Abstract
The objective of this study was to investigate the effects of the concentrate proportion and Fusarium toxin-contaminated triticale (FCT) in the diet on nutrient degradation, microbial protein synthesis and structure of the microbial community, utilising a rumen simulation technique and single-strand conformation polymorphism (SSCP) profiles based on PCR-amplified small subunit ribosomal RNA genes. Four diets containing 60% or 30% concentrates on a dry matter basis with or without FCT were incubated. The fermentation of nutrients and microbial protein synthesis was measured. On the last day of incubation, microbial mass was obtained from the vessel liquid, DNA was extracted and PCR-primers targeting archaea, fibrobacter, clostridia, bifidobacteria, bacillii, fungi, and bacteria were applied to separately study the individual taxonomic groups with SSCP. The concentrate proportion affected the fermentation and the microbial community, but not the efficiency of microbial protein synthesis. Neither the fermentation of organic matter nor the synthesis and composition of microbial protein was affected by FCT. The fermentation of detergent fibre fractions was lower in diets containing FCT compared to diets with uncontaminated triticale. Except for the clostridia group, none of the microbial groups were affected by presence of FCT. In conclusion, our results give no indication that the supplementation of FCT up to a deoxynivalenol concentration in the diet of 5 mg per kg dry matter affects the fermentation of organic matter and microbial protein synthesis. These findings are independent of the concentrate level in the diets. A change in the microbial community composition of the genus Clostridia may be the reason for a reduction in the cellulolytic activity.
1. Introduction
Deoxynivalenol (DON) is a naturally occurring mycotoxin produced by several species of Fusarium moulds in a variety of cereal grains. Due to high proportions in diets for dairy cows, these grains can contribute to the toxin exposure of the animal. Ruminants appear to be less affected by DON than other species (Trenholm et al. Citation1984). This higher tolerance to DON is attributed to the toxin metabolisation by rumen microorganisms (for review see Seeling and Dänicke Citation2005). The orientation value for critical concentrations of DON in diets of ruminants was reported at 5 mg per kg feed at 88% DM (Dänicke et al. Citation2001; Anonymous Citation2006). However, an acid pH value (<5.2) inhibits the complete transformation of DON to its metabolites (He et al. Citation1992). The intake of a diet low in structure and high in rapidly degradable carbohydrates can continuously decrease the rumen pH value below 5.5. Therefore, the concentrate proportion of a ruminant diet could influence the detoxifying potential of the rumen.
As shown in complementary studies with dairy cows (Keese et al. Citation2008a, Citation2008b), a DON concentration of up to 5.3 mg per kg affected neither the animal performances nor the fermentation parameters significantly. Most of the observed effects were related to an increase in DM intake that was attributed to the higher proportion of concentrates. Information about the amount and composition of the microbial protein are not available.
One aim of the present study was to determine the effects of the concentrate proportion in diets of dairy cows with or without Fusarium toxin-contaminated triticale (FCT) on fermentation and the capacity and quality of the microbial protein synthesis in vitro. In vitro methods have the advantage to define precisely the experimental conditions. Interferences like variable feed intake or environmental factors can be eliminated and the costs are much lower than in animal studies. A DON concentration of 5 mg per kg was targeted in the contaminated diets in order to be close to the orientation value for ruminants.
Under culture laboratory conditions, rumen microorganisms such as Ruminococcus albus and Methanobrevibacter ruminantium (May et al. Citation2000) as well as strains of Butyrivibrio fibrisolvens (Westlake et al. Citation1987a) were not inhibited by DON concentrations greater than 100 μg per ml. Because many microorganisms cannot yet be cultured, there has been considerable interest in developing cultivation independent methods to study the microbial community in the rumen. In recent years, the genetic diversity of the rumen bacterial community has been revealed by sequence analysis of 16S ribosomal RNA gene (16S rRNA) (Tajima et al. Citation1999). For applied studies such as this one, techniques based on conformation polymorphism and gel electrophoretic separation of PCR products from small subunits of the 16S rRNA (Muyzer et al. Citation1993; Schwieger and Tebbe Citation1998) are superior to cloning and subsequent sequencing. They provide an immediate display of the dominant constituents of a microbial community and allow direct comparison of communities by evaluating the similarities of the profiles; therefore, they are less time-consuming and laborious when larger numbers of samples need to be compared.
Thus, the second aim of the present study was the description of the effect of FCT on the structural diversity of the domains bacteria, archaea, and fungi as well as of specific groups of rumen microorganisms like fibrobacter, clostridia, bifidobacteria, and bacillii in a rumen simulation system.
2 Experimental methods
2.1 Diets
Four mixed diets containing maize silage, grass silage, soybean meal, maize, and triticale were used (). The diets differed in the proportions of concentrates (60% and 30% on DM basis; diets C60 and C30, respectively) and the mycotoxin concentration, especially DON. The increase in the DON concentration in the diets was achieved by substitution of an uncontaminated triticale by FCT.
Table 1. Composition and analysed crude nutrients and deoxynivalenol.*
The uncontaminated triticale and FCT contained 0.06 and 41 mg DON per kg DM, respectively (Keese et al. Citation2008a). Further mycotoxins in the contaminated triticale like zearalenone, nivalenol, scirpentriol, 15-acetyldeoxynivalenol, and 3-acetyldeoxynivalenol were analysed at 435, 0.97, 0.09, 0.52, and 0.20 mg per kg DM, respectively. The concentration of these Fusarium toxins was lower than the detection limit in the uncontaminated triticale (Keese et al. Citation2008a).
The DON concentrations of both diets without FCT were <0.03 mg DON per kg DM (). The diets with FCT contained 6.9 and 5.8 mg DON per kg DM. Other Fusarium toxins were not analysed in the diets.
