP from pRP or predict evolution to SSc.
1. Introduction
Raynaud’s phenomenon (RP) is an episodic vasospastic ischemic disorder first described by Maurice Raynaud in 1862 [Citation1]. It is characterized by an initial white discoloration (pallor) of the digits as a reaction to cold (or emotional stress), followed by cyanosis, pain, and numbness, then by post-ischemic red flush upon re-warming [Citation2]. RP has a prevalence of 3 to 21% worldwide, depending on weather conditions and ethnic origins [Citation3,Citation4]. It presents at a young age, may show a family clustering, associates with smoking [Citation5] and is more common in women: indeed, the incidence of RP is remarkably higher in premenopausal females or post-menopausal females on estrogen replacement therapy [Citation6], suggesting that different mechanisms influence the expression of RP in men and women [Citation7]. The distinction between primary RP (pRP) and secondary RP (sRP) is diagnostically and prognostically important, but their discrimination is quite impossible during the first months or years of presentation [Citation8,Citation9].
Systemic sclerosis (SSc) is a rare autoimmune disease characterized by small vessel vasculopathy, autoantibody production, and excessive collagen deposition within the skin and internal organs (lungs, heart, kidney, and gastro-intestinal tract) [Citation10]. The diagnosis of SSc and, consequently, appropriate treatment is often delayed until the appearance of skin involvement and/or clinically detectable internal organ involvement when microvascular remodeling, fibrosis, or atrophy are already irreversible, making SSc one of the most challenging autoimmune disorders to manage [Citation11].
Nailfold capillaroscopy (NFC) is an inexpensive and easy-to-perform noninvasive test which may detect early microvascular alterations in patients who present with RP [Citation12]. Although this technique has been used for decades, there are no homogeneous and widely accepted capillaroscopic criteria to distinguish pRP from sRP. Similarly, the ‘capillaroscopic scleroderma pattern’ is well defined when SSc is already established but the relevance of capillary abnormalities in predicting the development of SSc in a patient that presents only with RP remains unclear [Citation13].
As the ACR/EULAR revised their classification criteria [Citation14] and defined Very Early Diagnosis of Systemic Sclerosis (VEDOSS), placing NFC in a prominent place, it seemed useful to have a capillaroscopic scoring system that could discriminate sRP from pRP, and another which could predict the development of SSc in a patient with RP.
The aim of this study was to create and validate two NFC scores that could distinguish pRP from sRP and that could predict systemic sclerosis, respectively.
2. Patients and methods
This is a randomized observational prospective cohort study over a total period of 19 years. An independent researcher using computer-generated random numbers performed a 3:1 block randomization allowing the formation of the Derivation and the Confirmation cohorts of subjects with RP but no other associated symptoms or signs at allocation. The operator performing NFC was blind to the allocation made by the independent researcher.
2.1. Study groups
All subjects consecutively referred to our Internal Medicine and Autoimmune Diseases outpatient clinics from January 2004 to December 2016 were invited to participate in the study. The inclusion criterion was the diagnosis of RP (with no other associated signs or symptoms), defined as episodic, reversible vasospastic ischemia of the digits manifesting upon exposure to cold and/or in association with emotional stress, and characterized by well-demarcated blanching, possibly leading to cyanosis, followed by post-ischemic red flushing upon rewarming. The exclusion criteria were: RP in association with symptoms or signs already suggesting a connective tissue disease [i.e. skin/mucosal (pitting scars, gangrene, sclerodactyly, photosensitivity, rash, telangiectasias, orogenital ulcers), ocular (xerophthalmia, episcleritis, scleritis, uveitis), musculoskeletal (arthralgia, arthritis, myalgia, muscle weakness, fatigue), cardiopulmonary (serositis, dyspnea, pulmonary fibrosis and/or hypertension, heart ischemia, conduction disturbance), renal (nephrotic syndrome, glomerulonephritis), hematological (leukopenia, anemia, thrombocytopenia) or gastroenteric manifestations (xerostomia, dysphagia, esophageal hypomotility, malabsorption)], neuropathic, traumatic, or iatrogenic syndromes.
