Abstract
Fish and shrimp species, together with water quality data, were collected from two different stations located inside the Bakkhali river estuary of Bangladesh during winter, premonsoon and monsoon periods. Significant temporal differences were observed for water temperature, salinity and dissolved oxygen. The average catch of fish and shrimps per net between stations varied between 1.89±0.36kg at station 1 and 7.54±4.39kg at station 2, while the average catch in winter, premonsoon and monsoon periods found to be 2.79±1.08kg/net, 6.31±1.03kg/net and 5.06±2.89kg/net, respectively, with a significant difference in catch per net between stations although no significant difference in catch per net was observed between seasons. A total of 18,467 individuals of fish (35 species) and shrimp (10 species) were found in the present study. Three species of shrimps were observed to be dominant (>10.0%) and these were Metapenaeus lysianassa (17.07%), Ambassis dussumieri (14.54%) and Macrobrachium villosimanus (12.13%). Clear differences in faunal abundances were observed between seasons and stations with higher mean abundances during winter (1747.83±421.99 individual/5kg) and at Station 1 (1444±866.74 individuals/5kg). Similarly, the diversity indices, both Shannon–Wiener and Margalef, showed significant differences between stations and seasons (except Shannon for stations). Analyses of similarity (ANOSIM) results confirm both spatial and temporal differences in species community structure with a highly diverse assemblage. Canonical Correspondence Analysis results indicated that salinity and transparency were the main variables influencing fish and shrimp distribution in the Bakkhali river estuary.
Published in collaboration with the University of Bergen and the Institute of Marine Research, Norway, and the Marine Biological Laboratory, University of Copenhagen, Denmark
Introduction
Estuaries are transition zones between sea and freshwater; they are occupied by a combination of freshwater and marine species as well as juveniles (Claridge et al. Citation1986). They serve important economic functions including transportation, industry and tourism, but also drainage of waste from domestic, industrial and agriculture activities (Heip & Herman Citation1995; Raz-Guzman & Huidobro Citation2002). Simultaneously, these ecosystems offer protection, not only for resident species, but also for a wide range of marine and freshwater species which migrate there at certain stages of their life cycle (Weinstein Citation1985; Weisberg et al. Citation1996; Cowley and Whitfield Citation2002; McLusky and Elliott Citation2004; Blaber Citation2000). Fish assemblage structure of estuaries is characterized by high diversity and high abundance, especially for juveniles (Whitfield Citation1999). An examination of the ecological factors is important in defining habitats for fishes and has been the main focus of many previous studies (Able Citation1999; Martino & Able Citation2003). Most estuaries are characterized by high biological productivity associated with relatively extreme and varying environmental conditions (Day et al. Citation1989; Kennish Citation1990; Whitfield Citation1999). As boundary systems between watersheds and the sea, estuaries exhibit environmental gradients that favour the recruitment of a variety of species with diverse physical and trophic structures (Sánchez & Raz-Guzman Citation1997; Harris et al. Citation2001; Kimmerer et al. Citation2001). Since estuaries serve as nurseries for many commercially important fish and crustaceans (Shenker & Dean Citation1979; Weinstein Citation1979; Rakocinski et al. Citation1996; Blaber Citation2000; Elliott & Hemingway Citation2002; Akin et al. Citation2003), it is necessary to examine the environmental factors that shape the species assemblage structure. Fishes play an important role in estuaries as they constitute permanent and temporary community components, with marine species visiting these habitats for feeding, reproduction, growth and protection (Raz-Guzman & Huidobro Citation2002). The distributions of fish within biologically and physically complex estuarine systems may be influenced by many mechanisms. Several estuarine ecologists have pointed out that biotic processes, such as competition and predation, may be important in driving the occurrence of spatial and temporal patterns of fish abundance and assemblage in estuaries (Holbrook & Schmitt Citation1989; Ogburn-Matthews & Allen Citation1993; Lankford & Targett Citation1994; Barry et al. Citation1996).
By nature, estuarine habitats are highly productive (Nixon et al. Citation1986; Day et al. Citation1989) and their role as nursery grounds for fishes is well documented for temperate (Powles et al. Citation1984; Elliott et al. Citation1990; Kennish Citation1990; Drake & Arias Citation1991; Szedlmayer & Able Citation1996; Whitfield Citation1999; Blaber Citation2000; Shackell & Frank Citation2000; Elliott & Hemingway Citation2002) and tropical regions (Raynie & Shaw Citation1994; Sanvicente-Añorve et al. Citation2000; Harris et al. Citation2001; Cowley & Whitfield Citation2002; Franco-Gordo et al. Citation2003). Several biological and abiotic factors affect the occurrence and habitat of fish and shrimp within estuaries. These factors include salinity, temperature, turbidity, dissolved oxygen (DO), freshwater inflow, structural attributes of habitat, depth, geographic distance from the estuary mouth, and hydrography (Gunter Citation1961; Blaber & Blaber Citation1980; Weinstein et al. Citation1980; Rogers et al. Citation1984; Zimmerman & Minello Citation1984; Thorman Citation1986; Peterson & Ross Citation1991; Sogard & Able Citation1991; Cyrus & Blaber Citation1992; Rakocinski et al. Citation1992; Cowen et al. Citation1993; Everett & Ruiz Citation1993; Szedlmayer & Able Citation1996; Fraser Citation1997; Maes et al. Citation1998; Marshall & Elliott Citation1998; Araújo et al. Citation1999; Wagner & Austin Citation1999; Whitfield Citation1999; Hagan & Able Citation2003; Jaureguizar et al. Citation2003; Martino & Able Citation2003).
The assemblages of fish in estuaries are variable both in terms of species composition and distribution patterns (Harris et al. Citation1999). Changes in species assemblage are continuous, according to reproductive seasons of the species and the environmental fluctuations (Whitfield Citation1994; Harris & Cyrus Citation1995; Hettler & Hare Citation1998; Garcia et al. Citation2003). However, through discussion with local fisherman from the Bay of Bengal, Bangladesh, during the present study, there seems to be a general tendency for estuarine fish larvae to peak in abundance during the monsoon in this region. Furthermore, Hossain et al. (Citation2007) also reported a similar trend for juvenile fish species at Naaf river estuary. Fish and shrimp assemblage structure in the estuaries of Bangladesh has not been well studied; although there are some scattered works on different biological aspects of the coastal estuarine system of Bangladesh (Hossain et al. Citation2007), none of them examined the species assemblage structure.
The Bakkhali river estuary located at the southeastern part of Bangladesh is heavily supported by small scale and multigear fisheries. The coastal areas show a typical tropical multi-species fisheries ecosystem. There are about 490 species of fishes (Hossain Citation1971) and 19 species of shrimps/prawn (Chowdhury & Sanaullah Citation1991) available in this area. These fisheries are characterized by fishing households rather than commercial organizations and play a greater role in sustaining the livelihoods and ensuring the food security of large numbers of rural people throughout the developing world (Whitmarsh et al. Citation2003). The future of these potentially huge resources has not been well documented. However, for the sustainability of this fishery resource proper scientific study is an urgent task. Hence, the present study has been designed to provide an extensive report on the fish and shrimp assemblage structure of Bakkhali river estuary in relation to water quality parameters.
Materials and methods
Study area
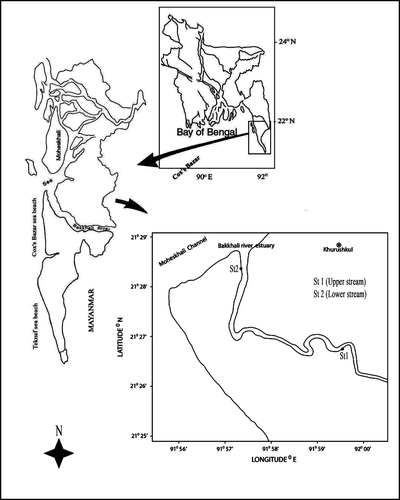
The Bakkhali river estuary is located at the southeastern coast of the Bay of Bengal in Bangladesh (). A number of small streams originating from the south-eastern hills of Mizoram (India) meets at the Naikhongchhari of Bandarban district and form the river Bakkhali. It flows through Naikhongchhari and Ramu of Cox's Bazar district and falls into the Moheshkhali channel of the Bay of Bengal. This river is relatively wide compared to other rivers of the Cox's Bazar district and has a length of about 67km. The Bakkhali river estuary has a semidiurnal tidal regime. Its hydrology is also heavily influenced by monsoon wind. The tidal range varied between 0.07m and 4.42m during neap and spring tide respectively (Hossain and Lin Citation2001). Salt intrusion extends up to 6km upstream where a rubber dam was constructed for irrigation purposes.