The digestibility of the nutrients from the diets were determined with four wether sheep each at the Institut für Tierernährung, Friedrich-Loeffler-Institut, Braunschweig, Germany, according to generally accepted German standards (AfBN Citation1991).
2.2 Rumen simulation and isolation of microbial mass
Each diet was incubated in triplicate using a rumen simulation system (Rusitec et al. Citation1977) with two times six reaction vessels. The experimental procedure has been described in detail by Boguhn et al. (Citation2006b). In brief, 15 g of the dried (65°C, 24 h) and ground (1 mm screen) diets were weighed into nylon bags and incubated in a rumen fluid-artificial saliva mixture. The inocula for starting the incubation were obtained from four rumen-cannulated sheep. Rumen contents from all sheep were mixed and filtered through two layers of linen cloth. Each feed container inside the reaction vessels contained two nylon bags. For the first 24 h of incubation, one bag was filled with pooled rumen solids (≈60 g) instead of feed. Daily, one bag was replaced by a new one, so that each bag was incubated for 48 h. The artificial saliva contained 0.7 mmol of ammonia per litre from ammonium chloride with a measured 15N excess of 11.5 atom% (Chemotrade Chemiehandelsgesellschaft mbH Leipzig, Germany) to quantify the de novo synthesis of microbial protein.
Samples of the feed residues and the liquid effluent were taken, beginning on day 7 of the incubation until the end of incubation on day 14. Each day, microbes were isolated from the liquid effluent by differential centrifugation (Boguhn et al. Citation2006b). The supernatant after the first step of centrifugation at 27,000 g and the microbial mass after two washing steps were pooled per reaction vessel, subsequently freeze-dried, and crushed with a pestle and mortar. The feed residues were dried at 65°C for 24 h, pooled per reaction vessel, and ground through a sieve with 1 mm pore size.
The calculation of fermented crude nutrients and detergent fibre fractions as well as of microbial protein synthesis was done according to Boguhn et al. (Citation2006b). The extent of fermentation was not corrected for the microbes attached to the feed residues. The breakdown of DON was determined as the difference between input with feed and output with feed residues in relation to the input of DON.
On the last day of incubation, the liquid content of each vessel was used to isolate microbial mass for DNA extraction. Forty millilitres of the liquid were centrifuged at 27,000 g and 4°C for 15 min. The remaining pellet was re-suspended in 10 ml of 0.9% (w/v) sodium chloride solution. Two millilitres of this suspension were stored in safe-lock tubes (Eppendorf AG, Hamburg, Germany) at −70°C until further preparation.
2.3 Chemical analysis
The crude nutrient and detergent fibre concentrations in the diets and feed residues after incubation were determined according to official methods (Naumann and Bassler Citation1976). The analysis of DON was conducted by high performance liquid chromatography with diode array detection after extraction by acetonitrile-water and clean-up with immunoaffinity columns (IAC; DONtest HPLC columns, VICAM, Watertown, USA) according to the modified method 16.12.1 (Naumann and Bassler Citation1976) described by Valenta et al. (Citation2002).
The 15N enrichment of N in dried samples from ammonium chloride, feed residues (ground through a sieve with 0.5 mm pore size), liquid effluents, and isolated microbes was measured by an isotope mass spectrometer (DELTA V Advantage; Thermo Fisher Scientific, Bremen, Germany) coupled with an elemental analyser (EuroEA; HEKAtech GmbH, Wegberg, Germany).
The analysis of ammonia content in the liquid effluent was done by steam distillation according to the procedure for Kjeldahl N analysis. The concentrations of short-chain fatty acids (SCFA) were analysed in the particle-free supernatant after the first step of centrifugation by gas chromatography (HP 6890 Plus, Hewlett-Packard, Acondale, USA) with a flame ionisation detector after treatment, as described by Geissler et al. (Citation1976).
Amino acid (AA) analysis of microbial protein was performed using an amino acid analyser (LC 3000, Eppendorf Biotronik, Maintal, Germany) following the method described by Rodehutscord et al. (Citation2004). In brief, after a performic acid oxidation step, samples were hydrolysed in 6 N HCl. Norleucine was used as the external standard. Tryptophan, histidine, and tyrosine were not determined. Amino acids were separated and detected using various citrate buffer solutions and ninhydrin. For comparison of the AA pattern, the relation between each individual AA and the sum of all analysed AA, expressed as g per 100 g of analysed AA, was used (Boguhn et al. Citation2006a).
2.4 DNA preparation and separation of PCR products using single-strand conformation polymorphism
DNA was extracted from 0.05–0.15 g of the suspensions by bead beating (FastPrep® Cell Disrupter FP120, Qbiogene, Inc., CA, USA; two times, speed 6.5 for 45 s) in the presence of sodium phosphate and a solution of sodium dodecylsulphate (10% (v/v)), sodium chloride (100 mmol), and tri(-hydroxymethyl)-aminomethane (500 mmol, pH 8.0). After centrifugation at 15,000 g and 4°C for 4 min, the DNA-containing supernatant was extracted with equal volumes of phenol-chloroform-isoamyl alcohol and chloroform-isoamyl alcohol (Lueders et al. Citation2004). Two hundred microlitres of the DNA extract were washed using EZNA® Tissue DNA Mini Kits (Peqlab Biotechnologie GmbH, Erlangen, Germany) following the manufacturer's protocol.
Aliquots of this preparation were visualised by ethidium bromide-stained 0.8% agarose gel to verify the quality of the extraction procedure. The amount of extracted DNA was quantified in the extracts using PicoGreen® (MoBiTec GmbH, Göttingen, Germany) at 485 nm (excitation) and 530 nm (emission) (Labsystems Fluoroscan II; GMI, Albertville, Minnesota, USA) according to the manufacturer's instructions.