2.2. Main outcome variable
Our main outcome was the generation of predictive scores as described in the Statistical section: score A for the prediction of sRP from pRP, and score B for the prediction of SSc or SSc-spectrum disorder (polymyositis, anti-synthetase syndrome, dermatomyositis, mixed connective tissue disease with SSc phenotype, overlap syndrome with SSc) from other RP-associated systemic rheumatic diseases. This was achieved via two primary end-points. End-point A was the appearance of a RP-associated systemic rheumatic disease that allowed discrimination of sRP from pRP; after the initial NFC, participants were followed-up in our clinic with periodic appointments (every 3 to 6 months) during which we performed a full set of diagnostic tests until it was possible to diagnose or to exclude an RP-associated systemic rheumatic disease: at this point, they were classified as having sRP or pRP, respectively. End-point B was the ability of NFC to predict the appearance of either SSc or a SSc-spectrum disorder in the participants who developed sRP.
2.3. Other variables
Age, gender, smoking habits, hormonal therapy, use of anti-platelets, anticoagulants, vasodilators and steroids were registered and used as covariates in the generation of score A and score B as indicated in the Statistical section.
2.4. Procedures
Each participant was asked to refrain from caffeine-containing beverages and smoking for 2 h prior to the NFC examination and to wait in a temperature-controlled room (20–22°C) for a minimum of 20 min before the test. After laying a drop of immersion oil onto the nailfold bed, eight fingers of each patient were examined using a Digital Videocap 200 (DS-Medica, Milan, Italy) with a 200× optic probe magnification. Fingers affected by recent trauma were not analyzed. Images were captured, coded and stored using Videocap 8.14 software. All of the nailfold areas where capillary visibility was good were scanned for 11 parameters: number of capillaries per field (defined as the mean number of capillaries in a 1-mm length of each finger), enlarged capillaries (≥20 µm loop width), giant capillaries (≥50 µm loop width), minor morphological abnormalities (crossed patterns), major morphological abnormalities (bushy and bizarre patterns), microhemorrhages, neoangiogenesis, avascular isolated areas, interstitial edema, and semi-quantitative changes in blood flow (velocity and erythrocyte stasis), as previously reported in the literature [Citation15]. NFC images were first analyzed and interpreted by the operator, confirmed later by another researcher, and data was recorded.
After NFC, participants were followed-up as described in main outcome variable section until December 2022 (recruitment ended in December 2016).
The ethics committee of our hospital approved the research protocol (2004/CEHFF, revised in 2018: 15/2018). Informed consent was obtained from all individual participants (or their legally authorized representative) included in the study, which was carried out according the Declaration of Helsinki.
2.5. Statistical analysis
In the Derivation Cohort, collected covariates (age, gender, smoking habits and current drugs) were screened for potential confounding through univariate tests for an association with the outcomes and capillaroscopic variables. A univariate logistic regression analysis was performed for each outcome. Capillaroscopic variables and covariates were selected for inclusion in a multivariate model based on a p-value <0,100. Multi-co-linearity was excluded through variance inflation factor calculation, and specification error through a link test. A simplified model was developed through backward elimination of non-significant variables for each outcome, as long as it did not significantly impair performance as evaluated by a likelihood-ratio test and by the Hosmer and Lemeshow’s goodness-of-fit test.
For both final simplified models, a score was developed from the regression coefficients, normalized by division to the lowest coefficient and rounded to the unit: score A for the prediction of sRP (versus pRP), and score B for the prediction of SSc or SSc-spectrum disorder (versus other RP-associated diseases). Each final score was accepted if the areas under the ROC curves for the score and its originating model were not significantly different when compared by a non-parametric ROC curve analysis [Citation16]. For each score/outcome we developed a classification table with sensitivity, specificity and positive and negative predictive values for each possible cut-point, as well as a ROC curve with the corresponding 95% confidence intervals. The scores were then applied to our Confirmation Cohort and the same methods were used to build classification tables and ROC curves.
