?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Pyrrolizidine alkaloid (PA) producing plants like Senecio jacobaea or Senecio vernalis are undesirable in fields for forage production, since PA are toxic to animals and humans. Previous studies have shown that ensiling can decrease the PA content in forages; however, no direct comparison of diverse PA from different Senecio spp. under various ensiling conditions has been made. Therefore, it was hypothesised that individual PA might react differently to ensiling, and silage inoculation with Lactobacillus will affect PA degradation because of a quick drop in pH, contrastingly to poor silage qualities resulting from contamination with soil. Laboratory scale grass silages were prepared in a multifactorial design with two levels of dry matter contents, four ensiling treatments and two storage durations (10 and 90 d). For each combination, four replicates were prepared individually. Ensiling treatments were (1) 10 ml water per kg fresh matter as control (CON), (2) 10 ml heterofermentative Lactobacillus buchneri strain LN4637 at 3 · 105 cfu/kg fresh matter plus 25 g molasses/kg fresh matter (LBHE), (3) 10 ml homofermentative lactobacilli at 3 · 105 cfu/kg fresh matter plus 25 g molasses/kg fresh matter (LBHO) and (4) 10 g soil/kg fresh matter (SOIL). Treatments affected formation of fermentation acids. Acetic acid was highest with treatment LBHE, and butyric acid was highest with treatment SOIL. All ensiling treatments effectively reduced total PA content by degrading the PA N-oxide (PANO) fraction. In parallel, though, the fraction of the tertiary base forms increased by around one-tenth of the original PANO content. Contents of jaconine and senkirkine were higher after ensiling than before, with regards to the sum of PA and PANO for jaconine, indicating higher stability or new formation through degradation of other PA. Overall, ensiling offers opportunities to decrease the PA-PANO content in feed and therefore lowers the risk of intoxication by Senecio in livestock.
1. Introduction
In many countries, the nutrition of dairy cows has largely changed during the last decades, away from grazing towards indoor housing with feeding preserved forages like silages (Wilkinson and Rinne Citation2018). Ensiling offers the opportunity to preserve forages for times when pasture growth is insufficient or climatic conditions do not allow preservation through field drying. Due to its dominant use in cattle feeding, the quality of silages is of concern as a transfer of putative toxic components to cow’s milk could occur (Kalač Citation2011; Driehuis Citation2013). This includes a number of secondary plant metabolites such as colchicine, tropane or pyrrolizidine alkaloids (PA) (Driehuis Citation2013). Several flowering plants can produce PA, but due to their wide distribution, Senecio species are particularly important for feed safety in Europe (Cortinovis and Caloni Citation2015). To date more than 1200 species of Senecio are recognised (Boppré Citation2011). In plants, several PA occur as a combination of a tertiary base form, in this study termed as PA, and their corresponding N-oxides (PANO). The latter represent the major fraction in the plant with around 90 % (Maedge et al. Citation2020).
In general, the highest PA-PANO contents are found in flowers and seeds, but total levels depend on factors such as climate, vegetation stage or season and can vary considerably (reviewed by Boppré Citation2011). Several PA are known to be hepatotoxic in humans as well as in experimental and farm animals, with their toxicity depending on chemical structure (Buchmüller et al. Citation2022). Therefore, their presence in feed as well as their potential transfer into foods of animal origin is undesirable (Dickinson et al. Citation1976; Molyneux et al. Citation2011; Mulder et al. Citation2020). A survey by Gottschalk et al. (Citation2015) revealed that 18% out of 115 grass silage samples from the state Bavaria (Germany) contained one or more PA at low concentrations (total PA-PANO on average 4.9 μg/kg dry matter (DM), highest concentration found 30 μg/kgDM). As levels in PA-PANO-producing plants in temperate European latitudes are in the range of 0.1–3% of DM, these data suggest that highly contaminated silages are rare. However, considering the fact that the appearance of Senecio spp. such as common ragwort (S. jacobaea), common groundsel (S. vulgaris), eastern groundsel (S. vernalis) or narrow-leaved ragwort (S. inaequidens) is increasing in Germany and other countries, a sound knowledge of the fate of PA in forage production is of interest (Gottschalk et al. Citation2015, Citation2018; Wiedenfeld Citation2011). While hot air-drying does not seem to affect PA-PANO content in contaminated grass (Gottschalk et al. Citation2018), a recent small scale ensiling experiment with S. vernalis contaminated grass could show that ensiling and ensiling treatments like the addition of fermentable carbohydrate sources (e.g. molasses) can promote the degradation of some PA-PANO (Klevenhusen et al. Citation2019). Currently, the most commonly used type of additive for ensiling, besides molasses addition, is inoculation of crops with homofermentative strains of lactic acid bacteria (e.g. Lactobacillus plantarum) at the time of harvest (Wilkinson and Rinne Citation2018), quickly reducing pH and shifting fermentation toward lactic acid. However, an inhibitory effect of pH on the enzymatic degradation of PA-PANO has been proposed (Gottschalk et al. Citation2015). Until now, there is a lack of studies systematically investigating the degradation of different PA-PANO from diverse Senecio spp. exposed to ensiling treatments typically used on farms, like pre-wilting and bacterial inocula and undesirable contamination with clostridia from soil. Therefore, the aim of this study was to prove whether individual PA and PANO from two prevailing Senecio spp. in Germany (S. vernalis and S. jacobaea) react differently during ensiling under the influence of differing ensiling conditions.