Group-specific 16S rRNA genes from DNA extracts were amplified using one of six different primer systems to amplify small subunit rRNA genes of archaea, fibrobacter, clostridia, bifidobacteria, bacillii, and bacteria, respectively (). For fibrobacter and clostridia, the unspecific reverse primer for bacteria was used. The used primer pairs for PCR were checked for their specificity on the selected microbial groups using the ARB-Probe library (version from 26 May 2005, Ludwig et al. Citation2004).
Table 2. Universal and specific primer pairs used for the PCR amplification of different regions of the 16S rRNA.
Each PCR was performed with a TGradient Thermocycler (Biometra Biomedizinische Analytik GmbH, Göttingen, Germany) in a total volume of 25 μl, as described in detail by Boguhn et al. (Citation2008). The cycling conditions were as follows: initial denaturation at 95°C for 15 min; 25–30 cycles of denaturation at 94°C for 45 s (for archaea 30 s and bacillii 60 s); annealing for 45 s (for archaea 30 s and bacillii 60 s); elongation at 72°C for 60 s (for bacillii 70 s); and a final elongation at 72°C for 5 min. The annealing temperatures of the specific primers were 50, 54, 55, 59, 55, and 60°C for bacteria, archaea, clostridia, fibrobacter, bacillii, and bifidobacteria, respectively.
The products of the first PCR were diluted (1:10 with pure PCR water) and used as templates in a following PCR carried out with universal primers (). The universal primer pairs for the domain bacteria were also used for their phylogenetic groups clostridia, fibrobacter, bacillii, and bifidobacteria. Archaea-specific PCR products were amplified with a modified primer pair. The PCR products of fungal DNA were amplified with the diluted DNA extract (1:10 with pure PCR water) directly with the unspecific primers. In these cases, the reverse primers were phosphorylated at the 5′-end. With the exception of the primer concentration (0.5 μM of each primer), the reaction mixtures were identical to those described earlier. The cycling conditions were as follows: initial denaturation at 95°C for 15 min; 25 (bacteria and archaea) or 30 (fungi) cycles of denaturation at 94°C for 45 s (fungi 60 s); annealing for 45 s (fungi 60 s); and elongation at 72°C for 60 s (fungi 70 s). PCR amplifications were completed by 5 min at 72°C. The annealing temperatures of the unspecific primers were 50, 56, and 55°C for bacteria, archaea, and fungi, respectively.
Each of the pooled PCR products was purified (PCR Purification Kit, Qiagen, Hilden, Germany) and analysed for its expected size in a 1% agarose gel stained with ethidium bromide. The DNA concentration was measured fluorometrically with PicoGreen® as indicated above and diluted with pure PCR water to equal amounts of double-strand DNA in the samples (approximately 500 ng).
In order to obtain single-stranded DNA from PCR products, the phosphorylated strand was removed by lambda exonuclease digestion and then purified as described by Schwieger and Tebbe (Citation1998).
Single-strand conformation polymorphism (SSCP) profiles were generated on polyacrylamide gels (Schwieger and Tebbe Citation1998; Dohrmann and Tebbe Citation2004; Boguhn et al. Citation2008). Clean genomic DNA from Bacillus licheniformis, Rhizobium trifolii, Flavobacterium johnsoneae, and Rhizobium radiobacter (Deutsche Sammlung von Mikroorganismem und Zellkulturen GmbH [German Collection of Microorganisms and Cell Cultures], Braunschweig, Germany) was supplementarily prepared and applied onto the gels in duplicate as SSCP markers.
2.5 Statistical and image analysis
For data from the in vitro experiment, the MIXED procedure of the software package SAS for Windows (Version 9.2, SAS Inst., Cary, NC, USA) was used to detect significant treatment effects (t-test, p ≤ 0.05). Analysis of variance was done for the two fixed effects, concentrate proportion and FCT, and their interaction.
The digitalisation of the SSCP patterns to a binary matrix and the calculation of a similarity matrix were done using the software TotalLab TL120 (Version 2006e, Nonlinear Dynamics Ltd, Newcastle upon Tyne, UK) based on the Jaccard coefficient. The statistical analysis of the distances between the band patterns followed the methods of Anderson (Citation2001) as described in detail by Boguhn et al. (Citation2008). Differences among treatments were declared significant at Monte Carlo p ≤ 0.05 (Anderson and Robinson Citation2003).
The distances between the treatments were visualised by non-metric multidimensional scaling (NMDS) plots based on the Jaccard coefficient using the PaSt program (Palaeontological Statistics, Version 1.92; Hammer et al. Citation2001). The “stress” according to Kruskal (Citation1964) was used to describe the goodness of fit of the reproduced distances to the observed distances.
3 Results
The organic matter digestibility differed between 80 and 85%, depending on the concentrate proportion (). Additionally, the digestibilities of crude fibre and acid detergent fibre were significantly higher in diets containing 30% concentrates. Only small differences were found for the digestibility of neutral detergent fibre. No other effects of FCT on the digestibilities of nutrients were observed.
Table 3. Digested and fermented nutrients, breakdown of deoxynivalenol, concentrations of ammonia and short-chain fatty acids, and efficiency of microbial crude protein synthesis (means ± SD, n = 3).
The breakdown of DON ranged between 75 and 83% and was not significantly different between the treatments (). The extent of fermentation of organic matter and crude protein ranged from 38.7–43.3% and 37.4–43.2%, respectively, and was significantly affected by the concentrate proportion. A higher concentrate proportion significantly increased the organic matter fermentation and decreased the crude protein fermentation. No treatment effects were detected for the fermented crude fibre (on average 6.0%). In contrast, the fermentation of neutral and acid detergent fibre was significantly lower for the treatments with FCT compared to the uncontaminated triticale (p < 0.05).