3. Results
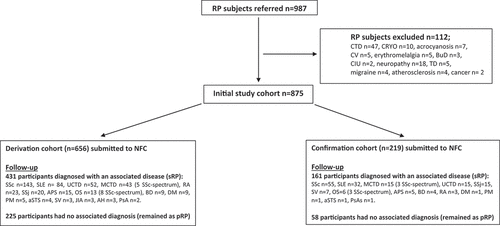
During the study period 987 subjects with RP were referred to our clinics but 112 were excluded as they did not meet our inclusion criteria: 47 had already signs or symptoms of a connective tissue disease, 32 had RP in the context of other acro-syndromes and 33 had RP associated with other diseases (see Flowchart); this left 875 participants who met the inclusion criteria and who contributed to our derivation and confirmation cohorts in the 3:1 ratio.
Flowchart leading to the formation of our derivation and confirmation cohorts.
3.1. Derivation cohort
The derivation cohort consisted of 656 RP participants (mean age 44.4 ± 17.1 years, 88.4% women) of whom 11.3% reported active smoking habits, 44% were on oral contraception, 5.5% were on anti-platelet/anticoagulant agents, 8.4% were taking vasodilator medication and 12.7% were on steroids. These last subjects have been medicated with steroids by their physician even in the absence of associated symptoms or complications of Raynaud’s phenomenon. After a median follow-up of 10 years (IQR 8–14), the diagnosis of pRP remained unchanged in 225 participants: this was supported by persistently negative clinical findings as well as persistent negativity of ANA either by immunofluorescence or by immunoassay (if the former test was borderline); at variance, 431 participants developed clinical and immunological features of a rheumatic disease and were labeled as sRP (see Flowchart).
By univariate analysis (Chi-square test) we found a significant association (p˂0.001) between sRP and: 1) age, 2) steroid use, 3) reduced number of capillaries per field (˂7/mm), 4) enlarged capillaries, 5) giant capillaries, 6) major morphological abnormalities, 7) microhemorrhages, 8) moderate to severe interstitial edema, 9) reduced red blood cell velocity and 10) intermittent flux with sludge; hormonal therapy was negatively associated (p˂0.001) with sRP (). Despite its significant association with sRP, steroids did not enter the following analysis as they were considered an indication bias.
Table 1. Univariate analysis of covariates and nailfold capillaroscopic variables in derivation cohort.
A multivariate logistic model was developed and optimized through backward elimination, resulting in the best model with four variables, without loss of performance as assessed by a likelihood-ratio test and Hosmer and Lemeshow’s goodness-of-fit test. Regression coefficients were normalized to generate the predictive score A (). We generated a ROC curve, and we calculated the AUC: 0.738 (StdE 0.019; 95% CI 0.700–0.776) (). We calculated sensitivity, specificity, negative and positive predictive values for each cutoff value. Score A showed low negative predictive values (NPV) with high positive predictive values (PPV) for any cut-point. As we can see in , cut-point 1 is the one that allows you to classify most correctly and this percentage decreases as points in score increase.
Figure 1. ROC curves and AUC of score A in derivation and confirmation cohorts. Left: Score A in derivation cohort; Right: Score A in confirmation cohort.
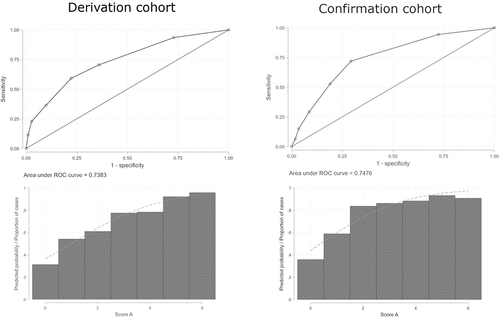
Table 2. Generation of scores A (left columns) and B (right columns) from derivation cohort.
Table 3. Score A in derivation and confirmation cohorts.
We generated score B from the 174 subjects with sRP associated with SSc (143) or one of its related diseases − 9 dermatomyositis, 8 overlap syndrome, 5 polymyositis, 5 mixed connective tissue disease, 4 anti-synthetase syndrome (see Flowchart).