2. Materials and methods
2.1. Plant material and ensilage protocol
S.vernalis and S. jacobaea were collected in Brandenburg, Germany, at flowering stage in 2019 by cutting plants approximately 2 cm above ground. Senecio spp. were harvested in full flowering stage on extensively used fields covering sandy soils in May (S. vernalis) and July (S. jacobaea), respectively and stored at −20°C prior to ensiling in August 2019. On the day of silage preparation, S. vernalis and S. jacobaea plants were taken from the freezer, chopped manually to ∼0.5 cm and separately mixed thoroughly. A second cut mixed grass sward (Lolium perenne, Dactylis glomerata, Phleum pretence, Alopecurus pratensis; cut to 4 cm particle length) was harvested at booting stage in Brandenburg, Germany, at 8:00 h and either directly used for silage preparation or pre-wilted over 24 h before being mixed with S. vernalis and S. jacobaea, respectively. For ensiling, each Senecio spp. and wilting condition was mixed with four different treatments and stored over 10 or 90 d. Each combination was replicated four times, resulting in a total of 128 individually prepared silages. In detail, for individual silage preparation, Senecio spp. were added at 3% on a fresh matter basis to 2.5 kg grass and thoroughly mixed in plastic troughs. The following ensiling treatments were added per kg fresh matter by spraying and thoroughly mixing: (1) CON, control treatment 10 ml water; (2) LBHE, 10 ml heterofermentative Lactobacillus buchneri strain LN4637 at 3 · 105 cfu/kg plus 25 g molasses/kg; (3) LBHO, 10 ml homofermentative lactobacilli at 3 · 105 cfu/kg plus 25 g molasses/kg; (4) SOIL, 10 g soil, obtained from a cow path between barn and pasture. Mixtures were ensiled in 1.5 l Weck® jars (approximately 900 g/jar), resulting in compaction densities of 178 ± 10.5 kg DM/m3, with densities averaging 175 kg DM/m3 for non pre-wilted material and 181 kg DM/m3 for pre-wilted material. Mixtures for each glass were individually prepared in large plastic troughs as indicated above.
One subsample of each prepared mixture was taken for analysis of PA-PANO before ensiling. An additional sample of each treatment, Senecio spp. and wilting condition combination was analysed by near-infrared spectroscopy (NIRS) for proximate composition showing an average DM content of 316 g/kg fresh matter, which increased to 356 g/kg fresh matter after pre-wilting. On average, the pre-siling material contained per kg DM crude protein (CP) at 146 g/kg DM, neutral detergent fibre (NDF) at 572 g/kg DM and acid detergent fibre (ADF) at 299 g/kg DM, total sugar at 86.1 g/kg DM and crude ash at 77.8 g/kg DM.
In total, four jars per treatment (128 jars in total) were prepared and stored either over 10 or over 90 d following the guidelines of the German Agricultural Society (DLG) for action category ACI (DLG TestServicen GmbH Citation2018; Pauly and Wyss Citation2019). The additional shorter storage time of 10 d was included to determine possible time effects on PA-PANO degradation. After preparation of silages and immediate closure, all jars were weighed and stored at room temperature. After 10 and 90 d of storage, four jars per treatment were weighed for determination of DM loss and subsequently opened and contents homogenised. Subsamples from each jar were taken and stored at −20°C for later analyses of nutrients, pH, fermentation acids and PA-PANO.
2.2. Analyses of chemical composition and fermentation characteristics of silages
Analyses of contents of DM, crude ash, CP, NDF and ADF were conducted with NIRS. Determination of organic acids, ethanol and ammonia-N (NH3-N) was performed in aqueous silage extracts, which were prepared by blending 50 g of the frozen material with 400 ml deionised water and 1 ml toluene. Extracts were kept at 4°C overnight and then filtered through MN 615 filter paper (Machery-Nagel, Düren, Germany), followed by micro-filtration (Minisart RC, 0.45 µm pore size, Sartorius, Göttingen, Germany). The pH was analysed potentiometrically by using a calibrated pH electrode. According to Weiß and Kaiser (Citation1995), lactic acid was detected by high-performance liquid chromatography (HPLC) using refractive index (RI) detection (LC-20 AB, Shimadzu Deutschland, Duisburg, Germany). Along with the detection of lactic acid, inulin-type fructans were analysed (Weiß and Alt Citation2017). Volatile organic acids and ethanol were determined by gas chromatography (GC) using a free fatty acid phase (Permabond FFAP 0.25 µm, Macherey-Nagel, Düren, Germany) column and flame ionisation detector (GC-2010, Shimadzu, Deutschland, Duisburg, Germany), as described by Weiß and Sommer (Citation2017). The limit of detection for each parameter was 0.01% of fresh matter. The NH3-N contents were analysed colorimetrically according to von Lengerken and Zimmermann (Citation1991) using a continuous flow analyser (CFA, San++, Skalar Analytical, Breda, the Netherlands). The DM content was corrected as described by Weissbach and Strubelt (Citation2008):
where DMmeas is the measured DM content, FM is fresh matter, SCFA is the sum of short chain fatty acids (C2 - C6), LAC is the lactic acid content, PD is the content of 1,2-propandiol, BD is the content of 2,3-butandiol and Alc is the sum of all other alcohols (C2 - C4) (all in g/kg FM).
2.3. Analyses of PA-PANO
For the analyses of PA-PANO samples were thawed and dried over night at 40°C. Samples were comminuted to a particle size below 1 mm using a knife mill (Grindomix GM 200, Retsch, Haan, Germany). From the pre-ensiling material, three samples per treatment were randomly selected for PA-PANO analyses (48 samples). From ensiled samples, each glass was analysed for PA-PANO (128 samples).
For the extraction of PA-PANO, 10.0 g ±0.1 g of comminuted dried sample material was weighed into a centrifuge tube. A duplicate extraction with a volume of 100 ml aqueous extraction solution containing 0.05 M H2SO4 was used. For extraction, an ultrasonic bath was used for 15 min, followed by 20 min shaking (overhead shaker). The samples were centrifuged (20°C 3218 g, 10 min), and the supernatant was passed through a 0.20 μm nylon membrane filter (VWR, Germany). As minor and major PA are unequally distributed in plants, each sample was 10-, 50-, 100-fold diluted and subsequently analysed by an external calibration applying a 14-point calibration curve in the range of 0.05–150 ng/ml.