The bottom of this river consists mainly of mud and sand particles. The estuarine zone is also characterized by long intertidal mudflats where mangrove vegetation (mainly Avicennia sp.), natural ullo grass Imperata cylindrica, cord grass Spartina sp. and sea grass Halophila beccarii are present (Hena et al. Citation2007). The lower part of this estuary is heavily influenced by anthropogenic and industrial activities including fish harbours, fish processing plants and a large number of fish and shrimp farms. The large amount of organic and inorganic waste changes the chemical characteristics of the water body by producing toxic substances, which ultimately affect the biodiversity. Samp took place at two stations (), one (St1) about 5km upstream from the estuary, which is protected from the sewage and anthropogenic intervention, and another (St2) at the lower stream near the mouth of the estuary, heavily influenced by domestic and industrial activities. Apart from these two stations, nets were not set on a regular basis in the other areas of the Bakkhali river estuary. Net setting and collection of samples was largely dependent on the local fishermen who have used these areas for generations. Therefore, these areas are allowed for fishing only by the local fishermen. Hence, through negotiation with the local fishermen these two stations were considered for the present study.
Sampling gear
The fish and shrimp samples were collected using barrier nets known locally as ‘Char jal’ (). In Cox's Bazar region, Char jal are used to catch various aquatic species from river banks inundated during high tide. Net fencing is made from bamboo poles which are submerged during high tide. Samples are collected during low tide. The net frame is around 2.5m in height and 150m in length, forming part of a circle so that there is approximately 120m distance between two ends of the net. The nylon net has a mesh size of 0.8cm. Bamboo poles are secured on the shore of the river during low tide. During high tide the water is allowed to enter and after 2–2.5h of the high tide the fishermen secure the upper portion of the net and create a barrier. At low tide all the animals inside the fence become trapped and the fishermen harvest the fish, shrimp and crab. Finally, the net is released from the bamboo and is ready for the next high tide.
Sampling periodicity
Samples were collected each month between December 2007 and August 2008. Of the four seasons specified by Mahmood et al. (Citation1994), three seasons were chosen – the winter (December, January and February), premonsoon (March, April and May) and monsoon (June, July and August) – to conduct the sampling. Sampling was done during the full moon and new moon, as during these periods higher abundance of fish and shrimps were reported by the fishermen. No samples were collected during the post monsoon period (September, October and November) as the fishermen become engaged in Hilsha fishery, which is the single largest commercial fishery of Bangladesh (Mazid Citation2002). Fishermen are engaged by Hilsha boat owners to leave this less-profitable Char jal fishing during the post monsoon season.
Sample collection
Sample catches from Char jal were taken directly from the nets. In the laboratory, samples were sorted and identified to species level (Fischer & Whitehead Citation1974; Shafi & Kuddus Citation1982a, Citation1982b; Talwar & Jhingran Citation1991; DeBruin et al. Citation1995). The total numbers of each species and their wet weight from each net were also recorded. During sampling, in situ water quality parameters were measured at each sampling site. The salinity, pH, temperature and dissolved oxygen were determined by using a refractrometer (NewS-100, TANAKA, Japan), a pen pH meter (s327535, HANNA Instruments), a thermometer in centigrade and a DO meter (HI 9142, HANNA Instruments), respectively. A Secchi disc (20cm diameter) was used to measure the water transparency.
Data analysis
Diversity of the species assemblage was expressed by the Shannon–Wiener index (H') (Shannon Citation1949; Shannon & Weaver Citation1963; Ramos et al. Citation2006) using the following formula:
Richness was measured by Margalef index (d) (Margalef Citation1968) using the following formula:
For environmental parameters (temperature, salinity, DO, pH and water transparency) one-way analysis of variance (ANOVA) was used to calculate if there is any difference between two stations. The same procedure was followed for seasons. Prior to ANOVA tests, all data were checked for normality using the Kolmogorov–Smirnov test and homogeneity of variances using Levene test (Sokal & Rohlf Citation1998). Furthermore, in the event of significance, a post hoc Tukey HSD test was used to determine which means were significantly different at a 0.05 level of probability (Spjotvoll & Stoline Citation1973). The Kruskal–Wallis test (Akin et al. Citation2005) was performed on data which did not satisfy the assumptions of normality and homogeneity, after performing diverse data transformations (Clarke & Warwick Citation1994). Except for salinity, all other water quality parameters met the criteria of normal distribution and homogeneity of variances. One-way analysis of similarity (ANOSIM) (Clarke & Warwick Citation1994) was used to conclude the significance of spatial and temporal variation in the fish and shrimp assemblage structure. This test is based on a Bray–Curtis rank similarity matrix and was calculated using log-transformed data. Similarity percentages analysis (SIMPER) (Clarke Citation1993) was used to observe the percentage contribution of each species to the average dissimilarity between samples of the various seasons and station-pair combinations. Hierarchical agglomerative clustering with group average linking and non-metric multi-dimensional scaling (nMDS) were performed to investigate similarities among stations and seasons (Clarke & Warwick Citation1994). This analysis was based on the Bray–Curtis similarity measure (Bray & Curtis Citation1957). Only species with more than 1% of the total species were included in the analysis to avoid any unusual effects of rare species. All the multivariate analyses were performed using the software PRIMER V6 (Plymouth Routines Multivariate Ecological Research) (Clarke & Warwick Citation1994). Associations between species and environmental variables were examined with the canonical correspondence analysis (CCA) using the ECOM 1.32 version (Environmental Community Analysis Citation2000) software. To reduce the effects of rare species, only species contributing >1% of the total based on all species and samples were included in CCA after log transformation (Log10(x+1)). CCA was proposed to constrain the axes in classical Correspondence Analysis (CA) to be linear functions of a-priori defined or measured variables associated with species records. The ordination axes of CA are termed Eigenvectors. Each Eigenvector has a corresponding Eigenvalue, often denoted by λ. The Eigenvalue is actually equal to the (maximized) dispersion of the species scores on the ordination axis, and is thus a measure of importance of the ordination axis. The first ordination axis has the largest Eigenvalue (λ1), the second axis the second largest Eigenvalue (λ2), and so on. The Eigenvalues of CA all lie between 0 and 1. Values over 0.5 often denote a good separation of the species along axis (Jongman et al. Citation1995).
Results
Environmental parameters
The measured environmental parameters are summarized in and illustrated in . Water temperature ranged between 21.00°C (in winter; January 2008 at St1) and 31.00°C (in premonsoon and monsoon; March and July 2008, respectively, at St2) with a mean of 27.16±3.33°C. No significant difference was observed in temperature between stations (F1,12=2.13, P=0.17). However, winter season showed a significant difference from monsoon and premonsoon (F2,12=66.65, P=0.001) although there was no significant difference between premonsoon and monsoon.
Figure 3. Temporal and spatial variations in mean environmental parameters at the study area (s, St, st, station).
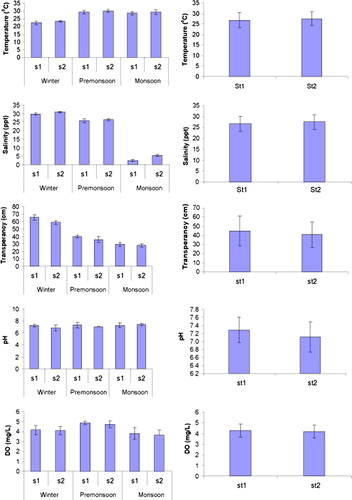
Table I. Mean of the different environmental parameters in different seasons and stations during the study period.