By univariate analysis (Chi-square test) we found a significant association (p˂0.001) between SSc/SSc-spectrum disorders and age as well as with all the NFC variables studied except for minor abnormalities (). A multivariate logistic regression model was developed and optimized through backward elimination, resulting in the best model with four variables. Regression coefficients were normalized to generate score B (). We generated a ROC curve and calculated the AUC: 0.861 (StdE 0.016; 95% CI 0.829–0.893) (). We calculated sensitivity, specificity, NPV, and PPV for each cut-point. Score B showed low PPV with high NPV for lower cut-points (≤3). As we can see in , a subject who scores below 3 has a low probability to be correctly classified.
Figure 2. ROC curves and AUC of score B in derivation and confirmation cohorts. Left: Score B in derivation cohort; Right: Score B in confirmation cohort.
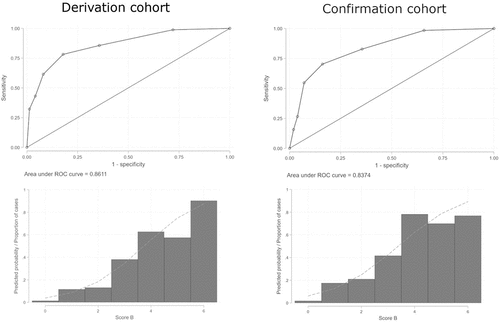
Table 4. Score B in derivation and confirmation cohorts.
3.2. Confirmation cohort
The confirmation cohort consisted of 219 RP participants (mean age 43.4 ± 16.1 years, 84.9% women) followed-up for a median of 11 years (IQR 9–15). At the end of this period, 58 participants remained as pRP and 161 developed a systemic autoimmune rheumatic disorder (see Flowchart).
We applied score A to this cohort, obtained a ROC curve and calculated the AUC: 0.748 (StdE 0.037; 95% CI 0.674–0.82) (). We calculated sensitivity, specificity, NPV, and PPV for each cut-point. As in derivation cohort, score A showed low NPV with high PPV for any cut-point ().
In a similar fashion, we applied score B to the 64 participants with sRP and SSc or SSc-spectrum disorder, which included 55 patients with SSc, 3 with MCTD, 3 with overlap syndrome, 1 with dermatomyositis, 1 with polymyositis, 1 with anti-synthetase syndrome (see Flowchart). We obtained a ROC curve for score B and calculated its AUC: 0.837 (StdE 0.029; 95% CI 0.781–0.894) (). We calculated sensitivity, specificity, NPV, and PPV for each cutoff. As in derivation cohort, score B showed low PPV with high NPV for lower cut-points (≤3) ().
4. Discussion
Having generated two nailfold capillaroscopy scoring systems, A and B, from our derivation cohort, we applied them to our confirmation cohort. Score A does not discriminate sRP from pRP, as a patient who scores ≥ 1 (out of 6) must be followed-up for years to come (low NPV) and therefore we cannot exclude almost anybody from further investigation. Thus, we still lack a universal NFC-based score to classify subjects into primary or secondary RP, implying that the current standardization of NFC patterns is still insufficient to discriminate between these two populations.
Primary RP is a functional vascular disorder [Citation17], but little is known about whether, when and why it becomes a structural disease. The transition rates from primary to secondary RP vary widely according to studies from 1% to 17%, and the incidence of the transition depends on the geographical origin of the patient cohort [Citation18,Citation19]. Moreover, previous studies identified various clinical and laboratory criteria that helped to explain the possible outcomes of patients with RP: criteria were set up to differentiate sRP from pRP but were non-diagnostic and simply reflected the result of a screening process [Citation2,Citation8,Citation9,Citation19–21]. However, it is important to diagnose sRP early on because it might be associated with a severe underlying disease that could shorten life expectancy. It is quite difficult to predict whether and when patients with isolated RP transit into SSc: hence, it would be of great clinical relevance to have a tool to identify at baseline those who will develop progressive disease. Contrary to previous studies [Citation5], smoking habits did not show any association with NFC parameters or with both primary and secondary outcomes. Similarly, the use of relevant medication like anti-platelets, anticoagulants, vasodilators and steroids did not act as covariates but for oral contraception/hormone replacement therapy, which showed significant association with primary RP, with no relevant changes in capillaroscopy, proving to be harmless in this context, as previously suggested [Citation6].