The mean ammonia concentration in the liquid effluent ranged from 7.3–5.0 mMol per l and no significant differences were observed between the different treatments (). The concentration of acetate was significantly lower for the diets containing 60% concentrates (p = 0.03), whereas the concentration of isovalerate was lower for the treatments with 30% concentrates (p < 0.01). The inclusion of FCT decreased the concentration of isobutyrate in the liquid effluent. No other effects of FCT on the SCFA were observed. The ratio of acetate to propionate was on a low level between 1.6 and 1.8.
The efficiency of microbial protein synthesis varied between 141 and 147 g microbial crude protein per kg fermented organic matter for all treatments (). Five out of fifteen AA of the microbial proteins, namely arginine, aspartic acid, lysine, methionine, and serine, were affected by the concentrate proportion of the diet (p ≤ 0.03), but not by FCT ().
Table 4. Amino acid contents of the crude protein from microbes isolated via differential centrifugation from the liquid effluent of the rumen simulation [g amino acid per 100 g analysed amino acid] (means ± SD, n = 3).
The “stress” of the NMDS plots (<0.20, with the exception of the plot for the Fibrobacter-specific gel) indicated an acceptable agreement between the observed and the reproduced distances of the band patterns (). In most cases, the variability in assemblages of the points within a treatment was smaller than between the different treatments. The allocation of the points indicated a greater similarity between the treatment with or without FCT than between the treatments with different concentrate proportion. Points describing the band patterns of the treatments with 60% concentrates in the diet were assembled on the left side (clostridia-, fibrobacter- and bifidobacteria-specific gels) and on the bottom (bacteria- and bacillii-specific gels) of the NMDS plots, whereas those for the treatments with 30% concentrates were located on the right side and on the top, respectively.
Figure 1. Two-dimensional graph of non-metric multidimensional scaling on Jaccard distance for the band pattern of SSCP gels based on 16S rRNA of microbes isolated from the liquid in the vessels of the rumen simulation (triangles: 60% concentrates in the diet; squares: 30% concentrates in the diet; open symbols: without Fusarium toxin-contaminated triticale; closed symbols: with Fusarium toxin-contaminated triticale; *two or more points are overlapping).
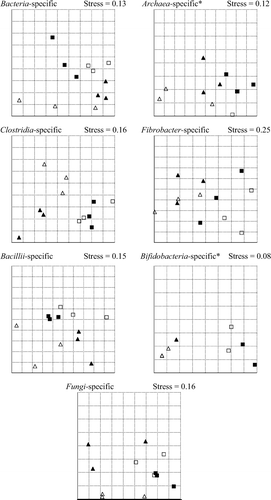
The statistical analysis confirmed these visual impressions (). For all microbial groups, significant differences were determined between the distances of band patterns of the respective SSCP gel depending on the concentrate proportion (p ≤ 0.03). With the exception of the clostridia-specific gel (p = 0.03), no significant effects were observed for the factor FCT. Interactions between the concentrate proportion and the DON contamination were detected for the bacillii-specific gel (p = 0.01).
Table 5. Two-factorial non-parametric MANOVA on Jaccard distance for the band pattern of SSCP gels using small subunit rDNA of microbes isolated via one-way centrifugation from liquid in the vessels of the rumen simulation.
4 Discussion
4.1 Ruminal fermentation and microbial protein synthesis
Besides the contamination with mycotoxins, Fusarium artificially inoculated feedstuffs can be modified in the content of nutrients and chemical and physical properties (Kang and Buchenauer Citation2000; Matthäus et al. Citation2004). In the present study, a naturally contaminated triticale was used to reach a DON concentration in the diet that did not clearly exceed the critical DON concentration of 5 mg per kg (Anonymous Citation2006). Therefore, only small differences in nutrient composition and digestibility of nutrients due to FCT inclusion were detected ().
When cereal grains are fed at high levels, like in the present study at 60% of DM, ruminal pH and fibre digestion could be reduced as a consequence of limited buffering capacity in the rumen (Sutton Citation1989). According to Keese et al. (Citation2008b), the individual minimum pH values of cows fed diet C60 were all higher than 6.3 and independent of the toxin supplementation. As expected, the pH value measured in the reaction vessel before the morning feeding varied in a very small range between 6.6 and 6.7 (data not shown). In consequence, the fermentation of fibre fractions was not affected by the concentrate level. As shown in studies with dairy cows, the ruminal fermentation of the acid detergent fibre was depressed, whereas the fermentation of organic matter was unchanged by using higher amounts of concentrates in the diets (Rode et al. Citation1985; Yang et al. Citation2001b). It was assumed that the similar degradation of organic matter between low and high forage to concentrate ratio resulted from the balance of higher ruminal degradation of starch and lower ruminal degradation of fibre fractions. The observed higher amounts of fermented organic matter by using 60% compared to 30% concentrates in the diet were caused by a greater availability of soluble carbohydrates. The observed lower amounts of fermented crude protein for the diets with 60% concentrates are contrary to a study with dairy cows (Rode et al. Citation1985). The potential extent of crude protein degradation in situ was higher by using alfalfa-based diets containing 33% compared to 66% concentrates (Faría-Mármol et al. Citation2002). The authors discussed a lower development of cellulolytic microorganisms with concentrate diets and therefore, a decrease in microbial colonisation of feed particles and degradation activity. Particle-associated microbes were not isolated in the present study and a correction of calculated fermentation could not be made.