All measurements were conducted on an Agilent 1290 infinity series UHPLC system (Agilent Technologies, Waldbronn, Germany). Chromatographic reversed-phase (RP) separation with 2.0 μl injection volume was performed on a C18 Hypersil Gold column (150 mm × 2.1 mm; 1.9 μm particle size) with guard column (Thermo Fisher Scientific, Waltham, USA) at a flow rate of 0.3 μl/min and with a column temperature of 40°C. The binary mobile phase was composed of water as mobile phase A and methanol as mobile phase B, both containing 0.1% formic acid and 5 mM ammonium formate. The gradient conditions were as follows: 0–0.5 min A: 95%/B: 5%, 7.0 min A: 50%/B: 50%, 7.5 min A: 20%/B: 80%, 7.6 min A: 0%/B: 100%, 10.1–15 min A: 95%/B: 5%. Electrospray ionisation tandem mass spectrometry (ESI-MS/MS) data were acquired in the positive ionisation mode on a QTRAP 6500 MS/MS system (AB Sciex, Foster City, CA, USA). The settings of the ESI source were as follows: source temperature 500°C curtain gas 35 psi, ion source gas 1 (sheath gas) 60 psi, ion source gas 2 (drying gas) 60 psi, ion-spray voltage +5500 V and collision gas (nitrogen) medium. Two MRM transitions were measured per analyte as follows: ([M + H]+ → quantifier/qualifier). Er (350 → 120/2138); ErN (366 → 120/136); Jb (352 → 120/155); JbN (368 → 296/120); Jl (370→ 120/138); Jl N (386→ 120/136); Jn (388 → 138/156); JnN (404 → 118/120); Re (352 → 120/138); ReN (368 → 120/118); Rd (350 → 120/138); RdN (366 → 136/120); Sc (336 → 120/138); ScN (352 → 118/136); Sp (334 → 120/138); SpN (350 → 118/136); Sk (366 → 150/168).
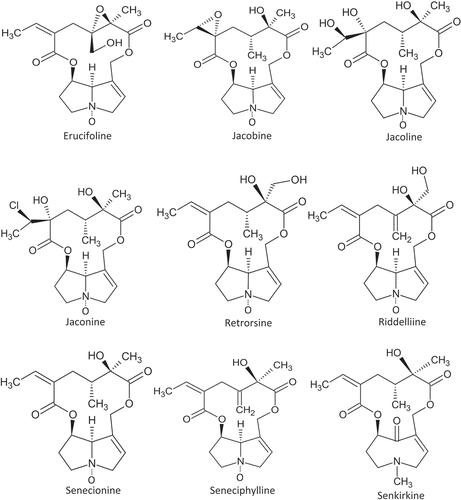
Total PA content was calculated as a sum of the following single PA: erucifoline/erucifoline N-oxide (N), jacobine/jacobine N (and isomers), jacoline/jacoline N (and isomers), jaconine/jaconine N (and isomers), retrorsine/retrorsine N (and isomers), riddelliine, riddelliine N, senecionine/senecionine N (and isomers), seneciphylline, seneciphylline N (and isomers) and senkirkine (). The PA occurring naturally as isomers were analysed as a group and expressed as the sum of individual isomers content. For instance, in case of co-elution, senecionine, senecivernine and integerrimine were summed up and expressed as senecionine.
2.4. Statistical analyses
Data were analysed by ANOVA in a multifactorial design followed by Tukey–Kramer post hoc test using SAS® software version 9.4 (SAS Institute GmbH, Cary, NC, USA) considering treatments (CON, LBHO, LBHE, SOIL), pre-wilting, duration of storage, Senecio spp. and their interactions as fixed effects. The model used to analyse the data set was as follows:
where Yijkl is an observation from the ith Senecio spp., jth wilting condition, kth duration of storage and lth ensiling treatment, μ is the grand mean, Di is effect of the ith Senecio spp., Bj is the effect of the jth wilting condition, Rk is the effect of the kth duration of storage, Kl is the effect of the lth ensiling treatment and εijklm the random residual error. In addition, (DK)il interactions between Senecio spp. and ensiling treatment, (DB)ij interactions between Senecio spp. and pre-wilting condition and (DR)ik interactions between Senecio spp. and storage duration were included. Differences were considered significant at p < 0.05. Pearson correlation coefficients between fermentation characteristics (i.e. fermentation acids, pH, DM content) and PA-PANO were calculated separately for S. vernalis and S. jacobaea contaminated silages, after 10 and 90 d together, using the CORR procedure in SAS. Since PANO were not detected after 10 d of ensiling, with the exception of the Senecionin N-oxide, they were not included in correlation analysis between silage quality traits and PA-PANO contents. Correlations between fermentation parameters and PA patterns in the silages were visualised by creating a correlation plot using R-studio.
