Abstract
The mullid genus Upeneus is highly diverse, with a considerable number of new species found only recently. Based on 16 specimens of Upeneus collected at the southeastern edge of the Seychelles Bank, a large oceanic platform in the Western Indian Ocean, a new species, Upeneus seychellensis, is described. Comparisons with and among populations of the closely related U. guttatus are made. By integration of an extensive, comparative data set consisting of 50 morphometric and meristic and several colour characters obtained from 25 additional Upeneus species, an updated key for the 18 Western Indian Ocean species is provided. The new species can be distinguished from all other congeners of the japonicus-species group, a complex of species with seven dorsal-fin spines, by a combination of number of pectoral fin rays and gill rakers, body depth at anal fin origin, caudal peduncle depth, anal fin size, first dorsal-fin height and colour of the lower caudal-fin lobe. Co-occurring specimens of U. guttatus are considerably differentiated in morphology and colour from other Indian Ocean populations and this was also found for a single specimen from the Gulf of Suez. These results indicate isolation and the formation of local adaptation in more remote areas of the Indian Ocean, but the influence of phenotypic plasticity also needs to be considered.
Introduction
The Seychelles Bank is a part of the Mascarene Plateau, Western Indian Ocean, that rises from depths between 1500 and 3000 m to a flat, nearly oval shelf platform of ca. 300 km maximum diameter and 50 m average depth (Gupta & Desa Citation2001). The fish fauna of the Seychelles Bank and associated islands has been studied during a number of surveys in the last 150 years and about 1000 fish species have been recorded, mostly from shallow reef habitats surrounding the islands (Jennings et al. Citation1999; Payet Citation2005).
In November 2008, during a cruise with the R/V Dr. F. Nansen, five bottom trawl hauls were made in the southern area of the Seychelles Bank at 60 m depth. Among the fishes collected were several goatfishes of the genus Upeneus (Mullidae) that could not be identified to species level on board and hence representative specimens of each distinct form were selected, photographed, and later included in the fish collection of the South African Institute of Aquatic Biodiversity (SAIAB).
The genus Upeneus is highly diverse, with 27 species, five of which have been described only recently (Uiblein & Heemstra Citation2010; Uiblein & Heemstra 2011). All five occur in the Western Indian Ocean, in which 17 Upeneus species have been found so far. Because more new species can be expected, an integrative approach has been adopted for the study of additional material using a large set of taxonomic characters obtained from 26 Upeneus species (Uiblein & Heemstra Citation2010; Uiblein & Heemstra 2011). In this way important information on intraspecific variation and phenotypic divergence among populations becomes available (Uiblein et al. Citation1998; Uiblein & Heemstra Citation2010; Uiblein & Heemstra 2011).
Among the Upeneus collected on the Seychelles Bank were 16 specimens belonging to the japonicus-species group with seven spines in the first dorsal fin (Uiblein & Heemstra Citation2010). We examined these specimens in detail based on body and fin colour patterns, 50 morphometric and meristic characters and comparative data from all valid species of the japonicus group. Based on three specimens, a new species is described. The other 13 specimens, identified as Upeneus guttatus (Day, Citation1868), are used for updating the species diagnosis and comparisons with specimens from other areas in the Indian Ocean in order to examine the extent of geographic variation and phenotypic differentiation among populations. In addition, an updated key for the now 18 valid Upeneus species from the Western Indian Ocean is provided.
Materials and methods
The data of specimens of Upeneus species published in Uiblein & Heemstra (Citation2010, 2011) were used for species comparisons and the preparation of the key. Additional material of U. australiae Kim and Nakaya, Citation2002 and U. guttatus that was examined specifically for this study is listed below.
The measuring and counting methods follow Uiblein & Heemstra (Citation2010). Examination of radiographs taken from the three specimens of Upeneus parvus Poey, 1852 used in our earlier account (Uiblein & Heemstra 2010) revealed the presence of a minute first dorsal spine hidden by skin (see also Kim & Nakaya 2002). In addition to the morphological studies, photographs of the freshly collected specimens were examined closely. Of the 13 Upeneus guttatus from the Seychelles Bank, 12 individuals were sexed to examine possible sexual differences in diagnostic characters using the aceto-carmine squash method (Guerrero & Shelton Citation1974).
In the comparisons, attention was paid to the most diagnostic characters, taking sample size into consideration. For the intraspecific comparison among Upeneus guttatus, Principal Component Analysis (PCA) based on 40 morphometric variables and 37 specimens, with size-adjustment based on the residuals gained from log–log regressions of the variables with standard length, was used to obtain information on optimal distinction (Uiblein & Winkler Citation1994).