Score B does not predict who will develop SSc (or a SSc-spectrum disorder). In fact, score B shows low PPV at lower cut-points: by the time a patient achieves a score ≥ 3 (out of 6) she/he may already have developed SSc or SSc spectrum disorder, negating the predictive value of NFC.
At a first glance our findings contradict what the literature has been trying to prove over the past decades. We have shown that capillaroscopic findings such as giant capillaries, traditionally described as specific for SSc, were consistently present in diseases quite distinct from SSc, like genetic and metabolic myopathies [Citation22]. Nevertheless, NFC is a widely accepted in vivo imaging technique that provides a detailed picture of skin microvasculature. It has been receiving increased attention as a potential prognostic tool because it is simple, noninvasive, and inexpensive. In fact, several groups not only confirmed the association between NFC alterations and disease duration but also highlighted a correlation between different capillaroscopic scleroderma patterns (early, active, late) and the presence of autoantibodies (anti-centromere, anti – topoisomerase I) as well as different cutaneous subsets [Citation23,Citation24]. Ingegnoli et al. created PRINCE, a prognostic model in which the variables giant loops, hemorrhages, and number of capillaries helped to identify patients at higher risk of developing SSc [Citation25]. However, in their subsequent studies, hemorrhages lost their importance, and autoantibodies had to be added to the prognostic model [Citation26,Citation27].
In SSc, NFC may also help in the evaluation of disease severity [Citation23,Citation28–30] as it correlates with visceral involvement [Citation31–34] and predicts death [Citation35]. Furthermore, a reduced number of capillaries per field and the presence of avascular areas had a high PPV for interstitial lung disease (ILD), and a significant association with a worse pulmonary function: these parameters correlated with forced vital capacity (FVC) and carbon monoxide diffusing capacity (DLCO) [Citation36]. Therefore, NFC may be used to evaluate SSc activity as well as response to treatment; some studies revealed that a change in capillaries match an improvement of SSc visceral involvement after immunosuppressive therapy [Citation37,Citation38]. Moreover, NFC may play a role in predicting the development of digital ulcers or lesions in patients with SSc as indicated by the proposed CSURI index [Citation39–41]. While optical coherence tomography has not met the expectations as a predictive tool [Citation42,Citation43], laser doppler perfusion imaging may provide better quantification of microvascular damage and improved standardization of capillaroscopic patterns [Citation44,Citation45]. Artificial intelligence in image interpretation will be a reality in the near future but for now there is still controversy about it [Citation46–49].
There is no consensus about the length of time one has to wait to surely exclude a secondary cause for RP, but an active median follow-up of 10 and 11 years in our derivation and confirmation cohorts, respectively, with no one being followed-up less than 5 years, can be considered a reasonable observational period. The large sample, the strict baseline enrollment rules, and the validation on the confirmation cohort overcame eventual weaknesses of the study.
5. Conclusions
Our study demonstrates that NFC not only lack discriminatory power between pRP and sRP, but also lacks discriminatory power between sRP associated SSc/SSc-spectrum of diseases and other systemic autoimmune rheumatic diseases. NFC is a great tool to monitor disease activity and response to treatment in established SSc and related diseases, but it may not help clinicians to decide whether to indefinitely follow-up patients with RP in the expectation they might develop any rheumatic disease. RP continues to be the most common clinical condition warranting a capillaroscopy [Citation12], but the result of this test should always be interpreted with caution and surely not to be taken as a conclusive test alone.
Article highlights
Distinction between primary and secondary Raynaud’s phenomenon can be challenging;
Nailfold capillaroscopy lacks discriminatory power between pRP and sRP;
In early stages nailfold capillaroscopy is unable to predict who will develop systemic sclerosis;
We must start looking at NFC in a different way, thinking how we can improve or complement it.