In contrast to the present results, Seeling et al. (Citation2006) found that the fermentation of organic matter, crude protein, and neutral detergent fibre was significantly higher when the DON contaminated diet was used. The authors supposed a higher availability of the nutrients in the Fusarium-contaminated wheat due to modifications in cell wall compounds. Because of the ten-fold lower DON concentration of the used diets, it was assumed that the modification of chemical and physical properties of the feedstuffs in response to the fungal invasion might have been lower in the present study. The lower fermentation of the detergent fibre fractions may be an effect of a reduced activity of cellulolytic microbes. A reduced amount of ruminally fermented organic matter was also reported by Dänicke et al. (Citation2005) after feeding a diet with Fusarium toxin-contaminated wheat to dairy cows at a level of 3.1 mg DON per kg (on 88% DM basis). Therefore, DON concentrations lower than 5 mg per kg seem to not affect rumen fermentation, with the exception of fibre fractions. This fact was reflected by the unchanged amounts of SCFA both in the present in vitro and earlier in vivo studies (Dänicke Citation2002; Dänicke et al. Citation2005). In contrast, higher fermentation rates (Seeling et al. Citation2006) and differences in feed intake (Keese et al. Citation2008b) changed the pattern of SCFA when toxin-contaminated grain was fed. The observed changes in the SCFA concentrations might reflect a change in the population of ruminal microorganisms, feed alteration induced by the fungus itself or feed preparation resulting in decreased particle sizes and different chemical properties (Matthäus et al. Citation2004).
The ammonia concentration in the rumen fluid (Korosteleva et al. Citation2009) and in the liquid effluent in the current study was not affected by DON-contaminated feedstuffs. A reason for this might be the feeding of mixed diets with moderate mycotoxin concentrations. As a result of the unaffected fermentation and ammonia supply, the efficiency of the microbial protein synthesis was found to be similar between all treatments independent of the FCT in the present study. Higher ammonia concentrations detected in the effluents of the treatments with Fusarium toxins compared to the control treatment was explained by an increase in the soluble protein fraction of the contaminated feedstuffs (Seeling et al. Citation2006). However, the increase in ruminal ammonia concentration could not be used by microbes for an enhanced protein synthesis (Dänicke et al. Citation2005). Moreover, the efficiency of microbial protein synthesis was decreased. These results would support the hypothesis of a selective antimicrobial effect of mycotoxins which affects the fermentative capacity of the rumen (Fink-Gremmels Citation2008).
4.2 Structural diversity of the microbial community
Specific antimicrobial properties were shown for the common mycotoxin fusaric acid, but not for DON (May et al. Citation2000) or any other toxins (Westlake et al. Citation1987a). This fact is attributed to the potential of rumen microbes for the metabolisation of these toxins. Since most environmental microorganisms are not easily culturable or not yet culturable at all, a cultivation-independent approach based on investigating the diversity of the universally abundant in bacteria 16S rRNA genes is now well established (Olsen et al. Citation1986; Amann et al. Citation1995). In recent in vitro studies, it was demonstrated that genetic profiles based on PCR-amplified rRNA gene fragments and SSCP allowed us to display the diversity of prokaryotes with high complexity and precision (Strobel et al. Citation2008). With the exception of the clostridia-specific SSCP gel, the FCT did not affect the band pattern of the gels. The genus Clostridium and its relatives are a very diverse group whose members exhibit a wide range of phenotypic characteristics. Therefore, some strains of Butyrivibrio have been identified that are involved in specialised activities in the rumen, for example mycotoxin modifications (Westlake et al. Citation1987b). It is likely that anaerobic cellulose and hemicellulose degradation is due primarily to bacteria related to the genera Clostridium and Eubacterium (Sleat and Mah Citation1985; van Gylswyk and van der Toorn Citation1985). Additionally, some species of this group showed proteolytic and amylolytic activity (Cotta and Forster Citation2006). Therefore, it is tempting to speculate that a reduction in cellulolytic species by the FCT and the probable modification of the chemical and physical properties of this grain caused the change in the band pattern of the clostridia-specific gel. This hypothesis is supported by an earlier study (Strobel et al. Citation2008). The authors found a specific band from a Clostridium relative only in a treatment without a Fusarium-toxin contaminated feed. However, one band in an SSCP profile may be composed of more than one sequence and does not necessarily indicate only one single organism (Dohrmann and Tebbe Citation2004; Witzig et al. Citation2010).
The increase in concentrate level in the diet which is used to improve the productivity of dairy cows often induces acidosis, coupled with pH decline and an increase in lactic acid levels. In consequence, cultivation studies have demonstrated that both lactate-producing and lactate-utilising bacteria accumulate in the rumen under conditions of acidosis (Mackie and Gilchrist Citation1979). While neither in the companion in vivo trial (Keese et al. Citation2008b) nor in the present in vitro experiment was an acidotic pH value observed, other factors than lactate may be the reason for the significant differences between the band patterns of all SSCP gels between the diets with high and low concentrate proportions. Yang et al. (Citation2001a) observed shifts in the bacterial colonisation of feed particles and the proportion of liquid and solid associated microbes by changing the ratio of concentrate to forage in a diet for dairy cows. As expected, the numbers of cellulolytic species like Fibrobacter succinogenes, Ruminococcus albus and Ruminococcus flavefaciens decreased by supplementation of readily fermentable carbohydrates to a hay-based diet (Mosoni et al. Citation2007). However, the extent of reduction seems to be species specific (Tajima et al. Citation2001) and the differences in cellulolytic populations that were attributable to individual cows were larger than those attributable to the diets (Weimer et al. Citation1999). The individual effects on the animals' microbial community could be reduced by using standardised in vitro methods. The stable environmental conditions caused by using in vitro techniques resulted in only small differences in the band pattern of SSCP gels between replicates within a treatment (Boguhn et al. Citation2008; Witzig et al. Citation2010).