Comparative material examined
Upeneus australiae: AMS 34384-012, 86 mm, holotype, Australia, Queensland, 2.5 km W of Island Head, FV Peggy, 22°19.73′ S 150°38.03′ E, depth 0–6 m, prawn trawl; AMS 34384-004, 2 (of 7): 86 mm, paratypes, same station data as holotype; AMS 34396-11, 89 mm, PT, Australia, Queensland, Port Clinton, FV Peggy, 22°34.24′ S 150°44.62′ E, depth 0–8 m, prawn trawl; Upeneus guttatus: AMS 22801-004, 1 of 7): 93 mm, Australia, Eastern Indian Ocean, NW Shelf, off Port Hedland, RV Soela, 19°32′ S, 118°09′ E, depth 50–52 m, Engel trawl; HIFIRE (Institute of Marine Research, Bergen) F5898, 92 mm, Andaman Islands, Eastern Indian Ocean; SAIAB 88872, 98 mm, Egypt, Red Sea, Gulf of Suez; BPBM 31272, 108 mm, Mozambique, IIOE expedition, RV Anton Bruun, st. 403A, 19°09′ S 26°20′ E, depth 27–30 m, shrimp trawl; SAIAB 84255, 10 (6 females, 3 males, 1 not sexed): 93–117, Seychelles, Seychelles Bank, R.V. Dr. F. Nansen, ACEP 08-26, 5°41′52.8′′ S, 56°42′07.2′′ E, depth 59 m, bottom trawl; SAIAB 84281, 3 (1 female, 2 males): 106–117 mm, Seychelles, Seychelles Bank, R.V. Dr. F. Nansen, ACEP 08-27; 5°24′25.2′′ S, 56°25′43.8′′ E, depth 60 m, bottom trawl.
For institutional abbreviations, see Eschmeyer (Citation2010).
Taxonomy
Family Mullidae
Genus Upeneus Cuvier, 1829
Upeneus Cuvier, Citation1829: 157. Type species Mullus vittatus (Forsskål, 1775) by subsequent designation of Desmarest (Citation1856).
Diagnosis
Dorsal fins VII or VIII + 9; anal fin I, 6; pectoral-fin rays 12–17; principal caudal-fin rays 7 + 8 (median 13 branched); gill rakers 4 − 9 + 14 − 24 = 18 − 33; lateral-line scales 28 − 39, lateral line complete; small scales present basally on second dorsal, anal and caudal fins; small teeth present on vomer, palatines and jaws, multiserial and villiform on jaws; body oblong, slightly compressed; barbel length in adults 4–7 times in SL, snout length 7–11 times in SL, subequal to postorbital length (7–10 times in SL); in fresh fish lateral body stripes and/or caudal-fin bars of differing colours, dark caudal-fin bars frequently retained on preserved fish.
Distribution
In all major oceans, tropical to subtropical, only a single species in the Atlantic and two in the Mediterranean, both immigrants from the Red Sea (Ben-Tuvia 1966).
Remarks
We recognize 28 species as valid. One single species, Upeneus filifer, and four species groups can be distinguished based on number of dorsal spines and gill rakers, length of longest dorsal-fin spine, length of pelvic and pectoral fins, and presence or absence of caudal-fin bars and mid-lateral body stripes (Uiblein & Heemstra Citation2010). Here Upeneus seychellensis sp. nov. is added to the japonicus group, which includes also U. asymmetricus Lachner, 1954, U. australiae Kim & Nakaya, Citation2002, U. francisi Randall & Guézé, 1992, U. guttatus (Day, Citation1868), U. japonicus (Houttuyn, 1782), and U. pori Ben-Tuvia & Golani, 1989. The other three groups are the moluccensis group (U. doriae (Günther, 1869), U. moluccensis (Bleeker, 1855), U. quadrilineatus Cheng & Wang, 1963, U. sulphureus Cuvier, Citation1829), the tragula group (U. luzonius Jordan & Seale, 1907, U. margarethae Uiblein & Heemstra, Citation2010, U. mouthami Randall & Kulbicki, Citation2006, U. oligospilus Lachner, 1954, Upeneus randalli Uiblein & Heemstra, 2011, U. sundaicus (Bleeker, 1855), U. taeniopterus Cuvier in Cuvier & Valenciennes, 1829, U. tragula Richardson, 1846), and the vittatus group (U. davidaromi Golani, 2001, U. indicus Uiblein & Heemstra, Citation2010, U. mascareinsis Fourmanoir & Guézé, Citation1967, U. parvus Poey, 1852, U. suahelicus Uiblein & Heemstra, Citation2010, U. subvittatus (Temminck & Schlegel, 1843), U. supravittatus Uiblein & Heemstra, Citation2010, U. vittatus (Forsskål, 1775)).
Key to species groups and Western Indian Ocean species of Upeneus
1a. Longest spine of first dorsal fin 2.5 times or less in SL, no caudal-fin bars or mid-lateral body stripes……………U. filifer
1b. Longest dorsal-fin spine more than 3 times in SL; caudal-fin bars and/or mid-lateral body stripes present in fresh fish [four species groups]……………2
2a. Dorsal-fin spines 7, first 2 spines usually longest; total gill rakers 21–32; pectoral-fin rays 12–15; bars on upper caudal-fin lobe of fresh fish in all species (bars present or absent on lower lobe) [japonicus group]……………3
2b. Dorsal-fin spines 8, first spine minute, recumbent, partly hidden by skin and scales at fin origin; total gill rakers 18–33; pectoral-fin rays 13–17; bars on caudal fin of fresh fish present or absent……………5
3a. Pectoral-fin rays 12–14; total gill rakers 23–25; first dorsal-fin height 4.1–5.1 times in SL; body red dorsally, preserved fish pale brown, not darker dorsally (Indo-Pacific)……………U. guttatus
3b. Pectoral-fin rays 14–15; total gill rakers 25–27; first dorsal-fin height 4.8–5.3 times in SL; body grey, reddish-brown or red dorsally, preserved fish darker dorsally or pale brown.……… 4
4a. Anal-fin base length 7.5–8.4 and anal-fin height 5.8–6.4 times in SL; orbit length 1.4–1.7 in snout; body depth at anal fin origin 4.6–5.1 times in SL; lower caudal-fin lobe with 5–9 grey or reddish brown bars ventrally; preserved fish darker dorsally (Red Sea to Oman, Madagascar, eastern Mediterranean……………U. pori
4b. Anal-fin base length 9.7–10 and anal-fin height 6.5–7.0 times in SL; orbit length 1.8–1.9 in snout; body depth at anal fin origin 5.4–5.5 times in SL, lower caudal-fin lobe with a red stripe and no bars in fresh fish; preserved fish pale brown (Seychelles)……………U. seychellensis sp. nov.