3. Results
3.1. Treatment effects on silage quality traits
Dry matter of silages was affected by treatment, pre-wilting and differed between Senecio spp. (). Across Senecio spp., DM was lower in silages treated with LBHO or LBHE in comparison to CON and SOIL (p < 0.001). The interaction of Senecio spp. × pre-wilting on DM content was significant (p < 0.001) because DM content was slightly higher in pre-wilted silages mixed with S. jacobaea compared to S. vernalis. Content of CP was not affected by Senecio spp. but significantly increased due to LBHE treatment (p < 0.001) and increased over time (p < 0.001). Likewise, NH3-N [g/kg N] did not differ between silages mixed with either Senecio spp. but was lowest in LBHO treated silages, followed by LBHE treated silages and SOIL (). In CON silages the NH3-N content was highest (p < 0.001). Pre-wilting increased NH3-N contents in silages (p < 0.001) and enhanced pH values (p < 0.001). As depicted in , treatment effects showed lowest pH values in silages treated with LBHO (pH = 3.98) and highest with SOIL (pH = 4.50; p < 0.001). Concentrations of lactic acid were highest in silages treated with LBHO (7.72% in DM) and lowest in silages contaminated with SOIL (3.34% in DM; p < 0.001) (). Pre-wilting led to lower lactic acid contents (p < 0.001) and tended to increase butyric acid contents (p < 0.001) but did not affect acetic acid contents. Acetic acid was two to three times higher with LBHE inoculum compared to the other treatments (p < 0.001). Concentration of butyric acid was highest in the silages, which had been mixed with SOIL (mean value 0.519% in DM; p < 0.001). Prolonged storage duration increased contents of all acids significantly. Ethanol contents averaged 1.03% in non pre-wilted and 0.776% in pre-wilted silages on DM basis (p < 0.001) and, similar to the fermentation acids, were higher after 90 d compared to 10 d of storage (p < 0.001). Treatment had an effect on ethanol formation (p < 0.001) with higher contents after treatment LBHO (1.07% in DM) and LBHE (0.995% in DM) and lower contents after treatment CON (0.739% in DM) and SOIL (0.795% in DM).
Figure 2. Mean pH values and NH3-N contents [g/kg N] after 10 d and 90 d of ensiling in pre-wilted and non pre-wilted silages, with either S. vernalis or S. jacobaea. Treatments were CON (control), LBHE (heterofermentative Lactobacillus buchneri strain LN4637 at 3 · 105 cfu/kg fresh matter plus 25 g molasses/kg fresh matter), LBHO (homofermentative lactobacilli at 3 · 105 cfu/kg fresh matter plus 25 g molasses/kg fresh matter) and SOIL (10 g soil/kg fresh matter).
![Figure 2. Mean pH values and NH3-N contents [g/kg N] after 10 d and 90 d of ensiling in pre-wilted and non pre-wilted silages, with either S. vernalis or S. jacobaea. Treatments were CON (control), LBHE (heterofermentative Lactobacillus buchneri strain LN4637 at 3 · 105 cfu/kg fresh matter plus 25 g molasses/kg fresh matter), LBHO (homofermentative lactobacilli at 3 · 105 cfu/kg fresh matter plus 25 g molasses/kg fresh matter) and SOIL (10 g soil/kg fresh matter).](/cms/asset/f04bebf2-fb04-4c9a-a95e-642d2a3360a5/gaan_a_2084321_f0002_b.gif)
Figure 3. Contents of fermentation acids (lactic acid, acetic acid and butyric acid) [% of DM] in pre-wilted and non pre-wilted silages, with either S. vernalis or S. jacobaea as means of day 10 and day 90 of ensiling. Treatments were CON (control), LBHE (heterofermentative Lactobacillus buchneri strain LN4637 at 3 · 105 cfu/kg fresh matter plus 25 g molasses/kg fresh matter) and LBHO (homofermentative lactobacilli at 3 · 105 cfu/kg fresh matter plus 25 g molasses/kg fresh matter) and SOIL (10 g soil/kg fresh matter).
![Figure 3. Contents of fermentation acids (lactic acid, acetic acid and butyric acid) [% of DM] in pre-wilted and non pre-wilted silages, with either S. vernalis or S. jacobaea as means of day 10 and day 90 of ensiling. Treatments were CON (control), LBHE (heterofermentative Lactobacillus buchneri strain LN4637 at 3 · 105 cfu/kg fresh matter plus 25 g molasses/kg fresh matter) and LBHO (homofermentative lactobacilli at 3 · 105 cfu/kg fresh matter plus 25 g molasses/kg fresh matter) and SOIL (10 g soil/kg fresh matter).](/cms/asset/5cabd6ea-a3a3-488f-9b19-9a1fd03921b2/gaan_a_2084321_f0003_b.gif)
Table 1. Nutrient contents [g/kg DM] of silages averaged across storage duration and for pre-wilted and non pre-wilted silages (except for DM content).
3.2. PA and PANO contents in pre-ensiling materials
Contents of PA-PANO were analysed in dried samples, and values are also given in µg/kg dried sample. Total contents of PA-PANO in plant materials before ensiling are shown in . Although all plant mixtures were contaminated with the same amount of dried Senecio plants prior ensiling (30 g Senecio plant/kg on DM basis) and thoroughly mixed, respective starting mixtures were not completely homogeneous. Therefore, the PA-PANO content showed a relative standard deviation of 6% and 17% for S. jacobaea and S. vernalis contaminated mixtures, respectively. On average, mixtures contaminated with S. jacobaea contained total PA-PANO at 20,931 ± 3,514 µg/kg, while contents of PA-PANO in plant materials contaminated with S. vernalis were considerably higher, averaging 114,444 ± 37,118 µg/kg. The majority of the total PA-PANO contents prior ensiling consisted of PANO, accounting for 97.7% in S. jacobaea and 91.5% in S. vernalis (). The PA-PANO profiles of S. jacobaea and S. vernalis contaminated mixtures comprised cyclic diester structures of senecionine type and contained individual compounds typical for the respective species (). The most abundant PA-PANO in S. jacobaea contaminated plant materials was erucifoline N-oxide, accounting for over 50% of total content, followed by senecionine N-oxide with 16%, retrorsine N-oxide with 12% and seneciphylline N-oxide with 7.7%. The most abundant PA-PANO in plant materials, contaminated with S. vernalis were senecionine N-oxide, accounting for almost 80% of total PA-PANO followed by senkirkine with 6.5% and retrorsine N-oxide with 3.4% (.)
Table 2. Contents and profile of pyrrolizidine alkaloids (PA) and their N-oxides (PANO) before ensiling.