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Author contributions
M Amaral, P Ames and J Delgado Alves contributed to the study conception and design. Material preparation, data collection and analysis were performed by M Amaral, F Seguro Paula, J Caetano and J Delgado Alves. The first draft of the manuscript was written by M Amaral and all authors commented on previous versions of the manuscript and on revision. All authors read and approved the final manuscript.
All authors take full responsibility for the integrity and accuracy of all aspects of the work.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Ethics Statement
The ethics committee of our hospital approved the research protocol (2004/CEHFF, revised in 2018: 15/2018). Informed consent was obtained from all individual participants (or their legally authorized representative) included in the study, which was carried out according the Declaration of Helsinki.
Additional information
Funding
References
- Raynaud M. De l’asphyxie locale et de la gangrène symétrique des extrémités. Edition originale ed. Paris: L. Leclerc; 1862. p. 1862.
- Block JA, Sequeira W. Raynaud’s phenomenon. Lancet. 2001 Jun 23;357(9273):2042–2048. doi: 10.1016/S0140-6736(00)05118-7
- Maricq HR, Carpentier PH, Weinrich MC, et al. Geographic variation in the prevalence of Raynaud’s phenomenon: a 5 region comparison. J Rheumatol. 1997 May;24(5):879–889.
- Brand FN, Larson MG, Kannel WB, et al. The occurrence of Raynaud’s phenomenon in a general population: the framingham study. Vasc Med. 1997 Nov;2(4):296–301. doi: 10.1177/1358863X9700200404
- Garner R, Kumari R, Lanyon P, et al. Prevalence, risk factors and associations of primary Raynaud’s phenomenon: systematic review and meta-analysis of observational studies. BMJ Open. 2015 Mar 16;5(3):e006389. doi: 10.1136/bmjopen-2014-006389
- Fardoun MM, Nassif J, Issa K, et al. Raynaud’s phenomenon: a brief review of the underlying mechanisms. Front Pharmacol. 2016;7:438. doi: 10.3389/fphar.2016.00438
- Fraenkel L, Zhang Y, Chaisson CE, et al. Different factors influencing the expression of Raynaud’s phenomenon in men and women. Arthritis Rheum. 1999 Feb;42(2):306–310.
- LeRoy EC, Medsger TA Jr. Raynaud’s phenomenon: a proposal for classification. Clin Exp Rheumatol. 1992 Sep;10(5):485–488.
- Maverakis E, Patel F, Kronenberg DG, et al. International consensus criteria for the diagnosis of Raynaud’s phenomenon. J Autoimmun. 2014 Feb;48–49:60–65.
- Coral-Alvarado P, Pardo AL, Castaño-Rodriguez N, et al. Systemic sclerosis: a world wide global analysis. Clin Rheumatol. 2009 Jul;28(7):757–765.
- Denton CP, Khanna D. Systemic sclerosis. Lancet. 2017 Oct 7;390(10103):1685–1699. doi: 10.1016/S0140-6736(17)30933-9
- Cutolo M, Pizzorni C, Sulli A. Capillaroscopy. Best Pract Res Clin Rheumatol. 2005 Jun;19(3):437–452. doi: 10.1016/j.berh.2005.01.001
- Paxton D, Pauling JD. Does nailfold capillaroscopy help predict future outcomes in systemic sclerosis? A systematic literature review. Semin Arthritis Rheum. 2018 Dec;48(3):482–494. doi: 10.1016/j.semarthrit.2018.02.005
- van den Hoogen F, Khanna D, Fransen J, et al. 2013 classification criteria for systemic sclerosis: an American college of rheumatology/european league against rheumatism collaborative initiative. Arthritis Rheum. 2013 Nov;65(11):2737–2747.
- Cutolo M, Grassi W, Matucci Cerinic M. Raynaud’s phenomenon and the role of capillaroscopy. Arthritis Rheum. 2003 Nov;48(11):3023–30. doi: 10.1002/art.11310
- DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988 Sep;44(3):837–845. doi: 10.2307/2531595
- Herrick AL. Pathogenesis of Raynaud’s phenomenon. Rheumatology (Oxford). 2005 May;44(5):587–96. doi: 10.1093/rheumatology/keh552
- Ziegler S, Brunner M, Eigenbauer E, et al. Long-term outcome of primary Raynaud’s phenomenon and its conversion to connective tissue disease: a 12-year retrospective patient analysis. Scand J Rheumatol. 2003;32(6):343–7. doi: 10.1080/03009740410005007
- Suter LG, Murabito JM, Felson DT, et al. The incidence and natural history of Raynaud’s phenomenon in the community. Arthritis Rheum. 2005 Apr;52(4):1259–63.