5 Conclusion
The concentration of up to 5 mg DON per kg feed (DM basis) from FCT had no significant effect on the fermentation of organic matter, the efficiency of microbial protein synthesis, and the amino acid pattern of microbial protein in vitro. The microbial community as described by band pattern of SSCP gels seems to be also unaffected by FCT, with the exception of the genus Clostridia. A reduction in the activity of cellulolytic species within this microbial group could explain the observed lower fermentation of detergent fibres in diets with FCT.
As expected, a higher concentrate proportion in a diet increased the fermented organic matter and the molar percentage of acetate. However, the efficiency of microbial protein synthesis was similar between the different treatments. The great differences in the band patterns of SSCP gels between the diets with 60% and 30% concentrates indicated a significant change in the microbial community.
Acknowledgements
The authors would like to thank their co-workers of the Animal Nutrition group at Martin-Luther-Universität Halle-Wittenberg and of the experimental station at the Friedrich-Loeffler-Institut in Braunschweig for their assistance with experiments and analyses.
References
- AfBN (Ausschuss für Bedarfsnormen) . 1991 . Leitlinien für die Bestimmung der Verdaulichkeit von Rohnährstoffen an Wiederkäuern . J Anim Physiol Anim Nutr , 65 : 229 – 234 .
- Amann , R I , Ludwig , W and Schleifer , K-H . 1995 . Phylogenetic identification and in situ detection of individual microbial cells without cultivation . Microbiol Rev , 59 : 143 – 169 .
- Anderson , M J . 2001 . A new method for non-parametric multivariate analysis of variance . Aust Ecol , 26 : 32 – 46 .
- Anderson , M J and Robinson , J J . 2003 . Generalized discriminant analysis based on distances . Aust N Z J Stat , 45 : 301 – 318 .
- Anonymous . 2006 . “ Empfehlungen der Kommission vom 17. August 2006 betreffend das Vorhandensein von Deoxynivalenol, Zearalenon, Ochratoxin A, T-2 und HAT-2-Toxin sowie von Fumonisinen in zur Verfütterung an Tiere bestimmten Erzeugnissen. Amtsblatt der Europäischen Union 200/576/EG. L 229/7-L229/9 ” .
- Blackwood , C B , Oaks , A and Buyer , J S . 2005 . Phylum- and class-specific PCR primers for general microbial community analysis . Appl Environ Microbiol , 71 : 6193 – 6198 .
- Boguhn , J , Kluth , H and Rodehutscord , M . 2006a . Effect of total mixed ration composition on amino acid profiles of different fractions of ruminal microbes in vitro . J Dairy Sci , 89 : 1592 – 1603 .
- Boguhn , J , Kluth , H and Rodehutscord , M . 2006b . Effect of total mixed ration composition on fermentation and efficiency of ruminal microbial crude protein synthesis in vitro . J Dairy Sci , 89 : 1580 – 1591 .
- Boguhn , J , Strobel , E , Witzig , M , Tebbe , C C and Rodehutscord , M . 2008 . Description of the structural diversity of rumen microbial communities in vitro using single-strand conformation polymorphism profiles . Arch Anim Nutr , 62 : 454 – 467 .
- Cotta , M and Forster , R . 2006 . The family Lachnospiraceae, including the genera Butyrivibrio, Lachnospira and Roseburia . Prokaryotes , 4 : 1002 – 1021 .
- Czerkawski , J W and Breckenridge , G . 1977 . Design and development of a long-term rumen simulation technique (Rusitec) . Brit J Nutr , 38 : 371 – 384 .
- Dänicke , S . 2002 . Effect of Fusarium toxin contaminated wheat grain and of a detoxifying agent on rumen physiological parameters and in sacco dry matter degradation of wheat straw and lucerne hay in wethers . J Anim Feed Sci , 11 : 437 – 451 .
- Dänicke , S , Gareis , M and Bauer , J . 2001 . Orientation values for critical concentrations of deoxynivalenol and zeralenone in diets for pigs, ruminants and gallinaceous poultry . Proc Soc Nutr Physiol , 10 : 171 – 174 .
- Dänicke , S , Matthäus , K , Lebzien , P , Valenta , H , Stemme , K , Ueberschär , K-H , Razzazi-Fazeli , E , Böhm , J and Flachowsky , G . 2005 . Effects of Fusarium toxin-contaminated wheat grain on nutrient turnover, microbial protein synthesis and metabolism of deoxynivalenol and zearalenone in the rumen of dairy cows . J Anim Physiol Anim Nutr , 89 : 303 – 315 .
- Dohrmann , A B and Tebbe , C C . 2004 . “ Microbial community analysis by PCR-single-strand conformation polymorphism (PCR-SSCP) ” . In Molecular microbial ecology manual , Edited by: Kowalchuk , G A , de Bruijn , F J , Head , I M , Akkermans , A DL and van Elsas , J D . 809 – 838 . Dordrecht : Kluwer Academic Publishers .
- Faría-Mármol , J , González , J , Rodríguez , C A and Alvir , M R . 2002 . Effect of diet forage to concentrate ratio on rumen degradability and post-ruminal availability of protein from fresh and dried lucerne . Anim Sci , 74 : 337 – 345 .
- Fink-Gremmels , J . 2008 . Mycotoxins in cattle feeds and carry-over to dairy milk: A review . Food Addit Contam A , 25 : 172 – 180 .
- Geissler , C , Hoffmann , M and Hickel , B . 1976 . Ein Beitrag zur gaschromatographischen Bestimmung flüchtiger Fettsäuren . Arch Anim Nutr , 26 : 123 – 129 .
- Großkopf , R , Janssen , P H and Liesack , W . 1998 . Diversity and structure of the methanogenic community in anoxic rice paddy soil microcosms as examined by cultivation and direct 16S rRNA gene sequence retrieval . Appl Environ Microbiol , 64 : 960 – 969 .