3.3. Treatment effects on PA and PANO contents after ensiling
The PA-PANO profiles and contents differed significantly between silages supplemented with different Senecio species (). After 90 d of ensiling, on average, 88% of the total PA-PANO was degraded in silages mixed with S. jacobaea and about 79% in silages mixed with S. vernalis. Recovery was lower in pre-wilted silages (p = 0.01) and increased slightly when silages were stored over 90 d instead of 10 (p = 0.04). This significant reduction can be explained mainly by the marked degradation of PANO. Only 0.012% (reflecting an absolute degradation of around 20,000 µg/kg) and 0.249% (reflecting an absolute degradation of around 104,000 µg/kg) of the originally detected PANO were still detectable in silages mixed with S. jacobaea and S. vernalis, respectively (). This decrease was observed for all treatments tested, but PA-PANO recovery was lowest in silages inoculated with LBHO (p < 0.001).
Table 3. Contents of pyrrolizidine alkaloids (PA) and their N-oxides (PANO) in silages and their proportions [%] after 90 d◊.
While the N-oxides were almost completely degraded, the corresponding tertiary base fraction increased during ensilage by 691% (reflecting an absolute formation of around 2,000 µg/kg) and 254% (reflecting an absolute formation of around 10,200 µg/kg). These data denote that after 90 d approximately one-tenth of the degraded PANO mass fraction is formed in parallel to the corresponding tertiary base fraction. In silages mixed with S. jacobaea, the final content of jacoline and jaconine was even higher than the sum of the free tertiary base and the corresponding N-oxide form in the pre-ensiling material. During ensiling the content of senkirkine increased from on average 14.9 to 262 µg/kg with S. jacobaea and from 7,501 µg/kg to 10,535 µg/kg with S. vernalis.
Interactions between effects of ensiling treatment and Senecio spp. (p < 0.001) were found for the PA belonging to the senecionine group and senkirkine (). Regarding the PA belonging to the senecionine group, treatment effects were significant only in S. vernalis mixed grass silages, where proportions were lower in silages treated with LBHE and LBHO in comparison to CON and SOIL. Similarly, the treatment effects for senkirkine were significant only in S. vernalis mixed grass, where proportions were higher in silages treated with LBHE and LBHO in comparison to CON and SOIL.
Table 4. p-Values of the interactions effects between Senecio spp. and ensiling treatments (p-values between Senecio spp. and pre-wilting conditions, storage duration as well ensiling treatment and storage duration).
Correlation analysis for silages mixed with S. vernalis is depicted in (compare to ) revealing positive correlations between pH value and total PA-PANO content (r = 0.430, p < 0.001). In particular, correlations were significant for pH value with contents of retrorsine (r = 0.294, p = 0.018), senecionine group (r = 0.412, p < 0.001), senecionine-NO-group (r = 0.703, p < 0.001), senecipylline (r = 0.434, p < 0.001) and senkirkine (r = 0.264, p = 0.035). In contrast, a negative correlation was observed between lactic acid and senecionine-NO-group (r = −0.622, p < 0.001). Contents of acetic acid were negatively correlated with PA of the senecionine group (r = −0.400, p = 0.001), senecipylline (r = −0.372, p = 0.002), retrorsine (r = −0.249, p = 0.047). Contents of butyric acid correlated with contents of PA of the senecionine group (r = 0.345, p = 0.005), of PA of the senecionine-NO-group (r = 0.568, p < 0.001) and senecipylline (r = 0.357, p = 0.004).
Figure 4. Correlation matrix of PA contents after ensiling [µg/kg dried sample] with quality traits from silages with either S. vernalis (A) or S. jacobaea (B). Data from 10 and 90 d of storage are included. Positive correlations are depicted in grey, and negative correlations in black. Areas of circles show the absolute value of corresponding correlation coefficients. Insignificant correlations with p > 0.05 were left blank. DM, dry matter; ADF, acid detergent fibre, NDF, neutral detergent fibre.
![Figure 4. Correlation matrix of PA contents after ensiling [µg/kg dried sample] with quality traits from silages with either S. vernalis (A) or S. jacobaea (B). Data from 10 and 90 d of storage are included. Positive correlations are depicted in grey, and negative correlations in black. Areas of circles show the absolute value of corresponding correlation coefficients. Insignificant correlations with p > 0.05 were left blank. DM, dry matter; ADF, acid detergent fibre, NDF, neutral detergent fibre.](/cms/asset/8ab8c359-4c7e-44bf-9093-84dbe5896889/gaan_a_2084321_f0004_b.gif)
Correlation analysis for silages mixed with S. jacobaea depicted in , revealed positive correlations between pH value and contents of erucifoline (r = 0.558, p < 0.001) and jacobine (r = 0.454, p < 0.001). A negative correlation was found for pH value and jaconine (r = −0.452, p < 0.001). In reverse, jaconine positively correlated with lactic acid content (r = 0.489, p < 0.001), while acetic acid was negatively correlated with erucifoline (r = −0.359, p = 0.004) and jacobine (r = −0.265, p = 0.036).
For several PA positive correlations were found with NH3-N resulting in correlations of NH3-N [g/kg N] with total PA for both, silages mixed with S. vernalis (r = 0.581, p < 0.001) and mixed with S. jacobaea (r = 0.460, p = 0.002). Total PA content also correlated with ADF content (r = 0.410, p = 0.003) and crude ash (r = 0.639, p < 0.001) for silages mixed with S. vernalis and with NDF content (r = 0.414, p = 0.006), ADF content (r = 0.463, p = 0.002) and crude ash (r = 0.476, p = 0.002) for silages mixed with S. jacobaea.