- Smith V, Vanhaecke A, Herrick AL, et al. Fast track algorithm: how to differentiate a “scleroderma pattern” from a “non-scleroderma pattern”. Autoimmun Rev. 2019 Nov;18(11):102394.
- Smith V, Herrick AL, Ingegnoli F, et al. Standardisation of nailfold capillaroscopy for the assessment of patients with Raynaud’s phenomenon and systemic sclerosis. Autoimmun Rev. 2020 Mar;19(3):102458.
- Seguro Paula F, Ferreira IA, Amaral MC, et al. Systemic sclerosis-related changes on nailfold videocapillaroscopy in genetic and metabolic myopathies. Rheumatology (Oxford). 2016 Oct;55(10):1911–2. doi: 10.1093/rheumatology/kew249
- Cutolo M, Pizzorni C, Tuccio M, et al. Nailfold videocapillaroscopic patterns and serum autoantibodies in systemic sclerosis. Rheumatology (Oxford). 2004 Jun;43(6):719–26.
- Caramaschi P, Canestrini S, Martinelli N, et al. Scleroderma patients nailfold videocapillaroscopic patterns are associated with disease subset and disease severity. Rheumatology (Oxford). 2007 Oct;46(10):1566–9.
- Ingegnoli F, Boracchi P, Gualtierotti R, et al. Prognostic model based on nailfold capillaroscopy for identifying Raynaud’s phenomenon patients at high risk for the development of a scleroderma spectrum disorder: PRINCE (prognostic index for nailfold capillaroscopic examination). Arthritis Rheum. 2008 Jul;58(7):2174–82. doi: 10.1002/art.23555
- Ingegnoli F, Gualtierotti R, Lubatti C, et al. Feasibility of different capillaroscopic measures for identifying nailfold microvascular alterations. Semin Arthritis Rheum. 2009 Feb;38(4):289–95.
- Ingegnoli F, Boracchi P, Gualtierotti R, et al. Improving outcome prediction of systemic sclerosis from isolated Raynaud’s phenomenon: role of autoantibodies and nail-fold capillaroscopy. Rheumatology (Oxford). 2010 Apr;49(4):797–805.
- Camargo CZ, Sekiyama JY, Arismendi MI, et al. Microvascular abnormalities in patients with early systemic sclerosis: less severe morphological changes than in patients with definite disease. Scand J Rheumatol. 2015;44(1):48–55. doi: 10.3109/03009742.2014.926566
- Ingegnoli F, Ardoino I, Boracchi P, et al. Nailfold capillaroscopy in systemic sclerosis: data from the EULAR scleroderma trials and research (EUSTAR) database. Microvasc Res. 2013 Sep;89:122–8.
- Arana-Ruiz JC, Silveira LH, Castillo-Martínez D, et al. Assessment of nailfold capillaries with a handheld dermatoscope may discriminate the extent of organ involvement in patients with systemic sclerosis. Clin Rheumatol. 2016 Feb;35(2):479–82.
- Sato LT, Kayser C, Andrade LE. Nailfold capillaroscopy abnormalities correlate with cutaneous and visceral involvement in systemic sclerosis patients. Acta Reumatol Port. 2009 Apr;34(2a):219–227.
- Marino Claverie L, Knobel E, Takashima L, et al. Organ involvement in Argentinian systemic sclerosis patients with “late” pattern as compared to patients with “early/active” pattern by nailfold capillaroscopy. Clin Rheumatol. 2013 Jun;32(6):839–43.
- Ong YY, Nikoloutsopoulos T, Bond CP, et al. Decreased nailfold capillary density in limited scleroderma with pulmonary hypertension. Asian Pac J Allergy Immunol. 1998 Jun;16(2–3):81–6.