Salinity values (mean 20.00±11.94) ranged from 2.00 ppt (during monsoon season; August 2008) to 31.00 ppt (during winter season; December 2007 and February 2008). No significant differences were found in salinity between the stations (H=0.788, P=0.375), while significant differences were observed among the seasons (H=15.316, P=0.001) with very low salinity during monsoon ().
Oxygen concentration (mean=4.21±0.58) attained a maximum in March (5.02mg/l at St1) and a minimum in June (3.24mg/l at St1). No significant differences were found in oxygen concentration throughout stations (F1,12=0.30, p=0.59); in contrast, significantly higher dissolved oxygen concentration was observed during premonsoon season (4.79±0.24mg/l) compared to winter (4.12±0.40mg/l) and monsoon (4.40±0.49mg/l) seasons (F2,12=9.22, P=0.001).
Water transparency varied from 26cm (during monsoon; July 2008 at St2) to 70cm (during winter season; January 2008 at St1) with a mean of 42.83±14.86cm. Significant differences were observed in water transparency between stations (F1,12=212.08, P=0.001). Similarly, water transparency exhibited a strong seasonal gradient (F2,12=9.06, P=0.01). Mean value in winter season (62.00±4.69cm) was noticeably higher than the premonsoon (37.83±3.63cm) and monsoon season (28.66±2.16cm).
The highest pH value (7.7) was observed during the premonsoon season at St1, while the lowest pH value (6.3) was observed during the winter season, also at St1. Mean pH value was observed to be 7.20±0.34. No significant differences were found for pH between stations (F1,12=1.20, P=0.29) and among the seasons (F2,12=1.28, P=0.31).
Species community composition by weight
The catch per net at St1 ranged between 1.31kg (during winter season) to 2.52kg during the premonsoon period with an average of 1.89±0.36kg. The average catch per net at St1 during winter, premonsoon and monsoon seasons was found to be 1.64±0.29kg, 1.94±0.57kg and 2.10±0.07kg, respectively. Of the fish species, Liza tade was found to be the most abundant (23.6%) followed by Mystus gulio (11.96%), Gerres filamentosus (9.34%) and Terapon jarbua (8.76%). Among the shrimps, Macrobrachium villosimanus was found to be moderately higher (7.98%) in species composition by weight. Only 12 species contribute about 90% of the total catch. No significant difference was observed in average catch per net between winter, premonsoon and monsoon (F2,6 =1.37, P=0.32).
The catch per net at St2 ranged between 2.71 and 14.77kg with an average of 7.54±4.39kg. The average catch per net St2 during winter, premonsoon and monsoon seasons was found to be 3.94±1.94kg, 10.67±2.12kg and 8.01±5.86kg, respectively. The highest percentage (27.40%) was found for the fish Mystus gulio followed by Acanthopagrus latus (10.25%), Cynoglossus cynoglossus (8.12%) and Macrobrachium villosimanus (7.47%). Only 13 species including those reported above contributed about 87.11% of the total catch (). No significant difference was observed in average catch per net between winter, premonsoon and monsoon (F2,6 =2.42, P=0.17).
Table II. Fish and shrimp species recorded in the Bakkhali river estuary from December 2007 to August 2008 showing relative contribution (%) to the total abundance by stations and seasons.
However, a significant difference was obtained in average catch per net between St1 and St2 (F1,16=14.74, P=0.001), although no significant difference was observed among the seasons (F2,15=1.09, P=0.35).
Species community composition by number
During the study period a total 18,467 fish, shrimp and crab were collected from the Char jal with a mean abundance of 1026±803 ind/5kg of species (). The maximum species abundance (2869 ind/5kg of species) was observed during the winter at St1 while the minimum (241 ind/5kg of species) was observed during the monsoon period at St2.
The species abundance per net in St1 ranged between 412 ind/5kg (during monsoon season) to 2869 ind/5kg during the winter period, with an average of 1444.00±886.74 ind/5kg. The average abundance during winter, premonsoon and monsoon seasons were found to be 2304.66±489.24 ind/5kg, 1585.66±341.70 ind/5kg and 441.66±25.73 ind/5kg, respectively. Ambassis dussumieri was found highest (16.83%) followed by Metapenaeus lysianassa (15.27%), Gerres filamentosus (12.45%) and Terapon jarbua (11.10%) in species abundance. Only 18 species contributed about 97.25% of the total catch. A significant difference was observed in average species abundance between winter, premonsoon and monsoon (F2,6 =22.26, P=0.002).
The species abundance per net in St2 ranged between 241 ind/5kg to 1601 ind/5kg with an average of 607.88±477.25 ind/5kg. The average abundance during winter, premonsoon and monsoon seasons was found to be 1191.00±357.31 ind/5kg, 368.66±98.77 ind/5kg and 264±19.92 ind/5kg, respectively. The highest percentage (23.49%) was found for Macrobrachium villosimanus followed by Metapenaeus lysianassa (21.35%), Mystus gulio (9.16%) and Ambassis dussumieri (9.10%). Only 13 species contribute about 92.56% of the total abundance (). A significant difference was observed between winter, premonsoon and monsoon (F2,6=16.83, P=0.003). A significant difference in average species abundance was also observed between stations (F1,16 =6.42, P=0.02) and seasons (F2,15=8.58, P=0.003).
Species diversity
The Shannon–Wiener diversity index ranged between 0.95 (at St2 during monsoon) and 2.62 (at St1 during premonsoon) with a mean diversity value of 1.91±0.46 (A). No significant difference was observed (F1,16=0.915, P=0.353) in the mean Shannon–Wiener diversity values between the stations. However, this difference was found significant between the seasons (F1,16=14.264, P<0.001) with higher mean diversity value (2.416±0.16) during the premonsoon period.
Figure 4. Temporal and spatial variations of (A) Shannon–Wiener index and Abundance and (B) Margalef diversity index and Abundance of the Bakkhali fish and shrimp assemblage. St, station. St, station.
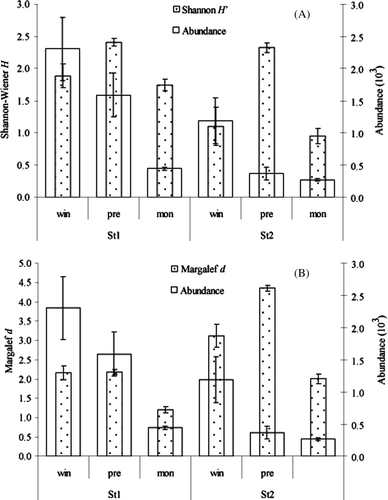
The minimum Margalef richness value (1.14) was observed at St1 during monsoon while the maximum value (4.50) was found in station St2 during premonsoon (B) with a mean richness value of 2.52±1.08. The mean species richness values at St1 and St2 was found to be 1.85±0.49 and 3.20±1.09, respectively. In the case of seasons, the highest mean richness value (3.30±1.22) was observed in premonsoon while the lowest mean value (1.56±0.40) was observed during monsoon. Significant differences were found for Margalef's index for both stations (F1,16=11.34, P=0.004) and seasons (F1,16=6.757, P=0.008).
Species assemblage
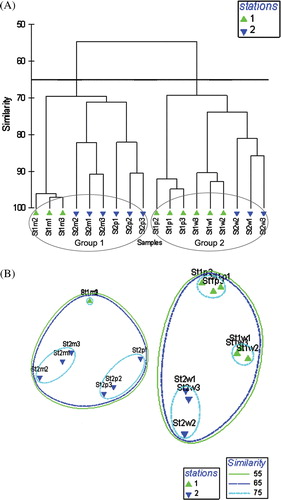
The analysis of similarity (ANOSIM) showed significant difference in assemblage structure between stations (Global R=0.365; p=0.008) (). Species assemblage at each station was found to be highly diverse and at St1 Liza tade (15.24%), Terapon jarbua (15.10%), Gerres filamentosus (13.45%) and Acanthopagrus latus (10.86%) were found to be the most dominant (>10%) species, while at St 2 Terapon jarbua (12.48%), Cynoglossus cynoglossus (11.57%) and Mystus gulio (11.16%) were found to be the dominant (>10%) species (). According to SIMPER results, other contributory species to the assemblage structure of the studied area were Glossogobius giurus, Metapenaeus monoceros, Metapenaeus lysianassa, Valamugil speigleri, Ambassis dussumieri and Butis butis ().