5a. Total gill rakers 18–26; pectoral-fin rays 13–15; pelvic-fin length 0.8–1.1 times in pectoral fins; bars on caudal fin in fresh fish of all species; bars retained or not retained in preserved fish [tragula group]……………6
5b. Total gill rakers 25–33; pectoral-fin rays 14–17; pelvic-fin length 1.1–1.5 times in pectoral fins; bars on caudal fin in fresh fish of several species; bars retained in preserved fish……11
6a. Dark dots or blotches on entire body and paired fins; first dorsal-fin tip dark; one brown to black mid-lateral body stripe in fresh and preserved fish; barbel yellow in fresh fish; upper-jaw length 7.1–9.2 times in SL……7
6b. No dark dots or blotches on body (except for area of lateral line in U. randalli) and paired fins; no dark dorsal-fin tip; 1–2 yellowish or pale brown lateral body stripes on fresh fish; barbel white or yellow in fresh fish; upper-jaw length 7.9–10 times in SL……………8
7a. Total number of caudal-fin bars 6–9 (6 or fewer in juveniles < 7 cm SL), 3–4 bars on lower caudal-fin lobe; caudal-fin length 3.5–4.1 times in SL and 1.0–1.3 times in head length; pelvic-fin length 4.7–6.0 times in SL (Persian Gulf)……………U. oligospilus
7b. Total number caudal-fin bars 10 or more (7–10 in juveniles <7 cm SL), 4–7 bars on lower caudal-fin lobe; caudal-fin length 3.1–3.6 times in SL and 0.9 – 1.1 times in head length; pelvic-fin length 4.2–5.0 times in SL (Indo-Pacific)……………U. tragula
8a. Pectoral-fin length 5.0–5.8 times in SL; lateral-line scales 35–39; upper caudal-fin lobe with 4–8 dark bars, distinct on preserved fish; fresh fish with a pale brown mid-lateral body stripe and a weaker, more yellowish stripe below (Indo-Pacific)……………U. taeniopterus
8b. Pectoral-fin length 4.0–4.9 times in SL; lateral-line scales 28–34; upper caudal-fin lobe with 4–6 red or grey bars, not or only traces retained on preserved fish; fresh fish with or without a yellow or pale brown mid-lateral body stripe……………9
9a. Total gill rakers 18–21; lateral-line scales 31–34; first dorsal-fin height 3.4–4.1 times in SL; caudal-peduncle depth 7.9–8.7 times in SL; barbel frequently yellow in fresh fish; 5–6 red or grey bars on upper caudal-fin lobe in fresh fish (Indo-Pacific)……………U. sundaicus
9b. Total gill rakers 21–25; lateral-line scales 28–30; first dorsal-fin height 4.3–5.3 times in SL; caudal-peduncle depth 9.0–10 times in SL; barbel white; 4 (rarely 5) red bars on upper caudal-fin lobe in fresh fish……………10
10a. Total gill rakers 21–24; caudal-peduncle width 17–28 times in SL and 4.9–7.9 in head length; anal-fin base 7.2–9.9 times in SL; pectoral-fin width 17–23 times in SL and 4.9–6.5 in head length; 4 (rarely 5) red bars on upper caudal-fin lobe in fresh fish, 3 (rarely 4) distally from fork; broad red band on lower caudal-fin lobe, covering up to 5 or 6 red bars, the latter only partly visible along ventral fin margin in fresh fish; mid-lateral body stripe running through eye, red from snout tip to eye and yellow from eye to caudal-fin base; stripe absent in preserved fish (Indian Ocean and Arafura Sea)……………U. margarethae
10b. Total gill rakers 23–25; caudal-peduncle width 27–34 times in SL and 7.5–10 in head length; anal-fin base 8.9–11 times in SL; pectoral-fin width 22–26 times in SL and 6.3–7.6 in head length; 5 red bars on upper caudal-fin lobe in fresh fish, 4 distally from fork; lower caudal-fin lobe with up to 8 dark red bars, bars not covered by a band; beige mid-lateral body stripe only faintly visible in fresh fish; stripe absent in preserved fish (Persian Gulf)……………U. randalli
11a. No bars on lower caudal-fin lobe; bars on upper caudal-fin lobe in some species; bars retained in preserved fish [moluccensis group]……………12
11b. Bars on both caudal-fin lobes in fresh and preserved fish [vittatus group]……………14
12a. No bars on entire caudal fin, also not in live or fresh fish; first dorsal-fin tip black or pale brown to yellowish in fresh fish, can be absent in preserved fish; body depth at first dorsal-fin origin 3.0–3.7 times in SL in adult fish (> 7 cm SL); head length 3.2–3.5 times in SL……………13
12b. Caudal fin with 6–8 red bars on upper lobe, no bars on lower lobe; bars conspicuous in live fish and mostly distinct in preserved fish; brown to black first dorsal-fin tip, retained in preserved fish; body depth at first dorsal-fin origin 3.9–4.2 times in SL in adult fish (>7 cm SL); head length 3.5–3.7 times in SL (Indo-Pacific, eastern Mediterranean)……………U. moluccensis