- Bredemeier M, Xavier RM, Capobianco KG, et al. Nailfold capillary microscopy can suggest pulmonary disease activity in systemic sclerosis. J Rheumatol. 2004 Feb;31(2):286–94.
- Kayser C, Sekiyama JY, Próspero LC, et al. Nailfold capillaroscopy abnormalities as predictors of mortality in patients with systemic sclerosis. Clin Exp Rheumatol. 2013 Mar;31(2 Suppl 76):103–8.
- Caetano J, Paula FS, Amaral M, et al. Nailfold videocapillaroscopy changes are associated with the presence and severity of systemic sclerosis-related interstitial lung disease. J Clin Rheumatol. 2019 Apr;25(3):e12–e15. doi: 10.1097/RHU.0000000000000815
- Aschwanden M, Daikeler T, Jaeger KA, et al. Rapid improvement of nailfold capillaroscopy after intense immunosuppression for systemic sclerosis and mixed connective tissue disease. Ann Rheum Dis. 2008 Jul;67(7):1057–9.
- Fleming JN, Nash RA, McLeod DO, et al. Capillary regeneration in scleroderma: stem cell therapy reverses phenotype? PLoS One. 2008 Jan 16;3(1):e1452. doi: 10.1371/journal.pone.0001452
- Sebastiani M, Manfredi A, Colaci M, et al. Capillaroscopic skin ulcer risk index: a new prognostic tool for digital skin ulcer development in systemic sclerosis patients. Arthritis Rheum. 2009 May 15;61(5):688–94. doi: 10.1002/art.24394
- Sebastiani M, Manfredi A, Vukatana G, et al. Predictive role of capillaroscopic skin ulcer risk index in systemic sclerosis: a multicentre validation study. Ann Rheum Dis. 2012 Jan;71(1):67–70.
- Cutolo M, Herrick AL, Distler O, et al. Nailfold videocapillaroscopic features and other clinical risk factors for digital ulcers in systemic sclerosis: a multicenter, prospective cohort study. Arthritis & Rheumat. 2016 Oct;68(10):2527–39.
- Pires NSM, Dantas AT, Duarte A, et al. Optical coherence tomography as a method for quantitative skin evaluation in systemic sclerosis. Ann Rheum Dis. 2018 Mar;77(3):465–466.
- Babalola O, Mamalis A, Lev-Tov H, et al. Optical coherence tomography (OCT) of collagen in normal skin and skin fibrosis. Arch Dermatol Res. 2014 Jan;306(1):1–9.
- Herrick AL, Berks M, Taylor CJ. Quantitative nailfold capillaroscopy-update and possible next steps. Rheumatology (Oxford). 2021 May 14;60(5):2054–2065. doi: 10.1093/rheumatology/keab006
- Herrick AL, Dinsdale G, Murray A. New perspectives in the imaging of Raynaud’s phenomenon. Eur J Rheumatol. 2020 Oct;7(Suppl –3):S212–s221. doi: 10.5152/eurjrheum.2020.19124
- Cutolo M, Trombetta AC, Melsens K, et al. Automated assessment of absolute nailfold capillary number on videocapillaroscopic images: proof of principle and validation in systemic sclerosis. Microcirculation. 2018 May;25(4):e12447.
- Garaiman A, Nooralahzadeh F, Mihai C, et al. Vision transformer assisting rheumatologists in screening for capillaroscopy changes in systemic sclerosis: an artificial intelligence model. Rheumatology (Oxford). 2023 Jul 5;62(7):2492–2500. doi: 10.1093/rheumatology/keac541
- Gracia Tello BC, Ramos Ibañez E, Saez Comet L, et al. External clinical validation of automated software to identify structural abnormalities and microhaemorrhages in nailfold videocapillaroscopy images. Clin Exp Rheumatol. 2023 Aug;41(8):1605–1611.
- Berks M, Dinsdale G, Murray A, et al. Automated structure and flow measurement - a promising tool in nailfold capillaroscopy. Microvasc Res. 2018 Jul;118:173–177.