Table III. Result of one-way ANOSIM (R value and significant levels) and SIMPER analysis of fish and shrimp abundance between stations and different seasons.
Table IV. Average similarity and discriminating fish and shrimp in each station using SIMPER analysis.
A highly diverse species assemblage was also observed among all seasons through SIMPER analysis (). Significant difference were observed for temporal community structure of the studied area (Global R=0.726; P=0.001) with a clear separation of different seasons. The pair-wise comparison of seasons also showed distinct separation (). Metapenaeus lysianassa (17.19%) and Ambassis dussumieri (13.43%) were found to be the most contributory species during winter, while during the premonsoon season it was Velamugil spaglari (11.23%) and Mystus gulio (10.38%). On the other hand, Mystas gulio (21.21%) and Terapon jarbua (17.12%) were found the most contributory species during the monsoon season ().
Table V. Average similarity and discriminating fish and shrimp in each season using SIMPER analysis.
At the similarity level of 65%, no marked separation, either for the stations or for the seasons, was observed by cluster analysis. Two clusters were identified – the first consists of St2 during monsoon and premonsoon period along with St1 during monsoon, and the second group consists of St1 during premonsoon and winter along with St2 during winter ().
Canonical correspondence analysis
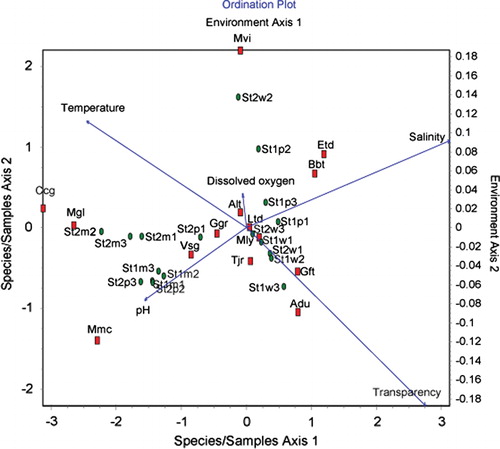
CCA eigenvalues of the first four axes were 0.36 (CCA1), 0.34 (CCA2), 0.11 (CCA3), and 0.10 (CCA4). Species–environment Pearson correlation coefficients for the first four axes were 0.96, 0.92, 0.82, and 0.79, respectively. The cumulative percentage variance of species for the first four axes (CCA 1–4) was 49.18. The first and second axes modelled 19.4 and 18.1% of species data, respectively. Therefore, the results obtained from the first two axes were plotted (). However, the vector length of a given variable indicates the importance of that variable in CCA analysis. Salinity (0.92), which has the longest vector along the first axis, was significantly correlated with premonsoon at St1 (). Furthermore, transparency was also significantly associated (0.81) with winter season of St1 and St2. As shown in CCA ordination (), high values of salinity concentration are the most significant water parameters for Butis butis and Eleutheronema tetradactylum. High values of water transparency was associated with occurrence of Ambassis dussumieri and Gerres filamentosus. High values of pH are associated with the occurrence of Valamugil speigleri, Glossogobius giurus and Metapenaeus monoceros. However, Acanthopagrus latus showed the highest association with dissolved oxygen (DO), while no species was found to be closely associated with temperature.
Discussion
Environmental parameters
During the present study, no significant spatial variation was observed for temperature, salinity, dissolved oxygen and pH. This is most probably due to the presence of a rubber dam upstream of this river, which prevents freshwater influx in this area during winter and premonsoon and ultimately the whole area, i.e. both St1 and St2, is similarly governed by the brackish water entering through tidal influence. In the same way, during monsoon, the rubber dam remains open and huge amount of freshwater is discharged through the dam. As a consequence, the water parameters in both stations remain the same. However, a significant difference in water transparency was observed between St1 and St2, where lower transparency was observed in St2 compared to St1. This may be due to the higher water turbulence in downstream (St2) due to strong tidal fluctuation and anthropogenic causes such as the presence of the fish harbour, jetty, etc., in the mouth of the river (St2). Comparatively lower dissolved oxygen was observed in both stations. Hena & Khan (Citation2009) also reported a lower level of DO in the same estuary (1.89–5.37mg/l). This may be due to the nearby domestic, agricultural and industrial waste water discharges which affect the water and sediment quality and lead to a hypoxic condition, as stated by Van Eck et al. (Citation1991).
Fluctuations in water transparency influence the primary productivity which ultimately affects the fish distribution (Arthington & Welcome Citation1995; McAllister et al. Citation2001). Rozengurt & Hedgepeth (Citation1989) reported changes in natural recruitment and species abundance in the Caspian Sea due to in increase in salinity. McAllister et al. (Citation2001) also reported changes in species abundance due to a salinity increase. According to Maes et al. (Citation2004), dissolved oxygen is one of the most important factors for fish abundance and distribution. Fish communities are highly affected by temperature within estuaries (Cyrus & Mclean Citation1996). A sudden increase or decrease in water temperature may cause fish mortality (Blaber Citation2000). Environmental parameters such as temperature, salinity, dissolved oxygen, water transparency and pH play an important role for species abundance and diversity (Whitfield Citation1999), especially for the tropical regions where the fluctuation of these parameters are frequently due to seasonal changes (Blaber Citation2000). The Bakkhali river estuary is no exception. Significant temporal differences were observed for temperature, salinity, water transparency and dissolved oxygen during the present study which may almost certainly affect the assemblage structure.
Species composition
The average catch per net at St2 (7.54±4.39kg) was found to be significantly higher compared to St1 (1.89±0.36kg). St2 is located in the river mouth and possesses a highly diverse type of larger-sized fish compared to St1. On the other hand, at St1 a small area of salt marsh is situated nearby, which acts as the nursery ground for small and juvenile fishes. As fish grew, their daily food requirement also increased and fish began to migrate to the river mouth/estuary in the search of food (Davies & Day et al. Citation1986). Hence, larger fish were caught and the total weight of the catch per net was higher were St2.
A total of 45 fish and shrimp species were recorded during the study. Among them are Metapenaeus lysianassa, Ambassis dussumieri, Macrobrachium villosimanus, Terapon jarbua, Gerres filamentosus, Liza tade, Mystus gulio, Metapenaeus monoceros, Butis butis, Acanthopagrus latus, Eleutheronema tetradactylum, Glossogobius giurus, Valamugil speigleri, and Cynoglossus cynoglossus, each contributing more than 1% of the composition. Islam et al. (Citation1992) reported about 185 species from the coastal waters of Bangladesh collected from the estuarine sey bagnet. On the other hand Hossain et al. (Citation2007) reported about 161 species collected by different types of net from Naaf river estuary located around 50km from the present study site. A smaller number of species observed in the present investigation is most probably due to the use of only one type of net, i.e. Char jal which catches only species living very near to the shore and closer to the bottom. Another reason is the controlled environment of the Bakkhali estuary by the rubber dam limiting the species abundance. Also, the dam-induced changes in water characteristics may have profound effects on species numbers in the river (McAllister et al. Citation2001).
During the study period, only one exclusively freshwater specimen fish (Labeo rohita) was collected. The rubber dam, constructed at the upper stream of the river, creates an unusual environment. During the monsoon season huge amounts of freshwater flow into the river and create a freshwater-influenced estuarine environment where salinity ranges were found to vary from 2.33±0.57 to 5.33±0.57. This supports the findings of the presence of a freshwater species in the investigated area. Freshwater fishes are usually incapable of osmoregulating in saltwater and consequently tend to be found in estuaries only when salinities decline to very low levels during periods of heavy freshwater discharge (Potter & Hyndes Citation1999).