- Hammer , Ø , Harper , D AT and Ryan , P D . 2001 . PAST: Paleontological statistics software package for education and data analysis . Palaeontol Electronica , 4 : 1 – 9 .
- He , P , Young , L G and Forsberg , C W . 1992 . Microbial transformation of deoxynivalenol (vomitoxin) . Appl Environ Microbiol , 58 : 3857 – 3863 .
- Kang , Z and Buchenauer , H . 2000 . Ultrastructural and cytochemical studies on cellulose, xylan and pectin degradation in wheat spikes infected by Fusarium culmorum . J Phytopathol , 148 : 263 – 275 .
- Kaufmann , P , Pfefferkorn , A , Teuber , M and Meile , L . 1997 . Identification and quantification of Bifidobacterium species isolated from food with genus-specific 16S rRNA-targeted probes by colony hybridization and PCR . Appl Environ Microbiol , 63 : 1268 – 1273 .
- Keese , C , Meyer , U , Rehage , J , Spilke , J , Boguhn , J , Breves , G and Dänicke , S . 2008a . On the effects of the concentrate proportion of dairy cow rations in the presence and absence of a Fusarium toxin-contaminated triticale on cow performance . Arch Anim Nutr , 62 : 241 – 262 .
- Keese , C , Meyer , U , Rehage , J , Spilke , J , Boguhn , J , Breves , G and Dänicke , S . 2008b . Ruminal fermentation patterns and parameters of the acid base metabolism in the urine as influenced by the proportion of concentrate in the ration of dairy cows with and without Fusarium toxin-contaminated triticale . Arch Anim Nutr , 62 : 287 – 302 .
- Korosteleva , S N , Smith , T K and Boermans , H J . 2009 . Effects of feed naturally contaminated with Fusarium mycotoxins on metabolism and immunity of dairy cows . J Dairy Sci , 92 : 1585 – 1593 .
- Kruskal , J B . 1964 . Nonmetric multidimensional scaling: A numerical method . Psychometrika , 29 : 115 – 129 .
- Lane , D J . 1991 . “ 16S/23S rRNA sequencing ” . In Nucleic acid techniques in bacterial systematics , Edited by: Stackebrandt , E and Goodfellow , M . 115 – 175 . Chichester, , UK : Wiley Intersience Publications .
- Ludwig , W , Strunk , O , Westram , R , Richter , L , Meier , H , Yadhukumar , Buchner , A , Lai , T , Steppi , S , Jobb , G , Förster , W , Brettske , I , Gerber , S , Ginhart , A W , Gross , O , Grumann , S , Hermann , S , Jost , R , König , A , Liss , T , Lüßmann , R , May , M , Nonhoff , B , Reichel , B , Strehlow , R , Stamatakis , A , Stuckmann , N , Vilbig , A , Lenke , M , Ludwig , T , Bode , A and Schleifer , K-H . 2004 . ARB: A software environment for sequence data . Nuc Acids Res , 32 : 1363 – 1371 .
- Lueders , T , Manefield , M and Friedrich , M W . 2004 . Enhanced sensitivity of DNA- and rRNA-based stable isotope probing by fractionation and quantitative analysis of isopycnic centrifugation gradients . Environ Microbiol , 6 : 73 – 78 .
- Mackie , R I and Gilchrist , F MC . 1979 . Changes in lactate-producing and lactate-utilizing bacteria in relation to pH in the rumen of sheep during stepwise adaptation to a high-concentrate diet . Appl Environ Microbiol , 38 : 422 – 430 .
- Matthäus , K , Dänicke , S , Vahjen , W , Simon , O , Wang , J , Valenta , H , Meyer , K , Strumpf , A , Ziesenib , H and Flachowsky , G . 2004 . Progression of mycotoxin and nutrient concentrations in wheat after inoculation with Fusarium culmorum . Arch Anim Nutr , 58 : 19 – 35 .
- May , H D , Wu , Q and Blake , C K . 2000 . Effects of the Fusarium ssp. mycotoxins fusaric acid and deoxynivalenol on the growth of Ruminococcus albus and Methanobrevibacter ruminantium . Can J Microbiol , 46 : 692 – 699 .
- McDougall , E I . 1948 . Studies on ruminant saliva, 1. The composition and output of sheep's saliva . Biochem J , 43 : 99 – 109 .
- Mosoni , P , Chaucheyras-Durand , F , Bera-Maillet , C and Forano , E . 2007 . Quantification by real-time PCR of cellulolytic bacteria in the rumen of sheep after supplementation of a forage diet with readily fermentable carbohydrates: Effect of a yeast additive . J Appl Microbiol , 103 : 2676 – 2685 .
- Muyzer , G , de Waal , E C and Uitierlinden , A G . 1993 . Profiling of complex microbial populations by denaturing gradient gel electrophoresis analysis of polymerase chain reaction-amplified genes coding for 16S rRNA . Appl Environ Microbiol , 59 : 695 – 700 .
- Naumann , C and Bassler , R . 1976 . VDLUFA-Methodenbuch, Vol. III. Die chemische Untersuchung von Futtermitteln; with supplements from 1983, 1988, 1993, 1997, 2004 and 2006 , Darmstadt : VDLUFA-Verlag .
- Olsen , G J , Lane , D J , Giovannoni , S J and Pace , N R . 1986 . Microbial ecology and evolution: A ribosomal RNA approach . Annu Rev Microbiol , 40 : 337 – 365 .
- Rode , L M , Weakley , D C and Satter , L D . 1985 . Effect of forage amount and particle size in diets of lactating dairy cows on site of digestion and microbial protein synthesis . Can J Anim Sci , 65 : 101 – 111 .