13a. Total gill rakers 29–33; rakers on lower limb 22–24; tip of first dorsal fin pale brown to yellowish in fresh fish, not or only faintly retained in preserved fish; fresh fish with a narrow, yellow mid-lateral body stripe; anal-fin length 6.7–7.0 times in SL; first dorsal-fin height 4.5–5.0 times in SL; second dorsal-fin height 6.6–7.3 times in SL (Persian Gulf, Gulf of Oman)……………U. doriae
13b. Total gill rakers 27–28; rakers on lower limb 19–21; tip of first dorsal fin black in fresh and preserved fish; fresh fish with 2 conspicuous, yellow lateral body stripes; anal-fin length 5.4–6.4 times in SL; first dorsal-fin height 3.9–4.4 times in SL; second dorsal-fin height 5.5–5.9 times in SL (Indo-Pacific)……………U. sulphureus
14a. Caudal-fin bars pale brown to brown, mostly uniformly coloured, pale spaces between bars of nearly equal width; bars on upper caudal-fin lobe curved; 2 yellow or pale brown lateral body stripes on fresh fish; body depth at anal-fin origin 3.7–4.6 times in SL; pectoral-fin length 3.5–4.4 times in SL and 1.1–1.3 in head length; total gillrakers 26–32……………15
14b. Caudal-fin bars at least partly black or dark brown, vary frequently in colour intensity, bars or the spaces between them vary often in width; upper caudal-fin lobe bars mostly straight and not curved; no or more than 2 yellow or pale-brown lateral body stripes on fresh fish; body depth at anal-fin origin 4.1–5.6 times in SL; pectoral-fin length 4.1–4.7 times in SL and 1.3–1.6 in head length; total gillrakers 26–29……………17
15a. Pectoral-fin length 4.2–4.4 times in SL and 1.3 times in head length, shorter than body depth at anal-fin origin, the latter 3.7–3.9 times in SL (SW India)……………U. indicus
15b. Pectoral-fin length 3.5–4.1 times in SL and 1.1–1.2 times in head length, subequal to or longer than body depth at anal-fin origin, the latter 3.8–4.6 times in SL……………16
16a. Total number of gill rakers 26–28; rakers on lower limb 19–21; pectoral-fin length 3.8–4.1 times in SL and subequal to body depth at anal-fin origin; head length 3.2–3.6 times in SL; barbel length 4.9–6.6 times in SL (East Africa, southern Red Sea)……………U. suahelicus
16b. Total number of gill rakers 29 (rarely 28)–32; rakers on lower limb 21–23; pectoral-fin length 3.5–4.0 times in SL and longer than body depth at anal-fin origin; head length 3.0–3.4 times in SL; barbel length 4.3–5.4 times in SL (South India, Sri Lanka)……………U. supravittatus
17a. Head depth through eye 4.6–4.8 times in SL; anal-fin length 5.8–6.3; second dorsal-fin height 5.8–6.1 times in SL; no lateral body stripe in fresh fish (Red Sea)……………U. davidaromi
17b. Head depth through eye 5.0–6.1 times in SL; anal-fin length 6.3–7.9; second dorsal-fin height 6.2–7.2 times in SL; no or more than 2 lateral body stripes on fresh fish……………18
18a. Body depth at anal-fin origin 4.6–5.6 times in SL; caudal-peduncle depth 11–12 times in SL; first dorsal-fin height 4.4–5.2 times in SL; vertical length of black tip on first dorsal fin and width of largest bar and/or interspace between distal bars of lower caudal-fin lobe narrower than orbit length; no lateral body stripes on fresh fish (Indian Ocean)……………U. mascareinsis
18b. Body depth at anal-fin origin 4.1–4.7 times in SL; caudal-peduncle depth 8.6–10 times in SL; first dorsal-fin height 4.0–4.5 times in SL; vertical length of black tip on first dorsal fin and width of largest bar and/or interspace between distal bars of lower caudal-fin lobe subequal to or wider than orbit length; 3 or 4 lateral body stripes on fresh fish: 2 yellow or coppery stripes mid-laterally and below, and one or 2 brown or pale brown stripes dorsally (Indo-Pacific)……………U. vittatus
Upeneus seychellensis sp. nov.
Tailstripe goatfish (; and )
Figure 1. A: Upeneus seychellensis sp. nov., holotype, SAIAB 96980, SL 115 mm, and (above) paratype, SAIAB 84280, SL 102 mm, Seychelles Bank (O. Alvheim); B: Upeneus guttatus, SAIAB 84281, female, SL 117 mm, and (below) male, SL 111 mm, Seychelles Bank (O. Alvheim).

Figure 2. Body depth at anal-fin origin against SL (A), first dorsal-fin height against caudal-peduncle depth (B), anal-fin height against number of pectoral-fin rays (C), and length of anal-fin base against total number of gill rakers (D), in the four Indian Ocean species of the japonicus group (morphometric variables expressed in % SL).
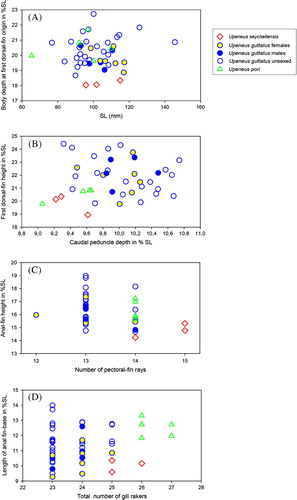
Table I. Measurements and counts for Upeneus seychellensis sp. nov. and the species of the japonicus group (morphometric characters in % SL; main differences with U. seychellensis are emphasized).