4. Discussion
The present study aimed at investigating PA-PANO degradation during ensiling from relevant Senecio spp. with diverse PA-PANO profile as affected by different ensiling treatments and silage conditions. Pre-wilting and ensiling treatments affected the chemical composition and characteristics of silages. While most of the results were expected, SOIL addition did not increase the contents of crude ash in silages in comparison to CON treatment. Strongest effects were seen after LBHO and LBHE treatments, which significantly improved silage quality, resulting in enhanced CP and decreased fibre contents. Treatment effects were found for lactate (highest for treatment LBHO), acetate (highest for treatment LBHE) and butyrate (highest for treatment SOIL). The latter one should not be detectable in well-fermented silages as it indicates the activity of clostridia during the ensiling process (Pahlow et al. Citation2003). Indeed, soil is the primary source of butyric acid producing clostridia (Kung et al. Citation2018). Overall pH and lactate concentrations were in the range considered as indicative for a sufficient fermentation and conservation (Kung et al. Citation2018). Pre-wilting resulted in slightly higher pH values and higher NH3-N contents, likely due to increased degradation of free amino acids (Scherer et al. Citation2019).
The present study confirms previous investigations demonstrating that PANO are largely degraded during ensiling (Candrian et al. Citation1984; Gottschalk et al. Citation2015; Klevenhusen et al. Citation2019). Besides during ensiling, degradation of PANO has also been observed under different conditions. For example, a decrease in PANO content has been observed during composting (Crews et al. Citation2009; Hough et al. Citation2010) and rumen fermentation (Mulder et al. Citation2020; Taenzer et al. Citation2022). Contrastingly, PANO degradation has not observed during the drying of plants for hay production (Candrian et al. Citation1984). Thus, the common denominator of PANO degradation seems to be the absence of oxygen (redox conditions). A recent study, which investigated the fate of PA-PANO during rumen fermentation in vitro and in vivo revealed that PANO were in a first step reduced to their corresponding PA, resulting in increased concentrations of the corresponding free base form in a short time frame of only few hours. Afterwards, the free base form underwent further degradation by a reduction of the double bonds present in the necic acid moieties (Taenzer et al. Citation2022). If similar processes happen during ensiling, the duration of silage storage and time of sampling is likely to affect individual PA contents, as indicated by differences observed between 10 and 90 d of storage. However, similar to ruminal degradation, major metabolisation might be expected to occur much earlier.
In the present silages about one-tenth of the original PANO content, appeared to have been converted to the corresponding tertiary base fraction (). Therefore, depending on the initial PA level and PA profiles, differences in extent of overall degradation can be observed. Wiedenfeld (Citation2011) reported that under normal conditions a reduction of the PA-PANO content down to 20% is possible during ensiling. Becerra-Jiminez et al. (Citation2013) found a decline of the initial total PA levels down to only 44 to 31% in silage samples with 75%, 50% and 25% S. jacobaea, respectively. In this study was stated that the degree of PA-PANO degradation depends on the initial level of contamination prior to ensiling and the authors suggested that decomposition of PA was negligible at S. jacobaea contents below 25% in the ensilage material. However, this conclusion is not entirely supported by other studies (Candrian et al. Citation1984; Berendonk and Hünting Citation2011), including the present one with a Senecio spp. content of 3% on fresh matter basis. In the present study, the initial PA content for S. jacobaea and S. vernalis grass mixtures varied by a factor of five but comparable degradation rates were observed. In both contamination scenarios, the PANO content declined by almost 100%, with a slight concomitant increase in the corresponding PA content, resulting in an overall reduction of 75–90%. This result exceeds the decomposition levels observed by Becerra-Jiminez et al. (Citation2013) and is close to the values reported by Wiedenfeld (Citation2011). Differences in overall reduction might further depend on factors like temperature, storage duration and amount of ensiling material (Becerra-Jiminez et al. Citation2013) as well as DM content and treatment effects, as seen in the present study. Gottschalk et al. (Citation2015) additionally presumed inhibitory pH effects on the enzymatic degradation of PA-PANO, as in their laboratory scale silages, which had very low pH of 3.9, the total contents of PA-PANO were reduced by only 24%. This is in agreement with observations made by Berendonk and Hünting (Citation2011). In a previous study, Klevenhusen et al. (Citation2019) observed a reduction of total PA-PANO in the range of 35–45% in silages with final pH of 3.8. In the present study, significant correlations were found between silage pH and certain PA. However, albeit statistically significant most correlations were rather low and positive, thus disproving the hypothesis of an inhibitory effect of pH on PA-PANO degradation.
Both bacterial inoculum preparations were prepared with the addition of molasses, which has previously been shown to lower seneciphylline and senecionine contents in contaminated silages (Klevenhusen et al. Citation2019). In S. vernalis mixed silages, bacterial inoculation resulted in lower final senecionine contents, while senkirkine concentration was increased. Reasons for this are unknown but might depend on the different silage microbiome hinting at different PA-metabolisation for CON and SOIL treatments, which might be considered as uncontrolled fermentation in contrast to LBHE and LBHO treatments. In silages mixed with S. vernalis, several PA additionally correlated with the butyric acid content indicating that the composition of the silage microbiome is of relevance for PA degradation.
The otonecine-type PA senkirkine has previously been shown to be resistant towards degradation during ensiling (Klevenhusen et al. Citation2019). While the other free PA bases are considered to be metabolites of their respective PANO degradation, no N-oxides exist for the otonecines, as they consist of an azacyclooctene ring system that is methylated at the N-atom (Mulder et al. Citation2018). Accordingly, the observed increased senkirkine content must derive from other precursors, like e.g. senecionine N-oxide (Hartmann and Dierich Citation1998).