Species abundance
The species abundance found in the Bakkhali river estuary is composed of small numbers of species with high contribution and a large number of species whose contributions are very negligible, a common feature of estuarine faunal populations (Gaughan et al. Citation1990; Harrison & Whitfield Citation1990; Drake & Arias Citation1991; Harris & Cyrus Citation1995; Whitfield Citation1999). The number of taxa in this study (45 species) was found to be lower than in the Naaf river estuary, another estuary close to the study area (Hossain et al. Citation2007). However, these types of judgements must be based on differences in sampling gear, sampling period and most importantly habitat characteristics. Moreover, each estuarine system may have a different abiotic environment (Blaber Citation1997), resulting from the tidal range, freshwater input, geomorphology and human pressure (Dyer Citation1997; McLusky & Elliott Citation2004) which also affects the species abundance. So a difference in species abundance is not likely to be the exception.
A remarkably lower number of species was observed in the upstream area (St1) of the Bakkhali river estuary, but the diversity index H′ was much higher. Out of total 45 species, 17 were relatively common in the upper stream which is characterized by juveniles of large-sized species and adults of small-sized species. The main reason for the high abundance of juvenile fishes is the presence of a salt marsh and seagrass bed at St1. These observations agree with the comments of Hena et al. (Citation2007) – seagrass and salt marsh habitats are among the most productive ecosystems in the world in terms of the quantity of vegetation production closely linked to the high production rates of associated fisheries.
The lower stream (St2) has a wider zone and is characterized by strong tidal influence and mangroves. As a consequence, relatively higher numbers of species with brackish to marine origin were captured in this zone. Downstream, water transparency showed higher values (28.00–65.33cm) when compared to other estuaries of Bangladesh (Mahmood Citation1986). This is because freshwater flows are scarce here for a major part of the year leading to the presence of marine species which have been described as being related to clearer waters (Blaber et al. Citation1997). The exception occurred during monsoon months (June, July) when the rubber dam is opened causing flushing effect by freshwater flows from the upper, hilly areas.
An estuarine water body along with mangrove plants is the most productive region for zooplankton especially for shrimps and prawns (Hena & Khan Citation2009). The Bakkhali river estuary is influenced by mangrove plants including Avicennia alba, A. marina, and Acanthus ilicifolius, which have created a huge potential habitat for phytoplankton, zooplankton, shellfish and fish larvae. Plankton communities living in mangrove waters are well adapted to the water motion (Alongi Citation2009). Whether a source of food, shade, or refuge, mangrove forests are an important habitat for coastal organisms that either float or swim on the ebb and flood of the tide (Alongi Citation2009). Following this pattern, the Bakkhali river estuary also supports a huge abundance of shellfish of both marine and brackish origin. Although mangroves support fisheries by playing a significant role as nursery ground for shrimps including the giant tiger shrimp (Penaeus monodon) which is the major species of the industrial bottom trawl fishery of Bangladesh (Islam & Haque Citation2004), the present study did not show the same result for the penaeid group where P. monodon, P. indicus, P. semisulcatus and P. japonicus were found to be 0.06, 0.35, 0.89 and 0.10%, respectively. The same was encountered for Macrobrachium rosenbergii (0.02%), although it is the major species found in different estuarine studies of Bangladesh. On the other hand, Metapenaeus lysianassa (bird shrimp), Macrobrachium villosimanus and Metapenaeus monoceros contributed 17.07, 12.13 and 4.39% of total catch, respectively. This may be due to the use of Char jal, a different gear compared with the previous study, and a higher average salinity gradient for most of sampling period.
Species diversity
Seasonal variation in species diversity is a very common phenomenon in tropical estuaries and the estuary studied here is not different. However, no significant difference for the diversity values (both Shannon–Wiener and Margalef) between the stations indicated that the ecosystem for both tations was unique. However, the diversity values were lower than the value reported by Hossain et al. (Citation2007) where the Shanon value was found to be 2.6. This difference may be due to the use of multigear fishing materials in the Naaf river estuary, the controlled environment of the Bakkhali estuary which limits the species interaction from the upstream communities and scarcity of marine species which normally come to the estuary for breeding.
Although several studies have reported the dominance of the resident species in the estuaries (Thompson Citation1966; Hotos & Vlahos Citation1998), in the case of the Bakkhali estuary no species was found to be dominant. Rather than a single species, five to six species were found dominant at different stations and different seasons. Blaber (Citation2000) also stated that the estuarine resident species are a relatively insignificant proportion of the fish fauna available in an estuary and are generally all relatively small-sized fish.
Metapenaeus lysianassa was found most abundant overall (17.07%). However, the species showed its maximum abundance during the winter season (27.57%) and at St2 (21.35%). As M. lysianassa is a marine-dominant species, it only appears during the winter season at St2 where the salinity is very high. Ambassis dussumieri also showed the same trend except of higher abundance (16.83%) at St1. This is most probably due to the presence of salt marshes at St1. A similar trend was observed for Macrobrachium villosimanus except for the fact that they were dominant during premonsoon (14.28%) and winter (13.38%). As this species is a brackish species, it shows a higher abundance during premonsoon and winter seasons. Mystus gulio and M. monoceros were more abundant during the monsoon season at St2, while M. monoceros is a brackish to marine habitat species. However, their higher abundance at St2 during the monsoon period may be due to spawning. The same is also probably true for freshwater to brackish-water species such as Mystus gulio.
Species assemblage
Regarding spatial and temporal fish and shrimp assemblage structural, two major groups were indicated by cluster analysis in the Bakkhali river estuary. Group 1 comprises the sample of the monsoon season from St1 and St2 along with the premonsoon season from St2 within a 65% similarity level. The capture of abundant large-sized species in the premonsoon and monsoon seasons and the absence of the major contributing species like A. lysianassa, M. villosimanus and Aetapaeneus dussumieri this. Substantial The sample was taken from the lower stream during the premonsoon season at St2 included large numbers of adult fish species. The abundant presence of A. dussumieri, Terapon jarbua, Gerres filamentosus, Liza tade and Butis butis also supports.
Importatnt group 2 comprises the samples from St1 and St2 during the winter season and samples from St1 during the premonsoon period. In general, samples from St1 and St2 during winter showed the same trend on the basis of catchability.
Seasonality is the most important feature among different studied parameters affecting the fish and shrimp assemblage, similar to results from other estuaries (Whitfield Citation1989; Loneragan & Potter Citation1990; Drake & Arias Citation1991; Barletta-Bergan et al. Citation2002; Young & Potter Citation2003). In general, differences between seasons were observed to be more pronounced in this study. According to Lam (Citation1983), the seasonal water variability in the spawning area has an important influence on the spawning activity, and on nursery areas (McErlean et al. Citation1973).
Canonical correspondence analysis
In CCA, species plotted closer to the vector, have stronger relationships with them. Species located near the origin either do not show a strong relationship to any of the variables or are found at average values of environmental variables (Marshall & Elliott, Citation1998). In this study, salinity, water transparency and pH were found to be three significant variables affecting species composition in the studied estuary. However, temperature and DO were not found to be significant. Half of the species in the estuary had average values in relation to environmental variables. Only two species, Eleutheronema tetradactylum and B. butis, indicated a strong response to the longitudinal salinity gradient. The five variables measured in this study explained species distributions well compared with most other estuarine studies where salinity and transparency were found to be the most influential factors for fish distribution patterns. For example, Marshall & Elliott (Citation1998) found that five environmental variables accounted for 18.4% of the total species variation even though they included bottom, mid and surface values of each variable in CCA. Rakocinski et al. (Citation1996) used 11 environmental variables that together explained only 21.9% of the total species variations in CCA. On the other hand, Martino & Able (Citation2003) explained 29.9% of the total species variation in Mullica River Estuary, New Jersey, using five environmental variables that included salinity and geographic distance. However, during this study, environmental variables accounted for 49.18% of the total species variation. These results are also in agreement with Akin et al. (Citation2005) for the Koycegiz Lagoon Estuary, Turkey.
Conclusion
In the Bakkhali river estuary, environmental influence was apparently more extensive during premonsoon and winter, when water transparency and salinity fluctuation leads to an increase in diversity. In this study, seasonality of the environmental conditions explained the major variations of the fish and shrimp assemblage. Seasonal variations occurred not only in total abundance and diversity, but also in the structure of the species assemblage of the Bakkhali river estuary. Besides seasonal variations, the assemblage also exhibited a defined spatial pattern. The migrating marine species Metapenaeus lysianassa was more abundant in the shallow salt marsh zones, while estuarine residents showed more or less equal distribution throughout the seasons except for Mystus gulio. The presence of this species seems to be more related to the spawning season during monsoons. In the Bakkhali river estuary, the four common fish species Ambassis dussumieri, Terapon jarbua, Gerres filamentosus, Liza tade were present for most of the sampling time, which is possibly due to their higher salinity tolerance. Favourable environmental conditions, mainly salinity, enable these fish species to spawn. Therefore, it can be said that these species use the Bakkhali estuary as a spawning ground.