Holotype
SAIAB 96980, 115 mm, Seychelles, Seychelles Bank, R.V. Dr. F. Nansen, ACEP08-27; 5°24′25.2′′ S, 56°25′43.8′′ E, depth 60 m, bottom trawl.
Paratypes
SAIAB 84280, 96–102 mm, same station data as holotype.
Diagnosis
Dorsal fins VII + 9; pectoral-fin rays 14–15; gill rakers 7 + 18–19 =25–26; lateral-line scales 29–31; measurements in % SL: body depth at first dorsal-fin origin 20–22; body depth at anus 18–19; caudal-peduncle depth 9.2–9.6; maximum head depth 18–20; head length 27–30; caudal-fin length 28–30; anal-fin height 14–15; pelvic-fin length 20–21; pectoral-fin length 21; pectoral-fin width 3.9–4.2; first dorsal-fin height 19–20; total bars on caudal fin 5, all on upper caudal-fin lobe, with 4 red bars distally from fork and one red bar close to fin base; lower caudal-fin lobe with a broad carmine stripe bordered by a thin white margin dorsally and by a wider pale margin ventrally; lower caudal-lobe tip black; dorsal fins pale with reddish stripes; fin pigmentation not retained in preserved fish apart from remains of lower caudal-fin lobe tip and upper caudal-fin lobe bars; barbels pale reddish; body and head dorsally reddish, snout reddish, lateral and ventral side of body and head white in fresh fish; entire body uniformly pale beige in preserved fish, with a weak pale brown mid-body band.
Description
Measurements in % SL and counts are given in ; morphometric data as ratios of SL for holotype, with data for paratypes in brackets: body elongate, body depth at first dorsal-fin origin 4.5 (4.7–5.0), subequal to pectoral-fin length 4.8 (4.7), body depth at anal-fin origin 5.4 (5.5), caudal-peduncle depth 10 (11), much larger than orbit length 15 (16–17), snout length 8.2 (8.4–9.1), slightly more than twice as long as orbit, head depth across eye 5.1 (5.1–5.5), head length 3.3 (3.6–3.7), larger than maximum depth of body and subequal to caudal-fin length 3.4 (3.4–3.5), barbel length 4.5 (5.9–6.0), pelvic-fin length 4.8 (4.8–5.0), subequal to pectoral-fin length, pectoral-fin width 26 (24–25), first dorsal-fin length 5.3 (4.9–5.0), second dorsal-fin length 6.0 (6.4).
Fresh colour ()
Head and body dorsally reddish; body becoming laterally paler and white from about mid body to ventral margin, partly overlain with weak red pigmentation; faint pale rose lateral band from caudal-fin base to operculum in the region between the dorsally red and ventro-laterally white body areas; paratypes with a few small red patches laterally on body, probably deriving from injuries; head white below a line through orbit parallel to head contour, snout reddish; a few small red patches on white area of head of types; first dorsal fin pale whitish with some tiny patches of red pigmentation, possibly the remains of two to three red stripes, fin tip pale; second dorsal fin pale white with four narrow red stripes; pectoral fins hyaline, rose at base; caudal-fin upper lobe pale whitish, with 4 red bars on lobe itself (lobe tip white in larger fish) and a fifth weaker bar at lobe base; bar width less than width of pale interspaces between bars; lower caudal-fin lobe with three different stripes: a thin white stripe along dorsal margin from fork to close to lobe tip, followed by a broad carmine stripe (> two-thirds of lobe width) from fin base to lobe tip and bordered ventrally by a pale to white ventral margin, wider than the dorsal margin (=about one-fourth of lobe width), running from base to close to lobe tip; lower-caudal-fin lobe tip black; barbels pale reddish.
Preserved colour
Head and body pale beige, belly somewhat darker anteriorly, a pale brown band at mid body from behind operculum to caudal-fin base; operculum transparent, gill cavity shining through; dorsal, ventral, and pectoral fins hyaline without any pigmentation; caudal fin mostly lacking pigmentation, only fin tip of lower lobe weakly pigmented and some remains of pigmentation deriving from 4 to 5 bars on upper caudal-fin lobes of all three types.
Distribution
Seychelles Bank, Western Indian Ocean.
Etymology
The name of this species ‘seychellensis’ derives from the tiny distributional range currently known for this species, which is limited to a single trawling station close to the southeastern edge of the Seychelles Bank.
Comparisons ( , and )
Upeneus seychellensis differs from U. guttatus in more pectoral-fin rays, more gill rakers, more slender body, longer postorbital, lower anal and first dorsal fin, no bars on lower caudal fin lobe and red stripe on lower lobe more conspicuous in fresh fish; from U. pori it differs in more total gill rakers, more slender body at anal-fin origin, shorter jaws, longer caudal peduncle, shorter second dorsal-fin base, smaller anal fin, narrower pectoral-fin width, no bars on lower caudal-fin lobe, and caudal-fin colour patterns not or only very weakly retained in preserved fish; from U. australiae it differs in more gill rakers, more slender body and head, thinner barbels, shorter dorsal-fin and anal-fin bases, smaller pectoral fins, and no bars on lower caudal lobe, and bars on upper caudal lobe not or only weakly retained; from U. asymmetricus it differs in more pectoral-fin rays, more gill rakers, more slender body, narrower interorbital, longer orbit, longer jaws, smaller anal fin, narrower pectoral fins, no bars on lower caudal-fin lobe, and caudal-fin colour mostly not retained in preserved fish; from U. francisi it differs in fewer gill rakers, shallower body depth at first dorsal-fin origin, shallower head depth through eye, shorter head, longer snout, shorter postorbital, smaller eyes, shorter anal-fin base, and shorter paired fins; and from U. japonicus it differs in more slender body and head, shorter second dorsal-fin base, smaller anal fin, lower first dorsal fin, the lower caudal-fin lobe more pointed, usually four vs. three red bars on the upper lobe distally from fork, and the red stripe on the lower caudal-fin lobe not rounded in fresh fish.