In the present study, certain PA such as jacoline, jaconine and senkirkine were poorly degraded by ensiling. The same PA were also slowly degraded in the rumen (Taenzer et al. Citation2022), which is congruent with their resistance to being metabolised in the liver (Geburek et al. Citation2020). The PA, which are more resistant to metabolic degradation, have previously been shown to pass into milk (Hoogenboom et al. Citation2011; Mulder et al. Citation2020). Generally, toxicity of PA is associated with their liver metabolism by cytochrome P450 monooxygenases (CYP), also called ‘bioactivation’ resulting in the formation of reactive metabolites, which can interact with DNA or proteins (Yan et al. Citation2008; Ruan et al. Citation2014). Accordingly, hepatic metabolism is a prerequisite for PA toxicity, and PA with a high metabolic stability may be of less concern to human and animal health. The framework of current risk assessment currently considers the individual PA and PANO as equipotent substances, and further research is needed to enlarge our knowledge on toxicity of individual PA. Nevertheless, it can be concluded that ensiling is an effective method to decrease the content of total PA-PANO, offering opportunities to decrease the risk of PA intoxication in livestock by especially reducing the content of metabolically active PA compounds.
5. Conclusions
The present study investigated the major Senecio-type PA-PANO during ensiling of grass mixed with two different Senecio spp. under different ensiling conditions. The study confirms that ensiling is an effective method to decrease the content of total PA-PANO. This is mainly achieved by an effective degradation of PANO. This reduction was detected for all treatments tested, with only minor differences among them and could be observed for silages mixed with S. jacobaea or S. vernalis with a fivefold higher initial PA-PANO content. The decrease in PANO was accompanied by an increase in free tertiary PA bases. For some PA, such as jacoline and jaconine, the content in silages was even higher than the sum of the free tertiary base and the corresponding N-oxide form in the starting material. This suggests that some PA are less affected by degradation and appear to be additionally formed by the conversion of other PA. This also holds true for the otonecine-type PA senkirkine that remained resistant to degradation and its content increased considerably during ensiling, regardless of ensiling treatment. However, due to their metabolic stability, these PA might be of lesser concern for animal and human health. Further research is needed to understand differences in toxicity and transfer of individual PA into the milk to allow the formulation of thresholds in animal feed.
Acknowledgement
The authors would like to thank Felicitas Koch, Janine Kowalczyk, Britta Ohlhoff and Marc Lorenzen for their help in making the silages as well as Anja Gessele, Jasmin Daniel, Anke Sjaba and Andreas Stock for PA analysis. We would like to thank the staff of the Institute of Animal Nutrition/Braunschweig (Federal Research Institute for Animal Health) for preparing the silages for analyses.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Anke S, Niemüller D, Moll S, Hänsch R, Ober D. 2004. Polyphyletic origin of pyrrolizidine alkaloids within the Asteraceae. Evidence from differential tissue expression of homospermidine synthase. Plant Physiol. 136:4037–4047.
- Becerra-Jiminez J, Kuschak M, Roeder E, Wiedenfeld H. 2013. Toxic pyrrolizidinalkaloids as undesired contaminants in food and feed: degradation of the PAs from Senecio jacobaea in silage. Pharmazie. 68:636–639.
- Berendonk C, Hünting K. 2011. Einfluss der Silierung auf den Gehalt an Pyrrolizidinalkaloiden von Senecio jacobaea in Abhängigkeit vom Seneciogehalt im Siliergut. In: Proceedings of the 55th annual meeting of the working group for Grassland and Forage Production (AGGF); August 25–27; Oldenburg, Germany, p. 159–162. [accessed 2021 Feb 10]. https://www.lfl.bayern.de/mam/cms07/ipz/dateien/aggf_2011_berendonk_huenting.pdf
- Boppré M. 2011. The ecological context of pyrrolizidine alkaloids in food, feed and forage: an overview. Food Add Contam A. 28:260–281.
- Buchmüller J, Enge AM, Peters A, Ebmeyer J, Küpper JH, Schäfer B, Brauening A, Hessel-Pras S. 2022. The chemical structure impairs the intensity of genotoxic effects promoted by 1,2-unsaturated pyrrolizidine alkaloids in vitro. Food Chem Toxicol. 164:113049. in press.
- Candrian U, Lüthy J, Schmid P, Schlatter C, Gallasz E. 1984. Stability of pyrrolizidine alkaloids in hay and silage. J Agric Food Chem. 32:935–937.
- Cortinovis C, Caloni F. 2015. Alkaloid-containing plants poisonous to cattle and horses in Europe. Toxins. 7:5301–5307.
- Crews C, Driffield M, Berthiller F, Krska R. 2009. Loss of pyrrolizidine alkaloids on decomposition of ragwort (Senecio jacobaea) as measured by LC-TOF-MS. J Agric Food Chem. 57:3669–3673.
- Dickinson JO, Cooke MP, King RR, Mohamed PA. 1976. Milk transfer of pyrrolizidine alkoloids in cattle. J Am Vet Med Assoc. 169:1192–1196.
- DLG TestService GmbH. 2018. DLG testing guidelines for the award and use of the DLG quality mark for ensiling agents. prepared under the auspices of the DLG commission for ensiling agents, at http://www.dlg.org/siliermittel.html
- Driehuis F. 2013. Silage and the safety and quality of dairy foods: a review. Agric Food Sci. 22:16–34.
- EFSA Panel on Contaminants in the Food Chain (CONTAM). 2011. Scientific opinion on pyrrolizidine alkaloids in food and feed. Efsa J. 9:2406.
- Geburek I, Preiss-Weigert A, Lahrssen-Wiederholt M, Schrenk D, These A. 2020. In vitro metabolism of pyrrolizidine alkaloids – metabolic degradation and GSH conjugate formation of different structure types. Food Chem Toxicol. 135:110868.
- Gottschalk C, Ronczka S, Preiß-Weigert A, Ostertag J, Klaffke H, Schafft H, Lahrssen-Wiederholt M. 2015. Pyrrolizidine alkaloids in natural and experimental grass silages and implications for feed safety. Anim Feed Sci Technol. 207:253–261.