Editorial responsibility: Geir Ottersen (St1, St2)
Notes
Published in collaboration with the University of Bergen and the Institute of Marine Research, Norway, and the Marine Biological Laboratory, University of Copenhagen, Denmark
References
- Able KW. 1999 . Measures of juvenile fish habitat quality: examples from a National Estuarine Research Reserve In : Beneka LR . Fish Habitat: Essential Fish Habitat and Rehabilitation American Fisheries Society Symposium 22 , Bethesda , MD : American Fisheries Society , p 207 – 32 .
- Akin , S , Buhan , E , Winemiller , KO and Yilmaz , H . 2005 . Fish assemblage structure of Koycegiz Lagoon Estuary, Turkey: Spatial and temporal distribution patterns in relation to environmental variation . Estuarine, Coastal and Shelf Science , 64 : 671 – 84 .
- Akin , S , Winemiller , KO and Gelwick , FP . 2003 . Seasonal and temporal variation in fish and macrocrustecean assemblage structure in Mad Island Marsh Estuary, Texas . Estuarine, Coastal and Shelf Science , 57 : 269 – 82 .
- Alongi DM. 2009 . The Energetics of Mangrove Forests . London : Springer . 212 pages .
- Arau′jo , FG , Bailey , RG and Williams , WP . 1999 . Spatial and temporal variations in fish populations in the upper Thames estuary . Journal of Fish Biology , 55 : 836 – 53 .
- Arthington AH Welcome RL. 1995 . The conditions of large river systems of the world In : Armantrout NB . Conditions of the World's Aquatic Habitats Proceedings of the World Fisheries Congress, Theme 1. Science Publishers Inc. p 45 – 75 .
- Barletta-Bergan , A , Barletta , M and Saint-Paula , U . 2002 . Structure and seasonal dynamics of larval fish in the Caeté river estuary in North Brazil . Estuarine, Coastal and Shelf Science , 54 : 193 – 206 .
- Barry , JP , Yoklavich , MM , Cailliet , GM , Ambrose , DA and Antrim , BS . 1996 . Trophic ecology of the dominant fishes in Elkhorn Slough, California, 1974–1980 . Estuaries , 19 : 115 – 18 .
- Blaber , SJM . 1997 . Fish and Fisheries of Tropical Estuaries (Fish and Fisheries Series, 22) , London : Chapman and Hall. 367 pages .
- Blaber , SJM . 2000 . Tropical Estuarine Fishes: Ecology, Exploitation and Conservation , Oxford : Blackwell Science. 372 pages .
- Blaber , SJM and Blaber , TG . 1980 . Factors affecting the distribution of juvenile estuarine and inshore fish . Journal of Fish Biology , 17 : 143 – 62 .
- Blaber , SJM , Farmer , MF , Milton , DA , Pang , J , Wong , P and Ong , B . 1997 . The ichthyoplankton assemblages of Sarawak and Sabah estuaries: composition, distribution and affinities . Estuarine, Coastal and Shelf Science , 45 : 197 – 208 .
- Bray , JR and Curtis , JT . 1957 . An ordination of the upland forest communities of southern Wisconsin . Ecology Monographs , 27 : 325 – 49 .
- Chowdhury , ZA and Sanaullah , M . 1991 . A check list of the shrimps/prawns of the Moheshkhali Channel, Cox's Bazar . Bangladesh, Journal of Zoology , 19 : 147 – 50 .
- Claridge , PN , Potter , IC and Hardisy , MW . 1986 . Seasonal changes in movements, abundance, size composition and diversity of the fish fauna of the Severn estuary . Journal of the Marine Biology Association of the UK , 66 : 229 – 58 .
- Clarke , KR . 1993 . Non parametric multivariate analyses of changes in community structure . Australian Journal of Ecology , 18 : 117 – 43 .
- Clarke KR Warwick RM. 1994 . Change in Marine Communities. An Approach to Statistical Analysis and Interpretation . Plymouth : Natural Environment Research Council . 144 pages .
- Cowen , RK , Hare , JA and Fahay , MP . 1993 . Beyond hydrography: Can physical processes explain larval fish assemblages within the Middle Atlantic Bight? . Bulletin of Marine Science , 53 : 567 – 87 .
- Cowley , PD and Whitfield , AK . 2002 . Biomass and production estimates of a fish community in a small South African estuary . Journal of Fish Biology , 61 : 74 – 89 .
- Cyrus , DP and Blaber , SJM . 1992 . Turbidity and salinity in a tropical Northern estuary and their influence on fish distribution . Estuarine, Coastal and Shelf Science , 35 : 545 – 63 .
- Cyrus , DP and McLean , S . 1996 . Water temperature and the 1987 fish kill at Lake St Lucia on the south eastern coast of Africa . Southern African Journal of Aquatic Sciences , 22 : 105 – 10 .
- Davies BR , Day JA . 1986 . The biology and conservation of South Africa's vanishing Waters CEMS, University of Cape Town and the Wildlife Society of Southern Africa . 487 pages .
- Day Jr , J.W , Hall CAS , Kemp WM , Yanez-Arancibia A. 1989 . Estuarine Ecology . New York , NY : Wiley . 558 pages .
- DeBruin GHP , Russell BC , Bogusch A. 1995 . FAO Species Identification Field Guide for Fishery Purposes . The Marine Fishery Resources of Sri Lanka . Rome : Food and Agricultural Organisation . 400 pages .
- Drake , P and Arias , AM . 1991 . Composition and seasonal fluctuations of the ichthyoplankton community in a shallow tidal channel of Cadiz bay (S.W. Spain) . Journal of Fish Biology , 39 : 245 – 63 .
- Dyer KR. 1997 . Estuaries. A Physical Introduction , 2nd ed . Chichester : John Wiley and Sons . 195 pages .
- Elliott M , Hemingway KL. 2002 . Fishes in Estuaries . Oxford : Blackwell . 636 pages .
- Elliott , M , O'Reilly , MG and Taylor , CJL . 1990 . The Forth estuary: A nursery and overwintering area for North Sea fishes . Hydrobiologia , 195 : 89 – 103 .
- Environmental Community Analysis 2000 . ECOM Version-1.32. Designed by Henderson PA, Seaby RMH, PISCES Conservation LTD, Pennington UK. Computer Program .
- Everett , RA and Ruiz , GM . 1993 . Coarse woody debris as a refuge from predation in aquatic communities: An experimental test . Oecologia , 93 : 475 – 86 .
- Fischer W , Whitehead PJP, . 1974 . FAO Species Identification Sheets for Fishery Purposes . Eastern Indian Ocean (fishing area 57) and Western Central Pacific (fishing area 71). Vols. 1–4 . Rome : FAO .
- Franco-Gordo , C , Godínez-Domínguez , E , Suárez-Morales , E and Vásquez-Yeomans , L . 2003 . Diversity of ichthyoplankton in the central Mexican Pacific: A seasonal survey . Estuarine, Coastal and Shelf Science , 57 : 111 – 21 .
- Fraser , TH . 1997 . Abundance, seasonality, community indices, trends and relationships with physicochemical factors of trawled fish in upper Charlotte Harbor, Florida . Bulletin of Marine Science , 60 : 739 – 63 .
- Garcia , AM , Vieira , JP and Winemiller , KO . 2003 . Effects of 1997e1998 El Nino on the dynamics of the shallow-water fish assemblage of the Patos Lagoon estuary (Brazil) . Estuarine, Coast and Shelf Science , 57 : 489 – 500 .
- Gaughan , DJ , Neira , FJ , Beckley , LE and Potter , IC . 1990 . Composition, seasonality and distribution of ichthyoplankton in the Lower Swan estuary, south-western Australia . Australian Journal of Marine and Freshwater Research , 41 : 529 – 43 .