Remarks
Upeneus seychellensis sp. nov. is the only species of the japonicus group from the Western Indian Ocean which has no bars on the lower caudal-fin lobe, like U. japonicus. Its close relationship with U. japonicus is also documented by considerable overlap in morphological characters (). Another closely related species, U. guttatus, co-ocurs with U. seychellensis (see also below).
Upeneus seychellensis attains 12 cm SL; depth 60 m.
Upeneus guttatus (Day 1868)
Two-tone goatfish ( and , to )
Upenoides guttatus Day, Citation1868: 938 (type locality: Madras, India).
Upeneus crosnieri Fourmanoir & Guézé, Citation1967: 52, figure I/c (type locality Mitsio, Pracel Bank, Madagascar); Bauchot et al. Citation1985: 7 (synonym of Upeneoides guttatus Day 1868).
Upeneus guttatus: Randall & Kulbicki Citation2006: 301, and (diagnosis and two colour photographs).
Figure 3. Upeneus guttatus: A: SAIAB 84255, female, SL 112 mm, Seychelles Bank (O. Alvheim); B: SAIAB 82714, SL 94 mm, Mozambique (P.C. Heemstra); C: SAIAB 13947, SL 100 mm, Kenya (P.C. Heemstra); D: BPBM 20658, SL 113 mm, Madras, India (J.E. Randall).

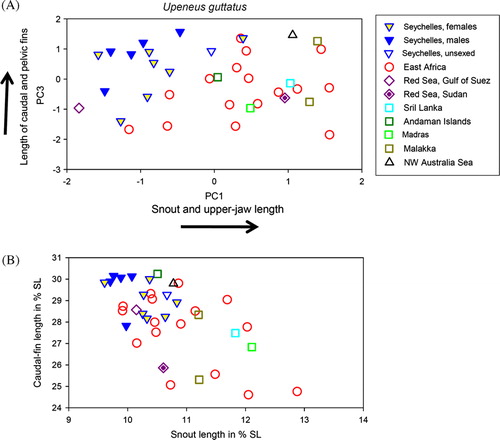
Figure 4. A: Scores of the first and third axis of Principal Component Analysis based on 40 morphometric characters in 37 Upeneus guttatus from different areas in the Indian Ocean, with trends for the highest-contributing characters indicated by arrows; B: caudal-fin length against snout length (both variables expressed in % SL).
Upeneus bensasi (non Temminck & Schlegel, 1843): De Bruin et al. Citation1994: 270, pl. 9, figure 136 (Sri Lanka).
Upeneus guttatus: Uiblein & Heemstra Citation2010: 42–43, tables 3, 4; pl. 1, 3.
Diagnosis
Dorsal fins VII + 9; pectoral-fin rays 12–14, mostly 13 (in 37 of 44 specimens); gill rakers 6 − 8 + 16 − 18 = 23 − 25; lateral-line scales 28–31; measurements in % SL: body depth at first dorsal-fin origin 22–26; body depth at anus 19–23; caudal-peduncle depth 9.3–11; maximum head depth 18–23; head length 26–30; caudal-fin length 25–30; anal-fin height 15–19; pelvic-fin length 19–22; pectoral-fin length 19–22; pectoral-fin width 3.7–5.0; first dorsal-fin height 20–24; total bars on caudal fin 7–13, upper caudal-fin lobe with 5 (rarely 4) reddish bars with 4 (rarely 3) bars distally from fork and one bar close to fin base, of similar width or narrower than the pale interspaces between bars; 2–8 faint, irregular red bars on exterior margin of lower caudal-fin lobe, sometimes extending to dorsal half of lobe, connecting to a red stripe which covers the lobe dorsally to two-thirds of its width at maximum; caudal-fin lobe bars and stripe fade away post mortem, only traces retained in preserved fish; first dorsal-fin tip pale; barbels yellow or white in fresh fish; body white below lateral line, covered by red pigmentation above lateral line which may also reach down ventrally, forming red patches or blotches; belly white; body pale brown and not dorsally darkened in preserved fish.
Distribution
Red Sea to Somalia, Kenya, Mozambique, South Africa, Madagascar, Réunion, Seychelles, India, Sri Lanka, Andaman Sea, Malaysia, Singapore, Northern Australia, New Caledonia, Philippines.
Comparison
See account of Upeneus seychellensis sp. nov. above and Uiblein & Heemstra (Citation2010) for comparisons with all other species of the japonicus group.