- Gottschalk C, Ostertag J, Meyer C, Gehring K, Thyssen S, Gareis M. 2018. Influence of grass pellet production on pyrrolizidine alkaloids occurring in Senecio aquaticus -infested grassland. Food Add Contam A. 35:750–759.
- Hantsch L, Bruelheide H, Erfmeier A. 2013. High phenotypic variation of seed traits, germination characteristics and genetic diversity of an invasive annual weed. Seed Sci Res. 23:27–40.
- Hartmann T, Dierich B. 1998. Chemical diversity and variation of pyrrolizidine alkaloids of the senecionine type: biological need or coincidence? Planta. 206:443–451.
- Hoogenboom LAP, Mulder PPJ, Zeilmaker MJ, van den Top HJ, Remmelink GJ, Brandon EFA, Klijnstra M, Meijer GAL, Schothorst R, Van Egmond HP. 2011. Carry-Over of pyrrolizidine alkaloids from feed to milk in dairy cows. Food Addit Contam A. 28:359–372.
- Hough RL, Crews C, White D, Driffield M, Campbell CD, Maltin C. 2010. Degradation of yew, ragwort and rhododendron toxins during composting. Sci Total Environ. 408:4128–4137.
- Kalač P. 2011. The effects of silage feeding on some sensory and health attributes of cow’s milk: a review. Food Chem. 125:307–317.
- Klevenhusen F, Pieper R, Winter J, Ronczka S, Speer K. 2019. Stability of pyrrolizidine alkaloids from Senecio vernalis in grass silage under different ensilage conditions. J Sci Food Agric. 99:6649–6654.
- Kung JL, Shaver RD, Grant RJ, Schmidt RJ. 2018. Silage review: interpretation of chemical, microbial, and organoleptic components of silages. J Dairy Sci. 101:4020–4033.
- Maedge I, Gehling M, Schone C, Winterhalter P, These A. 2020. Pyrrolizidine alkaloid profiling of four Boraginaceae species from Northern Germany and implications for the analytical scope proposed for monitoring of maximum levels. Food Addit Contam A. 37:1339–1358.
- Molyneux RJ, Gardner DL, Colegate SM, Edgar JA. 2011. Pyrrolizidine alkaloid toxicity in livestock: a paradigm for human poisoning? Food Addit Contam Part A. 28:293–307.
- Mulder PPJ, López P, Castelari M, Bodi D, Ronczka S, Preiss-Weigert A, These A. 2018. Occurrence of pyrrolizidine alkaloids in animal- and plant-derived food: results of a survey across Europe. Food Addit Contam A. 35:118–133.
- Mulder PPJ, Klijnstra MD, Goselink RMA, van Vuuren AM, Cone JW, Stoopen G, Hoogenboom RLAP. 2020. Transfer of pyrrolizidine alkaloids from ragwort, common groundsel and viper’s bugloss to milk from dairy cows. Food Addit Contam A. 37:1906–1921.
- Pahlow G, Muck RE, Driehus F, Elferink SJWH O, Spoelstra SF. 2003. Role of silage fermentation in forage conservation. In: Buxton D, Muck R and Harrison J, editors. Microbiology of ensiling. Madison, WI: American Society of Agronomy, Inc., Crop Science Society of America, Inc., Soil Science Society of America, Inc. Publications; p. 31–93.
- Pauly T, Wyss U. 2019. Efficacy testing of silage additives—methodology and existing schemes. Grass Forage Sci. 74:201–210.
- Ruan J, Yang M, Fu P, Ye Y, Lin G. 2014. Metabolic activation of pyrrolizidine alkaloids: insights into the structural and enzymatic basis. Chem Res Toxicol. 27:1030–1039.
- Scherer R, Gerlach K, Taubert J, Adolph S, Weiß K, Südekum K. 2019. Effect of forage species and ensiling conditions on silage composition and quality and the feed choice behaviour of goats. Grass Forage Sci. 74:297–313.
- Taenzer J, Gehling M, Klevenhusen F, Saltzmann J, Dänicke S, These A. 2022. Rumen metabolism of Senecio pyrrolizidine alkaloids may explain why cattle tolerate higher doses than monogastric species. J Agric Food Chem. accepted for publication.
- von Lengerken J, Zimmermann K. 1991. Handbuch Futtermittelprüfung. Berlin, Germany: Deutscher Landwirtschaftsverlag. (in German).
- Weiß K, Kaiser E. 1995. Milchsäurebestimmung in Silageextrakten mit Hilfe der HPLC. (Lactic acid determination in silage extracts by HPLC). Wirtscheig Futter. 41:69–80.
- Weiß K, Sommer G. 2017. Bestimmung von Gärsäuren und Alkoholen in Silageextrakten mittels Gaschromatographie [Determination of fermentation acids and alcohols in silage extracts by gas-chromatography]. VDLUFA-Schriftenreihe. 74:685–694.
- Weiß K, Alt M. 2017. Determination of single sugars, including inulin, in plants and feed materials by high-performance liquid chromatography and refraction index detection. Fermentation. 3:36.
- Weissbach F, Strubelt C. 2008. Correcting the dry matter content of grass silages as a substrate for biogas production. Landtechnik. 63:210–211.
- Wiedenfeld H. 2011. Plants containing pyrrolizidine alkaloids: toxicity and problems. Food Addit Contam A. 28:282–292.
- Wilkinson JM, Rinne M. 2018. Highlights of progress in silage conservation and future perspectives. Grass Forage Sci. 73:40–52.
- Yan J, Xia Q, Chou MW, Fu PP. 2008. Metabolic activation of retronecine and retronecine N-oxide – formation of DHP-derived DNA adducts. Toxicol Ind Health. 24:181–188.