- Rodehutscord , M , Kapocius , M , Timmler , R and Dieckmann , A . 2004 . Linear regression approach to study amino acid digestibility in broiler chickens . Brit Poult Sci , 45 : 85 – 92 .
- Schwieger , F and Tebbe , C C . 1998 . A new approach to utilize PCR-single-strand-conformation polymorphism for 16S rRNA gene-based microbial community analysis . Appl Environ Microbiol , 64 : 4870 – 4876 .
- Seeling , K , Boguhn , J , Strobel , E , Dänicke , S , Valenta , H , Ueberschär , K-H and Rodehutscord , M . 2006 . On the effects of Fusarium toxin contaminated wheat and wheat chaff on nutrient utilisation and turnover of deoxynivalenol and zearalenone in vitro (RUSITEC) . Toxicol In Vitro , 20 : 703 – 711 .
- Seeling , K and Dänicke , S . 2005 . Relevance of Fusarium toxins deoxynivalenol and zearalenone in ruminant nitrition. A review . J Anim Feed Sci , 14 : 3 – 40 .
- Sleat , R and Mah , R A . 1985 . Clostridium populeti sp. nov., a cellulolytic species from a woody-biomass digestor . Int J Syst Bacteriol , 35 : 160 – 163 .
- Stahl , D A and Amann , R I . 1991 . “ Development and application of nucleic acid probes ” . In Nucleic acid techniques in bacterial systematics , Edited by: Stackrandt , E and Goodfellow , M . 205 – 248 . New York : John Wiley and Sons Ltd. .
- Stahl , D A , Flesher , B , Mansfield , H R and Montgomery , L . 1988 . Use of phylogenetically based hybridization probes for studies of ruminal microbial ecology . Appl Environ Microbiol , 54 : 1079 – 1084 .
- Strobel , E , Seeling , K and Tebbe , C C . 2008 . Diversity responses of rumen microbial communities to Fusarium-contaminated feed, evaluated with rumen simulating technology (Rusitec) . Environ Microbiol , 10 : 483 – 496 .
- Sutton , J D . 1989 . Altering milk composition by feeding . J Dairy Sci , 72 : 2801 – 2814 .
- Tajima , K , Aminov , R I , Nagamine , T , Matsui , H , Nakamura , M and Benno , Y . 2001 . Diet-dependent shifts in the bacterial population of the rumen revealed with real-time PCR . Appl Environ Microbiol , 67 : 2766 – 2774 .
- Tajima , K , Aminov , R I , Nagamine , T , Ogata , K , Nakamura , M , Matsui , H and Benno , Y . 1999 . Rumen bacterial diversity as determined by sequence analysis of 16S rDNA libraries . FEMS Microbiol Ecol , 29 : 159 – 169 .
- Trenholm , H L , Hamilton , R MG , Friend , D W , Thompson , B K and Hartin , K E . 1984 . Feeding trials with vomitoxin (deoxynivalenol)-contaminated wheat: Effects on swine, poultry, and dairy cattle . J Am Vet Med Assoc , 185 : 527 – 531 .
- Valenta , H , Dänicke , S and Wolff , J . 2002 . Vergleich einer HPLC- und einer ELISA-Methode zur Bestimmung von Deoxynivalenol in Mühlenstäuben, Kleien und Getreide . VDLUFA-Kongressband 2002, VDLUFA-Schriftenreihe , 58 : 675 – 679 .
- van Gylswyk , N O and van der Toorn , J JTK . 1985 . Eubacterium uniforme sp. nov. and Eubacterium xylanophilum sp. nov., fiber-digesting Bacteria from the rumina of sheep fed corn stover . Int J Syst Bacteriol , 35 : 323 – 326 .
- Weimer , P J , Waghorn , G C , Odt , C L and Mertens , D R . 1999 . Effect of diet on populations of three species of ruminal cellulolytic bacteria in lactating dairy cows . J Dairy Sci , 82 : 122 – 134 .
- Weisburg , W G , Barns , S M , Pelletier , D A and Lane , D J . 1991 . 16S ribosomal DNA amplification for phylogenetic study . J Bacteriol , 173 : 697 – 703 .
- Westlake , K , Mackie , R I and Dutton , M F . 1987a . Effects of several mycotoxins on specific growth rate of Butyrivibrio fibrisolvens and toxin degradation in vitro . Appl Environ Microbiol , 53 : 613 – 614 .
- Westlake , K , Mackie , R I and Dutton , M F . 1987b . T-2 toxin metabolism by ruminal bacteria and its effect on their growth . Appl Environ Microbiol , 53 : 587 – 592 .
- White , T J , Burns , T , Lee , S and Taylor , J . 1990 . “ Amplification and direct sequencing of fungal ribosomal genes for phylogenetics ” . In PCR protocols. A guide to methods and applications , Edited by: Innis , M A , Gelfand , D H , Sninsky , J J and White , T J . 315 – 322 . San Diego : Academic Press .
- Witzig , M , Boguhn , J , Kleinsteuber , S , Fetzer , I and Rodehutscord , M . 2010 . Effect of the corn silage to grass silage ratio and feed particle size of diets for ruminants on the ruminal Bacteroides-Prevotella community in vitro . Anaerobe , 16 : 412 – 419 .
- Yang , W Z , Beauchemin , K A and Rode , L M . 2001a . Effect of dietary factors on distribution and chemical composition of liquid- or solid-associated bacterial populations in the rumen of dairy cows . J Anim Sci , 79 : 2736 – 2746 .
- Yang , W Z , Beauchemin , K A and Rode , L M . 2001b . Effects of grain processing, forage to concentrate ratio, and forage particle size on rumen pH and digestion by dairy cows . JDairy Sci , 84 : 2203 – 2216 .