Remarks
Upeneus guttatus shows considerable geographic variation within the Indian Ocean, both in morphology and colour patterns. The Seychelles Bank individuals can be distinguished from all other areas using Principal Components Analysis (A). The first and third principal components show the distinction, explaining 22 and 9% of the total variance, respectively. The characters showing the highest correlations with these two PCA axes reflect a trend towards longer caudal and pelvic fins in combination with shorter snout and upper jaw in the Seychelles Bank population. There is overlap with the single specimen from the Gulf of Suez, northern Red Sea, and one specimen from the East African population. If characters important for the multivariate separation are directly compared () or plotted singly against each other (B), considerably more overlap occurs. No indication for sexual dimorphism was found.
Table II. Measurements and counts for the Indian Ocean Upeneus guttatus populations (morphometric characters in % SL).
Comparisons of photographs of eight fresh fish from the two Seychelles Bank stations with those of six fish from the East African coast and one from each the Gulf of Suez (Red Sea) and Madras (NE Indian Ocean) revealed that the Seychelles population has fewer lower caudal-fin lobe bars (2–5 vs. 5–8). Because the lower caudal-fin lobe bars are not completely developed and less conspicuous in this species, counting can sometimes be difficult. However, the general presence or absence of these bars can be used to distinguish freshly collected U. guttatus from U. seychellensis.
The number of upper caudal-fin lobe bars is equal in U. guttatus in all areas (four red bars distally of fork, one bar on fin base), except for the single Gulf of Suez specimen which has only four bars on the upper caudal-fin lobe with three bars placed distally of the fork. This specimen has yellow barbels, like seven of the eight photographed Seychelles Bank specimens (barbels not visible in one specimen). Among the seven photographs of freshly collected specimens from other areas (East Africa, Madras) are two with white and five with yellow barbels ().
Upeneus guttatus attains 16 cm SL; depth 8–80 m.
Discussion
The Seychelles Bank is a large isolated shelf platform in the Indian Ocean surrounded by deep water, only the topographically similar Amirante Plateau and Saya de Malha Bank being relatively close. This biogeographical setting should enhance the potential for high endemism among the shallow-water fish fauna (Allen Citation2008). However, no clear estimates for the degree of endemism exist for this area due to the lack of a complete species inventory as it has been compiled for instance, for small oceanic islands like La Reunion (Fricke et al. Citation2009).
The encounter of a new Upeneus species on the Seychelles Bank in one of only five bottom trawl hauls may indicate that there are more undescribed fish species on the platform, as it can be also expected for the surrounding slopes and deep-water habitats (Bijoux et al. Citation2003). Only small subsamples from the trawl hauls were taken, but the inspection of additional photographs and on-board data recordings from the cruise suggest that U. seychellensis may be quite abundant close to the southern edge of the bank.
The geographic differentiation of Upeneus guttatus between the Seychelles Bank population, the single Gulf of Suez specimen, and the other areas of the Indian Ocean points to micro-evolutionary processes and local adaptation in body form and colour, but phenotypic plasticity needs to be considered, too (e.g. Uiblein Citation1995, Citation1996; Uiblein & Nielsen Citation2005). Earlier comparative morphological studies of the species Upeneus sundaicus, U. sulphureus, and U. tragula and two populations of U. sulphureus from Hainan Island, South China Sea, suggested phenotypic plasticity to be an important agent promoting rapid niche expansion and colonization of new habitats (Uiblein et al. Citation1998). Genetic studies of goatfishes have so far mainly dealt with species differences (see Uiblein Citation2007 for a review) and detailed investigations of gene flow and phylogeographic relationships among populations are still required.
With this and the two associated studies (Uiblein & Heemstra Citation2010; Uiblein & Heemstra 2011), we have now completed the taxonomic review of the genus Upeneus of the Western Indian Ocean. In total, six new species have been described and evidence for differentiation among populations and ontogenetic stages has been found. An identification key has been developed and comparisons with congeneric species from other ocean regions have been made, focusing on the practical need to base distinctions on a relatively small set of easily identifiable characters and to integrate both fresh and preserved fish. This approach shall now be extended towards other regional reviews and, ultimately, a revision of the genus.
Editorial responsibility: Peter R. Møller
Acknowledgements
For the loan or donation of specimens and/or making collection visits by the first author possible we thank Mark McGrouther, John Paxton and Amanda Hay (AMS) and Jack Randall, Lori O'Hara, and Arnold Suzumoto (BPBM). For providing photographs of fresh fish or other important information we thank Oddgeir Alvheim, Sergey Bogorodsky, Gavin Gouws, Elaine Heemstra, Bernard Mackenzie, Rajan P. Thomas, Jack Randall, and Denis Tweddle. The first author thanks the South African Institute for Aquatic Biodiversity and the Nansen Programme of the Center for Development Cooperation in Fisheries at the Institute of Marine Research (IMR), Bergen, for travel support. This paper is also a contribution to the Agulhas and Somali Current Large Marine Ecosystems ASCLME Project.
References
- Allen , GR. 2008 . Conservation hotspots of biodiversity and endemism for Indo-Pacific coral reef fishes . Aquatic Conservation of Marine and Freshwater Ecosystems , 18 : 541 – 56 .
- Bauchot ML , Desoutter M , Guézé P , Randall JE . 1985 . Catalogue critique des types de poisons du Muséum national d'Histoire naturelle . Bulletin du Muséum national d'histoire naturelle, Paris 4 7 , sect. A 2, supplement 1 – 125 .
- Ben-Tuvia A. 1966. Red Sea fishes recently found in the Mediterranean. Copeia 1966:254–75.