- Gunter , G . 1961 . Some relations of estuarine organisms to salinity . Limnology and Oceanography , 6 : 182 – 90 .
- Hagan , SM and Able , KW . 2003 . Seasonal changes of the pelagic fish assemblage in a temperate estuary . Estuarine, Coastal and Shelf Science , 56 : 15 – 29 .
- Harris , SA and Cyrus , DP . 1995 . Occurrence of larval fishes in the St. Lucia estuary, KwaZulu-Natal, South Africa . South African Journal of Marine Science , 16 : 333 – 50 .
- Harris , SA , Cyrus , DP and Beckley , LE . 1999 . The larval fish assemblage in nearshore coastal waters off the St Lucia estuary, South Africa . Estuarine, Coastal and Shelf Science , 49 : 789 – 811 .
- Harris , SA , Cyrus , DP and Beckley , LE . 2001 . Horizontal trends in larval fish diversity and abundance along an ocean estuarine gradient on the northern KwaZulu-Natal coast, South Africa . Estuarine, Coastal and Shelf Science , 53 : 221 – 35 .
- Harrison , TD and Whitfield , AK . 1990 . Composition, distribution and abundance of ichthyoplankton in the Sundays river estuary . South African Journal of Zoology , 25 : 61 – 168 .
- Heip , CHR and Herman , PMJ . 1995 . Major biological processes in European tidal estuaries: A synthesis of the JEEP-92 project . Hydrobiologia , 311 : 1 – 7 .
- Hena , MKA and Khan , MAA . 2009 . Coastal and estuarine resources of Bangladesh: Management and conservation issues . Maejo International Journal of Science and Technology , 3 : 313 – 42 .
- Hena , MKA , Short , FT , Sharifuzzaman , SM , Hasan , M , Rezowan , M and Ali , M . 2007 . Salt marsh and seagrass communities of Bakkhali Estuary, Cox's Bazar, Bangladesh . Estuarine, Coastal and Self Science , 75 : 72 – 78 .
- Hettler , W.F Jr and Hare , JA . 1998 . Abundance and size of larval fishes outside the entrance to Beaufort inlet, North Carolina . Estuaries , 21 : 476 – 99 .
- Holbrook , SJ and Schmitt , RJ . 1989 . Resource overlap, prey dynamics, and the strength of competition . Ecology , 706 : 1943 – 53 .
- Hossain , MM . 1971 . The commercial fishes of the Bay of Bengal (survey for the development of fisheries, East Pakistan, Chittagong) . UNDP Project publication. No 1 PAK , 22 : 1 – 6 .
- Hossain MS , Das NG , Chowdhury MSN 2007 . Fisheries management of the Naaf River . Coastal and Ocean Research Group of Bangladesh , Institute of Marine Sciences and Fisheries, University of Chittagong . 267 pages .
- Hossain S , Lin CK. 2001 . Land use zoning for integrated coastal zone management: remote sensing, GIS and RRA approach in Cox's bazaar coast, Bangladesh . ITCZM monograph, No. 3 , Asian Institute of Technology , Thailand . 25 pages .
- Hotos , GN and Vlahos , N . 1998 . Salinity tolerance of Mugil cephalus and Chelon labrosus (Pisces: Mugilidae) fry in experimental conditions . Aquaculture , 167 : 329 – 38 .
- Islam MS , Khan MG , Quaym SA , Chowdhury SZA. 1992 . Estuarine set bagnet fishery of Bangladesh . Marine Fisheries Survey Management and Development Project , Dept. of Fisheries, Chittagong , Bangladesh . 50 pages .
- Islam , MS and Haque , M . 2004 . The mangrove-based coastal and nearshore fisheries of Bangladesh: Ecology, exploitation and management . Reviews in Fish Biology and Fisheries , 14 : 153 – 80 .
- Jaureguizar , AJ , Menni , R , Bremec , C , Mianza , H and Lasta , C . 2003 . Fish assemblage and environmental patterns in the Río de la Plata estuary . Estuarine, Coastal and Shelf Science , 56 : 921 – 33 .
- Jongman RH , Ter Braak CJF , Tongeren OFR. 1995 . Data analysis in community and landscape ecology . 2nd edn . Cambridge : Cambridge University Press . 299 pages .
- Kennish MJ. 1990 . Biological sspects . In: Ecology of Estuaries, vol. II . Boca Raton , FL : CRC Press . 391 pages .
- Kimmerer , WJ , Cowan , JH Jr , Miller , LW and Rose , KA . 2001 . Analysis of an estuarine striped bass population: Effects of environmental conditions during early life . Estuaries , 24 : 556 – 74 .
- Lam , TJ . 1983 . “ Environmental influences of gonadal activity in fish ” . In Fish Physiology , Edited by: Hoar , WS , Randall , DJ and Donaldson , EM . Vol. 9 , 65 – 116 . London : Academic Press .
- Lankford Jr TE , Targett TE . 1994 . Suitability of estuarine nursery zones for juvenile weakfish (Cynoscion regalis): Effects of temperature and salinity on feeding, growth and survival . Marine Biology 119 : 611 – 20 .
- Loneragan , NR and Potter , IC . 1990 . Factors influencing community structure and distribution of different life-cycle categories of fishes in shallow waters of a large Australian estuary . Marine Biology , 16 : 25 – 37 .
- Maes , J , Van Damme , PA , Taillieu , A and Ollevier , F . 1998 . Fish communities along an oxygen-poor salinity gradient (Zeeschelde Estuary, Belgium) . Journal of Fish Biology , 52 : 534 – 46 .
- Maes JS , Damme VP , Meire F, Ollevier 2004 . Statistical modeling of seasonal and environmental influences on the population dynamics of an estuarine fish community . Marine Biology 145 : 1033 – 42 .
- Mahmood N. 1986 . Effects of shrimp farming and other impacts on mangroves of Bangladesh . Proceedings of ‘The Workshop of Strategies for the Management of Fisheries and Aquaculture in Mangrove Ecosystems’, Bangkok, Thailand, FAO Fisheries Report. No. 370. 248 pages .
- Mahmood NM , Chowdhury JU , Hossain MM , Haider SMB , Chowdhury SR. 1994 . A Review of the State of Environment Relating to Marine Fisheries of Bangladesh . In: Holmgren S, An Environmental Assessment of the Bay of Bengal Region. Report No. 67, Bay of Bengal Program. Madras, India. 247 pages .
- Margalef R. 1968 . Perspectives in Ecological Theory . Chicago , IL : University of Chicago Press . 111 pages .
- Marshall , S and Elliott , M . 1998 . Environmental influences on the fish assemblages of the Humber Estuary, U.K . Estuarine, Coastal and Shelf Science , 46 : 175 – 84 .
- Martino , EJ and Able , KW . 2003 . Fish assemblages across the marine to low salinity transition zone of a temperate estuary . Estuarine, Coastal and Shelf Science , 56 : 969 – 87 .
- Mazid MA . 2002 . Development of Fisheries in Bangladesh. Plans and Strategies for Income Generation and Poverty Alleviation Dhaka , , Bangladesh : Nasima Mazid Publications . 176 pages .
- McAllister DE , John F, Craig , Davidson N , Delany S , Seddon M. 2001 . Biodiversity Impacts of Large Dams Background Paper No. 1 prepared for IUCN/UNEP/WCD International Union for Conservation of Nature and Natural Resources and the United Nations Environmental Programme . 49 pages .
- McErlean , AJ , O'Connor , SG , Mihursky , JA and Gibson , CI . 1973 . Abundance, diversity and seasonal patterns of estuarine fish populations . Estuarine, Coastal and Marine Science , 1 : 19 – 36 .
- McLusky DS , Elliott M. 2004 . The Estuarine Ecosystem . Oxford: Oxford University Press. 214 pages .
- Nixon , SW , Oviatt , CA , Frithsen , J and Sullivan , BJ . 1986 . Nutrients and the productivity of estuarine and coastal marine ecosystems . Journal of the Limnological Society of South Africa , 12 : 43 – 71 .