- Bijoux JP , Adam PA , Alcindor R , Bristol R , Decommarmond A , Mortimer JA , et al. 2003 . Marine biodiversity in the Seychelles archipelago – The known and the unknown . In : Decker C , Griffiths C , Prochazka K , Ras C , Whitfield A , Marine Biodiversity in Sub-Saharan Africa: The Known and the Unknown . Census of Marine Life Programme in Sub-Saharan Africa Meeting Proceedings : Cape Town, , South Africa , 23 – 26 September 2003. 22 pages .
- Cuvier G . 1829 . Le règne animal, distribué d'après son organisation, pour servir de base à l'histoire naturelle des animaux et d'introduction à l'anatomie comparée , vol. 2 . Paris : Chez Déterville . xv + 406 pages .
- Day , F. 1868 . On some new or imperfectly known fishes of India . Proceedings of the General Meetings for Scientific Business of the Zoological Society of London , 1867 ( pt 3 ) : 935 – 42 .
- De Bruin GHP , Russell BC , Bogusch A. 1994 . The marine fishery resources of Sri Lanka. FAO species identification field guide for fishery purposes . Rome : Food and Agriculture Organization of the United Nations. 400 pages, 32 plates .
- Desmarest E. 1856 . Reptiles et poissons . In: J.G. Chenu Encyclopédie d'histoire naturelle; ou, Traité complet de cette science d'après les travaux des naturalistes les plus éeminents de toutes les époques 19:1–360 + 1 – 62 .
- Eschmeyer WN 2010 . Catalog of Fishes . Electronic version – 25 October 2010. California Academy of Sciences. Avalaible at: http://research.calacademy.org/ichthyology/catalog/fishcatmain.asp ( accessed 25 November 2010 ).
- Fourmanoir , P and Guézé , P. 1967 . Poissons nouveaux ou peu connus provenant de la Réunion et de Madagascar. Cahiers O.R.S.T.O.M . Série Oceéanographie , 5 : 47 – 58 .
- Fricke , R , y Mulochau , T , Durville , P , Chabanet , P , Tessier , E and Letourneur , Y. 2009 . Annotated checklist of the fish species (Pisces) of La Réunion, including a Red List of threatened and declining species . Stuttgarter Beiträge zur Naturkunde A, Neue Serie , 2 : 1 – 168 .
- Gupta RS , Desa E . 2001 . The Indian Ocean – A Perspective . Rotterdam : AA Balkema . 868 pages .
- Guerrero , RD and Shelton , WL. 1974 . An aceto-carmine squash technique for sexing juvenile fishes . The Progressive Fish-Culturist , 36 : 56
- Jennings , S , Marshall , S , Cuet , P and Naim , O. 1999 . “ The Seychelles ” . In Coral Reefs of the Western Indian Ocean: Their Ecology and Conservation , Edited by: McClanaham , TR , Sheppard , CS and Obura , DO . 399 – 432 . New York, NY : Oxford University Press, p 399–432 .
- Kim , B-J and Nakaya , K. 2002 . Upeneus australiae, a new goatfish (Mullidae: Perciformes) from Australia . Ichthyological Research , 49 : 128 – 32 .
- Payet , R. 2005 . Research, assessment and management on the Mascarene Plateau: a large marine ecosystem perspective . Philosophical Transactions of the Royal Society , 363 : 295 – 307 .
- Randall , JE and Kulbicki , M. 2006 . A review of the goatfishes of the genus Upeneus (Perciformes: Mullidae) from New Caledonia and the Chesterfield Bank, with a new species, and four new records . Zoological Studies , 45 : 298 – 307 .
- Uiblein , F. 1995 . Morphological variability between populations of Neobythites stefanovi (Pisces: Ophidiidae) from the deep Red Sea and the Gulf of Aden . Marine Ecology Progress Series , 124 : 23 – 29 .
- Uiblein , F. 1996 . Constraints and exploratory windows in light-reduced marine habitats. In: Uiblein F, Ott J, Stachowitsch M, editors. Deep-sea and Extreme Shallow-water Habitats: Affinities and Adaptations . Biosystematics and Ecology Series , 11 : 165 – 82 .
- Uiblein , F. 2007 . Goatfishes (Mullidae) as indicators in tropical and temperate coastal habitat monitoring and management . Marine Biology Research , 3 : 265 – 88 .
- Uiblein , F and Heemstra , PC. 2010 . A taxonomic review of the Western Indian goatfishes of the genus Upeneus (Family Mullidae) with descriptions of four new species . Smithiana Bulletin , 11 : 35 – 71 .
- Uiblein F , Heemstra PC . 2011 . Description of a new goatfish species , Upeneus randalli sp. nov. (Mullidae), from the Persian Gulf, with remarks on and identification keys for the genus Upeneus. Scientia Marina. 75(3):585–94 .
- Uiblein , F , Köhler , C and Tian , MC. 1998 . Quantitative examination of morphological variability among goatfishes of the genus Upeneus from the Malayan Province (Pisces: Perciformes: Mullidae) . Senckenbergiana Maritima , 28 : 123 – 32 .
- Uiblein , F and Nielsen , JG. 2005 . Ocellus variation and possible functions in the genus Neobythites (Teleostei, Ophidiidae) . Ichthyological Research , 52 : 364 – 372 .
- Uiblein , F and Winkler , H. 1994 . Morphological variability among Vimba in Austrian waters: Quantitative examination of a taxonomic and a functional hypothesis (Pisces: Cyprinidae) . Senckenbergiana Biologica , 73 : 57 – 65 .