- Ogburn-Matthews , V and Allen , DM . 1993 . Interactions among some dominant estuarine nekton species . Estuaries , 16 : 840 – 50 .
- Peterson , MS and Ross , ST . 1991 . Dynamics of littoral fishes and decapods along a coastal river–estuarine gradient . Estuarine, Coastal and Shelf Science , 33 : 467 – 83 .
- Potter , IC and Hyndes , GA . 1999 . Characteristics of the ichthyofaunas of southwestern Australian estuaries, including comparisons with Holarctic estuaries and estuaries elsewhere in temperate Australia: A review . Australian Journal of Ecology , 24 : 395 – 421 .
- Powles , H , Auger , F and Fitzgerald , GJ . 1984 . Nearshore ichthyoplankton of a north temperate estuary . Canadian Journal of Fisheries and Aquatic Sciences , 41 : 1653 – 63 .
- Rakocinski , CF , Baltz , DM and Fleeger , JW . 1992 . Correspondence between environmental gradients and the community structure in Mississippi Sound as revealed by canonical correspondence analysis . Marine Ecology Progress Series , 80 : 135 – 257 .
- Rakocinski , CF , Lyczkowski-Shultz , J and Richardson , SL . 1996 . Ichthyoplankton assemblage structure in Mississippi sound as revealed by canonical correspondence analysis . Estuarine, Coastal and Shelf Science , 43 : 237 – 57 .
- Ramos , S , Cowen , RK , Ré , P and Bordalo , AA . 2006 . Temporal and spatial distribution of larval fish assemblages in the Lima estuary (Portugal) . Estuarine, Coastal and Shelf Science , 66 : 303 – 14 .
- Raynie , CR and Shaw , RF . 1994 . Ichthyoplankton abundance along a recruitment corridor from offshore spawning to estuarine nursery ground . Estuarine, Coastal and Shelf Science , 39 : 421 – 50 .
- Raz-Guzman , A and Huidobro , L . 2002 . Fish communities in two environmentally different estuarine systems of Mexico . Journal of Fish Biology , 61 : 182 – 95 .
- Rogers , SG , Targett , TE and Von Sant , SB . 1984 . Fish nursery use in Georgia salt marsh estuaries: The influence of springtime freshwater conditions . Transactions of American Fisheries Society , 113 : 595 – 606 .
- Rozengurt , MA and Hedgepeth , JW . 1989 . The impact of altered river flow on the ecosystem of the Caspian Sea . Review of Aquatic Sciences , 1 : 337 – 62 .
- Sánchez , A and Raz-Guzman , A . 1997 . Distribution patterns of tropical estuarine brachyuran crabs in the Gulf of Mexico . Journal of Crustacean Biology , 17 : 609 – 20 .
- Sanvicente-Añorve , L , Flores-Coto , C and Chiappa-Carrara , X . 2000 . Temporal and spatial scales of ichthyoplankton distribution in the southern Gulf of Mexico . Estuarine, Coastal and Shelf Science , 51 : 463 – 75 .
- Shackell , NL and Frank , KT . 2000 . Larval fish diversity on the Scotian shelf . Canadian Journal of Fisheries and Aquatic Science , 57 : 1747 – 60 .
- Shafi M , Quddus MM. 1982a . Fisheries Resources of Bangladesh [ Bangladesher Matshaya Sampad, in Bengali ]. Dhaka : Bangla Academy . 444 pages .
- Shafi M , Quddus MM. 1982b . Fisheries Resources of Bay of Bengal [ Bonggoposagarer Matshaya Sampad, in Bengali ]. Dhaka : Bangla Academy . 426 pages .
- Shannon , CE . 1949 . Communication in the presence of noise . Proceedings of the Institute of Radio Engineers , 37 : 10 – 21 .
- Shannon CE , Weaver W. 1963 . The Mathematical Theory of Communications . Urbana , IL : University of Illinois Press . 125 pages .
- Shenker , JM and Dean , JM . 1979 . The utilization of an intertidal salt marsh creek by larval and juvenile fishes: Abundance, diversity and temporal variation . Estuaries , 2 : 154 – 63 .
- Sogard , SM and Able , KW . 1991 . A comparison of eelgrass, sea lettuce macroalgae, and marsh creeks as habitats for epibenthic fishes and decapods . Estuarine, Coastal and Shelf Science , 33 : 501 – 19 .
- Sokal RR , Rohlf FJ. 1998 . Biometry: The Principles and Practices of Statistics in Biological Research . New York , NY : WH Freeman and Company . 850 pages .
- Spjotvoll , E and Stoline , MR . 1973 . An extension of the T-method of multiple comparisons to include the cases with unequal sample sizes . Journal of the American Statistical Association , 68 : 976 – 78 .
- Szedlmayer , ST and Able , KW . 1996 . Patterns of seasonal availability and habitat use by fishes and decapod crustaceans in a southern New Jersey estuary . Estuaries , 19 : 697 – 709 .
- Talwar PK , Jhingran AG. 1991 . Inland fishes of India and adjacent countries . IBH publishing Co. Pvt. Ltd. New Delhi , Vol. 1–2. 1158 pages.
- Thomson , JM . 1966 . The grey mullets. Oceanography and Marine Biology . An Annual Review , 4 : 301 – 35 .
- Thorman , S . 1986 . Physical factors affecting the abundance and species richness of fishes in the shallow waters of the southern Bothnian Sea (Sweden) . Estuarine, Coastal and Shelf Science , 22 : 357 – 69 .
- Van Eck , GTM , Pauw , DN , Langenbergh , VM and Verreet , G . 1991 . Emissies, gehalten, gedrag en effecten van (micro) verontreinigingen in het stroomgebied van de Schelde en Schelde-estuarium . Water , 60 : 164 – 81 .
- Wagner , MC and Austin , HM . 1999 . Correspondence between environmental gradients and summer littoral fish assemblages in low salinity reaches of the Chesapeake Bay, USA . Marine Ecology Progress Series , 177 : 197 – 212 .
- Weinstein , MP . 1979 . Shallow marsh habitats as primary nursery for fishes and shellfish in Cape Fear River estuary, North Carolina, USA . US Fish and Wildlife Service Fishery Bulletin , 77 : 339 – 57 .
- Weinstein MP. 1985 . Distributional ecology of fishes inhabiting warm-temperate and tropical estuaries: community relationships and implications . In : Yanez-Arancibia A , Fish Community Ecology in Estuaries and Coastal Lagoons: Towards an Ecosystem Integration . Universidad Nacional Autónoma de México , México , p 285 – 309 .
- Weinstein , MP , Weiss , SL and Walters , MF . 1980 . Multiple determinants of community structure in shallow marsh habitats, Cape Fear River Estuary, North Carolina, USA . Marine Biology , 58 : 227 – 43 .
- Weisberg , SB , Wilson , HT , Himchak , P , Baum , T and Allen , R . 1996 . Temporal trends in abundance of fish in the tidal Delaware River . Estuaries , 19 : 723 – 29 .
- Whitfield , AK . 1989 . Ichthyoplankton in a Douthern African surf zone: Nursery area for the postlarvae of estuarine associated fish species? Estuarine . Coastal and Shelf Science , 29 : 533 – 47 .
- Whitfield , AK . 1994 . An estuary-association classification for the fishes of southern Africa . South African Journal of Science , 90 : 411 – 17 .
- Whitfield , AK . 1999 . Ichthyofaunal assemblages in estuaries: A South African case study . Review of Fish Biology and Fisheries , 9 : 151 – 86 .
- Whitmarsh , D , Pipitone , C , Badalamenti , F and D'Anna , G . 2003 . The economic sustainability of Artisanal fisheries: The case of the trawl ban in the Gulf of Castellammaare, NW Sicily . Marine Policy , 27 : 489 – 97 .
- Young , GC and Potter , IC . 2003 . Do the characteristics of the ichthyoplankton in an artificial and a natural entrance channel of a large estuary differ? . Estuarine, Coastal and Shelf Science , 56 : 765 – 79 .
- Zimmerman , RJ and Minello , TJ . 1984 . Densities of Penaeus aztecus, Penaeus setiferus and other natant macrofauna in a Texas salt marsh . Estuaries , 7 : 421 – 33 .